Abstract
Long-standing foot ulcers present a great challenge in diabetes care. Platelet products have been suggested as a possible therapeutic option. However, nor the effect of an injectable form of platelet lysate on the healing of ulcers nor that on primary cells of the epidermis have been studied. In the current study, we present two cases of an ongoing clinical trial showing the positive effect of autologous platelet lysate injected perilesional. Both clinical cases treated with injections of hPL showed complete healing of previously un-healed within 8 weeks of treatment. Further, we describe the in vitro effect of human platelet lysate (hPL) on primary human epidermal keratinocytes (HEK) in terms of chemotaxis, migration and proliferation. In vitro, HEK showed enhanced chemotaxis towards the hPL compared to keratinocyte-defined media (p < 0.0001). Their migration was also stimulated especially at hPL concentration of 10%V/V (p < 0.0001). In contrast, hPL significantly inhibited HEK proliferation measured through MTT assay (p < 0.0001). In conclusion, the findings presented here provide preliminary evidence of an explanatory mechanism for the effect of hPL on primary keratinocytes and therefore of their potential use in a clinical setting. hPL promotes keratinocyte migration and therefore closure of foot ulcers.
Keywords: Cell biology, Proteins, Biomedical engineering, Diabetes, Regenerative medicine, Platelet lysate, Primary keratinocytes, Migration, Wound healing
Cell biology; Proteins; Biomedical engineering; Diabetes; Regenerative medicine; Platelet lysate; Primary keratinocytes; Migration; Wound healing
1. Introduction
Diabetic foot ulcers (DFUs) are a chronic problem for 15% of diabetes mellitus (DM) patients. DM-associated wounds are slow or non-healing, frequently getting infected and leading to amputation. Defects in re-epithelialization, angiogenesis, as well as cytokine and growth factor deficits are the main impediments to proper healing [1, 2, 3, 4].
Wound healing is a multiplex and dynamic process requiring a series of fibroblast and keratinocyte functions to renovate the integrity of the skin barrier [5, 6, 7]. In response to chronic cutaneous wound injury, keratinocytes at the wound edges retreat from terminal differentiation and enter a status called “keratinocyte activation” [8]. This is distinguished by cell hypertrophy and changes in gene expression as keratinocytes initiate migration to close the wound between 6 and 24 h after injury [5, 9]. Cell migration and proliferation are initiated and regulated by growth factors and cytokines that are released from the wounded epithelium [10].
Numerous recombinant growth factors have been introduced into clinical practice for wound healing for more than two decades, yet few of them are reliable for clinical use [11]. Furthermore, the limited shelf-life of recombinant growth factors and their excessive cost are practical constraints for their routine clinical use [7].
Human platelet lysate (hPL) is a hemo-derivative that is rich in bio-substances and growth factors such as: platelet derived growth factor (PDGF), transforming growth factor-beta (TGFβ) and vascular endothelial growth factor (VEGF) [12, 13]. These growth factors are particularly involved in wound healing.
Prior studies have supported the use of platelet rich plasma in healing chronic lower extremity wounds and the treatment of diabetic foot ulcers and skin lesions [14, 15, 16, 17, 18]. These studies employed platelet rich plasma in the form of topical gel, and most of these studies excluded ulcers with challenging presentations such as in patients with severe limb ischemia. No previous work has been done with injectable hPL for wound healing in humans.
Currently, we are running two on-going clinical trials on the use of autologous and allogenic platelet lysate in diabetic foot ulcers (NCT02989961 and NCT02972528, respectively). In the current study, we present two cases enrolled in the autologous above-mentioned clinical trial, as we aimed to give an explanatory mechanism of the clinical effects observed. Therefore, we examined the effect of hPL on primary keratinocytes and primary fibroblasts in vitro as a potential mechanistic explanation for its in vivo effect.
2. Materials and methods
This study was approved by the Institutional Review Board (IRB) at the Cell Therapy Center, University of Jordan. A written informed consent was obtained from each participant in accordance with the declaration of Helsinki.
2.1. Patients and injection protocol
The cases described below are of participants enrolled in an on-going clinical trial at the Cell Therapy Center/University of Jordan (NCT02989961). Inclusion and exclusion criteria of the trial are summarized in Table 1.
Table 1.
Inclusion and exclusion criteria for patient selection.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
2.2. Case 1
A 58-year-old male patient with a 20-year history of type 2 diabetes mellitus with a non-healing diabetic foot ulcer of 10 months on the lateral aspect of patient's right foot. The patient's initial management included measures to optimize glycemic control and the use of moisture dressings and offloading pressure from the wound. His HbA1C was 7% with controlled fasting blood sugar levels. Conventional treatment failed to heal the ulcer. Autologous platelet lysate was used as detailed in the injection protocol below, in addition to standard of care.
2.3. Case 2
A 67-year-old female patient with a 25-year history of type 2 diabetes mellitus presented with 2 months non-healing diabetic foot ulcer on the planter aspect of patient's left heel. The patient's initial management included measures to optimize glycemic control and the use of moisture dressings and offloading pressure from the wound. Her HbA1C was 11.6% with controlled fasting blood sugar levels. Conventional treatment failed to heal the ulcer. Autologous platelet lysate was used as detailed in the injection protocol below, in addition to standard of care.
2.4. Platelet lysate
Platelet lysate (PL) was prepared as previously described [19, 20, 21]. Breifly, platelet rich plasma (PRP) was obtained from 20 ml of autologous whole blood collected in citrated tubes and 5ml of platelet lysate was prepared subsequently. The PRP preparation was frozen at -80 Cº, thawed once and refrozen as in first time. After thawing the second time, the sample was centrifuged at 1800g the supernatant was collected and then filtered with 0.22μl filter (BD, USA).
2.5. Injection protocol
Platelet lysate was injected peri-lesional at ulcer margins by multiple punctures per each session of therapy (a total of around 5–7 ml/session). A total of 4 sessions throughout the period of treatment were used (total blood taken from patient throughout treatment is 80 ml). The treatment was given on days 0, 14, 28, 42 and the final image was on day 56 (total duration is about 2 months).
3. In vitro experiments
3.1. Platelet parameters and growth factors
For in vitro experiments, pooled human platelet lysate (hPL) was prepared as described above with the exception of pooling PRP from at least 8 participants for each batch. Platelet count was normalized to 1 × 106 platelet/ml. Platelet count, plateletcrit, mean platelet volume was determined. Following freeze/thaw cycles, pooled platelet lysate was compared against autologous platelet lysate obtained from the two cases reported here using Luminex multiplex assay for the following growth factors: TGF-beta, PDGF-AB, FGF, EGF and VEGF-C.
3.2. Sample collection
Neonatal foreskin samples were acquired following new born males' routine circumcision surgery. Samples were obtained from the day care surgery unit of Jordan university hospital. For the purpose of this work, five different samples were collected. A written informed consent for each sample was acquired from the child's parents. Collected samples were transferred into 30ml of Dulbecco's modified Eagles' medium (DMEM) (Gibco, In vitrogen Life Technologies, USA) supplemented with 10% fetal bovine serum (FBS; Euroclone, Italy) and 100 IU penicillin/100 IU streptomycin (Gibco, In vitrogen Life Technologies, USA) and transported on ice to the processing facility at the cell therapy center within the same day.
3.3. Cell isolation and culture
Primary keratinocytes were isolated and expanded as per the protocol recommended by the media manufacturer (Invitrogen Life Technologies). Briefly, skin biopsies were incubated in dispase overnight to separate the epidermis from the dermis. A single cell suspension was obtained following incubation of the epidermis in 0.25% trypsin for 10–30 min. Keratinocytes were maintained in serum-free media (EpiLife®, Invitrogen Life Technologies, USA) as per manufacturers' recommendations. Only cells of passage three and lower were used in the following experiments.
For experiments involving the addition of hPL, keratinocyte media consisted of basal EpiLife® media supplemented with 0.1% W/V bovine serum albumin (BSA,Sigma-Aldrich,Germany), 1% antibiotic mixture (Gibco, In vitrogen Life Technologies,USA) and different amounts of hPL (5% V/V,10% V/V,15% V/V, and 20% V/V). Starvation media composed of basal EpiLife® medium supplemented with 0.1% W/V bovine serum albumin and 1% antibiotic mixture.
3.4. Chemotaxis assay
Keratinocyte chemotaxis was measured using a 6.5mm Transwell® with 8.0μm pore polycarbonate membrane insert (Corning Costar,USA). After starving cells cultured in a 25cm2 tissue culture flask for 24 h, cells were seeded at a density of 50,000 cells and maintained in 100μl of starvation medium in the upper chamber of each well. The lower chamber was filled with 650μl of medium containing different concentrations of hPL (5% V/V, 10% V/V, 15% V/V, and 20% V/V). For the control wells, lower chambers were filled with the same amount of starvation medium. Cells were allowed to transmigrate for 6 h in a 37 °C humidified CO2 incubator, the inserts were removed and membranes were fixed in 3.7% paraformadehyde (TEDIA) in phosphate buffered saline for 30 min. By using a cotton swab, non invading cells were removed from the upper side of the membrane. Finally, membranes were stained and mounted on microscope slides using ProLong® Gold Antifade Reagent with DAPI (molecular probes, Invitrogen Life Technologies, USA). Four fields per transwell membrane were assessed under inverted wide field fluorescent microscope (Carl Zeiss, Germany). Each treatment was performed in duplicate.
3.5. Scratch closure assay
Keratinocytes were seeded into 12 well plates. When cultures reached 80% confluence, cells were starved for 24 h in 37 °C humidified CO2 incubator. In confluent cell monolayers, scratch wounds were developed by using a 0.1–10 μL pipette tip. Following PBS washing of suspended cells, cultures were re-fed by media containing different concentrations of hPL (5% V/V, 10% V/V, 15% V/V, and 20% V/V). Control wells were re-fed by the starvation medium. Cell migration into the denuded area was evaluated at 0, 6 and 24 h, using an inverted microscope (Nikon ECLIPSE TS100, Japan), and measured (Nikon Digital Sight DS-L3). Time zero values were subtracted from measurements at 6 and 24 h, in order to achieve a net measurement of cell migration into the wound.
3.6. MTT assay
Keratinocytes were seeded on 96 well plates for 24 h before performing the experiment. Cells were seeded at a density of 5,000 cells and maintained in 100μl of complete EpiLife® medium, on the next day media on cultures were removed, and cells then were washed by PBS and media containing different concentrations of hPL (5% V/V, 10% V/V, 15% V/V, and 20% V/V) were then added, and basal EpiLife® medium was added to control wells. MTT assay was performed on three different time points 24, 48 and 72 h. MTT reagent (Sigma-Aldrich,Germany) was prepared from a powder as a (5 mg/ml) concentration in PBS. MTT was added to each 96 well plate at each time point. Briefly, 20μl of MTT reagent was added to cells and left for incubation in the dark in a 37 °C humidified CO2 incubator for 4 h. Media was removed and MTT solvent was added (Sigma-Aldrich,Germany). After 15 min incubation at room temperature in the dark, absorbance readings were obtained at 570nm using a microplate absorbance reader (sunrise basic-TECAN,Switzerland).
3.7. Data analysis
Document clinical cases were photographed and uploaded into Autocad software to measure the surface area of ulcers. Statistical analysis was performed using the Instat and Prism software package (GraphPad Software, Inc.,USA). To determine differences between five means, one-way ANOVA with Turkey's test for multiple comparisons was performed. All statistical analysis was performed at p < 0.05 level of significance.
4. Results
4.1. Case one
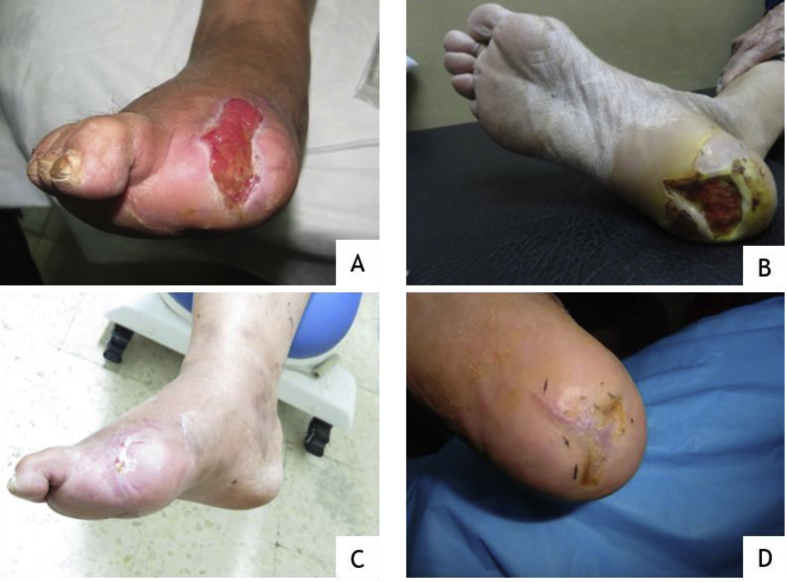
The patient presented to The Diabetic Foot Care Unit with a single, non-infected (4.98 ∗ 2.12cm) elliptical shaped ulcer located on the lateral aspect of patient's right foot, with inflamed margins (Figure 1-A). Two weeks after giving the first peri-lesional autologous hPL injection, the ulcer size and the margins showed early healing, the ulcer area was significantly reduced by 56% (from 33.15 cm2 to 14.46 cm2). Healing continued over the next 6 weeks until the ulcer was completely healed (Figure 1-C). Details of each follow up are summarized in Table 2.
Figure 1.
Representative photographs of case 1 at days 0 (A) and 56 (C), and case 2 at days 0 (B) and 56 (D).
Table 2.
Patients DFU area measurements before and after normalization on each follow-up day.
| Days | DFU area (cm2) |
Area after normalization to baseline |
||
|---|---|---|---|---|
| Case 1 | Case 2 | Case 1 | Case 2 | |
| Day 0 | 33.15 | 47.81 | 1.00 | 1.00 |
| Day 14 | 14.46 | 21.59 | 0.44 | 0.45 |
| Day 28 | 9.47 | 8.79 | 0.29 | 0.18 |
| Day 42 | unmeasurable | 0.79 | unmeasurable | 0.02 |
| Day 56 | 0.00 | 0.00 | 0 | 0.00 |
Two weeks after giving the 2nd injection, there was further decrease in the ulcer's area by 71%. Granulation tissue was noticed to fill the ulcer's floor, the edges became more slopped and the defect became unmeasurable after two weeks of giving the 3rd injection. Two weeks after the 4th injection, the ulcer was completely healed.
4.2. Case two
The patient presented to The Diabetic Foot Care Unit with a single, non-infected (3.5 ∗ 4.35cm) triangular shaped ulcer located on the planter aspect of patient's left heel (Figure 1-B). The DFU showed an ischemic appearance characterized by a dark red base, necrotic tissue and hyperkeratotic rim. Two weeks after giving the 1st peri-lesional Autologous hPL injection, a significant decrease in the ulcer size by 55% was observed with a healthy granulation tissue covered the base of the ulcer. The margins of the ulcer looked healthier. Two weeks after the second injection, the ulcer's surface area decreased by 82% (from 47.81 to 8.79 cm2). On the third follow up visit (two weeks after the 3rd injection), the ulcer's edges became more sloped and the ulcer's granulation tissue became more mature and the ulcer area was 0.02cm2. On day 56 (2 weeks after the 4th injection), the ulcer completely healed and the new skin became cosmetically more acceptable (Figure 1-D). Details of each follow up are summarized in Table 2.
4.3. Platelet parameters and growth factor content
Table 3 shows the platelet parameters of the two cases reported here, as well as those of the pooled hPL used in the in vitro experiments. No significant difference was observed in any of the parameters.
Table 3.
Platelet parameters for donors (mean), case 1 and case 2.
| Parameter | Donors (mean ± SD) | Case 1 | Case 2 |
|---|---|---|---|
| PLT (103/ul) | 252.9 ± 45.47 | 236 | 225 |
| PCT (%) | 0.27 ± 0.05 | 0.26 | 0.25 |
| MPV(fL) | 10.78 ± 0.79 | 10.7 | 10.8 |
| PDW (fL) | 13.2 ± 1.67 | 14 | 15 |
PLT, Platelet count; PCT, Plateletcrit; MPV, Mean platelet volume; PDW, Platelet distribution width.
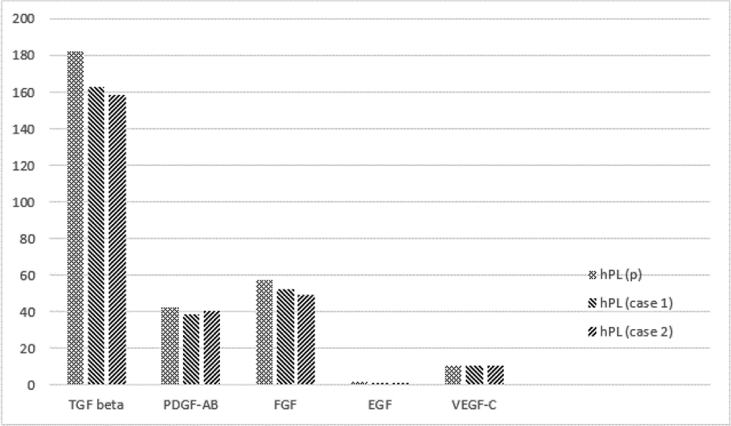
Similarly, the growth factor content did not differ significantly between the two cases and the pooled hPL used in the in vitro experiments, although slightly higher in the pooled hPL (Figure 2).
Figure 2.
Growth factor content of donors' pooled hPL (hPL(p)), case 1 and case 2 measured in ng/ml. TGF, transforming growth factor; PDGF, platelet derived growth factor; FGF, fibroblast growth factor; EGF, epidermal growth factor.
4.4. Chemotaxis assay
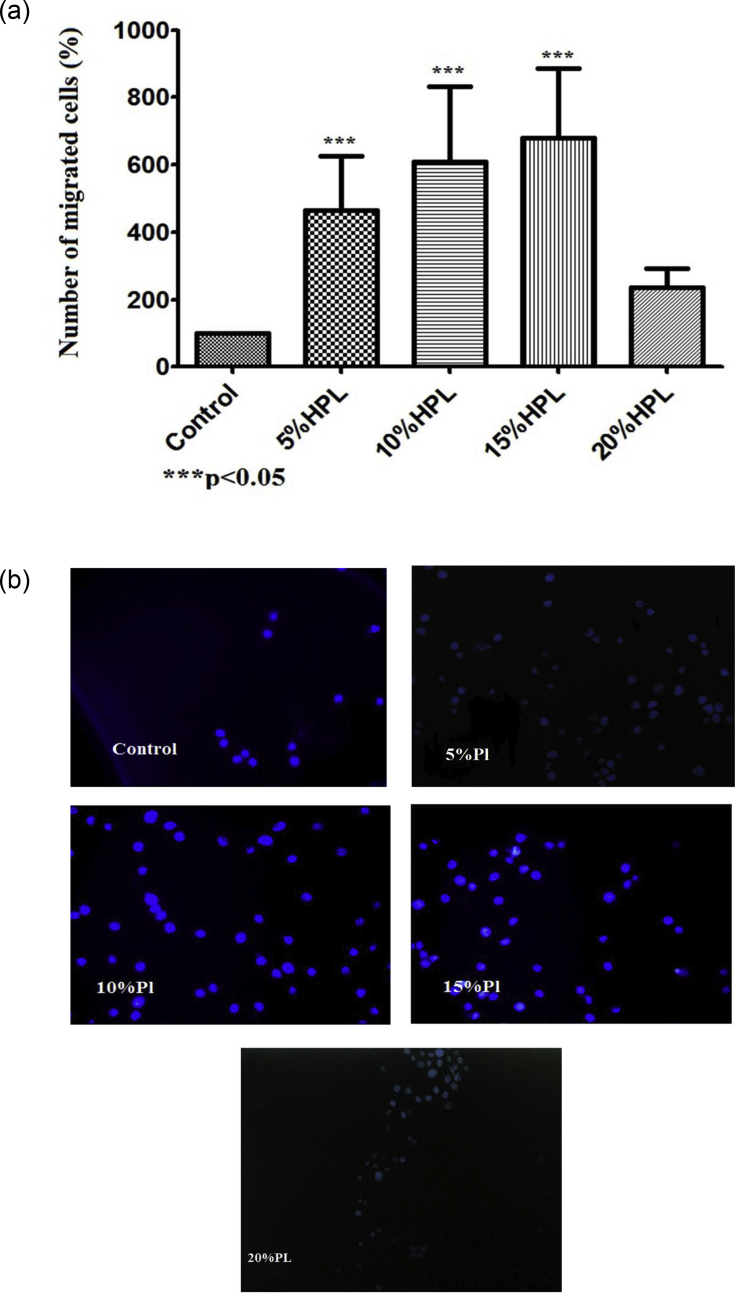
To investigate the chemotactic effect of different concentrations of hPL on primary human keratinocytes (HEK), we performed a transwell assay. It was shown that different concentrations of hPL have substantial chemotactic effects on HEK (p < 0.0001) (Figure 3). Notably, the 5%V/V hPL containing medium has fivefold higher chemotactic effect on HEK. Even higher chemotactic effect was observed reaching sevenfold increase in cell migration for both 10% and 15%V/V, in comparison with hPL-free medium. However, the 20%V/V hPL containing medium had no significant chemotactic effect on HEK compared to other hPL concentrations.
Figure 3.
Effect of platelet lysate on human primary keratinocyte chemotaxis. (a) The chemotactic effect of HPL on HEK cells was assessed following 6 h of cell migration towards different HPL concentrations (5, 10, 15 and 20%). Data (n = 5) are means ± SD of duplicates. (b) Representative photo micrographs of cells that crossed the transwell membrane stained with DAPI examined under fluorescence microscope (100x magnification).
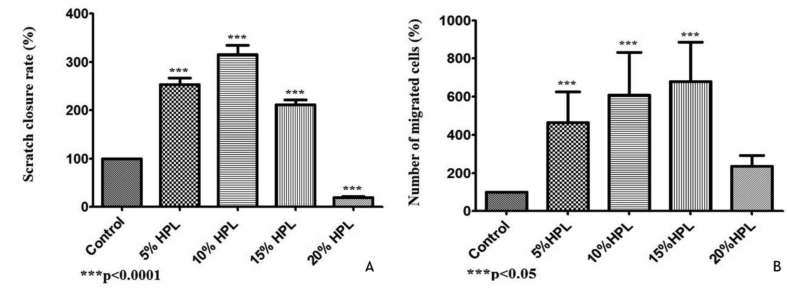
4.5. Scratch closure assay
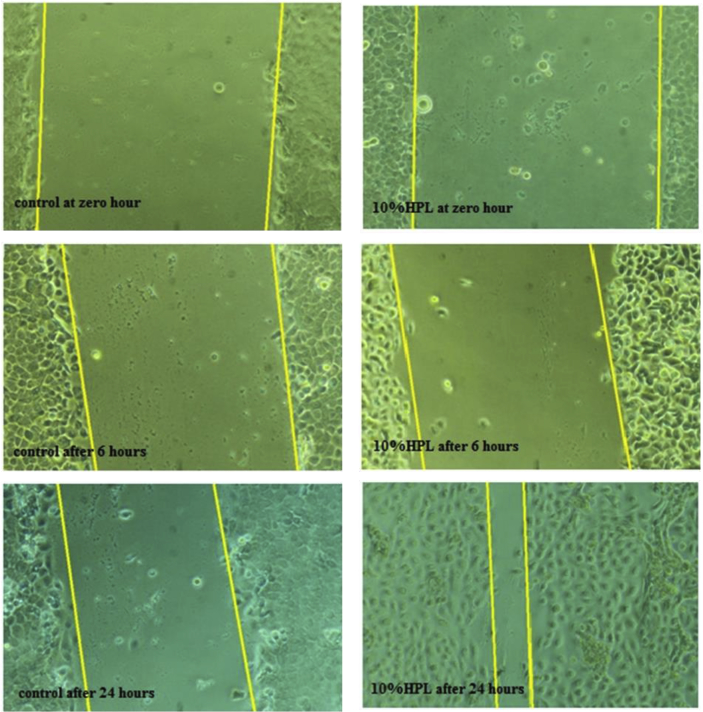
In vitro scratch assay was used to quantify the migration rate of the HEK in a monolayer cell model. As illustrated in Figure 4, a considerable stimulatory effect of the 5%, 10%and 15%V/V hPL containing media on HEK migration after 6 and 24 h of cells monolayer wounding compared to the control was observed; observed both as the percentage of scratch closure (Figure 4-A) and the percentage of cells migrated (Figure 4-B) (p < 0.0001). The 10%V/V of hPL concentration showed the best effect on wound closure after 24 h (Figure 5); the wound was fully closed in many regions. The 5%V/V hPL containing medium had a good stimulatory effect, but fully closed regions of the wound were much less in number compared to the 10%V/V concentration. While wounds that were exposed to 15%V/V hPL concentration showed a statistically significant difference in the appearance of wound closure however fully closed regions of wounds were not observed. As expected from the results of the chemotaxis and migration assays, the 20%V/V hPL containing medium showed an inhibitory effect on wound closure compared to the control.
Figure 4.
Effect of platelet lysate on human primary keratinocyte in wound closure assay. The chemotactic effect of HPL on HEK cells was assessed following 6 h of cell migration towards different HPL concentrations (5, 10, 15 and 20%). Data (n = 5) are means ± SD of duplicates. (∗p < 0.05; ∗∗∗p < 0.0001).
Figure 5.
Representative phase contrast micrograph of 10%V/V hPL treated HEKs at 0,6,24 h (100X magnification).
4.6. MTT assay
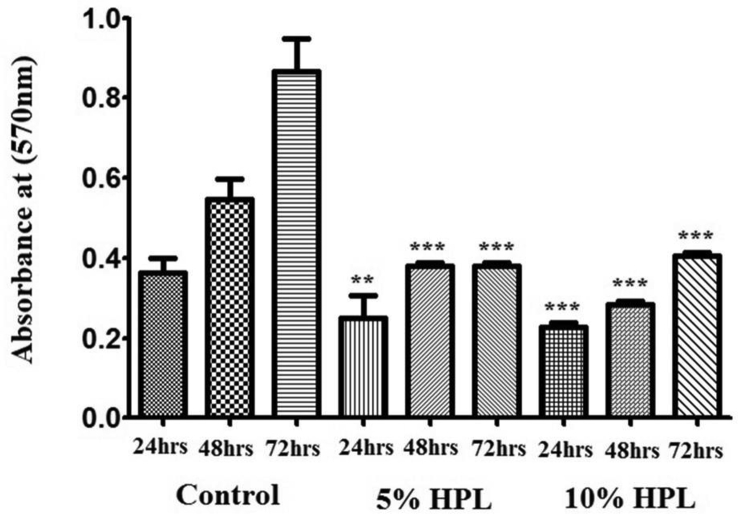
Cell viability and the proliferation of HEK cells exposed to increased hPL dilutions at different time points 24, 48 and 72 h were assessed by MTT assay. As illustrated in Figure 6, 5% and 10%V/V hPL containing cultures demonstrated a significant reduction in cell proliferation compared to control cultures on all time points (p < 0.0001).
Figure 6.
MTT results reflecting HEKs proliferation in the presence of hPL. Following 24, 48, 72 h of incubation with different hPL concentrations (5, 10 %V/V). Data (n = 5) are means ± SD of duplicates. (∗∗∗P < 0.0001).
5. Discussion
Only few reports have been published on the use of autologous platelets derivatives for the treatment of diabetic foot ulcers [8, 9]. Most of these studies excluded ulcers with "challenging presentations" such as severe limb ischemia. On account of paucity of information in the literature about the use of injectable hPL for diabetic foot ulcers, we have initiated a clinical trial to investigate this effect. In the current study, we report 2 of these case and attempt to offer an explanatory mechanism for the positive clinical impact observed so far, Needless to say, our clinical trial is still on-going and therefore, statistical significance of the results is yet to be calculated for the entire cohort.
Photographs of representative diabetic foot ulcer cases are shown in Figure 1. A DFU was observed in a 58-year-old man (case 1): the duration of the ulcer was 10 months, the ulcer measured 33.15cm2. Time to complete healing was 28 days. A 67-year-old woman (case 2) showed a DFU that persisted for 2 months, the ulcer measured 47.81cm2. Time to complete healing was 42 days.
After the administration of first hPL injection, the healing process was quite visible (at day 14) and at day 56 for both cases, the wound was completely healed. The skin surrounding the ulcer area became healthier, cosmetically more acceptable and more youthful in appearance. In addition, patients did not report any side effects during or after treatment.
Previous studies have shown that 10% and 20% (v/v) of pooled allogenic hPL have significant effects on cell migration and proliferation, in vitro [6.7]. However, these studies were conducted on cell lines rather than primary cells. Our in vitro results are in slight contradiction to these reports as our data revealed that lower percentages of autologous hPL (5% and 10%) showed the highest stimulatory effect on HEK migration and lower inhibitory effect on cell proliferation. This may be attributed to the preparation method of hPL we used. Higher concentrations like the 20%V/V may have cytotoxic effects on HEK, altering their pattern of migration.
In contrast to other studies that were performed by adding hPL to FBS-containing media, we investigated the role of hPL on HEK proliferation in the absence of serum and growth factors. MTT assay results denoted that HPL containing media showed significant lower cell proliferation compared to control.
The 5% and 10% V/V concentrations showed the least inhibitory effect on HEK proliferation compared to higher hPL concentrations, despite the fact that cell proliferation increased along the 72 h. This can be explained by the fact that hPL has a major effect on cell migration, so cells were induced more to migrate and less to proliferate. We showed that in vitro, low concentrations of hPL (5%, 10%) have a significant stimulatory effect on the migration of HEK cultures. In that regard, we considered the level of VEGF-C in hPL, as this growth factor was observed to promote migration of certain cancer [22] and mesenchymal stem cells [23]. Further work is needed to support this hypothesis. Overall, our results supported the idea that autologous hPL could be a potent therapeutic agent for the treatment of chronic diabetic foot ulcers. Further evidence to support this preliminary data is needed.
Declarations
Author contribution statement
H. Jafar: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
M. Hasan: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
D. Al-Hattab: Conceived and designed the experiments; Analyzed and interpreted the data.
M. Saleh and L. Ameereh: Performed the experiments; Analyzed and interpreted the data.
S. Khraisha: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
N. Younes: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
A. Awidi: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
A. Awidi was supported by the Scientific Research Support Fund (SRF), Ministry of Higher Education/Jordan (2009/01/1year 2009.h). N. Younes was supported by the Deanship of Scientific Research/The University of Jordan.
Competing interest statement
The authors declare no conflict of interest.
Additional information
The clinical trial described in this paper was registered at ClinicalTrials.gov under the registration number NCT02989961.
Acknowledgements
The authors wish to thank Arch. Rawan Jafar for her technical assistance in the production of figures.
Contributor Information
Hanan Jafar, Email: hanan.jafar@ju.edu.jo, hanan.jafar@gmail.com.
Abdalla Awidi, Email: aabbadi@ju.edu.jo, abdalla.awidi@gmail.com.
References
- 1.Reiber G.E., Boyko E.J., Smith D.G. Diabetes in America. second ed. DIANE Publishing; USA: 1995. Lower extremity foot ulcers and complications in diabetes. [Google Scholar]
- 2.Reiber G.E., Vileikyte L., Boyko E.J., del Aguila M., Smith D.G., Lavery L.A., Boulton A.J. Causal pathways for incident lower- E xtremity ulcers in patients with diabetes from two settings. Diabetes Care. 1999;22(1):157–162. doi: 10.2337/diacare.22.1.157. [DOI] [PubMed] [Google Scholar]
- 3.FalangaV Wound healing and its impairment in the diabetic foot. Lancet. 2005;366(9498):1736–1743. doi: 10.1016/S0140-6736(05)67700-8. [DOI] [PubMed] [Google Scholar]
- 4.Brem H., Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J. Clin. Invest. 2007;117(5):1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Usui M.L., Mansbridge J.N., Carter W.G., Fujita Mayumi, Olerud John E. Keratinocyte migration, proliferation, and differentiation in chronic ulcers from patients with diabetes and normal wounds. J. Histochem. Cytochem. 2008;56(7):687–696. doi: 10.1369/jhc.2008.951194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee S.-H., Zahoor M., Hwang J.-K., Min do S., Choi K.Y. Valproic acid induces cutaneous wound healing in vivo and enhances keratinocyte motility. PloS One. 2012;7(11):1–10. doi: 10.1371/journal.pone.0048791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barsotti M.C., Losi P., Briganti E., Sanguinetti E., Magera A., Al Kayal T., Feriani R., Di Stefano R., Soldani G. Effect of platelet lysate on human cells involved in different phases of wound healing. PloS One. 2013;8(12):1–11. doi: 10.1371/journal.pone.0084753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grinnell F. Wound repair, keratinocyte activation and integrin modulation. J. Cell Sci. 1992;101(pt 1):1–5. doi: 10.1242/jcs.101.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Patel G., Wilson C., Harding K., Harding K.G., Finlay A.Y., Bowden P.E. Numerous keratinocyte subtypes involved in wound re-epithelialization. J. Invest. Dermatol. 2006;126(2):497–502. doi: 10.1038/sj.jid.5700101. [DOI] [PubMed] [Google Scholar]
- 10.Lubin F.D., Segal M., Mcgee D.W. Regulation of epithelial cell cytokine responses by the a3b1 integrin. Immunology. 2003;108(2):204–210. doi: 10.1046/j.1365-2567.2003.01577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fu X., Li X., Cheng B., Chen W., Sheng Z. Engineered growth factors and cutaneous wound healing: success and possible questions in the past 10years. Wound Repair Regen. 2005;13(2):122–130. doi: 10.1111/j.1067-1927.2005.130202.x. [DOI] [PubMed] [Google Scholar]
- 12.Fante C.D., Perotti C., Bonferoni M.C., Rossi S., Sandri G., Ferrari F., Scudeller L., Caramella C.M. Platelet lysate mucohadesive formulation to treat oral mucositis in graft versus host disease patients: a new therapeutic approach. AAPS PharmSciTech. 2011;12(3):893–899. doi: 10.1208/s12249-011-9649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shanskii Y.D., Sergeeva N.S., Sviridova I.K., Kirakozov M.S., Kirsanova V.A., Akhmedova S.A., Antokhin A.I., Chissov V.I. Human platelet lysate as a promising growth-stimulating additive for culturing of stem cells and other cell types. Bull. Exp. Biol. Med. 2013;156(1):146–151. doi: 10.1007/s10517-013-2298-7. [DOI] [PubMed] [Google Scholar]
- 14.Corte P.D., Verween G., Verbeken G., Barone M., Guizzardi M., Campanati B., Moroni M., Carabelli A. Platelet gel for healing cutaneous chronic wounds. Transfus. Apher. Sci. 2004;30(3):145–151. doi: 10.1016/j.transci.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 15.McAleer J.P., Sharma S., Kaplan E.M., Persich G. Use of autologous platelet concentrate in a nonhealing lower extremity wound. Adv. Skin Wound Care. 2006;19(7):354–363. doi: 10.1097/00129334-200609000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Salemi S., Rinaldi C., Manna F., Guarneri G.F., Parodi P.C. Reconstruction of lower leg skin ulcer with autologous adipose tissue and platelet-rich plasma. J. Plast. Reconstr. Aesthetic Surg. 2008;61(12):1565–1567. doi: 10.1016/j.bjps.2008.04.048. [DOI] [PubMed] [Google Scholar]
- 17.Singh N., Armstrong D., Lipsky B. Preventing foot ulcers in patients with diabetes. JAMA. 2005;293(2):217–228. doi: 10.1001/jama.293.2.217. [DOI] [PubMed] [Google Scholar]
- 18.Driver V., Hanft J., Fylling C. A prospective, randomized, controlled trial of autologous platelet-rich plasma gel for the treatment of diabetic foot ulcers. Ostomy Wound Manage. 2006;52(6):68–70. [PubMed] [Google Scholar]
- 19.Al-Ajlouni J., Awidi A., Samara O., Al-Najar M., Tarwanah E., Saleh M.…Dweik M. Safety and efficacy of autologous intra-articular platelet lysates in early and intermediate knee osteoarthrosis in humans: a prospective open-label study. Clin. J. Sport Med. 2015;25(6):524–528. doi: 10.1097/JSM.0000000000000166. [DOI] [PubMed] [Google Scholar]
- 20.Jafar H., Abuarqoub D., Ababneh N., Hasan M., Al-Sotari S., Aslam N. hPL promotes osteogenic differentiation of stem cells in 3D scaffolds. PloS One. 2019;14(5) doi: 10.1371/journal.pone.0215667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abu-Ameerh M.A., Jafar H.D., Hasan M.H., Al Bdour M.D., Msallam M., Ababneh O.H.…Awidi A.S. Platelet lysate promotes re-epithelialization of persistent epithelial defects: a pilot study. Int. Ophthalmol. 2019;39(7):1483–1490. doi: 10.1007/s10792-018-0968-1. [DOI] [PubMed] [Google Scholar]
- 22.Deng Y., Yang Y., Yao B., Ma L., Wu Q., Yang Z.…Liu B. Paracrine signaling by VEGF-C promotes non-small cell lung cancer cell metastasis via recruitment of tumor-associated macrophages. Exp. Cell Res. 2018;364(2):208–216. doi: 10.1016/j.yexcr.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Ishii M., Takahashi M., Murakami J., Yanagisawa T., Nishimura M. Vascular endothelial growth factor-C promotes human mesenchymal stem cell migration via an ERK-and FAK-dependent mechanism. Mol. Cell. Biochem. 2019;455(1-2):185–193. doi: 10.1007/s11010-018-3481-y. [DOI] [PubMed] [Google Scholar]