Abstract
Aims
High prevalence and lack of pharmacological treatment are making heart failure with preserved ejection fraction (HFpEF) a growing public health problem. No algorithm for the screening of asymptomatic patients with risk for HFpEF exists to date. We assessed whether HFA/ESC 2007 diagnostic criteria for HFpEF are helpful to investigate the cardiovascular outcome in asymptomatic patients.
Methods and results
We performed an analysis of the Diagnostic Trial on Prevalence and Clinical Course of Diastolic Dysfunction and Heart Failure (DIAST-CHF) that recruited patients with cardiovascular risk factors. All patients underwent a comprehensive diagnostic workup at baseline. Asymptomatic patients with preserved LVEF (>50%) were selected and classified according to HFA/ESC surrogate criteria for left ventricular elevated filling pressure (mean E/e’ >15 or E/e’ >8 and presence of either NT-proBNP > 220 ng/l, BNP > 200 ng/l or atrial fibrillation) into elevated filling pressure (FPe) or controls. Cardiovascular hospitalizations and all-cause death were assessed for both groups over a 10-year-follow-up.
851 asymptomatic patients (age 65.5 ± 7.6 years, 44% female) were included in the analysis. FPe-patients were significantly older (p < 0.001), more often female (p = 0.003) and more often had a history of coronary artery disease, atrial fibrillation and renal dysfunction (p < 0.001, respectively) compared to controls. Incidence of death was significantly higher in the FPe group after a 10-year follow-up (p < 0.001), whereas cardiovascular hospitalization did not differ between groups.
Conclusion
Asymptomatic patients that fulfill HFA/ESC diagnostic criteria for HFpEF are at higher risk of symptomatic HFpEF and have a worse 10-year-outcome than those who do not fulfill criteria.
Keywords: Heart failure with preserved ejection fraction, Asymptomatic, Left ventricular diastolic dysfunction, Outcome, Elevated left ventricular filling pressure
1. Introduction
Heart failure (HF) with preserved left ventricular ejection fraction (HFpEF) is a common disease with high morbidity and mortality. Prevalence of HFpEF is high [1], [2] and a specific pharmacological treatment has not been found to date [3], [4], [5], [6]. This makes HFpEF a growing public health problem [1], [2]. Therefore, an intensified search for prevention and treatment strategies in HFpEF is of global interest.
To date, programs on prevention or screening for patients at risk for HFpEF are scarce [7], [8]. First symptoms, like fatigue and reduced exercise capacity, are often unspecific [2] and may lead to delayed consultation of practitioners. In overt HFpEF no pharmacological treatment has been identified to reduce hospitalization and mortality [2].
On echocardiography, many patients present with preserved left ventricular (LV) systolic function and LV diastolic dysfunction, but report no signs or symptoms of HF [9]. This asymptomatic LV diastolic dysfunction is associated with development of HF in the future [10], [11].
Investigation of prognosis in patients at risk of HFpEF has previously been a challenge [9]. Data from the Framingham Heart Study showed that diastolic dysfunction is associated with increased risk of HF [12]. Left atrial diameter has shown prognostic value in a small cohort with preserved LV ejection fraction (LVEF) [13]. However, since it has been debated for years how to properly diagnose HFpEF there is also no consensus on how to screen for patients with high risk for developing HFpEF in the future. These patients might benefit from an early and more aggressive therapy of comorbidities or from more frequent follow-ups to prevent or delay the development of HFpEF.
A number of risk factors and comorbidities contribute to development of HFpEF: obesity [14], advancing age, diabetes [15], hypertension, coronary artery disease and obstructive sleep apnea [16], [17], [18]. Therefore, several screening approaches have been suggested, including these risk factors and natriuretic peptides (BNP, NT-proBNP) [15], [19], [20], [21]. Also, several new biomarkers may indicate an elevated risk for HFpEF (e.g. markers of myocardial fibrosis or mitochondrial dysfunction) [17], [18], [19].
In 2007 a working group of the HFA/ESC presented a consensus algorithm on how to diagnose HFpEF [22]. The following conditions have to be fulfilled for diagnosis of HFpEF: signs and symptoms of HF, LVEF > 50% and normal or only mildly abnormal LV dimension, and evidence of diastolic dysfunction (e.g. obtained by tissue Doppler measurement of E/e’ ratio) and/or elevated natriuretic peptides (NT-proBNP or BNP) and/or atrial fibrillation. Although these criteria aim for diagnosing symptomatic HFpEF, they may also serve to identify asymptomatic patients at risk for progression to overt HFpEF and adverse cardiovascular events.
In this work, we investigate whether HFA/ESC criteria for HFpEF are suitable for screening patients at risk of developing overt HFpEF within a cohort with asymptomatic LV diastolic dysfunction. Also, we assessed whether these criteria may be of prognostic value in asymptomatic patients with risk for HFpEF.
2. Methods
2.1. DIAST-CHF study
The Diagnostic Trial on Prevalence and Clinical Course of Diastolic Dysfunction and Heart Failure (DIAST-CHF) was a multicenter, prospective cohort study initiated in 2004 as part of the nationwide German Competence Network Heart Failure [23]. Patients were eligible to participate in the DIAST-CHF study if they fulfilled all following inclusion criteria: age 50–85 years, presence of at least one cardiovascular risk factor (history of hypertension, diabetes mellitus, sleep apnea syndrome or atherosclerotic disease) or had a previous diagnosis of HF. Patients underwent a comprehensive non-invasive clinical assessment, including ECG, blood pressure measurement, detailed echocardiography, 6-minute walk test (6MWT) and blood analysis at baseline and were frequently followed up in person in a clinical trial center and via telephone for 10 years of follow-up. Hospitalizations and death were assessed by acquiring information from treating physicians. The study complies with the Declaration of Helsinki, and all patients gave written informed consent before being included in the study.
2.2. Identification of asymptomatic patients for analysis
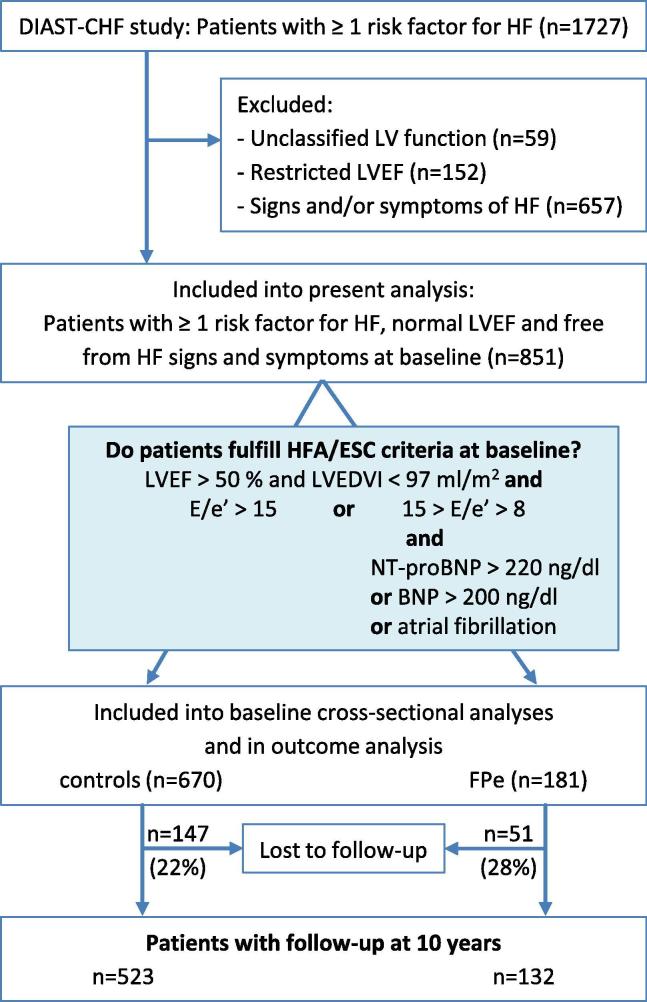
A total of 1727 patients with at least one risk factor for HF were selected from all patients included in the DIAST-CHF study. A flowchart of the selection process is shown in Fig. 1. After excluding all patients with unclassified or restricted LVEF or signs/symptoms of HF, 851 asymptomatic patients with LVEF > 50% (calculated by Simpson) were included into the baseline analyses. Patients were further assessed whether they fulfilled criteria of HFA/ESC recommendations on diagnosis of HFpEF [22]. Patients were categorized as FPe (elevated LV filling pressure) if tissue doppler derived mean E/e’ ratio (mean of septal and lateral) was > 15 or if E/e’ ratio was > 8 but < 15 and either NT-proBNP levels > 220 ng/l or BNP levels > 200 ng/l or atrial fibrillation were present. All patients that did not fulfill these criteria were categorized as controls. For outcome analysis, all patients with a known follow-up status after 10 years were included.
Fig. 1.
Flowchart for selection of patients for analysis: At baseline asymptomatic patients with preserved left ventricular ejection fraction (LVEF) were classified according to HFA/ESC criteria for HFpEF. Outcome was assessed over ten years after baseline. FPe = asymptomatic patients that fulfill HFA/ESC criteria (elevated LV filing pressure), E/e’ >15 or 15 > E/e’ >8 and either atrial fibrillation or NT-proBNP > 220 ng/l or BNP > 200 ng/l. From 181 patients who fulfilled the HFA/ESC criteria 82 were hat E/e’ > 15 (45.3%). 75 patients had E/e’ > 8 and NT-proBNP > 220 (41.4%), 16 patients had E/e’ > 8 and atrial fibrillation (8.8%) and 8 patients had E/e’ > 8 and both NT-proBNP > 220 and atrial fibrillation (4.4%). controls = all asymptomatic patients that did not fulfill criteria.
2.3. Statistics
Baseline characteristics were analyzed using mean and standard deviation with t test (metric variables) and cross tabulation with chi-square-test (categorical variables). For analysis of NT-proBNP and BNP, median with quartiles and Wilcoxon-Mann-Whitney U test were used. P values were adjusted for multiple testing by the method of Bonferroni and Holm. For baseline characteristics, raw p values are presented but those that remained significant are marked bold.
To find baseline characteristics multiply associated with FPe classification, we performed a backward variable selection based on Akaikes information criterion using the R function step. The logistic regression procedure started with the variables age, sex, pulse pressure, coronary heart disease, atrial fibrillation, renal dysfunction, beta-blocker use and anticoagulation. The remaining variables built a final model for estimating the effects with 95% confidence interval (CI). We depicted the results by means of a forest plot.
Similar, the dependency of all-cause mortality, death or cardiovascular hospitalization, the occurrence of HF signs and symptoms and the combined endpoint of all three events from FPe classification was investigated. For mortality and hospitalization, we used Cox regression. Including the patients with status at their study end enabled us to use all patients in time-to-event analysis. Logistic regression was performed for the first occurrence of HF signs and symptoms. We present hazard and odds ratios with 95% CI for FPe, first unadjusted, second adjusted by age and sex and finally adjusted by all variables independently associated with the endpoint.
For the competing events death and cardiovascular hospitalization, we performed the analysis of cumulative incidences following Gray et al. 1988[24]. Cumulative incidence curves were generated, group comparisons were performed for both events.
A p-value < 0.05 (two tailed) was considered statistically significant. SPSS Statistics Version 24.0 (IBM, Chicago, IL, USA) and R inclusive the packages survival, glm2, and Hmisc was applied for the analyses.
3. Results
Baseline characteristics of all asymptomatic patients are shown in Table 1. Age, sex, heart rate, as well as systolic, diastolic and pulse pressure showed significant differences between FPe and controls. FPe were older (69.7 years vs. 64.4 years, p < 0.001), more frequently female, had a lower heart rate, higher systolic and lower diastolic blood pressure and thus higher pulse pressure (p < 0.001). The cohort is obese with mean BMI 28.1 kg/m2. Coronary artery disease and atrial fibrillation were significantly more often reported in FPe than in controls (29.8% vs. 11.9% and 16% vs. 1.9%, p < 0.001, respectively). Consequently, FPe patients were significantly more often treated with anti-platelet drugs and anti-coagulants (p = 0.001 and p < 0.001), as well as with blood pressure lowering drugs (p = 0.002), especially with beta-blockers (p < 0.001). However, systolic blood pressure was high on baseline examination (153 ± 22 mmHg in FPe, 149 ± 20 mmHg in controls, p = 0.017).
Table 1.
Baseline characteristics of all asymptomatic patients.
| Variable | All subjects | FPe | controls | P-value |
|---|---|---|---|---|
| Number of subjects | 851 | 181 | 670 | – |
| Age [years] | 65.5 (7.6) | 69.7 (7.7) | 64.4 (7.2) | <0.001* |
| Female | 378 (44.4%) | 98 (54.1%) | 280 (41.8%) | 0.003 |
| Physical examination | ||||
| BMI [kg/m2] | 28.1 (4) | 27.7 (3.9) | 28.2 (4) | 0.11 |
| Systolic blood pressure [mmHg] | 150 (21) | 153 (22) | 149 (20) | 0.017 |
| Diastolic blood pressure [mmHg] | 85 (11) | 83 (12) | 86 (11) | 0.010 |
| Pulse pressure [mmHg] | 64 (17) | 70 (18) | 63 (16) | <0.001* |
| Mean arterial pressure [mmHg] | 107 (13) | 106 (14) | 107 (13) | 0.81 |
| Heart rate [1/min] | 66 (11) | 63 (12) | 66 (11) | 0.001 |
| Cardiac diagnoses and risk factors | ||||
| History of diagnosis of heart failure | 28 (3.3%) | 8 (4.4%) | 20 (3%) | 0.34 |
| Coronary heart disease | 134 (15.7%) | 54 (29.8%) | 80 (11.9%) | <0.001* |
| Atrial fibrillation | 42 (4.9%) | 29 (16%) | 13 (1.9%) | <0.001* |
| Hypertension | 751 (88.2%) | 166 (91.7%) | 585 (87.3%) | 0.10 |
| Hyperlipidaemia | 350 (41.1%) | 74 (40.9%) | 276 (41.2%) | 0.94 |
| Obesity | 228 (26.8%) | 40 (22.1%) | 188 (28.1%) | 0.11 |
| Diabetes mellitus | 194 (22.8%) | 41 (22.7%) | 153 (22.8%) | 0.96 |
| Sleep apnoea | 47 (5.5%) | 9 (5%) | 38 (5.7%) | 0.72 |
| Current smoker | 112 (13.2%) | 18 (9.9%) | 94 (14%) | 0.15 |
| Depression | 90 (0.1%) | 22 (12.2%) | 68 (10.1%) | 0.61 |
| Medication | ||||
| Any blood pressure lowering agent | 698 (82%) | 163 (90.1%) | 535 (79.9%) | 0.002 |
| Diuretic agent | 370 (43.5%) | 89 (49.2%) | 281 (41.9%) | 0.08 |
| - Loop diuretic | 34 (4%) | 12 (6.6%) | 22 (3.3%) | 0.041 |
| - Thiazide | 344 (40.4%) | 81 (44.8%) | 263 (39.3%) | 0.18 |
| - Potassium sparing diuretic | 44 (5.2%) | 11 (6.1%) | 33 (4.9%) | 0.54 |
| - Aldosterone receptor antagonist | 5 (0.6%) | 2 (1.1%) | 3 (0.4%) | 0.31 |
| Other blood pressure lowering agent | 678 (79.7%) | 158 (87.3%) | 520 (77.6%) | 0.004 |
| - ACE inhibitor | 362 (42.5%) | 90 (49.7%) | 272 (40.6%) | 0.028 |
| - Angiotensin receptor antagonist | 129 (15.2%) | 31 (17.1%) | 98 (14.6%) | 0.41 |
| - Beta-blocker | 400 (47%) | 112 (61.9%) | 288 (43%) | <0.001* |
| - Calcium channel blocker | 157 (18.4%) | 42 (23.2%) | 115 (17.2%) | 0.063 |
| Insulin | 62 (7.3%) | 18 (9.9%) | 44 (6.6%) | 0.12 |
| Oral antidiabetic | 111 (13%) | 27 (14.9%) | 84 (12.5%) | 0.40 |
| Anti-platelet therapy | 274 (32.2%) | 76 (42%) | 198 (29.6%) | 0.001 |
| Anti-coagulant | 31 (3.6%) | 19 (10.5%) | 12 (1.8%) | <0.001* |
| Statin | 215 (25.3%) | 55 (30.4%) | 160 (23.9%) | 0.074 |
| Laboratory measurements | ||||
| NTpro-BNP [ng/L] | 85 (47; 169) | 262 (165; 434) | 70 (42; 122) | <0.001* |
| BNP [ng/L] | 49 (25; 95) | 116 (65; 181) | 40 (22; 72) | <0.001* |
| Hemoglobin [g/dL] | 14.7 (9.2) | 15.1 (17) | 14.6 (5.5) | 0.67 |
| Anaemia1 | 46 (5.4) | 18 (9.9) | 28 (4.2) | 0.002 |
| eGFR [mL/min/1.73 m2 BSA] | 73.5 (18.2) | 67.6 (19.4) | 75 (17.5) | <0.001* |
| Renal dysfunction2 | 132 (15.5) | 45 (24.9) | 87 (13.0) | <0.001* |
| Echocardiography | ||||
| LVEF [%] | 61.5 (6.3) | 61.1 (7) | 61.6 (6.1) | 0.39 |
| LVEDD [mm] | 49.1 (5.6) | 48.7 (5.8) | 49.2 (5.5) | 0.33 |
| LVEDVI [mL/m2 BSA] | 49 (12.5) | 48.8 (13.8) | 49 (12.1) | 0.85 |
| IVST [mm] | 12.2 (1.9) | 12.6 (1.9) | 12.1 (1.8) | 0.002 |
| LVPWT [mm] | 11.3 (1.7) | 11.5 (1.5) | 11.2 (1.7) | 0.063 |
| LVMI [g/m2 BSA] | 84.4 (47.5) | 88.1 (40.8) | 83.5 (49.2) | 0.20 |
| LAVI [mL/m2 BSA] | 47.2 (16.6) | 55.9 (20) | 45.2 (15.1) | <0.001* |
| Ventricular filling | ||||
| - Mitral E wave peak velocity [cm/s] | 71.8 (18.1) | 84.7 (20.3) | 68.3 (15.7) | <0.001* |
| - Mitral A wave peak velocity [cm/s] | 78.9 (18.3) | 85.9 (21.3) | 77.2 (17) | <0.001* |
| - Tissue Doppler e' [cm/s]3 | 7.2 (1.9) | 6.4 (1.8) | 7.4 (1.8) | <0.001* |
| - Tissue Doppler a' [cm/s]3 | 10.9 (2) | 10.1 (2) | 11.1 (2) | <0.001* |
| - E/e' ratio (mean of septal/lateral) | 10.6 (3.4) | 14.1 (4.2) | 9.6 (2.4) | <0.001* |
| 6-minute walk test (6MWT) | ||||
| Distance [m] | 542 (91) | 521 (87) | 547 (92) | 0.001 |
* p-values, significant after correction for multiple testing.
FPe = asymptomatic patients that fulfil HFA/ESC criteria for HFpEF and have elevated left ventricular filling pressure; controls = asymptomatic patients that do not fulfil HFA/ESC criteria.
Pulse pressure is defined as the difference between systolic and diastolic pressure. IQR = interquartile range; LVEF = left ventricular ejection fraction; LVEDD = left ventricular end-diastolic diameter; LVEDVI = left ventricular end-diastolic volume index; IVST = inter-ventricular septum thickness; LVPWT = left ventricular posterior wall thickness; LAVI = left atrial volume index; LVMI = left ventricular mass index; Mean (standard deviation) is presented for continuous variables, count (%) for categorical and median [interquartile range] for the markers of neurohumoral activity.
Anaemia, WHO classification: Hb < 13 g/dL (m), <12 g/dL (f).
Renal dysfunction: eGFR < 60 mL/min/1.73 m2 BSA.
Mean of lateral and medial measurement.
Laboratory measurements showed significantly higher levels of NT-proBNP and BNP (p < 0.001, respectively), more cases of manifest anemia (p = 0.002), as well as lower eGFR and more cases of diagnosed renal dysfunction (p < 0.001, respectively) among FPe.
On echocardiography, FPe presented with a significantly thicker interventricular septum (p = 0.002) but no significant difference in posterior wall thickness or left ventricular mass index. Left atrial volume index (LAVI), and mitral E and A wave peak velocity were higher and tissue Doppler derived mean e’ and a’ were lower in FPe, resulting in a higher mean E/e’ ratio.
Although all patients were asymptomatic, FPe patients had a significantly lower walking distance than controls (521 m vs. 547 m, p = 0.001).
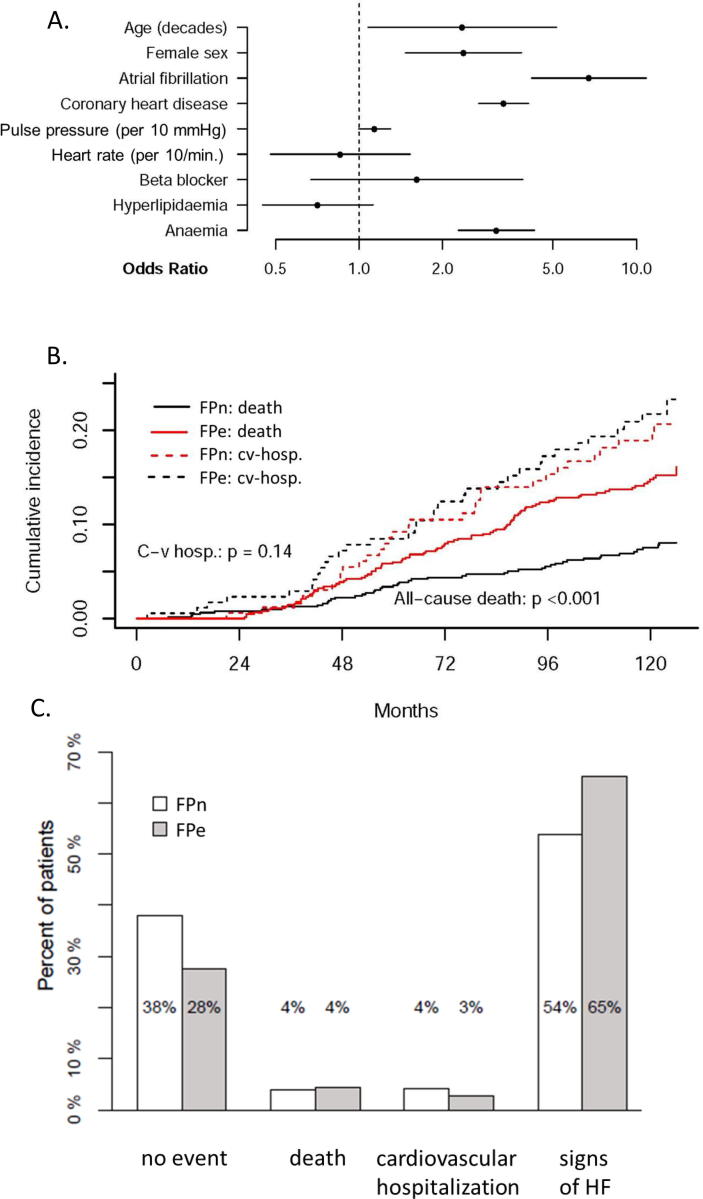
After correction for multiple testing several baseline characteristics were shown to be associated with FPe classification (see Fig. 2A and table 4 supp): age, female sex, atrial fibrillation, history of coronary artery disease and anemia. Atrial fibrillation (OR 9.07) and coronary artery disease (OR 3.24) showed the strongest association to FPe.
Fig. 2.
(A) Characteristics associated with FPe classification: Multiple logistic model for elevated left ventricular filling pressures according to HFA/ESC criteria (FPe) in asymptomatic patients at baseline. Odd’s ratio and 95% CI. Anaemia is defined according to WHO: Hb < 13 g/dL (m), <12 g/dL (f). (B) Cumulative incidences for death and cardiovascular hospitalization within a 10-year follow-up: Asymptomatic patients who fulfill HFA/ESC criteria for HFpEF (FPe) significantly more often showed events of death than controls (p < 0.001) but did not differ in the amount of cardiovascular hospitalization within a 10-year follow-up. (C) Outcome status after 10 years (first event): Within a 10-year follow-up, patients with elevated left ventricular filling pressures according to HFA/ESC criteria (FPe, n = 181, grey) showed a significantly higher number of total events (p = 0.005) than controls (n = 670, white). However, this difference is mainly a consequence of 11.3% more patients with signs and symptoms of heart failure (HF) in FPe. Only in 4% death was the first occurring event (e.g prior to occurrence of HF signs/symptoms). In controls 38% of all patients did not show any event within a 10-year follow-up. Event = new occurence of HF signs/symptoms, cardiovascular hospitalization, death.
Outcome analysis was performed for 851 patients and showed that within ten years follow-up FPe had significantly more cumulative events of all-cause death than controls (p < 0.001, Fig. 2B). Cumulative incidences for first cardiovascular hospitalization did not differ between FPe and controls. Interestingly, only in 4% death was the first occurring event with no difference between FPe and controls (Fig. 2C). However, FPe showed a significantly higher number of total events than controls (p = 0.005), which is accounted for by significantly more new-onset of HF signs and symptoms in FPe, whereas significantly more controls (38%) did not show any event within a 10-year follow-up. No difference was seen for cardiovascular hospitalization as first event between the groups.
FPe classification was associated with increased all-cause mortality even after adjustment for age and sex, heart rate and renal impairment (p = 0.004, Table 2). Also, after adjustment for covariables age, sex, renal impairment and beta blocker therapy FPe was associated with significantly higher risk for death or cardiovascular hospitalization (HR 1.43, 95% CI 1.04–1.97, Table 2). Occurrence of HF signs and symptoms was not shown to be significantly associated with FPe after adjustment for age, coronary artery disease, pulse pressure and heart rate. In a sensitivity analysis of the 646 patients (76%) with a 10-year follow-up showed good accordance to the main cohort with only minimal deviation in values (see table 3 supp).
Table 2.
Logistic and Cox regression to predict outcomes after 10 years for FPe and covariates.
| Endpoint variable and regression model | Risk ratio for FPe (95% CI) | P value |
|---|---|---|
| Combined endpoint of either death, cardiovascular hospitalization or occurrence of HF signs/symptoms | OR | |
| Unadjusted | 1.61 [1.12 – 2.31] | 0.010 |
| Adjusted for age and sex | 1.41 [0.97 – 2.06] | 0.072 |
| Adjusted for age, betablocker and anticoagulant therapy | 1.13 [0.76 – 1.67] | 0.550 |
| All-cause mortality | HR | |
| Unadjusted | 3.13 [2.05 – 4.79] | <0.001 |
| Adjusted for age and sex | 1.90 [1.21 – 2.99] | 0.005 |
| Adjusted for age and sex, heart rate and renal impairment | 1.98 [1.25 – 3.14] | 0.004 |
| Death or cardiovascular hospitalization | HR | |
| Unadjusted | 2.08 [1.55 – 2.79] | <0.001 |
| Adjusted for age and sex | 1.61 [1.18 – 2.21] | 0.003 |
| Adjusted for age, sex, renal impairment and betablocker therapy | 1.43 [1.04 – 1.97] | 0.026 |
| Occurrence of HF signs/symptoms | OR | |
| Unadjusted | 1.60 [1.14 – 2.26] | 0.007 |
| Adjusted for age and sex | 1.47 [1.03 – 2.10] | 0.034 |
| Adjusted for age, sex, coronary artery disease, pulse pressure and heart rate | 1.22 [0.84 – 1.77] | 0.310 |
FPe = asymptomatic patients that fulfill HFA/ESC criteria for HFpEF (elevated left ventricular filling pressure); HF = heart failure.
4. Discussion
The present work demonstrates that asymptomatic patients with preserved LVEF and at least one cardiovascular risk factor have a worse outcome if they meet HFA/ESC criteria for HFpEF [22] as compared with asymptomatic patients who do not meet the criteria. For the first time, this work shows the potential prognostic value of the HFA/ESC criteria and their importance for early identification of asymptomatic patients with risk of HF and cardiovascular death.
4.1. Study population
The DIAST-CHF study included well-characterized patients with risk of HF and a long-term follow-up. Patients are representative according to age and comorbidities. In comparison to other cohorts [11], [17], women are represented almost adequately (44.4% of all patients were female). Although all patients in this analysis were asymptomatic and had current echocardiography revealing preserved LVEF, baseline characteristics were significantly different between FPe and controls. This offers valuable insight into the need of more differentiated characterization of asymptomatic patients with risk factors for HF.
4.2. Arterial hypertension
Among all patients 88.2% have known arterial hypertension, but blood pressure control is insufficient. Additionally, FPe patients show a higher systolic blood pressure at baseline than controls. Since blood pressure management in HFpEF is complicated by comorbidities despite of guidelines [25] further research is needed to investigate whether more consequent treatment of arterial hypertension in the FPe group may decrease hospitalization and mortality.
4.3. Coronary artery disease
Our data show the clear association between coronary artery disease, increasing age and female sex and HFA/ESC criteria. Previous analyses present similar data: In a cohort with coronary artery disease and no history of HF, moderate to severe LV diastolic dysfunction was predictive of incident hospitalization for HF in a 3-year-follow-up [17]. Although the cohort was smaller than DIAST-CHF, the investigators did not apply HFA/ESC criteria for HFpEF and diastolic dysfunction was assessed without using e’-value it still underlines our results and states the importance of coronary artery disease as risk factor for HFpEF. In the DIAST-CHF cohort all patients were clinically stable and adequately treated according to guidelines at baseline. Coronary artery disease was assessed by the investigator and status of revascularization was not evaluated invasively. Therefore, it should be investigated whether coronary artery disease including coronary microvascular disease needs further treatment or more frequent follow-ups the FPe group to delay or even prevent onset of HF.
4.4. Diastolic dysfunction
Asymptomatic diastolic dysfunction and its progression to HF have previously been assessed. In a single-center trial Kane et al. reported an HF incidence of 22.6% in patients with asymptomatic diastolic dysfunction and progression in diastolic dysfunction over a 6-year-follow-up [10]. In our cohort occurrence of HF in 10-years of follow-up was higher (65% in FPe, 54% in controls, n = 851). Since Kane et al. did not apply HFA/ESC criteria for HFpEF, included younger patients (≥45 years) and excluded atrial fibrillation from analysis, their study population may have been healthier with less severe diastolic dysfunction. Also, due to study design Kane et al. may have underestimated worsening of diastolic dysfunction [10]. A meta-analysis recently demonstrated a relative risk of HF of 1.7 in asymptomatic LV diastolic dysfunction in 7.9 years of average follow-up compared to asymptomatic patients without LV diastolic dysfunction [11]. This is comparable with our data (OR 1.60 for occurrence of HF signs or symptoms in FPe), although diastolic dysfunction was not assessed using HFA/ESC criteria in the meta-analysis.
4.5. Non-cardiovascular risk factors
Lund et al. showed that in patients with HFpEF prognosis was determined by non-cardiovascular co-morbidities including anemia, valve disease and non-cardiovascular syncope [26]. Our data underline this finding: Overall 5.4% of our patients reported anemia, 15.5% had history of renal dysfunction, and both co-morbidities were significantly more often present in FPe than in controls (9.9% vs. 4.2%, p = 0.002 and 24.9% vs. 13.0%, p < 0.001, respectively).
Interestingly, prevalence of diabetes mellitus did not show any difference between FPe and controls in our cohort whereas previous data suggest that asymptomatic diabetic patients have a high incidence of diastolic dysfunction (E/e’ > 15) [27]. Also, patients with type 2 diabetes have a higher risk of developing HF [28]. However, in our data presence of diabetes was not associated with FPe classification although 22.8% of our study population presented with diabetes. This suggests that HFA/ESC criteria for HFpEF may be valuable for outcome assessment in asymptomatic patients independent of presence of diabetes.
Depression is a known prognostically relevant comorbidity in HF [29], [30]. In our cohort depression was reported in only 0.1% of all patients whereas other cohorts with asymptomatic patients report higher prevalence of depression [30]. In out cohort, at baseline 7.8% of all patients reported a PHQ-9 score of at least 10 with no significant difference between FPe and controls, and 5.8% of patients reported use of antidepressants (8.9% for FPe vs. 5.0% in controls, p = 0.043). Since in DIAST-CHF depression was primarily assessed by the investigator and only secondarily by validated questionnaires, PHQ-9 data suggest that undetected depression might be higher in our cohort.
Also, in future studies a more detailed assessment of pulmonary and peripheral vascular disease, as well as potential inflammatory abnormalities should be investigated, since these pathomechanisms are known to be involved in HFpEF [31].
4.6. Natriuretic peptides
Many studies have stated the importance of natriuretic peptides as indicator of diastolic dysfunction in patients with preserved LVEF [15], [19], [20], [32], [33]. However, whether natriuretic peptides may be used solely has been discussed since data were conflicting [20], [33]. Natriuretic peptides may be of different significance in males and females: Ahmadi et al. compared patients with regular and with impaired diastolic function and showed a significant difference in NT-proBNP levels in males whereas no difference was observed in females [32]. In our cohort, FPe showed significantly higher NT-proBNP and BNP levels, significantly higher LAVI and E/e’ ratio than controls. Because of this careful phenotypization, we believe that our patients were more characteristic of a cohort at risk of HFpEF than previous data. Natriuretic peptides show poor specificity and wide biological variability and may therefore not be used alone for screening for diastolic dysfunction but may be more meaningful when combined with other clinical parameters.
4.7. Outcome analysis
In patients with normal LVEF and risk of HF outcome has previously been assessed [10], [11], [17]. However, those trials focused on hospitalization and onset of HF and did not report incidence of all-cause death in asymptomatic diastolic dysfunction. In patients with coronary artery disease, LV diastolic dysfunction and no history of HF, death occurred in 7% of cases within a 3-year-follow-up [17]. Our study clearly states the difference in outcome depending on HFA/ESC criteria: within a 10-year follow-up 14.8% of FPe and 7.5% of controls died (p < 0.001, see Fig. 2B). These unique data demonstrate for the first time that HFA/ESC criteria on HFpEF are valuable for risk assessment in asymptomatic patients with diastolic dysfunction. Further data are necessary to explore whether asymptomatic patient that fulfill these criteria benefit from a more intensive treatment of their risk factors and comorbidities.
4.8. Limitations
Some important limitations should be mentioned. Since this work constitutes a retrospective analysis it is to be viewed as exploratory. Statistically significant associations should be assessed on clinical importance in the future. Nevertheless, this analysis illuminates the important value of HFA/ESC criteria on HFpEF which should be applied in future cohorts.
The DIAST-CHF study was powered for a cumulative endpoint of manifestation or worsening of heart failure, occurrence of cardiovascular events or cardiovascular death in patients with reduced and preserved LVEF. Our subgroup analysis on asymptomatic patients with preserved LVEF and LV diastolic dysfunction may offer valuable insights but in the future results should be reevaluated in an adequately powered cohort. Also, not all variables of the HFA/ESC criteria were assessed within the DIST-CHF trial since the trial started 2004 and the HFA/ESC criteria were published in 2007: No invasive hemodynamic measurements were available in our cohort.
Patients were classified as asymptomatic by absence of signs and symptoms of HF at baseline. However, patients with early stages of HFpEF often report unspecific symptoms like fatigue and impaired exercise capacity [2]. Due to feasibility, unspecific HF symptoms were not investigated in the DIAST-CHF study. Also, only submaximal exercise capacity was assessed at baseline by using the 6-minute walk test (6MWT). More detailed assessment of exercise capacity (including maximal exercise capacity assessed by cardiopulmonary exercise testing) could have demasked early symptoms of HF. In addition, although significant differences in 6MWT-distance were observed between FPe and controls at baseline, long-term assessment of exercise capacity is missing, since 6MWT was performed by only very few patients at 10-year follow-up. Previous data show that LV diastolic dysfunction correlated with 6MWT results in hypertensive patients [34].
An important limitation is that hospitalization, cardiovascular cause of hospitalization and death were assessed by the investigator. Patients may have not reported hospitalizations properly and the study investigator may have categorized them inadequately. For further studies, data collection should be performed in collaboration with health insurances to be more certain of data completeness.
Several newer scores were developed to enable a more precise diagnosis of HFpEF, including the H2FPEF score by Reddy et al.[7] and the HFA-HEFF diagnostic algorithm by Pieske et al.[35]. We believe that these algorithms may be valuable to assess outcome in asymptomatic patients, but both scores reflect different aspects of the disease. We exemplary analyzed how the H2FPEF score was distributed in our cohort and found a moderate correlation between the FPe group and high H2FPEF score (Kendall's tau_b = 0.21). Furthermore, the AUC (ROC analyses between the scores) was 0.66 suggesting that the interpretation of the scores should also consider the different components of the score itself.
The present work underlines the potential prognostic value of HFA/ESC criteria on HFpEF in asymptomatic patients at risk for developing HFpEF. Asymptomatic patients with preserved LVEF and LV diastolic dysfunction may benefit from a more frequent follow-up or from earlier or even more aggressive therapy of comorbidities to prevent or delay the development of HF. HFA/ESC criteria may be used for a systematic screening of patients at risk of developing HFpEF. Whether additional parameters (e.g. novel biomarkers, non-cardiovascular risk factors) may enrich these criteria for more precise identification of high-risk patients should be investigated in the future.
Asymptomatic patients with preserved LVEF that fulfill HFA/ESC criteria on HFpEF early develop overt HFpEF and have a worse 10-year outcome than those who do not fulfill criteria. Therefore, these criteria should be considered for outcome assessment and prevention of HFpEF in asymptomatic patients with risk factors for heart failure.
5. Author’s contribution
Authorship: AB, MM, RW and FE contributed to the conception of the work. All contributed to the acquisition, analysis, or interpretation of data for the work. AB drafted the manuscript. MM, FE, CHL, BP and RW critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Funding
This work was supported by a grant from the German Federal Ministry of Education and Research (Competence Network of Heart Failure, TP 7, FKZ 01GI0205).
Declaration of Competing Interest
Dr. Bobenko, Dr. Duvinage, Dr. Duengen and Dr. Nolte have no conflict of interest to declare.
Dr. Mende and Dr. Holzendorf report grants from Kompetenznetz Herzinsuffizienz, Berlin, during the conduct of the study.
Dr. Herrmann-Lingen reports grants from German Ministry of Education and Research, during the conduct of the study; personal fees from Hogrefe Huber Publishers, personal fees from Servier, personal fees from Novartis, personal fees from Heel, grants from German Ministry of Education and Research, grants from Grun foundation, outside the submitted work.
Dr. Binder reports that Roche Diagnostics sponsered NT-proBNP reagents.
Dr. Hasenfuss reports personal fees from Corvia, personal fees from Servier, personal fees from Impulse Dynamics, personal fees from Novartis, personal fees from AstraZeneca, personal fees from Vifor Pharma, personal fees from Springer, outside the submitted work.
Dr. Pieske reports speakers fees and advisory/steering committee honoraria from pharmaceutical companies (such as Bayer Healthcare, Novartis, MSD, Servier).
Dr. Wachter reports grants from Boehringer Ingelheim, during the conduct of the study; personal fees and other from Bayer, personal fees and other from Berlin Chemie, personal fees and other from Boehringer Ingelheim, other from Boston Scientific, personal fees from Bristol-Myers-Squibb, personal fees and other from CVRx, other from Gilead, other from Johnson&Johnson, personal fees and other from Medtronic, personal fees and other from Novartis, personal fees from Pfizer, other from Relypsa, personal fees from Sanofi, personal fees and other from Servier, outside the submitted work.
Dr. Edelmann reports grants from DFG (German Research Foundation), grants and non-financial support from BG Medicine, personal fees from Novartis, Berlin Chemie, Boehringer Ingelheim, Servier, AMGEN, Bayer, Merck, MSD Sharpe & Dohme, Biotronik, Medtronic, grants from Servier, outside the submitted work.
Acknowledgements
This work was supported by a grant from the German Federal Ministry of Education and Research (Competence Network of Heart Failure, TP 7, FKZ 01GI0205).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2020.100525.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Owan T.E., Hodge D.O., Herges R.M., Jacobsen S.J., Roger V.L., Redfield M.M. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N. Engl. J. Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P., Voors A.A., Anker S.D., Bueno H., Cleland J.G.F., Coats A.J.S., Falk V., González-Juanatey J.R., Harjola V.P., Jankowska E.A., Jessup M., Linde C., Nihoyannopoulos P., Parissis J.T., Pieske B., Riley J.P., Rosano G.M.C., Ruilope L.M., Ruschitzka F., Rutten F.H., Van Der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016;37:2129–2200m. doi: 10.1016/j.rec.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S., Pfeffer M.A., Swedberg K., Granger C.B., Held P., McMurray J.J.V., Michelson E.L., Olofsson B., Ostergren J. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–781. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A., Rich M.W., Fleg J.L., Zile M.R., Young J.B., Kitzman D.W., Love T.E., Aronow W.S., Adams K.F., Gheorghiade M. Effects of digoxin on morbidity and mortality in diastolic heart failure. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cleland J.G., Tendera M., Adamus J., Freemantle N., Polonski L., Taylor J. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur. Heart J. 2006;27:2338–2345. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 6.Massie B.M., Carson P.E., McMurray J.J.V., Komajda M., McKelvie R., Zile M.R., Anderson S., Donovan M., Iverson E., Staiger C., Ptaszynska A. Irbesartan in patients with heart failure and preserved ejection fraction. N. Engl. J. Med. 2008;359:2456–2467. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 7.Reddy Y.N.V., Carter R.E., Obokata M., Redfield M.M., Borlaug B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;138:861–870. doi: 10.1161/CIRCULATIONAHA.118.034646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah S.J. Precision medicine for heart failure with preserved ejection fraction: an overview. J. Cardiovasc. Transl. Res. 2017;10:233–244. doi: 10.1007/s12265-017-9756-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redfield M.M., Jacobsen S.J., Burnett J.C., Jr, Mahoney D.W., Bailey K.R., Rodeheffer R.J. Burden of systolic and diastolic ventricular dysfunction in the community. JAMA Am. Med. Assoc. 2003;289:194. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 10.Kane G.C., Karon B.L., Mahoney D.W., Redfield M.M., Roger V.L., Burnett J.C., Jacobsen S.J., Rodeheffer R.J. Progression of left ventricular diastolic dysfunction and risk of heart failure. JAMA Am. Med. Assoc. 2011;306:191–198. doi: 10.1001/jama.2011.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Echouffo-Tcheugui J.B., Erqou S., Butler J., Yancy C.W., Fonarow G.C. Assessing the risk of progression from asymptomatic left ventricular dysfunction to overt heart failure. JACC Hear Fail. 2016;4:237–248. doi: 10.1016/j.jchf.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Lam C.S.P., Lyass A., Kraigher-Krainer E., Massaro J.M., Lee D.S., Ho J.E., Levy D., Redfield M.M., Pieske B.M., Benjamin E.J., Vasan R.S. Cardiac dysfunction and noncardiac dysfunction as precursors of heart failure with reduced and preserved ejection fraction in the communityclinical perspective. Circulation. 2011;124:24–30. doi: 10.1161/CIRCULATIONAHA.110.979203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi A., Cicoira M., Florea V.G., Golia G., Florea N.D., Khan A.A., Murray S.T.M., Nguyen J.T., O’Callaghan P., Anand I.S., Coats A., Zardini P., Vassanelli C., Henein M. Chronic heart failure with preserved left ventricular ejection fraction: Diagnostic and prognostic value of left atrial size. Int. J. Cardiol. 2006;110:386–392. doi: 10.1016/j.ijcard.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Paulus W.J., Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction. J. Am. Coll. Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 15.Görmüş U., Özmen D., Özmen B., Parıldar Z., Özdoğan Ö., Mutaf I., Bayındır O. Serum N-terminal-pro-brain natriuretic peptide (NT-pro-BNP) and homocysteine levels in type 2 diabetic patients with asymptomatic left ventricular diastolic dysfunction. Diabetes Res. Clin. Pract. 2010;87:51–56. doi: 10.1016/j.diabres.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Lee D.S., Gona P., Vasan R.S., Larson M.G., Benjamin E.J., Wang T.J., Tu J.V., Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the framingham heart study of the national heart, lung, and blood institute’s Framingham Heart Study. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren X., Ristow B., Na B., Ali S., Schiller N.B., Whooley M.A. Prevalence and prognosis of asymptomatic left ventricular diastolic dysfunction in ambulatory patients with coronary heart disease. Am. J. Cardiol. 2007;99:1643–1647. doi: 10.1016/j.amjcard.2007.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abdullah A., Eigbire G., Salama A., Wahab A., Nadkarni N., Alweis R. Relation of obstructive sleep apnea to risk of hospitalization in patients with heart failure and preserved ejection fraction from the national inpatient sample. Am. J. Cardiol. 2018;122:612–615. doi: 10.1016/j.amjcard.2018.04.052. [DOI] [PubMed] [Google Scholar]
- 19.Hung T.-C., Wang K.-T., Yun C.-H., Kuo J.-Y., Hou C.J.-Y., Liu C.-Y., Wu T.-H., Bezerra H.G., Cheng H.-Y., Hung C.-L., Yeh H.-I. Value of serum N-terminal B-type natriuretic peptide in asymptomatic structural heart disease in Taiwanese population: comparisons with current ESC Guidelines. Int. J. Cardiol. 2017;231:195–200. doi: 10.1016/j.ijcard.2016.12.180. [DOI] [PubMed] [Google Scholar]
- 20.Collier P., Watson C.J., Voon V., Phelan D., Jan A., Mak G., Martos R., Baugh J.A., Ledwidge M.T., McDonald K.M. Can emerging biomarkers of myocardial remodelling identify asymptomatic hypertensive patients at risk for diastolic dysfunction and diastolic heart failure? Eur. J. Heart Fail. 2011;13:1087–1095. doi: 10.1093/eurjhf/hfr079. [DOI] [PubMed] [Google Scholar]
- 21.Romano S., Di Mauro M., Fratini S., Guarracini L., Guarracini F., Poccia G., Penco M. Early diagnosis of left ventricular diastolic dysfunction in diabetic patients: a possible role for natriuretic peptides. Cardiovasc. Diabetol. 2010;2010(9):89. doi: 10.1186/1475-2840-9-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulus W.J., Tschöpe C., Sanderson J.E., Rusconi C., Flachskampf F.A., Rademakers F.E., Marino P., Smiseth O.A., De Keulenaer G., Leite-Moreira A.F., Borbély A., Édes I., Handoko M.L., Heymans S., Pezzali N., Pieske B., Dickstein K., Fraser A.G., Brutsaert D.L. How to diagnose diastolic heart failure: A consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur. Heart J. 2007;28:2539–2550. doi: 10.1093/eurheartj/ehm037. [DOI] [PubMed] [Google Scholar]
- 23.Mehrhof F., Löffler M., Gelbrich G., Özcelik C., Posch M., Hense H.-W., Keil U., Scheffold T., Schunkert H., Angermann C., Ertl G., Jahns R., Pieske B., Wachter R., Edelmann F., Wollert K.C., Maisch B., Pankuweit S., Erbel R., Neumann T., Herzog W., Katus H., Müller-Tasch T., Zugck C., Düngen H.-D., Regitz-Zagrosek V., Lehmkuhl E., Störk S., Siebert U., Wasem J. A network against failing hearts—Introducing the German “Competence Network Heart Failure”. Int. J. Cardiol. 2010;145:135–138. doi: 10.1016/j.ijcard.2009.06.061. [DOI] [PubMed] [Google Scholar]
- 24.Gray R.J. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann. Stat. 1988;16:1141–1154. [Google Scholar]
- 25.Pinho-Gomes A.C., Rahimi K. Management of blood pressure in heart failure. Heart. 2019;105:589–595. doi: 10.1136/heartjnl-2018-314438. [DOI] [PubMed] [Google Scholar]
- 26.Lund L.H., Donal E., Oger E., Hage C., Persson H., Haugen-Lofman I., Ennezat P.-V., Sportouch-Dukhan C., Drouet E., Daubert J.-C., Linde C. Association between cardiovascular vs. non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014;16:992–1001. doi: 10.1002/ejhf.137. [DOI] [PubMed] [Google Scholar]
- 27.Patil V.C., Patil H.V., Shah K.B., Vasani J.D., Shetty P. Diastolic dysfunction in asymptomatic type 2 diabetes mellitus with normal systolic function. J. Cardiovasc. Dis. Res. 2011;2:213–222. doi: 10.4103/0975-3583.89805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nichols G.A., Gullion C.M., Koro C.E., Ephross S.A., Brown J.B. The incidence of congestive heart failure in type 2 diabetes. Diabetes Care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 29.Norra C., Skobel E.C., Arndt M., Schauerte P. High impact of depression in heart failure: early diagnosis and treatment options. Int. J. Cardiol. 2008;125:220–231. doi: 10.1016/j.ijcard.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Yang H., Nolan M., Burgess J., Negishi K., Marwick T.H. Association of depression with evolution of heart failure in patients with type 2 diabetes mellitus. Cardiovasc. Diabetol. BioMed. Central. 2018;17:19. doi: 10.1186/s12933-018-0664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Del Buono M.G., Arena R., Borlaug B.A., Carbone S., Canada J.M., Kirkman D.L., Garten R., Rodriguez-Miguelez P., Guazzi M., Lavie C.J., Abbate A. Exercise intolerance in patients with heart failure: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019;73:2209–2225. doi: 10.1016/j.jacc.2019.01.072. [DOI] [PubMed] [Google Scholar]
- 32.Ahmadi N.S., Bennet L., Larsson C.A., Andersson S., Månsson J., Lindblad U. Clinical characteristics of asymptomatic left ventricular diastolic dysfunction and its association with self-rated health and N-terminal B-type natriuretic peptide: a cross-sectional study. ESC Hear Fail. 2016;3:205–211. doi: 10.1002/ehf2.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lubien E., DeMaria A., Krishnaswamy P., Clopton P., Koon J., Kazanegra R., Gardetto N., Wanner E., Maisel A.S. Utility of B-natriuretic peptide in detecting diastolic dysfunction. Circulation. 2002;105:595–601. doi: 10.1161/hc0502.103010. [DOI] [PubMed] [Google Scholar]
- 34.Farag E.S., Dydamony M.A.L., Gad M. What is the association between left ventricular diastolic dysfunction and 6-minute walk test in hypertensive patients? J. Hypertens. 2016;34 doi: 10.1016/j.jash.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 35.Pieske B., Tschöpe C., de Boer R.A., Fraser A.G., Anker S.D., Donal E., Edelmann F., Fu M., Guazzi M., Lam C.S.P., Lancellotti P., Melenovsky V., Morris D.A., Nagel E., Pieske-Kraigher E., Ponikowski P., Solomon S.D., Vasan R.S., Rutten F.H., Voors A.A., Ruschitzka F., Paulus W.J., Seferovic P., Filippatos G. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) Eur. Heart J. 2019;40:3297–3317. doi: 10.1093/eurheartj/ehz641. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.