Abstract
Mononuclear osteoclast precursor cells fuse with each other to become mature multinucleated osteoclasts, which is regulated by dendritic cell-specific transmembrane protein (DC-STAMP). We evaluated the effects of tea extract and catechins on cell-cell fusion and DC-STAMP expression to elucidate their relationship with osteoclast development. When tea extract or epigallocatechin gallate (EGCg) was applied to RAW264.7 cells, multinucleated cells were increased significantly, while tartrate-resistant acid phosphatase (TRAP) activity was hardly upregulated. Flow cytometric analysis revealed that EGCg suppressed DC-STAMP expression on the cell surface, which is similar to osteoclast development. These observations suggest that TRAP activity is not activated even when suppression of both surface DC-STAMP expression and multinucleation occurs, which might be mediated by another pathway.
Keywords: Epigallocatechin gallate, Catechin, DC-STAMP, Tea extract, RAW264.7
Abbreviations: TEx, tea extract; EC, (−)-epicatechin; EGC, (−)-epigallocatechin; ECg, (−)-epicatechin gallate; EGCg, (−)-epigallocatechin gallate; DC-STAMP, dendritic cell-specific transmembrane protein
Graphical abstract
Highlights
-
•
We revealed tea extract (TEx) and catechin effects on cell fusion under non-RANKL conditions.
-
•
TEx or catechin increased cell fusion without upregulation of TRAP enzyme activity.
-
•
Cell fusion occurred via DC-STAMP downregulation on the cell surface.
1. Introduction
Bone metabolism is maintained through regulation of bone resorption by osteoclasts and bone formation by osteoblasts [1,2]. Osteoclast functions are crucial for the maintenance, repair, and remodelling of bone tissue. Osteoclast precursor cells are mononuclear and fuse with each other to form large multinucleated osteoclasts during osteoclast differentiation. Mature multinucleated osteoclasts secrete tartrate-resistant acid phosphatase (TRAP) to digest proteins and minerals for bone resorption. Osteoclasts express receptor activator for nuclear factor (NF)-κB ligand (RANKL) that activates the RANK receptor to regulate the differentiation, activation, and survival of osteoclasts [3]. In addition to the RANK signalling pathway, dendritic cell-specific transmembrane protein (DC-STAMP) is essential for cell fusion to develop mature osteoclasts [4,5]. In addition to osteoclasts, DC-STAMP is expressed in dendritic cells and macrophages, which has different functions in each cell type [6,7]. In osteoclasts, DC-STAMP is localized on the cell membrane as a dimer and has an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic tail [8,9]. When DC-STAMP on the cell surface is blocked by a monoclonal antibody, cell fusion of osteoclasts is inhibited. During RANKL-induced osteoclast differentiation, surface DC-STAMP is downregulated and internalized from the cell surface to the cytoplasm. DC-STAMP downregulation on the cell surface is essential for cell-cell fusion and abundant multinucleation of mature osteoclasts [10]. In DC-STAMP-deficient mice, expression of osteoclast-related markers, TRAP activity, and the proportion of osteoclast precursor cells are the same as those in wild-type mice [4]. However, the bone-absorbing area decreases compared with wild-type mice, suggesting that mononucleated osteoclasts have less bone-absorbing activity than multinucleated osteoclasts. Thus, DC-STAMP is more important for cell fusion of mononucleated osteoclasts than osteoclastogenesis.
To avoid abnormal bone resorption, regulation of osteoclast differentiation is critical for mammals. Decreased bone mass, resulting from an imbalance of osteoclast and osteoblast activities, leads to osteoporosis [11], a severe disease that causes bones to become weak and brittle, which increases the risk of bone fracture [12]. Intake of compounds beneficial for bone by diet management is recommended to improve bone metabolism and prevent osteoporosis. Green tea is a functional food that prevents osteoporosis, and tea catechins (generally EC, EGC, ECg and EGCg) have been reported to protect against bone loss by inhibiting osteoclast differentiation [[13], [14], [15]]. In general, the effects of tea extract have been elucidated using ovariectomized animals or RANKL-induced cultured cells [16]. One of the main active components in tea extract (TEx) is EGCg that has anti-osteoporotic effects on osteoclast development [17,18]. In addition to these catechins, theaflavins in tea extract affect cell growth and development [19]. Previously, we reported that tea extract modulates DC-STAMP mRNA expression in RAW264.7 cells [20]. Although a suppressive effect on osteoclast differentiation by tea extract is well reported, studies on the cellular effects of tea extract and catechins under non-RANKL conditions are lacking. In this study, we investigated the effects of tea extract on the fusion of RAW264.7 cells without RANKL-induced osteoclast development.
2. Materials and methods
2.1. Preparation of green tea extract
Green tea leaves (Taki-gun, Mie, Japan), which were cultivated in Mie prefecture, were used to prepare tea extract [20]. The leaves were finely ground before extraction with hot water at 80 °C for 10 min, and then the solution was centrifuged at 4000×g for 15 min to collect the supernatant. After removing water by evaporation, the remaining dry matter was used as the TEx in this study. A stock solution was prepared by dissolving in ultrapure water at 4 mg/mL.
2.2. Determination of catechin contents in tea extract by HPLC
The amount of tea catechins in the extract was measured by HPLC under the following conditions: column, Develosil ODS-HG-5 (4.6 × 150 mm) (Nomura Chemical Co., Ltd., Aichi, Japan); guard column, Develosil ODS-HG-5 (4 × 10 mm) (Nomura Chemical Co., Ltd); flow rate, 1 mL/min; injection volume, 10 μL; absorbance detection wavelength, 230 nm; column temperature, 40 °C; eluents, (A) 0.1% phosphoric acid in H2O, and (B) 60% eluent A and 40% acetonitrile; gradient conditions, 20% B (0–10 min) and 20%–64% B (10–40 min).
2.3. Cell culture
RAW264.7 cells (American Type Culture Collection, Manassas, VA, USA, passages 6–8) were grown in alpha-modified minimum essential medium (Gibco, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Biowest, Nuaillé, France) at 37 °C with 5% CO2. A cell scraper was used to harvest the RAW264.7 cells. Cells were cultured until 80% confluence before use in each assay. For reagent treatments, cells were seeded at the indicated cell number and cultured for 24 h. After 3 days of incubation, the medium was replaced with fresh medium containing the same reagent for further experiments. TRAP staining and a TRAP activity assay were performed at day 5, and immunofluorescence and flow cytometry were performed at day 3.
2.4. Reagents
EC (Nacalai Tesque, Kyoto, Japan), ECg (Nacalai Tesque), EGC (Nacalai Tesque), GCg (Nacalai Tesque), and EGCg (Taiyo Kagaku, Yokkaichi, Japan) were used in experiments. UPW was used to dissolve the reagents at 5 mM for stock solutions and as a vehicle control. Caffeine (Nacalai Tesque) was dissolved in UPW at 10 mM for the stock solution. For osteoclast differentiation, cells were treated with RANKL (Oriental Yeast, Tokyo, Japan) and macrophage colony-stimulating factor (M-CSF; Affymetrix Japan, Tokyo, Japan) at final concentrations of 50 and 10 ng/mL, respectively.
2.5. Cell viability assay
RAW264.7 cells (2 × 103 cells/well; 96-well plate) were cultured for 24 h. Cells in the exponential growth phase were treated with the indicated reagents for 48 h. Then, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazalium bromide (MTT; Nacalai Tesque) solution (5 mg/mL in ultrapure water) was added to each well, followed by incubation for a further 4 h at 37 °C. Formazan crystals were dissolved in 100 μL of 0.01 M HCl/10% SDS. Absorbance was quantitated as a percentage compared with the untreated control using a Microplate Reader (CHROMATE4300; Practical Japan, Chiba, Japan) at 600 nm.
2.6. TRAP staining
RAW264.7 cells were treated with reagents in a 96-well plate (2 × 103 cells/well) for 5 days until TRAP staining [21]. In brief, cells were fixed with 10% formalin for 5 min at room temperature, followed by washing with phosphate-buffered saline (PBS). Then, the cells were treated with a TRAP staining solution for 40 min at 37 °C. TRAP-positive osteoclasts with three or more nuclei were considered as mature osteoclasts.
2.7. TRAP activity assay
Cells were cultured in a 96-well plate (2 × 103 cells/well) for 5 days, and then TRAP enzyme activity was measured using a TRAP activity kit (Takara Bio, Shiga, Japan), in accordance with the manufacturer's protocol. In brief, cells were lysed with 1% NP40 and incubated with the TRAP activity solution for 30 min at 37 °C. Then, 0.5 M NaOH was added to stop the reaction. Absorbance was quantitated as the percentage compared with the vehicle control using the Microplate Reader (Practical Japan) at 405 nm.
2.8. Immunofluorescence staining
Reagent-treated cells were cultured on glass coverslips (Matsunami Glass, Osaka, Japan) in a 6-well plate (4 × 104 cells/well) for immunofluorescence staining of DC-STAMP [10]. The cells on coverslips were fixed with 4% paraformaldehyde in PBS for 10 min at room temperature, permeabilized with 0.1% saponin in PBS for 10 min on ice, and then blocked with 1% bovine serum albumin in PBS for 30 min. The cells were then incubated at 37 °C for 2 h with an anti-DC-STAMP mAb (clone 1A2, 1:200, Becton Dickinson, Mountain view, CA, USA) as the primary antibody. Goat anti-mouse Alexa Fluor 555 (A21425, 1:200, Life Technologies, Carlsbad, CA, USA) was used as the secondary antibody. For F-actin ring staining, phalloidin-iFluor488 Reagent (ab176753, abcam, Cambridge, UK) was added for 30 min at room temperature, in accordance with the manufacturer's protocol. After washing with PBS, 4′6-diamidino-2-phenylindole (DAPI; Life Technologies) was used to stain DNA, and the cells on coverslips were mounted on slides for imaging.
2.9. Western blot
Reagent-treated cells were cultured in a 6-well plate (4 × 104 cells/well) for 72 h. The cells were washed twice with PBS before collection followed by dissolved in certain quantity of lysis buffer. The cell lysates were loaded onto a 10% gel for SDS-PAGE and blotted onto a PVDF membrane using a wet-transfer method. The membrane was blocked with 1% bovine serum albumin in TBS with 0.05% tween 20 (TBST) for 1 h, and then incubated at 4 °C for overnight with an anti-DC-STAMP mAb (clone 1A2, 1:500, Becton Dickinson) as the primary antibody. Goat anti-mouse IgG horse-radish peroxidase conjugate (1:3000, Biorad, Hercules, CA, USA) was used as the secondary antibody at room temperature for 1 h. The membrane was incubated with ECL prime (GE Healthcare, Chicago, IL, USA) at room temperature for 5 min in accordance with the manufacturer's protocol, and LAS imaging system (Wako, Osaka, Japan) was used for imaging.
2.10. Flow cytometry
Cells were cultured in a 6-well plate (4 × 104 cells/well) and then harvested using a cell scraper before flow cytometric analysis of cell surface DC-STAMP [10]. The cells were washed with PBS and then centrifuged at 150×g for 15 min. As the primary antibody, an anti-DC-STAMP mAb (clone 1A2, 1:200; Merck Millipore, Burlington, MA, USA) was applied for 30 min on ice to detect DC-STAMP. To avoid detecting nonspecific antibody reactions, purified mouse IgG1 (1:200; BioLegend, San Diego, CA, USA) was used. After washing with PBS, goat anti-mouse Alexa Fluor 555 (A21425, 1:200, Invitrogen) was applied for 15 min on ice as the secondary antibody. After washing with PBS, flow cytometry was performed using a FACSCantoII (Becton Dickinson). Data were analysed using FACS DIVA software (Becton Dickinson).
2.11. Microscopy and imaging
An inverted microscope (IX51; Olympus, Tokyo, Japan) equipped with a digital camera (DP26; Olympus) was used to image TRAP-stained cells. A fluorescence microscope (Axioplan 2 MOT; Carl Zeiss, Inc., Oberkochen, Germany) equipped with an ORCA-R2 camera (Hamamatsu, Shizuoka, Japan) was used for fluorescence imaging using a × 63 objective lens. Z-stacked three-dimensional images were then assembled, pseudo coloured, and overlaid using MetaMorph software (version 7.7; MDS Analytical Technologies, Sunnyvale, CA, USA).
2.12. Statistical analysis
All experiments were conducted at least twice independently. Data are expressed as the mean ± SD of results from at least three different cultures, or as the mean ± SE of results. The data were analysed using the Student's t-test. P-values of less than 0.01 and 0.05 were considered as statistically significant.
3. Results
3.1. Preparation and analysis of TEx
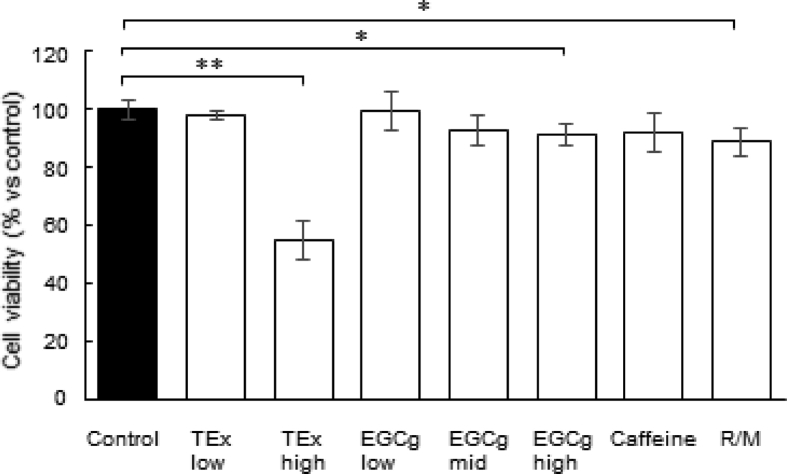
We prepared TEx from 20 g green tea leaves, and the yield of dried TEx was 4.55 g (22.75%). HPLC analysis was performed to confirm the amount of tea catechins based on the calibration curve of a standard control, as same as our previous study [20]. We found that the amounts of catechins were as follows: EC, 3.6%; EGC, 11.8%; ECg, 1.6%; EGCg, 11.5% (Table 1). Based on these results, we adjusted the concentration of EGCg to 5, 25 or 50 μM for experiments. We examined the cytotoxicity of TEx and EGCg in cultured RAW264.7 cells. MTT assays were performed to measure cell viability based on the mitochondrial activity of cells at 48 h of culture. Compared with the untreated control, 40 μg/ml TEx suppressed cell growth, and 50 μM EGCG suppressed that slightly (Supplementary material 1).
Table 1.
Calculation of contained catechins in TEx by HPLC analysis.
| EC | EGC | ECg | EGCg | |
|---|---|---|---|---|
| Content (%): | 3.6 | 11.8 | 1.6 | 11.5 |
3.2. Effect of TEx and EGCg on cell fusion and TRAP activity
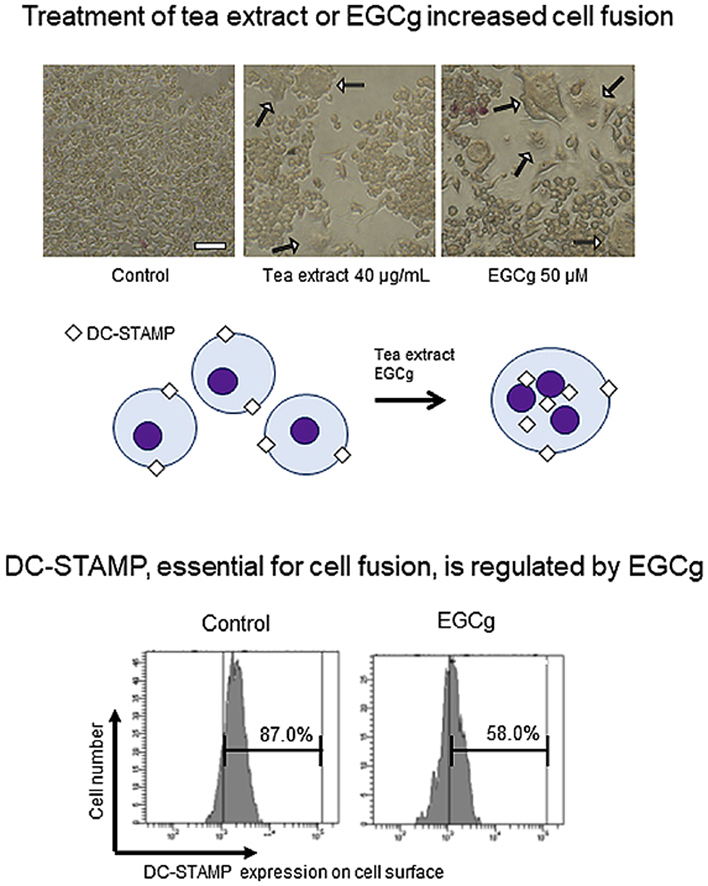
We determined whether TEx treatment alone affected cell fusion of RAW264.7 cells by evaluating multinucleated cells. Because RAW264.7 cells undergo repeated cell fusion to differentiate into multinucleated cells (MNCs), we used RANKL and M-CSF (R/M) as a cell fusion positive control in the following experiments. In addition, we applied caffeine, which is present in TEx, as a potential osteoclast-inducing compound. MNCs with more than three nuclei were counted as fused cells (Fig. 1A). As a result, MNCs were significantly increased by treatment with TEx or EGCg in a dose dependent manner (Fig. 1B). The average number of MNCs in the vehicle control was 65. In contrast, the numbers of MNCs obtained by treatment with 4 or 40 μg/mL TEx were 83 and 136, those by 5, 25, or 50 μM EGCg were 69, 121, and 181, that by 100 mM caffeine was 70, and that by R/M was 223, respectively. Then, TRAP enzyme activity was measured, but no enhancement of TRAP activity was observed, except for R/M treatment (Fig. 1C). In addition, the numbers of MNCs obtained by treatment with the vehicle control, 50 μM EC, EGC, ECg, or EGCg were 44, 54, 89, 65, and 113, respectively (Fig. 1D), without upregulating TRAP activity in any tea catechin-treated condition (Fig. 1E).
Fig. 1.
Effects of TEx and catechins on cell fusion and TRAP activity. (A) Representative images of 40 μg/mL TEx, 50 μM EGCg, or both 50 ng/mL RANKL and 10 ng/mL M-CSF (R/M)-treated RAW264.7 cells after TRAP staining at day 5. White arrows indicate multinucleated osteoclasts with three or more nuclei. Scale bar: 50 μm. (B) RAW264.7 cells were treated with 4 or 40 μg/mL TEx, 5, 25, or 50 μM EGCg, 100 μM caffeine, or R/M for 5 days. The numbers of multinucleated osteoclasts were then counted. Data are presented as the mean ± SD (n = 3). *p < 0.05, **p < 0.01 compared with the vehicle control by the Student's t-test. (C) TRAP activity was measured by a microplate reader at 405 nm. Data are presented as the mean ± SD (n = 3). *p < 0.05, **p < 0.01 compared with the vehicle control by the Student's t-test. (D) RAW264.7 cells were treated with 50 μM EC, EGC, ECg, or EGCg for 5 days. The numbers of multinucleated osteoclasts were then counted. (E) TRAP activity was measured by the microplate reader at 405 nm.
3.3. Effect of EGCg on DC-STAMP localization
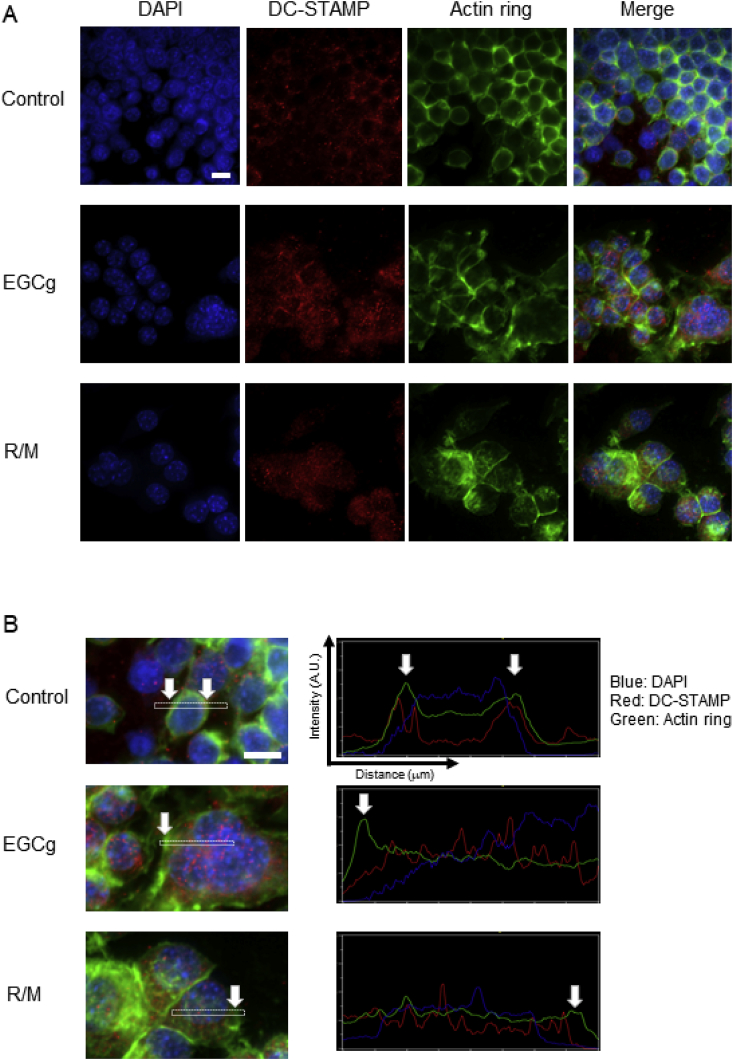
To confirm expression and localization of DC-STAMP protein, we performed immunofluorescence in RAW264.7 cells treated with EGCg for 3 days. Fig. 3 shows fixed cells stained with DAPI (blue, nuclei), Alexa Fluor 555 (red, DC-STAMP), and phalloidin (green, actin ring). Vehicle control-treated cells expressed DC-STAMP that was colocalized with the actin ring along the cell membrane (Fig. 2A, upper panels). After EGCg treatment, intracellular DC-STAMP expression was increased (Fig. 2A, middle panels), and RANKL-treated cells expressed a relatively high level of intracellular DC-STAMP (Fig. 2A, lower panels). Then, line scan analysis was performed to quantify overlaps of the three signals in a representative region (Fig. 2B). White arrows indicate the cell membrane corresponding to the line graph. EGCg-treated cells had less overlap between DC-STAMP and F-actin compared with the vehicle control. These results suggested that EGCg induced DC-STAMP translocation from the cell membrane to inside of cells.
Fig. 3.
Flow cytometric analysis of DC-STAMP expression on the cell surface. (A) Representative flow cytometric histograms showing surface DC-STAMP expression. RAW264.7 cells were treated with 40 μg/mL TEx, 50 μM catechins, or R/M for 3 days and then stained with anti-DC-STAMP antibody 1A2 followed by an Alexa Fluor 555-conjugated secondary antibody for flow cytometric analysis. (B) Histogram of the mean fluorescence intensity (MFI) levels of surface DC-STAMP expression on EC-, EGC-, ECg-, EGCg-, TEx-, or R/M-treated cells. Relative MFIs calculated by FACSDiva software compared with the untreated control are shown. Data are presented as the mean ± SE (n ≥ 3).
Fig. 2.
Visualization of DC-STAMP expression and localization. (A) RAW264.7 cells were treated with 50 μM EGCg or RM for immunostaining. Nuclear DNA was stained with DAPI (blue), DC-STAMP was detected with an anti-DC-STAMP monoclonal antibody 1A2 and Alexa Fluor 555-conjugated secondary antibody (red), and the F-actin ring was stained with phalloidin-iFluor 488 (green). Images were obtained at × 63 magnification by fluorescence microscopy, and then z-stacked and merged by MetaMorph software. Scale bar: 10 μm. (B) Quantified overlap of the three signals in a representative region using the line scan function by MetaMorph software. White arrows indicate the corresponding region of the cell and line graph. Intensity of the signal (A.U.) on the vertical axis and distance (μm) on the horizon axis are shown. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. Western blot analysis for DC-STAMP detection
Reagent-treated cells were used for western blot analysis to quantify changes of DC-STAMP expression. We prepared vehicle control, TEx-treated, EGCg-treated and R/M-treated cells, however, there were no significant differences among these 4 samples. We shows representative images of detected DC-STAMP (Supplemental material 2). We expected that the changes of DC-STAMP expression is too small to detect by western blot analysis in this condition. Thus, we performed FACS analysis to detect DC-STAMP expression precisely.
3.5. Flow cytometric analysis of surface DC-STAMP levels
Flow cytometry was performed to quantify changes in surface DC-STAMP expression. The ratio of surface DC-STAMP-expressing cells was decreased by EGCg or TEx treatment (Fig. 3A). Similar to a previous study, the surface DC-STAMP level was quite low in RANKL-treated cells compared with the untreated control. We analysed the surface DC-STAMP level and assessed the mean fluorescence intensity (MFI) of the expression (Fig. 3B). Relative MFI was significantly decreased to 76.9% and 55.1% by EGCg and R/M treatments, respectively. EGCg had a stronger effect on downregulation of DC-STAMP expression than the other tea catechins, which corresponded to the increase in the number of MNCs shown in Fig. 1.
4. Discussion
Osteoporosis is a systemic skeletal disorder with various symptoms caused by bone loss [11,12]. Anti-osteoporotic compound-containing foods, including green tea, can prevent the symptoms. Thus, anti-osteoporotic diets have attracted attention to prevent osteoporosis [22,23]. In this study, we evaluated the effects of TEx and catechins on cell fusion and DC-STAMP expression to elucidate the mechanisms of osteoclast development. Previous reports, including our own study, have identified four major catechins in tea extract: EC (MW = 290.27), ECg (MW = 442.37), EGC (MW = 306.27), and EGCg (MW = 458.37). In particular, EGCg suppresses osteoclastogenesis that involves cell fusion during osteoclast development [15,24]. RAW264.7 cells undergo morphological changes and cell fusion during early osteoclastogenesis. Therefore, we used R/M treatment as a positive control for multinucleation and cell fusion in this study.
As shown in Fig. 1, although R/M was the untreated condition, TEx or EGCg-treated RAW264.7 cell cultures showed increases in MNCs. Therefore, we assume that EGCg is one of the main active components in tea extract for cell-cell fusion. However, both reagents did not increase TRAP activity, suggesting that this multinucleation occurs via a bone resorption activity-independent pathway. Next, we confirmed the relationship of DC-STAMP expression with this increased multinucleation by focusing on changes of localization. In a previous report, MNCs expressed low levels of DC-STAMP protein on their cell surface, whereas mononuclear cells expressed a high level of surface DC-STAMP that was colocalized with cortical actin [10]. Our Z-stacked three-dimensional imaging revealed that treatment by EGCg increased intracellular DC-STAMP expression and decreased colocalization of DC-STAMP and the F-actin ring (Fig. 2A and B). We quantified the changes in DC-STAMP expression by flow cytometry and found downregulation of the cell surface level of DC-STAMP by EGCg treatment. Because internalization of DC-STAMP is a critical process for multinucleation of RAW264.7 cells, suppression of surface DC-STAMP expression leads to activation of DC-STAMP. Furthermore, EGCg treatment was most effective to decrease surface DC-STAMP levels compared with treatment by other catechins (Fig. 3B). These results are in good agreement with our previous report indicating that EGCg exhibits higher activity to modulate DC-STAMP mRNA expression [20]. EGCg suppresses osteoclastogenesis by regulation of MMP9 [25] under RANKL-induced osteoclastogenesis. In contrast, EGCg enhanced multinucleation via DC-STAMP activation under the RANKL-untreated condition. Nevertheless, TRAP activity was not increased (Fig. 1C, E). Our results indicate that increased multinucleation by EGCg treatment is independent of the RANKL signalling pathway. DC-STAMP is an essential protein for this multinucleation, but the complete pathway remains unknown, and other molecules may be involved in the multinucleation [5]. Transmembrane DC-STAMP has an ITIM tail in the cell membrane and regulates phosphorylation of SH2 domain-containing tyrosine phosphatase 1 [9]. DC-STAMP regulates calcium and NFATC1 to activate osteoclast differentiation [26]. Although further study is needed to identify the fusogenic gene crucial for cell fusion, some genes have been identified as potentially downstream of DC-STAMP [27].
In summary, we investigated the effect of an extract from tea leaves on cell fusion and DC-STAMP expression. Treatment with TEx or EGCg increased multinucleated osteoclast formations, but not TRAP activity in RAW264.7 cells. Fluorescence microscopic imaging and flow cytometry showed internalization of surface DC-STAMP by EGCg treatment, which mimics R/M treatment. These results indicate that increased cell fusion by TEx and EGCg results from localization changes of DC-STAMP, and upregulation of TRAP activity does not occur even when both decreased surface DC-STAMP and increased multinucleation, suggesting that bone resorption might be regulated by another pathway. Although further studies are required for elucidation, our results support a DC-STAMP signalling pathway that regulates osteoclast development.
CRediT authorship contribution statement
Kenji Kuriya: Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Masahiro Nishio: Supervision, Project administration, Funding acquisition. Tomoko Matsuda: Conceptualization, Methodology, Formal analysis. Hayato Umekawa: Writing - original draft, Writing - review & editing, Supervision.
Acknowledgements
We thank Mitchell Arico from Edanz Group for editing a draft of this manuscript. This work was supported partly by a Local Creation Accelerated Grant, Agriculture, Forestry and Fisheries Research Council from the Minister of Agriculture, Forestry and Fisheries of Japan (27028C, to Masahiro Nishio, Hayato Umekawa).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100759.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary material 1.
Effects of TEx or EGCg on cell survival. Cells were treated with TEx, EGCg, caffeine, or R/M at the indicated concentrations for 48 h before MTT assays. The absorbance of dissolved formazan crystals was then measured at 600 nm *p < 0.05, **p < 0.01 compared with the vehicle control by the Student's t-test.
Supplementary material 2.
Representative images of western blot analysis. RAW264.7 cells were treated with 40 μg/mL TEx, 50 μM EGCg, or R/M for 3 days, and then prepared for western blot analysis. Upper panel is detected DC-STAMP, and lower one is detected β-actin on the same membrane, respectively.
References
- 1.Parfitt A.M. The cellular basis of bone remodeling: the quantum concept reexamined in light of recent advances in the cell biology of bone. Calcif. Tissue Int. 1984;36 doi: 10.1007/BF02406132. [DOI] [PubMed] [Google Scholar]
- 2.Lacey D.L., Timms E., Tan H.L., Kelley M.J., Dunstan C.R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y.X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W.J. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/S0092-8674(00)81569-X. [DOI] [PubMed] [Google Scholar]
- 3.Sobacchi C., Frattini A., Guerrini M.M., Abinun M., Pangrazio A., Susani L., Bredius R., Mancini G., Cant A., Bishop N., Grabowski P., Del Fattore A., Messina C., Errigo G., Coxon F.P., Scott D.I., Teti A., Rogers M.J., Vezzoni P., Villa A., Helfrich M.H. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat. Genet. 2007;39:960–962. doi: 10.1038/ng2076. [DOI] [PubMed] [Google Scholar]
- 4.Yagi M., Miyamoto T., Sawatani Y., Iwamoto K., Hosogane N., Fujita N., Morita K., Ninomiya K., Suzuki T., Miyamoto K., Oike Y., Takeya M., Toyama Y., Suda T. DC-STAMP is essential for cell–cell fusion in osteoclasts and foreign body giant cells. J. Exp. Med. 2005;202:345–351. doi: 10.1084/jem.20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwasaki R., Ninomiya K., Miyamoto K., Suzuki T., Sato Y., Kawana H., Nakagawa T., Suda T., Miyamoto T. Cell fusion in osteoclasts plays a critical role in controlling bone mass and osteoblastic activity. Biochem. Biophys. Res. Commun. 2008;377:899–904. doi: 10.1016/j.bbrc.2008.10.076. [DOI] [PubMed] [Google Scholar]
- 6.Hartgers F.C., Vissers J.L.M., Looman M.W.G., Van Zoelen C., Huffine C., Figdor C.G., Adema G.J. DC-STAMP, a novel multimembrane-spanning molecule preferentially expressed by dendritic cells. Eur. J. Immunol. 2000;30:3585–3590. doi: 10.1002/1521-4141(200012)30:12<3585::AID-IMMU3585>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 7.Staege H., Brauchlin A., Schoedon G., Schaffner A. Two novel genes FIND and LIND differentially expressed in deactivated and Listeria-infected human macrophages. Immunogenetics. 2001;53:105–113. doi: 10.1007/s002510100306. [DOI] [PubMed] [Google Scholar]
- 8.Kukita T., Wada N., Kukita A., Kakimoto T., Sandra F., Toh K., Nagata K., Iijima T., Horiuchi M., Matsusaki H., Hieshima K., Yoshie O., Nomiyama H. RANKL-induced DC-STAMP is essential for osteoclastogenesis. J. Exp. Med. 2004;200:941–946. doi: 10.1084/jem.20040518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiu Y.H., Mensah K.A., Schwarz E.M., Ju Y., Takahata M., Feng C., McMahon L.A., Hicks D.G., Panepento B., Keng P.C., Ritchlin C.T. Regulation of human osteoclast development by dendritic cell-specific transmembrane protein (DC-STAMP) J. Bone Miner. Res. 2012;27:79–92. doi: 10.1002/jbmr.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mensah K.A., Ritchlin C.T., Schwarz E.M. RANKL induces heterogeneous DC-STAMPlo and DC-STAMPhi osteoclast precursors of which the DC-STAMPlo precursors are the master fusogens. J. Cell. Physiol. 2010;223:76–83. doi: 10.1002/jcp.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonet W.S., Lacey D.L., Dunstan C.R., Kelley M., Chang M.S., Luthy R., Nguyen H.Q., Wooden S., Bennett L., Boone T., Shimamoto G., DeRose M., Elliott R., Colombero A., Tan H.L., Trail G., Sullivan J., Davy E., Bucay N., Renshaw-Gegg L., Hughes T.M., Hill D., Pattison W., Campbell P., Sander S., Van G., Tarpley J., Derby P., Lee R., Boyle W.J. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 12.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Heal. Organ. Tech. Rep. Ser. 1994;843:1–129. [PubMed] [Google Scholar]
- 13.Chen C.-H., Ho M.-L., Chang J.-K., Hung S.-H., Wang G.-J. Green tea catechin enhances osteogenesis in a bone marrow mesenchymal stem cell line. Osteoporos. Int. 2005;16:2039–2045. doi: 10.1007/s00198-005-1995-0. [DOI] [PubMed] [Google Scholar]
- 14.Ko C.H., Lau K.M., Choy W.Y., Leung P.C. Effects of tea catechins, epigallocatechin, gallocatechin, and gallocatechin gallate, on bone metabolism. J. Agric. Food Chem. 2009;57:7293–7297. doi: 10.1021/jf901545u. [DOI] [PubMed] [Google Scholar]
- 15.Oka Y., Iwai S., Amano H., Irie Y., Yatomi K., Ryu K., Yamada S., Inagaki K., Oguchi K. Tea polyphenols inhibit rat osteoclast formation and differentiation. J. Pharmacol. Sci. 2012;118:55–64. doi: 10.1254/jphs.11082FP. [DOI] [PubMed] [Google Scholar]
- 16.Wu X., Xie C., Zhu Q., Wang M., Sun B., Huang Y., Shen C., An M., Zhao Y., Wang X., Sheng J. Green tea (Camellia sinensis) aqueous extract alleviates postmenopausal osteoporosis in ovariectomized rats and prevents RANKL-induced osteoclastogenesis in vitro. Food Nutr. Res. 2018;62:1–11. doi: 10.29219/fnr.v62.1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vali B., Rao L.G., El-Sohemy A. Epigallocatechin-3-gallate increases the formation of mineralized bone nodules by human osteoblast-like cells. J. Nutr. Biochem. 2007;18:341–347. doi: 10.1016/j.jnutbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 18.Morinobu A., Biao W., Tanaka S., Horiuchi M., Jun L., Tsuji G., Sakai Y., Kurosaka M., Kumagai S. (-)-Epigallocatechin-3-gallate suppresses osteoclast differentiation and ameliorates experimental arthritis in mice. Arthritis Rheum. 2008;58:2012–2018. doi: 10.1002/art.23594. [DOI] [PubMed] [Google Scholar]
- 19.Ilacqua A.N., Shettler J.A., Wernke K.M., Skalla J.K., McQuade K.J. Theaflavins from black tea affect growth, development, and motility in Dictyostelium discoideum. Biochem. Biophys. Res. Commun. 2017;491:449–454. doi: 10.1016/j.bbrc.2017.07.058. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda T., Fujita A., Hara M., Nishio M., Umekawa H. Tea extract modulates the expression of DC-STAMP mRNA in RAW264.7 cells. Food Sci. Technol. Res. 2015;21:869–873. doi: 10.3136/fstr.21.869. [DOI] [Google Scholar]
- 21.Shevde N., Anklesaria P., Greenberger J.S., Bleiberg I., Glowacki J. Stromal cell-mediated stimulation of osteoclastogenesis. Proc Soc Exp Biol Med. 1994;205:306–315. doi: 10.3181/00379727-205-43711. [DOI] [PubMed] [Google Scholar]
- 22.Cashman K.D. Diet , Nutrition , and Bone Health. 2007;1(2):2507–2512. doi: 10.1093/jn/137.11.2507S. [DOI] [PubMed] [Google Scholar]
- 23.Levis S., Lagari V.S. The role of diet in osteoporosis prevention and management. Curr. Osteoporos. Rep. 2012;10:296–302. doi: 10.1007/s11914-012-0119-y. [DOI] [PubMed] [Google Scholar]
- 24.Zhao R., Kamon M., Sakamoto K. (–)-Epigallocatechingallate interferes RANKL/RANK signal pathway and induces apoptosis during osteoclastogenesis in RAW264 cell. Food Nutr. Sci. 2014;5:107–116. doi: 10.4236/fns.2014.52014. [DOI] [Google Scholar]
- 25.Yun J.-H., Pang E.-K., Kim C.-S., Yoo Y.-J., Cho K.-S., Chai J.-K., Kim C.-K., Choi S.-H. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J. Periodontal. Res. 2004;39:300–307. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 26.Chiu Y.H., Schwarz E., Li D., Xu Y., Sheu T.R., Li J., de Mesy Bentley K.L., Feng C., Wang B., Wang J.C., Albertorio-Saez L., Wood R., Kim M., Wang W., Ritchlin C.T. Dendritic cell-specific transmembrane protein (DC-STAMP) regulates osteoclast differentiation via the Ca2+/NFATc1 Axis. J. Cell. Physiol. 2017;232:2538–2549. doi: 10.1002/jcp.25638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yagi M., Ninomiya K., Fujita N., Suzuki T., Iwasaki R., Morita K., Hosogane N., Matsuo K., Toyama Y., Suda T., Miyamoto T. Induction of DC-STAMP by alternative activation and downstream signaling mechanisms. J. Bone Miner. Res. 2007;22:992–1001. doi: 10.1359/jbmr.070401. [DOI] [PubMed] [Google Scholar]