Abstract
Ureaplasma urealyticum is a commensal of the female genital tract and can be detected as a pathogen in urethritis and vaginitis. Its importance as a respiratory pathogen beyond the field of neonatology remains controversial. We report a case of Ureaplasma-pneumonia in a recently lung-transplanted patient, with hyperammonemic syndrome.
The 51-year-old lung-transplanted female was admitted to the intensive care unit with new-onset reduction of her mental state due to hyperammonemia. A diagnostic bronchoscopy showed purulent bronchitis and multiple superficial ulcerations of the bronchial mucosa. The DNA-PCR from bronchoalveolar lavage confirmed the presence of Ureaplasma urealyticum in low concentration (about 5 * 104 copies/ml), which was interpreted as evidence of infection and treated with Doxycycline intravenously. Ureaplasma was also identified by DNA-PCR in the biopsy specimens of the inflammatory enlarged mediastinal lymph nodes. Bilateral pleural effusions were found to be transudative and culturally sterile.
Ureaplasma-pneumonia can cause fatal hyperammonemia in lung-transplant patients and should be considered in the differential diagnosis of every unclear hyperammonemia with normal liver function. The early identification and treatment of the infection leads to clinical and biochemical resolution.
Keywords: Atypical pneumonia, Immunosuppression, Hyperammonemic syndrome
1. Introduction
Infections are a major cause of early and late mortality after Lung Transplantation (LT) and chemoprophylaxis is an important component in the prevention of post-LT infections. According to a monocentric epidemiological study by Ji Hyun Yun et al., the most common bacterial infection in the first post-transplant month is the catheter-associated bacteremia. Bacterial pneumonia occurs typically between the second and the sixth month, as well as six months after transplantation. Multi-bacterial infections and multidrug-resistant pathogens are significantly likely to be detected. Fungal infections of the lung have been observed six months after transplantation and the most common viral agent of pneumonia was cytomegalovirus (CMV). Atypical pathogens, such as mycoplasma, were detected rarely and occurred over six months of transplantation time [1].
Ureaplasma urealyticum, Ureaplasma parvum and Mycoplasma hominis are known as "genital mycoplasmas". Ureaplasma urealyticum can be detected as a causative agent of urethritis and vaginitis, Ureaplasma parvum is usually saprophytic and can rarely cause urethritis and Mycoplasma hominis is considered to be facultative pathogenic [2].
Ureaplasma species is considered to be a low virulence commensal of the female genital tract. The clinical significance of Ureaplasma infections remains unclear. Ureaplasma colonization has a confirmed etiological association with infertility, stillbirth, premature birth, histological chorioamnionitis, and neonatal morbidities, including congenital pneumonia, meningitis, bronchopulmonary dysplasia, and perinatal death [[3], [4], [5], [6]]. Recently, Ureaplasma was divided into 2 different biovars and 14 different serotypes. Ureaplasma parvum is known as Biovar 1 and concludes the serotypes 1, 3, 6 and 14 and Ureaplasma urealyticum is known as Biovar 2 and contains the serotypes 2, 4, 5 and 7–13. The virulence of the serotypes and biovars remains controversial [7].
The importance of Ureaplasma species as an agent of pulmonary pathogenicity in adults is still under discussion and further investigations are required before recommendations to guide clinical practice can be made. We report a rare case of Ureaplasma-pneumonia in a recently lung-transplanted patient, being diagnosed in the setting of hyperammonemia.
2. Case presentation
The intensive care acquisition of the 51-year-old female followed after a new-onset alteration of her mental state. Two weeks earlier, the patient underwent a double lung transplantation, due to a secondary lung fibrosis by rheumatoid arthritis. The postoperative course was uneventful. Suddenly, the patient developed agitation and was disoriented to place, time and person. In the context of cerebrospinal fluid (CSF) diagnostics, bacterial and viral pathogens of the central nervous system were excluded and neurological evaluation suggested that metabolic encephalopathy could be the cause of her symptoms.
At the point of intensive care admission, the patient was hemodynamically and respiratory stable, but not meaningfully contactable. Because of progressive reduction of her mental state, endotracheal intubation and mechanical ventilation had to be initiated. An emergency computed tomography of the brain was performed in order to exclude ischemic brain injury or intracranial hemorrhage. Although Posterior Reversible Encephalopathy Syndrome (PRES) could be ruled out by magnetic resonance imaging (MRI), current immunosuppression with Tacrolimus (12ng/ml by submission) was discontinued. Laboratory parameters were uneventful, except of an isolated hyperammonemia (serum ammonia 387 mmol/l, normal range 26–47 mmol/l). As all liver function parameters were normal, we hypothesized that hyperammonemia was unlikely linked to a hepatic dysfunction and started a targeted search for urease-producing microorganisms.
The bronchoscopy showed a pronounced purulent bronchitis and multiple white-yellow non flushable mucosal lines, as well as superficial ulcerations with necrotic mucosa, especially in the area of anastomosis in both sides. DNA-PCR (DNA-Polymerase Chain Reaction) of the extracted bronchoalveolar lavage (BAL) was positive for Ureaplasma urealyticum in low concentration (5 * 104 copies/ml). Based on the overall clinical presentation of the patient and the severity of bronchoscopic findings, we evaluated this evidence as an Ureaplasma urealyticum-induced infection, and initiated an antibiotic treatment with doxycycline 200mg once a day intravenously. All blood cultures obtained prior to antibiotic therapy were sterile after 5 days of incubation. More interestingly, Ureaplasma could be detected by DNA-PCR in the biopsy specimens of the inflammatory enlarged mediastinal lymph nodes.
Several cultures of the bronchoalveolar lavage were negative for conventional bacteria, as well as for slow growing bacteria (such as Legionella pneumophila), mycobacteria and fungi. Pneumocystis jurovecii, Toxoplasma gondii, Aspergillus spp., Mycoplasma pneumoniae, Chlamydia spp. and Bordetella pertussis were negative. Cytomegalovirus (CMV), Epstein-Barr virus (EBV), Varicella-Zoster Virus (VZV) and Herpes Simplex Virus (HSV) were excluded by PCR in blood and BAL. Multiplex PCR of BAL samples was negative for respiratory viruses.
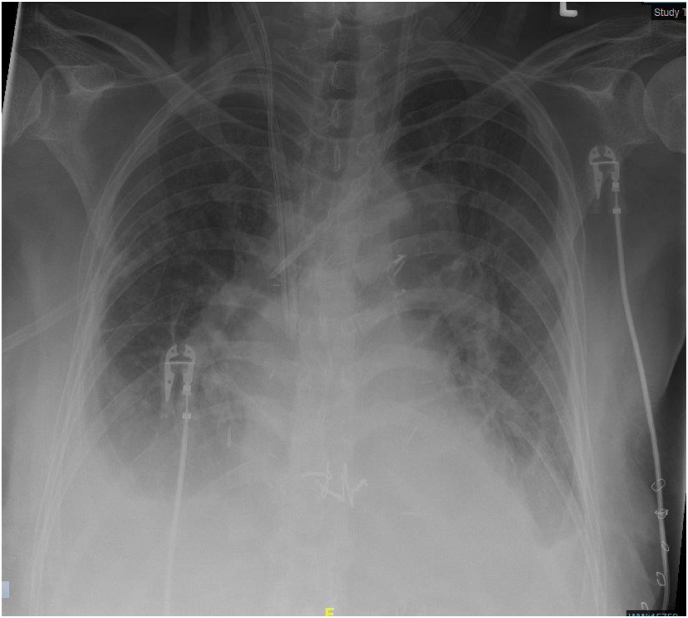
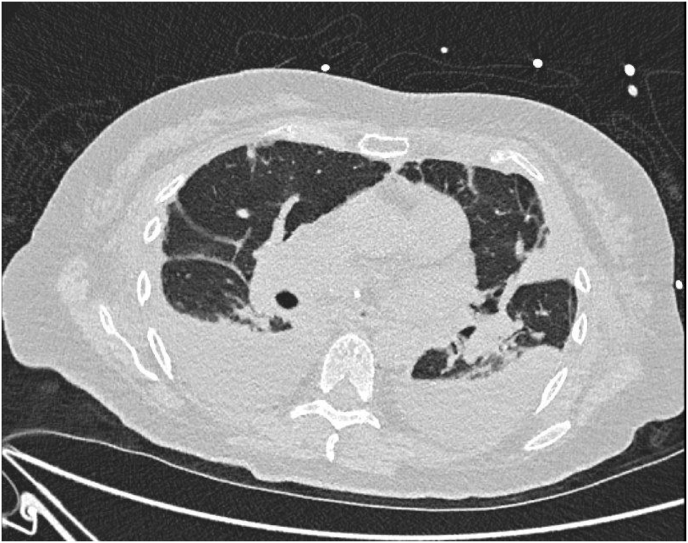
Under continuous renal replacement treatment, serum ammonia was rapidly eliminated, mental status was normal again and the patient could be extubated a few days later. Intermittent hemodialysis had to be performed, as bilateral pleural effusions persisted and renal retention parameters remained significantly high. The diagnostic puncture of the pleura confirmed transudate in both sides and no molecular or cultural evidence of Ureaplasma was detected. Fig. 1, Fig. 2 show the bilateral pleural effusions in conventional radiology and computed tomography, 10 days after submission to the intensive care unit.
Fig. 1.
Posteroanterior chest radiograph showing bilateral increased opacity of the lower fields, 10 days after submission to the intensive care unit.
Fig. 2.
Thorax CT-scan with evidence of bilateral pleural effusions, 10 days after submission to the intensive care unit.
3. Discussion
We describe the unusual case of Ureaplasma-pneumonia after LT in an adult patient. Ureaplasma was detected in BAL samples and transbronchial biopsies of mediastinal lymph nodes. The concentration of microorganism was low (about 5 * 104 copies/ml) and its role as a respiratory pathogen remains unclear. However, taking account of the adequate response to doxycycline, and the absence of other pathogens, we believe that Ureaplasma-infection was present. Thus, we discuss our case in comparison to similar reports in immunosuppressed patients, especially lung-transplant recipients.
Ureaplasma-infections in immunosuppressed patients are often presented as co-infections with other atypical bacteria, such as Nocardia farcinica [8] and Mycoplasma hominis [9]. The likelihood of a co-infection in our case was ruled out by extensive molecular diagnostics.
The early identification of Ureaplasma was achieved, as unclear hyperammonemia occurred. Hyperammonemia with encephalopathy was the only clinical manifestation. Although bronchoscopy findings were pronounced, respiratory symptoms were absent. As liver function parameters were normal, we supposed that hyperammonemia was not linked to a liver dysfunction but to urease-producing microorganisms and performed a targeted search for urease-producing bacteria by extensive sampling and specific molecular investigations.
The appearance of atypical pneumonia in the early post-transplant period is very rare, as such a pulmonary infection occurs more often after six months of transplantation time [1]. There is some evidence that Ureaplasma-pneumonia in lung recipients can be a donor-derived opportunistic infection, which causes refractory hyperammonemia [10]. Transplantation experts suggest that a routine donor screening should be initiated for this reason [10]. Our case supports the theory of a donor-derived Ureaplasma-infection, as the elapsed time between LT and illness was very short.
A similar case of Ureaplasma-pneumonia with hyperammonemia in a lung-transplant recipient has been recently described by Matson KM et al. [11]. The patient was treated with doxycycline, along with other measures to lower ammonia levels, and got clinically improved. Ureaplasma species were identified using 16S ribosomal RNA PCR/sequencing of pleural fluid, and by culture of bronchoalveolar lavage.
Parapneumonic pleural effusions with Ureaplasma-growth are often in immunosuppressed patients with Ureaplasma-pneumonia [8,9,11,12]. In our case, parapneumonic pleuritic involvement could be excluded, as bilateral pleural effusions were transudative and culturally sterile. However, our patient was already treated with antibiotics over 10 days before pleural puncture and microbial growth could have been eliminated for that reason. After a short period of volume recompensation, pleural effusions completely vanished.
A refractory hyperammonemia following Ureaplasma-infection after LT has been already described in the literature [13]. The 65-year-old patient presented on day 7 after LT with refractory status epilepticus and normal computed tomography of the brain. Hyperammonemia >1000 μmol/l was detected and despite aggressive treatment, the patient developed global cerebral edema and died. Postmortem investigations revealed Ureaplasma parvum.
A refractory hyperammonemia was not observed in our patient, as a continuous renal replacement therapy resulted in a rapid biochemical and clinical resolution of the disorder. An explanation for this outcome may have been the identification and successful treatment of the underlying cause of hyperammonemia early in the clinical course of the disease. A second reason could have been the absence of a disseminated Ureaplasma-infection. According to Bharat A et al. [14], disseminated Ureaplasma-infections with bacteremia can cause a fatal hyperammonemia in lung-transplant patients and empiric antimicrobial treatment, while awaiting microbiological confirmation, can be lifesaving in this condition.
4. Conclusion
Ureaplasma-pneumonia can cause fatal hyperammonemia in lung-transplant patients, and should be considered as a differential diagnosis by every unclear hyperammonemia with normal liver function. This disorder may occur early in the post-transplant time, as it seems to be donor-derived. The early identification and treatment of the infection leads to clinical and biochemical resolution. However, disseminated Ureaplasma-infections in lung-recipients may lead to refractory hyperammonemia and death to due cerebral edema.
Ethics approval and consent to participate
A case report is intended to develop information to be shared for educational purposes and do not meet the definition of “research”. Ethical approval was not necessary. Written informed consent was obtained from the legal guardian of the patient.
Consent for publication
Written consent for publication was obtained from the legal guardian of the patient.
Funding
Not applicable.
Declaration of competing interest
The Authors have disclosed that they have no significant relationship with, or financial interest in, any commercial companies pertaining to this article.
References
- 1.Yun J.H., Lee S.O., Jo K.W. Infections after lung transplantation: time of occurrence, sites, and microbiologic etiologies. Korean J. Intern. Med. 2015;30(4):506–514. doi: 10.3904/kjim.2015.30.4.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nenoff P., Manos A., Ehrhard I. Non-viral sexually transmitted infections - epidemiology, clinical manifestations, diagnostics and therapy: Part 2: Chlamydia and mycoplasma. Hautarzt. 2017;68(1):50–58. doi: 10.1007/s00105-016-3906-z. [DOI] [PubMed] [Google Scholar]
- 3.Latino M.A., Botta G., Badino C. Association between genital mycoplasmas, acute chorioamnionitis and fetal pneumonia in spontaneous abortions. J. Perinat. Med. 2018;46(5):503–508. doi: 10.1515/jpm-2016-0305. [DOI] [PubMed] [Google Scholar]
- 4.Kotecha S., Hodge R., Schaber J.A. Pulmonary Ureaplasma urealyticum is associated with the development of acute lung inflammation and chronic lung disease in preterm infants. Pediatr. Res. 2004;55(1):61–68. doi: 10.1203/01.PDR.0000100757.38675.50. [DOI] [PubMed] [Google Scholar]
- 5.Skevaki C., Kafetzis D.A. Ureaplasma urealyticum airway colonization and pulmonary outcome in neonates. Expert Rev. Anti Infect. Ther. 2003;1(1):183–191. doi: 10.1586/14787210.1.1.183. [DOI] [PubMed] [Google Scholar]
- 6.Waites K.B., Crouse D.T., Cassell G.H. Systemic neonatal infection due to Ureaplasma urealyticum. Clin. Infect. Dis. 1993;17(Suppl 1):S131–S135. doi: 10.1093/clinids/17.supplement_1.s131. [DOI] [PubMed] [Google Scholar]
- 7.Sung T.J. Ureaplasma infections in pre-term infants: recent information regarding the role of Ureaplasma species as neonatal pathogens. Korean J. Pediatr. 2010;53(12):989–993. doi: 10.3345/kjp.2010.53.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Canouï E., Blanc K., Loubinoux J. The value of molecular techniques to diagnose Ureaplasma urealyticum and Nocardia farcinica pleuropneumonia in a patient with diffuse large B-cell lymphoma. Int. J. Infect. Dis. 2017;64:93–95. doi: 10.1016/j.ijid.2017.09.015. [DOI] [PubMed] [Google Scholar]
- 9.García-de-la-Fuente C., Miñambres E., Ugalde E. Post-operative mediastinitis, pleuritis and pericarditis due to Mycoplasma hominis and Ureaplasma urealyticum with a fatal outcome. J. Med. Microbiol. 2008;57(Pt 5):656–657. doi: 10.1099/jmm.0.47632-0. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez R., Ratliff A., Crabb D. Ureaplasma transmitted from donor lungs is pathogenic after lung transplantation. Ann. Thorac. Surg. 2017;103(2):670–671. doi: 10.1016/j.athoracsur.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Matson K.M., Sonetti D.A. Successful treatment of Ureaplasma-induced hyperammonemia syndrome post-lung transplant. Transpl. Infect. Dis. 2019;21(1) doi: 10.1111/tid.13022. [DOI] [PubMed] [Google Scholar]
- 12.Deverrière G., Lemée L., Grangé S. Life-threatening pneumopathy and U urealyticum in a STAT3-deficient hyper-IgE syndrome patient. Pediatrics. 2017;139(6) doi: 10.1542/peds.2016-0845. [DOI] [PubMed] [Google Scholar]
- 13.McLaughlin D.C., Mallea J.M., Ng L.K. Hyperammonemia presenting as refractory status epilepticus after lung transplant in a patient positive for Ureaplasma parvum. Indian J. Crit. Care Med. 2018;22(6):463–465. doi: 10.4103/ijccm.IJCCM_356_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharat A., Cunningham S.A., Scott Budinger G.R. Disseminated Ureaplasma infection as a cause of fatal hyperammonemia in humans. Sci. Transl. Med. 2015;7(284):284re3. doi: 10.1126/scitranslmed.aaa8419. [DOI] [PMC free article] [PubMed] [Google Scholar]