Abstract
The present study evaluated the histopathological features, biological nature, anatomical location, sex, age and breeds of dogs affected by spontaneous gastrointestinal epithelial tumor. Biopsy samples of gastrointestinal tumors, from 95 dogs were examined and classified according to the WHO histological classification. A total of 131 samples, including 38 gastric, 13 small intestinal, and 80 large intestinal tumors were examined. The study observed that Jack Russell Terriers and Miniature Dachshunds were the breeds with the highest predisposition for gastrointestinal tumors. Gastric tumors included 5 adenomas, 30 adenocarcinomas (12 tubular, 2 papillary, 4 tubulopapillary and 12 signet-ring cell carcinomas) and 3 undifferentiated carcinomas. Intestinal tumors included 35 adenomas, 57 adenocarcinomas (43 acinar, 4 papillary, 7 mucinous and 3 signet-ring cell carcinomas), and 1 undifferentiated carcinoma. The study did not detect any difference among the incidence rates of invasion/metastasis in the tubular (44%), papillary (33%) and tubulopapillary (25%) adenocarcinomas. Additionally, the tubular (acinar), papillary and tubulopapillary adenocarcinomas were further divided into 48 polypoid and 17 non-polypoid types, based on their growth patterns. Invasion/metastasis was detected in 21% of the polypoid type and 100% of the non-polypoid type of adenocarcinomas. A correlation was detected between the occurrence of invasion/metastasis and the type of histopathological growth pattern in adenocarcinomas. The study demonstrated that Jack Russell terriers and Miniature Dachshunds are the most common breeds affected by gastrointestinal tumors and the entire group of the canine adenocarcinomas with non-polypoid growth pattern has greater malignant potentials, compared to the adenocarcinomas with polypoid growth patterns.
Keywords: dog, gastric tumor, histopathology, intestinal tumor, spontaneous epithelial tumor
Introduction
Spontaneous canine gastrointestinal tumors are rather common in veterinary medicine and such tumors are believed to be a suitable model, for their human counterparts. This is because various types of mutations of Adenomatous polyposis coli gene (APC), a tumor suppressor gene, are commonly found in human and canine colorectal tumors1, 2, APC gene-mutated or -transgenic mice are most widely used for investigating the tumorigenesis of this particular tumor. These rodent models may reflect the pathogenesis of APC-associated gastrointestinal tumors, but may not explain the pathological features seen in human tumors. Thus, research on spontaneous canine gastrointestinal tumors will provide useful information, which can provide assistance in understanding the tumorigenesis of their human counterparts. Histopathological classification of canine gastrointestinal tumors was based on the WHO histological classification of tumors in domestic animals3. Despite the presence of well-described morphological characteristics in the classification, to date, the biological and/or clinicopathological features of canine gastrointestinal tumors have not yet been established properly.
There are two major concepts to explain the carcinogenesis of alimentary tract tumors in humans, especially in the colorectum: the adenoma-carcinoma sequence4, 5, 6 and de novo carcinoma theories7, 8, 9, 10. Carcinogenesis, by the adenoma-carcinoma sequence, progresses from benign precursor lesions6. This theory indicates a progressive malignant transformation, induced by stepwise genetic alterations2. The concept is mainly based on the phenomenon of frequent occurrence of colorectal carcinoma in adenomatous lesions. On the other hand, de novo carcinogenesis is characterized by primary tumor lesions, which exhibit a flat or dome-shaped appearance. Since small carcinomatous lesions occur without any antecedent adenomatous elements in their vicinity, it can be assumed that these malignant lesions do not originate from precursor adenomatous lesions10, 11, 12. In addition, flat-shaped tumor lesions are reported to be highly malignant, even though they are very small in size13, 14.
The aim of the present study was to explain the clinicopathological and histopathological features of canine gastrointestinal epithelial tumors. Biopsy samples of canine gastrointestinal epithelial tumors were classified according to the “WHO histological classification of tumors in domestic animals.” The results obtained from this study may provide beneficial knowledge regarding the histopathological nature, age, sex, breed and tumor location of spontaneous canine tumors.
Materials and Methods
Cases
A total of 131 biopsy samples of gastrointestinal epithelial tumors (38 gastric, 13 small intestinal, and 80 large intestinal tumors) from 95 dogs were collected through endoscopy or surgical excision, at three institutions (Veterinary Medical Center of the University of Tokyo, Japan Small Animal Medical Center and Japan Animal Referral Medical Center), between 2013 and 2016. We recorded the breed, age, and sex of the study subjects, as well as the anatomical locations, presence of metastasis/invasion and histopathological classification of the lesions.
Histological examinations
All the samples were fixed in 10% neutral buffered formalin and routinely embedded in paraffin. The tissue sections, which were four-micrometers in thickness, were stained with hematoxylin and eosin (HE) stains. The specimens were examined by three pathologists: one pathologist was certified by the Japanese Society of Toxicologic Pathology (Tsubasa Saito) and the other two pathologists were certified by the Japanese College of Veterinary Pathology (James K. Chambers and Kazuyuki Uchida). Each lesion was classified according to the “WHO histological classification of tumors of the alimentary system of domestic animals”3. In addition, histological patterns of adenocarcinoma (excluding signet-ring cell carcinomas and mucinous adenocarcinomas) were classified into polypoid growth (PG) type and non-polypoid growth (NPG) type, according to the histological classifications of human intestinal tumor11. Briefly, they defined PG carcinomas as those with marked intramucosal proliferation and NPG carcinomas as those without protuberant intramucosal growth.
Results
Age, sex, and breed of the cases
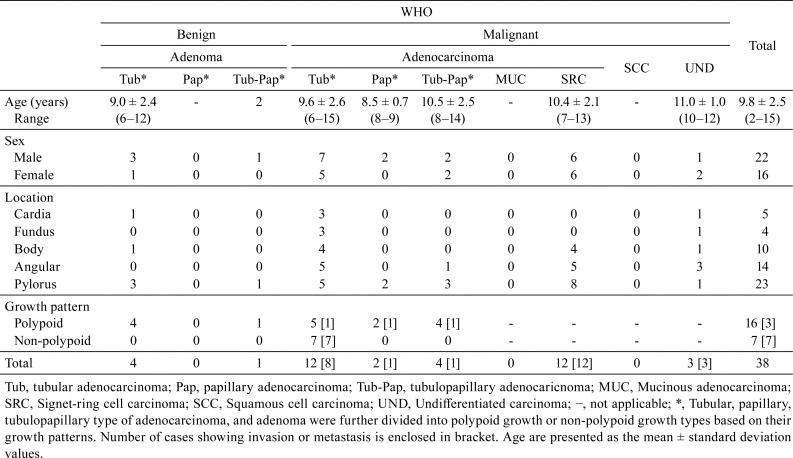
The average ages of the canine patients with gastric, small intestinal, and large intestinal tumors were 9.8 years, 11.9 years and 9.3 years, respectively (Table 1 and 2). The average ages of canine patients with gastric adenoma (7.6 years) and large intestinal adenoma (8.5 years) were observed to be lesser, compared to the average ages of canine patients with malignant tumors (10.1 years in gastric carcinoma and 10.0 years in large intestinal carcinoma).
Table 1. Histopathological Diagnosis and Age, Sex and Location of Canine Gastric Epithelial Tumors.
Large intestinal tumors were observed to be more frequent in the male canine patients, compared to the females: the male/female ratio was 51/26 (Table 2). However, there was no significant gender predilection observed in the incidence of gastric or small intestinal tumors (Table 1 and 2).
Table 2. Histopathological Diagnosis and Age, Sex and Location of Canine Intestinal Epithelial Tumors.
Jack Russell terriers and Miniature Dachshunds were the most commonly affected breeds, with regards to with gastric (Table 3) and intestinal (Table 4) tumors, respectively. These tumors were observed to have a tendency to occur in multiple regions throughout the gastrointestinal tract. No cases of signet-ring cell carcinoma, mucinous adenocarcinoma, or undifferentiated carcinoma were detected in Jack Russell terriers.
Table 3. Histopathological Diagnosis and Breeds of Dogs of Canine Gastric Epithelial Tumors.
Table 4. Histopathological Diagnosis and Breeds of Dogs of Canine Intestinal Epithelial Tumors.
Anatomical location of the tumors
In the current study, the most common anatomical location, for gastric tumors, was the angular and the pyloric regions (Table 1). There were no particular areas in the small intestine, where tumors developed frequently (Table 2). However, adenoma and adenocarcinoma were observed to have a tendency to develop in the lower parts of the large intestine, such as the colorectal junction and the rectum (Table 2).
Histopathological classification of gastric tumors
The 38 gastric tumors, involved in the current study, included five adenomas, thirty adenocarcinomas and three undifferentiated carcinomas. There were no cases of mucinous adenocarcinoma or squamous cell carcinoma. Adenoma lesions displayed a focal polypoid growth pattern, composed of tubulopapillary structures (Fig. 1A) and were further classified into four tubular adenomas and one tubulopapillary adenoma; on the other hand, no cases of papillary adenoma were detected (Table 1). The tubulopapillary structures were composed of single-layered columnar epithelial tumor cells, with a slightly enlarged and unevenly located nucleus (Fig. 1B). The 30 adenocarcinomas were further classified into 12 tubular adenocarcinomas, two papillary adenocarcinomas, four tubulopapillary adenocarcinomas and 12 signet-ring cell carcinomas (Table 1). A disorganized cell layer, with loss of cellular polarity, anisokaryosis, and prominent nucleoli were observed in the lesions of papillary, tubulopapillary (Fig. 1C and D) and/or tubular (Fig. 1E and F) adenocarcinoma. Papillary lesions were observed to be accompanied by a fibrovascular stalk, whereas tubular lesions were accompanied by irregular branching tubules. All adenomas (5/5), papillary adenocarcinomas (2/2), tubulopapillary adenocarcinomas (4/4) and less than half of the tubular adenocarcinomas (5/12) exhibited polypoid growth. Submucosal invasion or metastasis was detected in 27% (3/11) of the PG type adenocarcinomas and 100% (7/7) of the NPG type adenocarcinomas. Lesions of signet-ring cell carcinoma displayed a focal proliferation, just beneath the mucosal epithelium, occasionally. The tumor cells, which possessed abundant intracytoplasmic mucin (Fig. 1G), commonly invaded into the deep mucosa or the submucosa and often metastasized into the regional lymph nodes. Undifferentiated carcinomas did not show any tubular or papillary structures; however, a diffuse invasive growth of pleomorphic tumor cells, with irregular-shaped nuclei and prominent nucleoli, was observed frequently (Fig. 1H).
Fig. 1.
Canine gastric epithelial tumors; HE stained. (A) Tubulopapillary adenoma with polypoid proliferation. Bar=1,000 μm. (B) Higher magnification of Fig. 1A; tumor cells of tubulopapillary adenoma on the right. Bar=40 μm. (C) Polypoid proliferation of tubulopapillary adenocarcinoma with focal submucosal invasion. Bar=1,000 μm. (D) Higher magnification of Fig. 1C; focal invasion of malignant papillary adenocarcinoma cells to the muscularis. Bar=80 μm. (E) Non-polypoid growth of tubular adenocarcinoma within the lamina propria. Bar=160 μm. (F) Higher magnification of Fig. 1E; tumor cells of tubular adenocarcinoma with irregular tubular structures. Bar=40 μm. (G) Tumor cells of signet-ring cell carcinoma, with eccentric nuclei due to abundant intracytoplasmic mucin. Bar=40 μm. (H) Tumor cells of undifferentiated carcinoma without specific differentiation. Bar=40 μm.
Histopathological classification of intestinal tumors
The current study involved 93 intestinal tumors: 13 small intestinal tumors and 80 large intestinal tumors (Table 2). Tumors of the small intestine were histologically classified into six adenocarcinomas (five acinar and one papillary adenocarcinoma), five mucinous adenocarcinomas, one signet-ring cell carcinoma and one undifferentiated carcinoma. No cases of adenoma or adenosquamous carcinoma were detected in the small intestine. A half (3/6) of the adenocarcinomas exhibited polypoid growth: acinar (2/5) and papillary (1/1) types. Submucosal invasion or metastasis was detected in 33% (1/3) of PG type adenocarcinomas and 100% (3/3) of NPG type adenocarcinomas.
The 80 large intestinal tumors, in the current study, were histologically classified into 35 adenomas, 41 adenocarcinomas (38 acinar and three papillary adenocarcinomas), two mucinous adenocarcinomas and two signet-ring cell carcinomas. No cases of adenosquamous carcinoma or undifferentiated carcinoma were detected in the large intestine. Adenoma showed a focal polypoid growth, composed of papillary and/or tubular structures (Fig. 2A). A single layer of the columnar epithelium consisted of epithelial tumor cells, with slightly enlarged and unevenly located nuclei (Fig. 2B). The most common type of intestinal adenocarcinoma (Fig. 2C–E), observed in the current study, was the acinar adenocarcinoma (tubular carcinoma). The lesion was composed of multi-layered acinar tubular structures and the nuclei of the tumor cells were heterogeneous and irregularly-shaped, with prominent nucleoli (Fig. 2D), or the basement membrane of the neoplastic glands were not clear (Fig. 2F). Most of the colorectal adenocarcinomas (34/41), including 31 acinar and three papillary types, showed the polypoid growth pattern (Fig. 2C), whereas the only tumor that showed the non-polypoid growth pattern (7/41) was the acinar adenocarcinoma (Fig. 2E). Submucosal and muscular invasion was detected in 18% (6/34) of the PG type adenocarcinomas and 100% (7/7) of the NPG type adenocarcinomas. Mucinous adenocarcinoma and signet-ring cell carcinoma showed lesions, which were predominantly seen in the submucosa. Mucinous adenocarcinoma showed severe mucinous lesions in the submucosa, with sporadically formed irregular glandular structures (Fig. 2G and H). Intracytoplasmic mucin was observed in the tumor cells of signet-ring cell carcinoma. Mucinous adenocarcinoma and signet ring cell carcinoma were occasionally observed in the same specimen. In undifferentiated carcinoma, the tumor cells that lacked the cytological features of the typical epithelial tumor and had a high nuclear-cytoplasmic ratio with indistinct cellular borders were observed within the mucosa, submucosa and the muscular layer. This lesion was accompanied by partial lesions that displayed tubular, signet-ring and mucinous differentiations.
Fig. 2.
Canine intestinal epithelial tumors; HE stained. (A) Colorectal adenoma with polypoid proliferation. Bar=1,000 μm. (B) Higher magnification of Fig. 2A; tumor cells of colorectal adenoma with a slight atypia. Bar=40 μm. (C) Polypoid proliferation of papillary adenocarcinoma with focal submucosal invasion. Bar=1,000 μm. (D) Higher magnification of Fig. 2C; tumor cells of papillary adenocarcinoma with anisokaryosis and prominent nucleoli. Bar=40 μm. (E) Non-polypoid growth of acinar adenocarcinoma in the mucosa and invasion into the submucosa. Bar=1,000 μm. (F) Higher magnification of Fig. 2F; tumor cells of acinar adenocarcinoma with irregular tubular structures. Bar=40 μm. (G) Mucinous adenocarcinoma, with abundant mucin within the submucosa and muscularis. Bar=1,000 μm. (H) Higher magnification of Fig. 2G; tubular structures and signet-ring cells, with excess mucin in the lesion of mucinous adenocarcinoma. Bar=40 μm.
Discussion
Canine gastrointestinal epithelial tumors have attracted the interest of human tumorigenesis researchers because spontaneous gastrointestinal tumors are rare in experimental animals. However, the biological behaviors and clinicopathological and histopathological features of canine tumors are not yet well known. In canine medicine, gastrointestinal polyps are frequently detected, during endoscopic examinations. The present study, we reviewed 131 canine gastrointestinal epithelial tumors. While, information on the average age, location of tumor lesions, or predisposition towards a particular gender is already well-established15, 16, 17, the present study revealed that in Japan, gastrointestinal epithelial tumors occur more frequently in Jack Russell Terriers and Miniature Dachshunds. Previous reports on the subject revealed diverse results, with some authors stating that there was no predisposition towards any particular breed, in canine gastric neoplasms15, 18, whereas others showed that Belgian Shepherd Dogs19, Collies15, German Shepherds15, 20 and West Highland White terriers20 have increased risks of gastric or intestinal carcinoma. However, Jack Russell Terriers and Miniature Dachshunds were not considered by any of these reports. In the current study, Jack Russell Terriers were frequently observed to develop ‘multiple polyp’ lesions throughout the gastrointestinal tract, especially in the stomach and rectum, from an early age (two years). The gastrointestinal polypoid lesions, observed in in this breed, are similar to the lesions seen in human familial adenomatous polyposis (FAP). FAP is known to form a considerable number of gastrointestinal polyps in young patients21, 22, 23, 24, while several authors have also reported patients with relatively fewer polyps25, 26. The age and location of polypoid lesions in the present Jack Russell terrier cases were similar to those reported in human FAP22, 24, 26; however, the high incidence of gastric tumors may be a characteristic unique to the breed of Jack Russell terriers. In contrast, the large intestine is of importance, while considering the tumorigenesis of gastrointestinal tumors in Miniature Dachshunds because the breed frequently develops inflammatory polyposis in the colorectum27, 28. A previous study, by the authors, showed that inflammatory polyposis in Miniature Dachshunds may subsequently develop into adenoma or adenocarcinoma29. Hence, these two breeds of dog could possibly provide useful information as models in the study of tumorigenesis of gastrointestinal tumors.
According to the “WHO histological classification of tumors of the alimentary system of domestic animals,” malignant intestinal epithelial tumors are classified into acinar (tubular) adenocarcinoma, papillary adenocarcinoma, mucinous adenocarcinoma, signet-ring cell carcinoma, undifferentiated carcinoma and adenosquamous carcinoma3. Although there have been a few reports on canine gastrointestinal squamous cell carcinoma30, 31, the tumor was not detected in the present study. Moreover, these tumors frequently display mixed histopathological features within a specimen and hence, it would be difficult to make a differential diagnosis between acinar and papillary adenocarcinomas, based on the cell morphology alone. Thus, the histological growth pattern is of importance, while diagnosing an adenocarcinoma. The current study focused on the histologic growth patterns of adenocarcinomas, namely polypoid growth pattern and non-polypoid growth pattern. Invasion and/or metastasis were detected in 21% (10/48) of the PG type adenocarcinomas and 100% (17/17) of the NPG type adenocarcinomas.
Considerable research has been performed on the topic of human polypoid and non-polypoid lesions. Adenocarcinomas with the non-polypoid pattern of growth are considered as “de novo carcinomas,” from a histogenesis perspective and are different from adenocarcinomas with the polypoid growth pattern, indicative of an adenoma-carcinoma sequence11. The de novo carcinoma theory is based on the recognition of small lesions of colorectal cancer of the superficial type, forming NPG type or depressed lesions11, 13, 32, 33, which often lack adenomatous components34. NPG type adenocarcinomas frequently infiltrate into the submucosa and readily permeate the lymphatics and veins11, 13, 14, 32, 35, growing rapidly and resulting in advanced carcinomas34. These histological and biological characteristics may be similar between humans and canines.
In conclusion, the present study provided the status of the occurrence of canine gastrointestinal tumors in Japan; reported the Jack Russell terriers and Miniature Dachshunds as the most susceptible breeds and explained the histopathological features of the tumors. Histological growth patterns are important, in order to understand the histopathological and biological features of canine gastrointestinal tumors. In particular, most of the canine adenocarcinomas with non-polypoid growth pattern have a higher malignant potential, when compared to canine adenocarcinomas with polypoid growth pattern.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no conflicts of interest.
Acknowledgments
The authors would like to thank Mr. Pate Aughton, D.A.B.T., Dip.R.C.Path., ITR Laboratories Canada Inc., for language editing of this paper.
References
- 1.Youmans L, Taylor C, Shin E, Harrell A, Ellis AE, Séguin B, Ji X, and Zhao S. Frequent alteration of the tumor suppressor gene APC in sporadic canine colorectal tumors. PLoS One. 7: e50813 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelstein B, Fearon ER, Hamilton SR, Kern SE, Preisinger AC, Leppert M, Nakamura Y, White R, Smits AM, and Bos JL. Genetic alterations during colorectal-tumor development. N Engl J Med. 319: 525–532. 1988. [DOI] [PubMed] [Google Scholar]
- 3.Head KW, Cullen JM, Dubielzig RR, Else RW, Misdrop W, Patnaik AK, Tateyama S, and van der Gaag I. Histological classification of tumors of the alimentary system of domestic animals. In: World Health Organization International Histological Classification of Tumors of Domestic Animals. Second series. FY Schulman (ed). Armed Forces Institute of Pathology in cooperation with the American Registry of Pathology, Washington, D.C. 73–110. 2003. [Google Scholar]
- 4.Muto T, Bussey HJ, and Morson BC. The evolution of cancer of the colon and rectum. Cancer. 36: 2251–2270. 1975. [DOI] [PubMed] [Google Scholar]
- 5.Morson BC. Precancerous and early malignant lesions of the large intestine. Br J Surg. 55: 725–731. 1968. [DOI] [PubMed] [Google Scholar]
- 6.Morson B. President’s address. The polyp-cancer sequence in the large bowel. Proc R Soc Med. 67: 451–457. 1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ackerman LV, and Spratt JS, Jr . Do adenomatous polyps become cancer? Gastroenterology. 44: 905–908. 1963. [PubMed] [Google Scholar]
- 8.Ackerman LV. Malignant potential of polypoid lesions of the large intestine. Trans Stud Coll Physicians Phila. 32: 5–14. 1964. [PubMed] [Google Scholar]
- 9.Castleman B, and Krickstein HI. Do adenomatous polyps of the colon become malignant? N Engl J Med. 267: 469–475. 1962. [DOI] [PubMed] [Google Scholar]
- 10.Kuramoto S, and Oohara T. Minute cancers arising de novo in the human large intestine. Cancer. 61: 829–834. 1988. [DOI] [PubMed] [Google Scholar]
- 11.Shimoda T, Ikegami M, Fujisaki J, Matsui T, Aizawa S, and Ishikawa E. Early colorectal carcinoma with special reference to its development de novo. Cancer. 64: 1138–1146. 1989. [DOI] [PubMed] [Google Scholar]
- 12.Spratt JS, Jr , Ackerman LV, and Moyer CA. Relationship of polyps of the colon to colonic cancer. Ann Surg. 148: 682–696, discussion 696–698. 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kudo S, Kashida H, and Tamura T. Early colorectal cancer: flat or depressed type. J Gastroenterol Hepatol. 15(Suppl): D66–D70. 2000. [DOI] [PubMed] [Google Scholar]
- 14.Iishi H, Tatsuta M, Tsutsui S, Imanishi K, Otani T, Okuda S, Ishiguro S, and Taniguchi H. Early depressed adenocarcinomas of the large intestine. Cancer. 69: 2406–2410. 1992. [DOI] [PubMed] [Google Scholar]
- 15.Patnaik AK, Hurvitz AI, and Johnson GF. Canine gastrointestinal neoplasms. Vet Pathol. 14: 547–555. 1977. [DOI] [PubMed] [Google Scholar]
- 16.Frgelecová L, Škorič M, Fictum P, and Husník R. Canine gastrointestinal tract tumours: a restrospective study of 74 cases. Acta Vet Brno. 82: 387–392. 2013. [Google Scholar]
- 17.Sullivan M, Lee R, Fisher EW, Nash AS, and McCandlish IA. A study of 31 cases of gastric carcinoma in dogs. Vet Rec. 120: 79–83. 1987. [DOI] [PubMed] [Google Scholar]
- 18.Sautter JH, and Hanlon GF. Gastric neoplasms in the dog: a report of 20 cases. J Am Vet Med Assoc. 166: 691–696. 1975. [PubMed] [Google Scholar]
- 19.Fonda D, Gualtieri M, and Scanziani E. Gastric carcinoma in the dog: A clinicopathological study of 11 cases. J Small Anim Pract. 30: 353–360. 1989. [Google Scholar]
- 20.Holt PE, and Lucke VM. Rectal neoplasia in the dog: a clinicopathological review of 31 cases. Vet Rec. 116: 400–405. 1985. [DOI] [PubMed] [Google Scholar]
- 21.Al-Sukhni W, Aronson M, and Gallinger S. Hereditary colorectal cancer syndromes: familial adenomatous polyposis and lynch syndrome. Surg Clin North Am. 88: 819–844, vii. 2008. [DOI] [PubMed] [Google Scholar]
- 22.Half E, Bercovich D, and Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 4: 22 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groden J, Thliveris A, Samowitz W, Carlson M, Gelbert L, Albertsen H, Joslyn G, Stevens J, Spirio L, Robertson M, et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 66: 589–600. 1991. [DOI] [PubMed] [Google Scholar]
- 24.Nakamura Y, Nishisho I, Kinzler KW, Vogelstein B, Miyoshi Y, Miki Y, Ando H, Horii A, and Nagase H. Mutations of the adenomatous polyposis coli gene in familial polyposis coli patients and sporadic colorectal tumors. Princess Takamatsu Symp. 22: 285–292. 1991. [PubMed] [Google Scholar]
- 25.Hernegger GS, Moore HG, and Guillem JG. Attenuated familial adenomatous polyposis: an evolving and poorly understood entity. Dis Colon Rectum. 45: 127–134, discussion 134–136. 2002. [DOI] [PubMed] [Google Scholar]
- 26.Soravia C, Berk T, Madlensky L, Mitri A, Cheng H, Gallinger S, Cohen Z, and Bapat B. Genotype-phenotype correlations in attenuated adenomatous polyposis coli. Am J Hum Genet. 62: 1290–1301. 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohmi A, Tsukamoto A, Ohno K, Uchida K, Nishimura R, Fukushima K, Takahashi M, Nakashima K, Fujino Y, and Tsujimoto H. A retrospective study of inflammatory colorectal polyps in miniature dachshunds. J Vet Med Sci. 74: 59–64. 2012. [DOI] [PubMed] [Google Scholar]
- 28.Uchida E, Chambers JK, Nakashima K, Saito T, Ohno K, Tsujimoto H, Nakayama H, and Uchida K. Pathologic features of colorectal inflammatory polyps in Miniature Dachshunds. Vet Pathol. 53: 833–839. 2016. [DOI] [PubMed] [Google Scholar]
- 29.Saito T, Chambers JK, Nakashima K, Uchida E, Ohno K, Tsujimoto H, Uchida K, and Nakayama H. Histopathologic features of colorectal adenoma and adenocarcinoma developing within inflammatory polyps in Miniature Dachshunds. Vet Pathol. 55: 654–662. 2018. [DOI] [PubMed] [Google Scholar]
- 30.Kamano T, Kurihara M, Kishino H, Mizukami K, Kidokoro T, Wakabayashi K, and Kuwabara N. Experimental colonic cancer in a dog. Jpn J Surg. 11: 214–218. 1981. [DOI] [PubMed] [Google Scholar]
- 31.Patnaik AK, and Lieberman PH. Gastric squamous cell carcinoma in a dog. Vet Pathol. 17: 250–253. 1980. [DOI] [PubMed] [Google Scholar]
- 32.Kuramoto S, and Oohara T. Flat early cancers of the large intestine. Cancer. 64: 950–955. 1989. [DOI] [PubMed] [Google Scholar]
- 33.Ikegami M. A pathological study on colorectal cancer. From de novo carcinoma to advanced carcinoma. Acta Pathol Jpn. 37: 21–37. 1987. [PubMed] [Google Scholar]
- 34.Matsui T, Yao T, Yao K, Takenaka K, Sakurai T, Iwashita A, Fuchigami T, Aoyagi K, and Date H. Natural history of superficial depressed colorectal cancer: retrospective radiographic and histologic analysis. Radiology. 201: 226–232. 1996. [DOI] [PubMed] [Google Scholar]
- 35.Kurisu Y, Shimoda T, Ochiai A, Nakanishi Y, Hirata I, and Katsu KI. Histologic and immunohistochemical analysis of early submucosal invasive carcinoma of the colon and rectum. Pathol Int. 49: 608–616. 1999. [DOI] [PubMed] [Google Scholar]