Abstract
Desmoglein-3 (DSG3) is a potential target of cytotoxic antibody therapy for squamous cell carcinomas but is also expressed in various normal squamous epithelia. We obtained information about DSG3 distribution in mouse tissues by immunohistochemistry and conducted an intravenous multiple-dose study in mouse to estimate the toxic potential of anti-DSG3 therapy. DSG3 was expressed in the squamous epithelium of several organs including the skin, esophagus, tongue, forestomach, eye, and vagina. It was expressed at all estrous cycles of the vagina with changes in distribution patterns along with the structural changes in each cycle, and expression was reduced in ovariectomized (OVX) mice. On the administration of the antibody, there was disarrangement of the vaginal mucosal epithelium with formation of miroabscess, increased granulocyte infiltration, and single cell necrosis. Despite similar expression levels of DSG3 in other tissues, histopathological changes were limited to the vagina. The severity of the changes was reduced by ovariectomy. From these findings, the lesions were thought to be related to the drastic change in the histological structure of the vaginal mucosa accompanying the estrous cycle. Thus, we have shown that the changing expression of target antigen distribution and its relationship with physiological changes in tissue structure are important features for estimating the toxic potential of cytotoxic antibody therapy.
Keywords: cytotoxic antibody, desmoglein-3, tissue distribution, estrous cycle, vagina
Introduction
When developing therapeutic antibodies, it is important to understand the tissue distribution of the target antigen to estimate the potential of the therapy1, 2. Desmoglein-3 (DSG3) is overexpressed in the human squamous cell carcinoma3, 4, 5 and is thought to be a promising target for anti-tumor therapy with therapeutic antibodies. However, DSG3 is also expressed in normal squamous epithelium such as skin and stratified squamous mucosa6, 7, and is expected to be widely distributed in many organs. Additionally, anti-DSG3 autoantibodies cause a potentially fatal autoimmune disease called pemphigus vulgaris (PV) in humans, which involves the formation of blisters that easily rupture causing painful erosions in the skin and mucosa8, 9. Hence, there was a concern that targeting DSG3 for antibody therapy would cause severe toxicity. In fact, in our preliminary experiment in mice using an antibody with PV-like effects, there were severe toxic changes in squamous epithelium such as the esophagus and vagina, causing lethality.
Funahashi et al. deemed that modifying the function of an anti-DSG3 antibody to eliminate PV-like effects and to exert effects through antibody-dependent cellular cytotoxicity (ADCC) would be effective in avoiding severe toxicity while retaining robust anti-tumor effects10. ADCC activation is largely dependent on antigen expression level11, 12, 13; therefore, they assumed that compared to tumor tissues, normal tissues express DSG3 at lower levels. Thus, they would be able to separate the efficacy from toxicity of the antibody. By addressing this point, they succeeded in generating antibodies with robust anti-tumor activity and no severe toxicity10. However, as DSG3 was expected to be expressed in a variety of organs and tissues, there was a risk of unexpected toxicity caused by the novel antibody function.
Thus, a further understanding of the relationship between the distribution of DSG3 and its physiological features in vivo was thought necessary to evaluate the potential of toxic effects to normal tissues by anti-DSG3 therapy with an ADCC antibody. Therefore, we conducted immuno-histochemical analysis of DSG3 in mice to elucidate its distribution and a detailed pathological analysis in mice administered the18-1m mouse anti-DSG3 antibody that has ADCC functions as previously described by Funahashi et al.10. As changes were found in the vagina, we also evaluated the effects in ovariectomized (OVX) mice.
Materials and Methods
Animals
For the analysis of DSG3 distribution in mouse, normal or OVX BALB/cAnNCrlCrlj mice were obtained by Forerunner Pharma Research Co., Ltd. (Kanagawa, Japan) from Charles River Japan, Inc. (Kanagawa, Japan) at the age of eight (intact) or nine (OVX) weeks, and they were acclimatized before subjecting to the study at ten weeks of age at Forerunner Pharma Research Co., Ltd. The animals were fed pelleted chow (MF 30kGy, Oriental Yeast Co., Ltd., Tokyo, Japan), given mineral water with 2 ppm sodium hypochlorite ad libitum, and housed in plastic cages with wood chip bedding at a temperature of 20–26°C, humidity 30–70%, and a 12 h light/12 h dark cycle. Animal procedures were approved by the Institutional Animal Care and Use Committee at Forerunner Pharma Research Co., Ltd.
For the multiple-dose study, BALB/cAnNCrlCrlj mice were obtained by Chugai Pharmaceutical Co., Ltd. (Kanagawa, Japan) from Charles River Japan, Inc. at the age of five weeks and were acclimatized before subjecting to the study at six weeks of age at Chugai Pharmaceutical Co., Ltd. The animals were fed pelleted chow (CE-2, CLEA Japan, Inc.), given tap water with 2 ppm sodium hypochlorite ad libitum, and housed in plastic cages with wood chip bedding at a temperature of 20–26°C, humidity 30–70%, and a 12 h light/12 h dark cycle. A sham operation group and an OVX group were designated to compare the effects in animals with an intact or arrested estrous cycle. An abdominal incision was surgically performed for the sham operation group. For the OVX group, both ovaries were surgically removed. The operations were performed under pentobarbital anesthesia. The multiple-dose study was conducted in 2009 and was approved according to the guidelines at the time by the Institutional Animal Care and Use Committee at Chugai Pharmaceutical Co., Ltd.
Anti-DSG3 antibodies
Two types of anti-DSG3 antibodies were used in this study. A commercially available mouse anti-mouse DSG3 antibody (clone AK18, Medical & Biological Laboratories Co., Ltd., Aichi, Japan) was used as the primary antibody for immunohistochemistry in mouse tissues. A mouse anti-mouse DSG3 antibody (clone 18-1m, Forerunner Pharma Research Co., Ltd., Tokyo, Japan) obtained in a previous study10 was used for the multiple-dose study in the mouse. This antibody exhibits ADCC activity but does not inhibit cell–cell interaction in vitro10. The antibody also has robust anti-tumor activity in a syngeneic mouse tumor model derived by subcutaneous inoculation of LC-12 cells into Balb/c mice10.
Study designs
Study 1: Evaluation of DSG3 distribution in mouse tissue
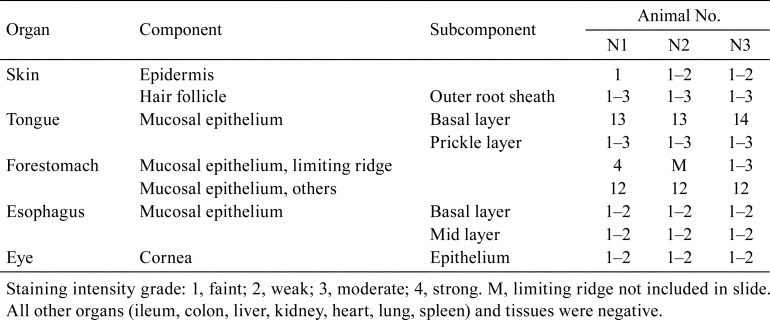
To determine the distribution of DSG3 in normal mice, the following tissues were evaluated (n=3): the skin, tongue, stomach, ileum, colon, esophagus, eye, liver, kidney, heart, lung, and spleen (Table 1). As the tissue structure of the vagina changes in association with the estrous cycle, tissues at the stages of proestrus (n=3), estrus (n=3), metestrus (n=3), and diestrus (n=3) were evaluated (Table 1). The animals were euthanized under deep isoflurane anesthesia at the end of the acclimation period, and the tissues were collected and fixed for 24 h in 4% PFA and embedded by the AMeX method as previously described14, 15.
Table 1. Study Groups.
Study 2: Effects of multiple dosing with the 18-1m mouse anti-DSG3 antibody
To evaluate the effects of the 18-1m mouse anti-DSG3 antibody in vivo, the antibody was intravenously administered into the tail vein of mice (Fig. 1) once a week for 3 weeks. Additionally, to elucidate the correlation of the changes with the estrous cycle, sham operation and OVX group were designated for each antibody. The animals were allocated into 3 groups each for the sham and OVX groups by body weight. Based on previous in vivo efficacy studies10, the antibody was administered weekly at 0, 10, 50 mg/kg (n=5) for each operation group for 3 weeks, starting at 26 days after the surgical procedures (Table 1). The body weight of all animals was measured twice in the week before the first administration, and 4 times a week, including the days of the administration, and at necropsy. The animals were euthanized by exsanguination from the abdominal artery under deep isoflurane anesthesia at 3 days after the 3rd administration. Gross examination was performed and the skin, tongue, stomach, esophagus, eye, liver, kidney, heart, lung, spleen, and vagina were sampled, fixed in 10% neutral buffered formalin, and embedded into paraffin by a routine method. One animal of the OVX group administered 10 mg/kg was excluded from the study because the OVX procedure was considered unsuccessful.
Fig. 1.
Study design for antibody administration.
Tissue preparation
For both the DSG3 distribution study and the antibody administration study, hematoxylin and eosin-stained slides were prepared by a routine method. For the DSG3 distribution study, immunohistochemistry for mouse DSG3 was conducted. Briefly, the tissue sections were deparaffinized and treated with microwave heating in Target Retrieval Solution (Agilent Technologies Inc., Santa Clara, CA, USA). Then the sections were treated with 0.3% H2O2 in methanol to quench endogenous peroxidase and then blocked with a mouse-on-mouse blocking reagent (Vector Laboratories, Burlingame, CA, USA) and with 5% bovine serum albumin in Tris-buffered saline. Next, the slides were incubated with the primary antibody and then subsequently with a rat anti-mouse IgG1 heavy chain antibody (Abcam, Cambridge, UK), and a rat IgG heavy and light chain antibody (Bethyl Laboratories Inc., Montgomery, TX, USA). Finally, the slides were incubated with streptavidin-HRP (Vector Laboratories) and the reaction was visualized with a 3, 3’-diaminobenzidine (FUJIFILM Wako Pure Chemical Co., Osaka, Japan) solution, counterstained with hematoxylin. The slides were read evaluated under a light microscope.
Histopathological evaluation of the effects of antibody administration
All the tissues sampled at necropsy were histopathologically evaluated. As there were only findings in the vagina, grading by severity (0, not observed; 1, very mild; 2, mild; 3, moderate; 4, severe) of the changes in this organ was conducted for each finding. A histology score was designated for each animal by adding up the histology grades.
Statistical analysis
The Dunnet’s test was performed to compare the body weights between dose groups. P<0.05 was judged to be statistically significant.
Results
Study 1: Evaluation of DSG3 distribution in mouse tissue
Expression patterns in squamous epithelium
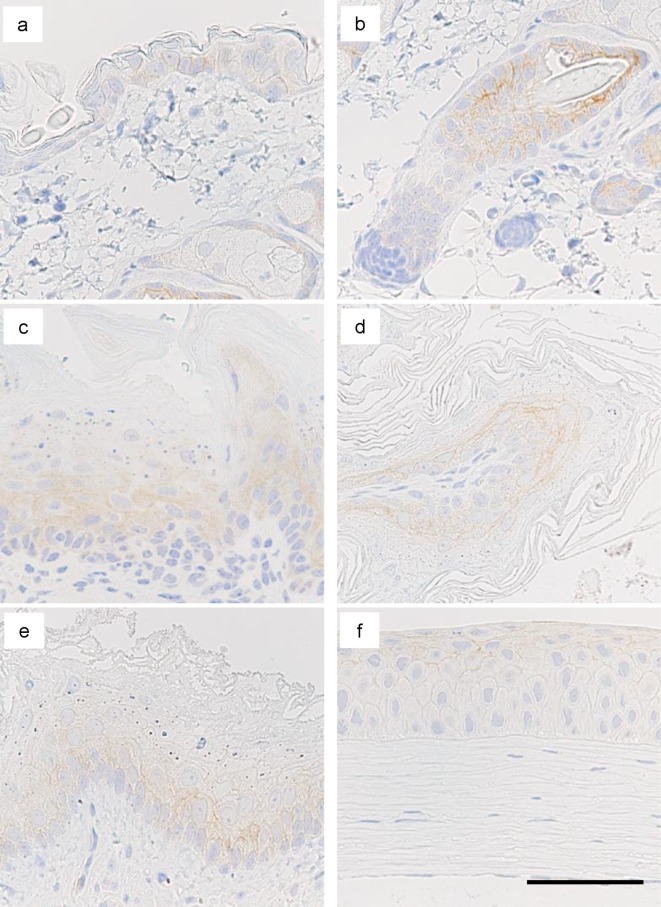
Positive staining for DSG3 was observed in the cytoplasmic membrane of squamous epithelium including the tongue, esophagus, cornea, skin, and forestomach (Fig. 2, Table 2). In the tongue and esophagus, positive staining was localized in the basal to prickle cell layers; whereas, in the cornea, positive staining was observed throughout the layer (Fig. 2, Table 2). There was no clear trend for localization in the layers of the epidermis and forestomach, but there tended to be stronger staining in the limiting ridge of the forestomach and in the outer root sheath of hair follicles (Fig. 2). There were no other positive organs or tissues.
Fig. 2.
Immunohistochemical staining for desmoglein-3 (DSG3) in squamous epithelium of mouse. Representative images of the epidermis (a), hair follicle (b), tongue (c), limiting ridge of the forestomach (d), esophagus (e), and cornea (f) are shown. Bar=100 μm.
Table 2. Results of Immunohistochemical Staining for Desmoglein-3 (DSG3) in Mouse Tissues.
DSG3 expression in the vagina in relation with the estrous cycle
The expression of DSG3 was evaluated in the vagina at the stages of proestrus, estrus, metestrus, and diestrus and of OVX animals (Fig. 3, Table 3). At proestrus and estrus, there was positive staining in the basal to prickle cell layer of the mucosa, as with the tongue and esophagus (Fig. 3, Table 3). At metestrus and diestrus, positive staining was observed in the whole mucosal layer, as in the cornea (Fig. 3, Table 3). There was no difference in staining intensity in relation with the changes of the estrous cycle (Fig. 3, Table 3). In the vagina of OVX animals, the mucosa consisted of 1–2 layers of small cuboidal epithelial cells (Fig. 3). There was very weak staining in the cytoplasm of the epithelium (Fig. 3).
Fig. 3.
Immunohistochemical staining for desmoglein-3 (DSG3) in mouse vaginal epithelium. Represenative images of normal and ovariectomized (OVX) mice are shown. Bar=100 μm.
Table 3. Results of Immunohistochemical Staining for Desmoglein-3 (DSG3) in Mouse Vaginal Tissues of Each Estrous Cycle Stage.
Study 2: Effects of multiple dosing with 18-1m mouse anti-DSG3 antibody
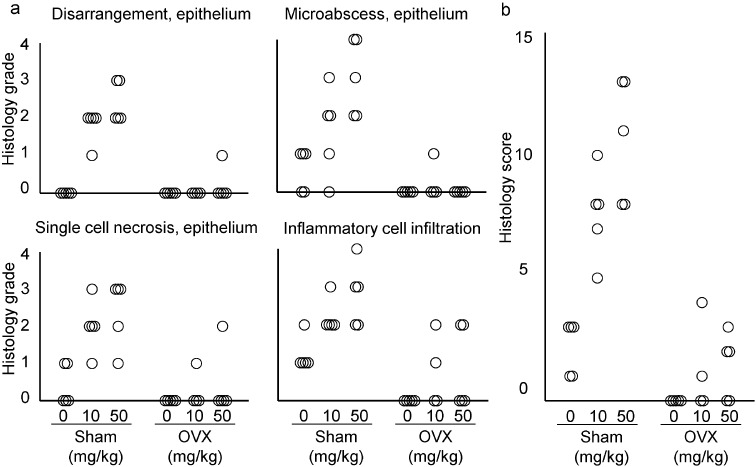
On administration of the 18-1m antibody to mice, there were no unscheduled deaths in any of the groups or doses. In the sham group, there was no difference in body weight between the vehicle and antibody administered groups (Fig. 4). In the OVX group, there was an increase in body weight from approximately 5 weeks after OVX in the vehicle group (Fig. 4). In the OVX antibody-administered groups, there was a slight transient decrease from day 1 to 3 after the first administration, and an absence of weight gain until the day of necropsy (Fig. 4). There were no gross findings in any of the animals at necropsy except for the changes related to OVX in the OVX group. Histopathological findings were observed only in the vagina. There were no findings in other organs (Fig. 5a) besides OVX-related changes and changes in the spleen related to non-specific immune activation caused by antibody administration. In the OVX animals, the uterine mucosal epithelium consisted of a single layer of cuboidal cells, showing that the operation was successful. In the vagina of the sham group animals administered the antibody, there was disarrangement of the vaginal epithelium (Fig. 5b, 6a) at both doses. Formation of microabscess in the epithelium was also notably increased, as well as infiltration of granulocytes in the submucosa, muscular layer, and in the lumen of the vagina (Fig. 5b, 6a). Single-cell necrosis was slightly increased in the antibody administered groups. There was a variation in the findings between animals that was thought related to the estrous cycle ranging from a non-keratinized to keratinized epithelium. However, as the structure of the epithelium was disrupted, the precise estrous cycle stage in animals treated with antibody could not be distinguished. The highest histology scores were found in the 50 mg/kg group, which suggests that there is a dose dependency (Fig. 6b). Compared to the sham groups, changes in the OVX groups were markedly less severe (Fig. 6). There were mild changes in some of the OVX animals of the antibody administered groups, and in these cases, there were areas of stratified epithelium. As there were no gross or histopathological findings except for slight changes in the vagina, there were thought to be no effects of the antibody related to the reduced weight gain in the OVX animals.
Fig. 4.
Body weight of Sham and ovariectomized (OVX) group animals. Each symbol shows the mean ± S.D. Arrows show the days of antibody administration. There was a statistically significant (P<0.05) difference between the OVX vehicle and 50 mg/kg group at days 34, 40, and 42 (*).
Fig. 5.
Representative images of tissues from mice administered the 18-1m mouse anti- desmoglein (DSG) antibody. Representative images of the tongue (a, left) and esophagus (a, right) are shown. HE stain. Bar=100 μm. In the vaginal epithelium of the sham group, there was disarrangement of mucosal structure, marked formation of microabscess, single cell necrosis (arrows) in the epithelium, and increased inflammatory cell infiltration throughout the mucosa (b). HE stain. Left, bar=200 μm. Right, high magnification of left, bar=100 μm.
Fig. 6.
Severity of changes in the vaginal mucosa of mice treated with the 18-1m mouse anti-desmoglein-3 (DSG3) antibody. Each symbol represents one animal.
Discussion
In the current study, we have studied the distribution of DSG3 in mouse tissues by immunohistochemistry and the effects of antibody administration in mice to evaluate the potential of toxic effects in normal tissues by anti-DSG3 therapy with an ADCC antibody.
By immunohistochemical analysis, DSG3 was expressed in the squamous epithelium of multiple organs in the mouse. There were two distinct expression patterns of DSG3 in relatively well stratified squamous epithelium: a basal to a spinous pattern (tongue, esophagus, proestrus and estrus vagina) and a whole-layer pattern (cornea, metestrus and diestrus of vagina). The two patterns were largely consistent with the previous reports in humans8, 16. Thus, we confirmed that DSG3 is widely expressed in mouse organs, and from these results we expected that organs expressing DSG3 would be affected by antibody administration.
In the multiple-dose study in mouse, although there were tissues with similar or higher expression levels of DSG3 compared with the vagina, changes were observed only in the vagina and no changes were observed in the other squamous epithelium. The drastic change in tissue structure of the vaginal mucosa in relation to the estrous cycle is well known in rodents17. Additionally, DSG3-dependent signaling is thought to have a role in wound healing18. Thus, we deemed that antibody administration only affects tissues with structures that are under reconstruction.
The structure of the vaginal epithelium was distorted by antibody administration, suggesting that DSG3 is necessary for maintaining the epithelial structure. To our knowledge, there are no reports concerning the changing expression of DSG3 in the mouse vagina, so how it functions during structural changes is unknown. However, DSG3 is known to be important in maintaining the orientation and integrity of stratified squamous epithelium19, 20, and the evidence of altered tissue structure by antibody-treatment in the current study was thought consistent with these facts. Thus, it was thought that DSG3 has a role in maintaining the structure of the vaginal mucosa during the estrous cycle in mice.
As for the mode of action of the antibody against vaginal mucosa and its relationship with the estrous cycle, the fact that the structure of the vaginal mucosa was altered indicates an interference with DSG3 function during structural changes. Moreover, the inflammatory changes in the vagina, along with single-cell necrosis of the mucosa suggest a role for ADCC, but no definitive evidence was obtained in the current study. Further studies such as time-course observation after single dose administration in animals tested for vaginal swabs to determine the estrous cycle stage at the time of administration or by administering an antibody engineered to eliminate ADCC effects are thought to be effective in further elucidating the mechanisms related to the physiological role of DSG3 in the mouse vagina and the modifying effects of the current antibody.
In the current study, there was vaginal atrophy in OVX mice with reduced expression of DSG3 in the epithelium. Vaginal atrophy is associated with reduced expression of DSG1 in humans, and in part can be recovered by the administration of estrogens21. It is also known that estrogens enhance the formation of desmosomes and upregulate desmosomal proteins22. The above facts suggest the involvement of estrogen in the regulation of DSG3 expression in the mouse vagina. Thus, studying the relationship between sex hormones and DSG3 expression may be valuable information for estimating the effects of anti-DSG3 antibody administration.
In the current study, the changes were largely reduced in OVX mice, which was thought to be due to the reduced expression of DSG3 in the atrophic vaginal epithelium. Thus, OVX was effective in avoiding severe changes. In mice, with slight changes, the mucosal epithelium may have still been in a changing state from stratified epithelium to a mono-layered epithelium at the time of administration. It is known that there is a gradual change in tissue structure after OVX23 in mice. As in mice the human vagina also shows drastic changes in tissue structure, such as changes in mucosal thickness and parakeratosis24; hence, there may be toxic effects by anti-DSG3 therapy. However, the target patient segment for DSG3 therapy is in cancer patients, and expected in older age groups25, so further studies to determine the relationship between the distribution of DSG3 and menopause in humans may enable a therapeutic strategy in minimizing toxic effects to normal tissue.
Expression analysis of target antigens has provided valuable information for prediction or retrospective analysis of efficacy and toxicity with therapeutic antibodies26, 27, 28, but analysis of a single time point only provides a snapshot-view. Our current results show that if the target antigen is expressed in tissues with dynamic changes, the temporal aspects should be considered. Thus, we have shown that the changing expression of target antigen distribution and its relationship with physiological changes in tissue structure are important features for estimating the toxic potential of cytotoxic antibody therapy.
Disclosure of Potential Conflicts of Interest
The authors declare that they have no competing interests.
Acknowledgments
We would like to thank Ms. Asuka Ikeda of Chugai Research Institute for Medical Science, Inc. for assisting in histopathological procedures.
References
- 1.Suzuki M, Kato C, and Kato A. Therapeutic antibodies: their mechanisms of action and the pathological findings they induce in toxicity studies. J Toxicol Pathol. 28: 133–139. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leach MW, Halpern WG, Johnson CW, Rojko JL, MacLachlan TK, Chan CM, Galbreath EJ, Ndifor AM, Blanset DL, Polack E, and Cavagnaro JA. Use of tissue cross-reactivity studies in the development of antibody-based biopharmaceuticals: history, experience, methodology, and future directions. Toxicol Pathol. 38: 1138–1166. 2010. [DOI] [PubMed] [Google Scholar]
- 3.Fukuoka J, Dracheva T, Shih JH, Hewitt SM, Fujii T, Kishor A, Mann F, Shilo K, Franks TJ, Travis WD, and Jen J. Desmoglein 3 as a prognostic factor in lung cancer. Hum Pathol. 38: 276–283. 2007. [DOI] [PubMed] [Google Scholar]
- 4.Chen YJ, Chang JT, Lee L, Wang HM, Liao CT, Chiu CC, Chen PJ, and Cheng AJ. DSG3 is overexpressed in head neck cancer and is a potential molecular target for inhibition of oncogenesis. Acta Histochem. 116: 803–809. 2014. [DOI] [PubMed] [Google Scholar]
- 5.Fang WK, Chen B, Xu XE, Liao LD, Wu ZY, Wu JY, Shen J, Xu LY, and Li EM. Altered expression and localization of desmoglein 3 in esophageal squamous cell carcinoma. Acta Histochem. 116: 803–809. 2014. [DOI] [PubMed] [Google Scholar]
- 6.Shirakata Y, Amagai M, Hanakawa Y, Nishikawa T, and Hashimoto K. Lack of mucosal involvement in pemphigus foliaceus may be due to low expression of desmoglein 1. J Invest Dermatol. 110: 76–78. 1998. [DOI] [PubMed] [Google Scholar]
- 7.Kitajima Y. 150(th) anniversary series: Desmosomes and autoimmune disease, perspective of dynamic desmosome remodeling and its impairments in pemphigus. Cell Commun Adhes. 21: 269–280. 2014. [DOI] [PubMed] [Google Scholar]
- 8.Kasperkiewicz M, Ellebrecht CT, Takahashi H, Yamagami J, Zillikens D, Payne AS, and Amagai M. Pemphigus. Nat Rev Dis Primers. 3: 17026 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amagai M, and Stanley JR. Desmoglein as a target in skin disease and beyond. J Invest Dermatol. 132: 776–784. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Funahashi SI, Kawai S, Fujii E, Taniguchi K, Nakano K, Ishikawa S, Aburatani H, and Suzuki M. Generation of an anti-desmoglein 3 antibody without pathogenic activity of pemphigus vulgaris for therapeutic application to squamous cell carcinoma. J Biochem. 164: 471–481. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishiguro T, Sugimoto M, Kinoshita Y, Miyazaki Y, Nakano K, Tsunoda H, Sugo I, Ohizumi I, Aburatani H, Hamakubo T, Kodama T, Tsuchiya M, and Yamada-Okabe H. Anti-glypican 3 antibody as a potential antitumor agent for human liver cancer. Cancer Res. 68: 9832–9838. 2008. [DOI] [PubMed] [Google Scholar]
- 12.Kawai S, Koishihara Y, Iida S, Ozaki S, Matsumoto T, Kosaka M, and Yamada-Okabe H. Construction of a conventional non-radioisotope method to quantify HM1.24 antigens: correlation of HM1.24 levels and ADCC activity of the humanized antibody against HM1.24. Leuk Res. 30: 949–956. 2006. [DOI] [PubMed] [Google Scholar]
- 13.Kawai S, Yoshimura Y, Iida S, Kinoshita Y, Koishihara Y, Ozaki S, Matsumoto T, Kosaka M, and Yamada-Okabe H. Antitumor activity of humanized monoclonal antibody against HM1.24 antigen in human myeloma xenograft models. Oncol Rep. 15: 361–367. 2006. [PubMed] [Google Scholar]
- 14.Sato Y, Mukai K, Watanabe S, Goto M, and Shimosato Y. The AMeX method. A simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am J Pathol. 125: 431–435. 1986. [PMC free article] [PubMed] [Google Scholar]
- 15.Suzuki M, Katsuyama K, Adachi K, Ogawa Y, Yorozu K, Fujii E, Misawa Y, and Sugimoto T. Combination of fixation using PLP fixative and embedding in paraffin by the AMeX method is useful for histochemical studies in assessment of immunotoxicity. J Toxicol Sci. 27: 165–172. 2002. [DOI] [PubMed] [Google Scholar]
- 16.Stanley JR, and Amagai M. Pemphigus, bullous impetigo, and the staphylococcal scalded-skin syndrome. N Engl J Med. 355: 1800–1810. 2006. [DOI] [PubMed] [Google Scholar]
- 17.Westwood FR. The female rat reproductive cycle: a practical histological guide to staging. Toxicol Pathol. 36: 375–384. 2008. [DOI] [PubMed] [Google Scholar]
- 18.Rötzer V, Hartlieb E, Winkler J, Walter E, Schlipp A, Sardy M, Spindler V, and Waschke J. Desmoglein 3-dependent signaling regulates keratinocyte migration and wound healing. J Invest Dermatol. 136: 301–310. 2016. [DOI] [PubMed] [Google Scholar]
- 19.Merritt AJ, Berika MY, Zhai W, Kirk SE, Ji B, Hardman MJ, and Garrod DR. Suprabasal desmoglein 3 expression in the epidermis of transgenic mice results in hyperproliferation and abnormal differentiation. Mol Cell Biol. 22: 5846–5858. 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elias PM, Matsuyoshi N, Wu H, Lin C, Wang ZH, Brown BE, and Stanley JR. Desmoglein isoform distribution affects stratum corneum structure and function. J Cell Biol. 153: 243–249. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotreau MM, Chennathukuzhi VM, Harris HA, Han L, Dorner AJ, Apseloff G, Varadarajan U, Hatstat E, Zakaria M, Strahs AL, Crabtree JS, Winneker RC, and Jelinsky SA. A study of 17beta-estradiol-regulated genes in the vagina of postmenopausal women with vaginal atrophy. Maturitas. 58: 366–376. 2007. [DOI] [PubMed] [Google Scholar]
- 22.Maynadier M, Chambon M, Basile I, Gleizes M, Nirde P, Gary-Bobo M, and Garcia M. Estrogens promote cell-cell adhesion of normal and malignant mammary cells through increased desmosome formation. Mol Cell Endocrinol. 364: 126–133. 2012. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki A, Enari M, Abe Y, Ohta Y, and Iguchi T. Effect of ovariectomy on histological change and protein expression in female mouse reproductive tracts. In Vivo. 10: 103–110. 1996. [PubMed] [Google Scholar]
- 24.Farage MA, and Maibach HI. Morphology and physiological changes of genital skin and mucosa. Curr Probl Dermatol. 40: 9–19. 2011. [DOI] [PubMed] [Google Scholar]
- 25.Lagiou P, Trichopoulos D, and Adami HO. Chapter 26. Epidemiology in the identification of cancer causes. In: The Cancer Handbook, Volume 1, 2nd ed. MR Alison (ed). John Wiley & Sons Ltd., West Sussex. 644–665. 2007. [Google Scholar]
- 26.Fujii E, Watanabe K, Nishihara K, Suzuki M, and Kato A. Hazard characterization of an anti-human tissue factor antibody by combining results of tissue cross-reactivity studies and distribution of hemorrhagic lesions in monkey toxicity studies. Regul Toxicol Pharmacol. 90: 289–296. 2017. [DOI] [PubMed] [Google Scholar]
- 27.Kato A, Watanabe T, Yamazaki M, Deki T, and Suzuki M. IL-6R distribution in normal human and cynomolgus monkey tissues. Regul Toxicol Pharmacol. 53: 46–51. 2009. [DOI] [PubMed] [Google Scholar]
- 28.Furukawa S, Nagaike M, and Ozaki K. Databases for technical aspects of immunohistochemistry. J Toxicol Pathol. 30: 79–107. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]