Abstract
Introduction
Endothelial nitric oxide synthase (eNOS) polymorphisms might influence predisposition to hemorrhagic cerebral vascular diseases, but the results of already published studies regarding relationship between eNOS polymorphisms and hemorrhagic cerebral vascular diseases were still controversial.
Methods
The authors performed this meta‐analysis to estimate relationship between eNOS polymorphisms and hemorrhagic cerebral vascular diseases in a larger pooled population by combing the results of already published related studies. The authors searched Pubmed, Embase, Web of Science, and CNKI for already published studies.
Results
Eighteen already published studies were pooled analyzed in this meta‐analysis. The pooled meta‐analyses results showed that eNOS rs2070744 polymorphism was significantly associated with predisposition to hemorrhagic cerebral vascular diseases in East Asians (dominant comparison: OR = 0.77, p = .01; overdominant comparison: OR = 1.24, p = .04; allele comparison: OR = 0.78, p = .006) Nevertheless, the pooled meta‐analyses did not reveal any positive results for eNOS rs1799983 and rs869109213 polymorphisms.
Conclusions
This meta‐analysis suggested that eNOS rs2070744 polymorphism, but not rs1799983 and rs869109213 polymorphisms, might influence predisposition to hemorrhagic cerebral vascular diseases in East Asians.
Keywords: East Asians, endothelial nitric oxide synthase (eNOS), gene polymorphisms, hemorrhagic cerebral vascular diseases, meta‐analysis
This is a comprehensive meta‐analysis about eNOS polymorphisms and hemorrhagic cerebral vascular diseases. We found that eNOS rs2070744 polymorphism might influence predisposition to hemorrhagic cerebral vascular diseases in East Asians. Our findings indicated that eNOS may involve in the development of hemorrhagic cerebral vascular diseases.
1. INTRODUCTION
Hemorrhagic cerebral vascular disease, characterized by structural or functional abnormalities of cerebral vessels, and associated spontaneous bleeding into brain tissue, is one of the leading causes of death and disability all over the world (Global Burden of Disease Study 2013 Collaborators, 2015; Caceres & Goldstein, 2012). Although its definite etiologies and pathogenesis mechanisms are still unclear, accumulating evidence suggested that genetic architecture greatly influence its development. First, the incidences of hemorrhagic cerebral vascular diseases in different populations differed significantly (Backhaus et al., 2015; Krishnan et al., 2018), and genetic background was probably the underlying reason of this phenomenon. Second, previous association studies also detected numerous predisposing gene loci of hemorrhagic cerebral vascular diseases in different populations (Carpenter, Singh, Gandhi, & Prestigiacomo, 2016; Chen, Chang, & Chen, 2018). However, the etiologies and pathogenesis mechanisms of hemorrhagic cerebral vascular diseases are extremely sophisticated, and genetic factors that contribute to the development of hemorrhagic cerebral vascular diseases still need intensively explorations.
Endothelial nitric oxide synthase (eNOS) is essential for maintaining vascular homeostasis and plays vital roles in modulating vascular endothelial function (Besler et al., 2011; Tsutsui, Shimokawa, Morishita, Nakashima, & Yanagihara, 2006). Therefore, if a polymorphism can impact gene expression or protein structure of eNOS, it is likely that this polymorphism might lead to severe vascular endothelial dysfunction and influence predisposition to hemorrhagic cerebral vascular diseases.
In the last two decades, investigators across the world extensively explored relationship between eNOS polymorphisms and hemorrhagic cerebral vascular diseases, especially for intracranial aneurysm (IA) and its associated aneurysmal subarachnoid hemorrhage (aSAH), yet the relationship between eNOS polymorphisms and hemorrhagic cerebral vascular diseases is still controversial. Thus, we performed this meta‐analysis to get a more statistically reliable conclusion regarding relationship between eNOS polymorphisms and hemorrhagic cerebral vascular diseases by pooling the results of already published relevant studies.
2. MATERIALS AND METHODS
The PRISMA guideline was followed by the authors when conducting this meta‐analysis (Moher, Liberati, Tetzlaff, Altman, & PRISMA group, 2009).
2.1. Literature search and inclusion criteria
Literature searching of Pubmed, Web of Science, Embase, and CNKI was performed by the authors using the following terms: (endothelial nitric oxide synthase or nitric oxide synthase type III or eNOS or NOS3) and (polymorphism or variant or variation or mutation or SNP or genome‐wide association study or genetic association study or genotype or allele) and (hemorrhagic cerebral vascular disease or hemorrhagic cerebrovascular disease or cerebral hemorrhage or basal ganglia hemorrhage or putaminal hemorrhage or subarachnoid hemorrhage or cerebrum hemorrhage or brain hemorrhage or intracranial hemorrhage or intracranial aneurysm or cerebral aneurysm). When searching CNKI, the authors translated searching terms into Chinese. The authors also checked the references of obtained articles manually for additional related studies.
Eligible studies must meet all of three inclusion criteria: I. formally published case–control studies evaluating relationship between eNOS polymorphisms and hemorrhagic cerebral vascular diseases; II. provide sufficient genotypic data of eNOS polymorphisms in patients with hemorrhagic cerebral vascular diseases and controls; III. the whole manuscript is available in English or Chinese. Articles were excluded when at least one of the following three conditions was fulfilled: I. studies not concerning eNOS polymorphisms and hemorrhagic cerebral vascular diseases; II. reviews or expert comments; III. case series only involved participants with hemorrhagic cerebral vascular diseases. When duplicate reports were observed during literature searching, only the most recent one was included for pooled meta‐analyses. The literature searching was performed by the first author and the second author, and the latest literature searching update was performed on 10 November 2019.
2.2. Data extraction and quality assessment
We extracted following items from included studies: I. surname of the first author; II. year of online publication; III. country and ethnicity of involved participants; IV. number of patients and controls; V. genotypic distributions of eNOS polymorphisms in patients and control subjects. We also calculated p values of Hardy–Weinberg equilibrium (HWE) based on genotypic distributions of eNOS polymorphisms using chi‐square test.
The authors used Newcastle–Ottawa scale (NOS) to assess the quality of included studies (Stang, 2010). The NOS's score range is from zero to nine, and the methodology quality of an article was considered to be good when it got a score of more than seven.
Data extraction and quality assessment of included studies were performed by the first author and the second author separately. The authors wrote to the corresponding authors for additional data when they failed to extract necessary information from included studies. In case of disagreement between the first author and the second author, the two authors would consult the corresponding author for suggestions, and a consensus must be reached among three authors.
2.3. Statistical analyses
The authors used Review Manager to pool meta‐analyses results (Version 5.3.3, The Cochrane Collaboration, Software Update). The authors calculated ORs and 95% CIs of Z test to evaluate relationship between eNOS polymorphisms and predisposition to hemorrhagic cerebral vascular diseases in dominant, recessive, overdominant, and allele models (Keat Wei, Griffiths, Irene, & Kooi, 2019; Wei, Griffiths, Kooi, & Irene, 2019). The authors set the statistical significant threshold at 0.05. The authors used I 2 statistics to estimate heterogeneity. I 2 values of 25%, 50%, and 75% represented low, moderate, and high heterogeneities, respectively. The authors used DerSimonian–Laird method (random‐effect model) to pool the results if I 2 is larger than 50%. Otherwise, the authors used Mantel–Haenszel method (fixed‐effect model) to pool the results. The authors also conducted subgroup analyses by ethnicity. The authors examined stabilities of pooled results through omitting one study each time and pooling the results of the other studies. The authors examined publication biases through funnel plots.
3. RESULTS
3.1. Characteristics of included studies
One hundred and sixty articles were retrieved by the authors through our literature searching strategy. The authors assessed thirty‐six articles for eligibility after omitting unrelated or repeated reports. Twelve reviews and six case series were further excluded by the authors. Totally eighteen studies were finally pooled in our meta‐analyses (Figure 1). Extracted data of eligible studies were summarized in Table 1.
Figure 1.
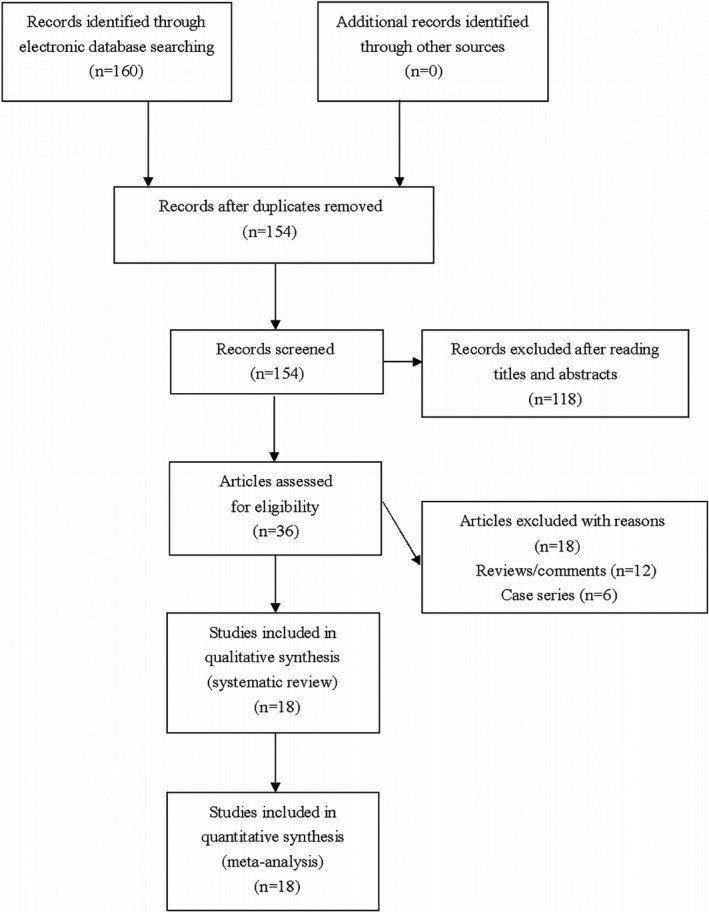
Flowchart of study selection for this meta‐analysis
Table 1.
The characteristics of included studies in this meta‐analysis
| First author (year) | Country | Ethnicity | Type of disease | Sample size | Genotype distribution | p value for HWE | NOS score | |
|---|---|---|---|---|---|---|---|---|
| Cases | Controls | |||||||
| rs1799983 G/T | GG/GT/TT | |||||||
| Du (2014) | China | East Asian | Aneurysmal subarachnoid hemorrhage | 60/60 | 27/19/14 | 39/16/5 | .097 | 7 |
| Khurara (2004) | USA | Caucasian | Aneurysmal subarachnoid hemorrhage | 51/90 | 20/25/6 | 20/55/15 | .032 | 7 |
| Kim (2011) | Korea | East Asian | Intracranial aneurysms | 149/121 | 125/24/0 | 98/23/0 | .248 | 8 |
| Konar (2019) | India | Mixed | Aneurysmal subarachnoid hemorrhage | 100/100 | 71/23/6 | 76/23/1 | .607 | 7 |
| Koshy (2008) | India | Mixed | Aneurysmal subarachnoid hemorrhage | 122/224 | 85/35/2 | 159/61/4 | .501 | 8 |
| Krex (2006) | Germany | Caucasian | Intracranial aneurysms | 142/190 | 64/67/11 | 96/76/18 | .602 | 7 |
| Krischek (2006) | Japan | East Asian | Intracranial aneurysms | 405/176 | 349/50/6 | 145/23/8 | <.001 | 8 |
| Liu (2013) | China | East Asian | Intracranial aneurysms | 82/82 | 49/25/8 | 36/38/8 | .656 | 8 |
| Ozum (2008) | Turkey | Caucasian | Aneurysmal subarachnoid hemorrhage | 53/60 | 26/23/4 | 34/22/4 | .863 | 8 |
| Staalsø (2014) | Denmark | Caucasian | Aneurysmal subarachnoid hemorrhage | 331/498 | 151/153/27 | 233/216/49 | .918 | 8 |
| Xu (2009) | China | East Asian | Aneurysmal subarachnoid hemorrhage | 58/67 | 27/17/14 | 45/15/7 | .005 | 7 |
| Zhao (2008) | China | East Asian | Hypertensive intracerebral hemorrhage | 70/112 | 53/15/2 | 105/7/0 | .732 | 7 |
| Zhe (2019) | China | East Asian | Aneurysmal subarachnoid hemorrhage | 169/156 | 90/50/29 | 106/38/12 | .003 | 8 |
| rs2070744 T/C | TT/TC/CC | |||||||
| Akagawa (2005 a) | Japan | East Asian | Intracranial aneurysms | 220/214 | 176/41/3 | 179/34/1 | .648 | 8 |
| Akagawa (2005 b) | Korea | East Asian | Intracranial aneurysms | 191/191 | 144/46/1 | 149/41/1 | .304 | 8 |
| Deng (2018) | China | East Asian | Aneurysmal subarachnoid hemorrhage | 71/142 | 70/1/0 | 130/11/1 | .177 | 7 |
| Khurara (2003) | USA | Caucasian | Aneurysmal subarachnoid hemorrhage | 52/90 | 12/35/5 | 28/46/16 | .699 | 7 |
| Kim (2011) | Korea | East Asian | Intracranial aneurysms | 149/121 | 122/24/3 | 99/22/0 | .271 | 8 |
| Konar (2019) | India | Mixed | Aneurysmal subarachnoid hemorrhage | 100/100 | 71/23/6 | 76/23/1 | .607 | 7 |
| Koshy (2008) | India | Mixed | Aneurysmal subarachnoid hemorrhage | 122/224 | 68/51/3 | 136/81/7 | .219 | 8 |
| Krex (2006) | USA | Caucasian | Intracranial aneurysms | 135/184 | 48/60/27 | 71/86/27 | .908 | 7 |
| Krischek (2006) | Japan | East Asian | Intracranial aneurysms | 405/176 | 326/72/7 | 145/23/8 | <.001 | 8 |
| Liu (2013) | China | East Asian | Intracranial aneurysms | 82/82 | 59/19/4 | 69/12/1 | .569 | 8 |
| Song (2006) | Korea | East Asian | Aneurysmal subarachnoid hemorrhage | 132/113 | 106/26/0 | 100/13/0 | .516 | 7 |
| Staalsø (2014) | Denmark | Caucasian | Aneurysmal subarachnoid hemorrhage | 331/498 | 145/147/39 | 197/233/68 | .946 | 8 |
| Zhe (2019) | China | East Asian | Aneurysmal subarachnoid hemorrhage | 169/156 | 82/60/27 | 95/47/14 | .029 | 8 |
| rs869109213 VNTR | 4b4b/4b4a/4a4a | |||||||
| Bi (2010) | China | East Asian | Aneurysmal subarachnoid hemorrhage | 80/107 | 59/18/3 | 91/16/1 | .753 | 8 |
| Fang (2011) | China | East Asian | Hypertensive intracerebral hemorrhage | 62/236 | 52/10/0 | 187/48/1 | .257 | 7 |
| Khurara (2004) | USA | Caucasian | Aneurysmal subarachnoid hemorrhage | 51/90 | 25/25/1 | 70/16/4 | .029 | 7 |
| Kim (2011) | Korea | East Asian | Intracranial aneurysms | 149/121 | 122/24/3 | 96/25/0 | .205 | 8 |
| Konar (2019) | India | Mixed | Aneurysmal subarachnoid hemorrhage | 100/100 | 65/30/5 | 72/25/3 | .648 | 7 |
| Koshy (2008) | India | Mixed | Aneurysmal subarachnoid hemorrhage | 122/224 | 77/40/5 | 143/77/4 | .077 | 8 |
| Krex (2006) | Germany | Caucasian | Intracranial aneurysms | 142/189 | 98/41/3 | 126/55/8 | .525 | 7 |
| Krischek (2006) | Japan | East Asian | Intracranial aneurysms | 405/176 | 325/70/10 | 143/30/3 | .341 | 8 |
| Staalsø (2014) | Denmark | Caucasian | Aneurysmal subarachnoid hemorrhage | 332/498 | 254/71/7 | 344/145/9 | .155 | 8 |
Abbreviations: HWE, Hardy–Weinberg equilibrium; NA, not available; NOS, Newcastle–ottawa scale.
3.2. Meta‐analyses of eNOS rs1799983 polymorphism and hemorrhagic cerebral vascular diseases
Thirteen studies involving 1792 cases and 1936 control subjects were eligible for estimation of relationship between eNOS rs1799983 polymorphism and hemorrhagic cerebral vascular diseases. The pooled meta‐analyses did not reveal any significant associations for eNOS rs1799983 polymorphism and hemorrhagic cerebral vascular diseases (see Table 2).
Table 2.
Pooled meta‐analyses results of the current study
| Polymorphisms | Population | Sample size | Dominant comparison | Recessive comparison | Overdominant comparison | Allele comparison | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| p value | OR (95%CI) | p value | OR (95%CI) | p value | OR (95%CI) | p value | OR (95%CI) | |||
| rs1799983 G/T | Overall | 1792/1936 | .21 | 0.84 (0.64–1.10) | .33 | 1.26 (0.80–1.98) | .24 | 1.09 (0.94–1.27) | .14 | 0.83 (0.64–1.06) |
| Caucasian | 577/838 | .71 | 0.96 (0.78–1.19) | .26 | 0.81 (0.56–1.17) | .30 | 1.12 (0.90–1.39) | .82 | 1.02 (0.87–1.20) | |
| East Asian | 993/774 | .20 | 0.71 (0.42–1.20) | .20 | 1.66 (0.76–3.61) | .55 | 1.13 (0.76–1.68) | .15 | 0.69 (0.42–1.14) | |
| aSAH | 944/1255 | .10 | 0.77 (0.56–1.05) | .10 | 1.57 (0.92–2.70) | .24 | 1.11 (0.93–1.34) | .06 | 0.75 (0.55–1.01) | |
| IA | 848/681 | .85 | 0.95 (0.55–1.64) | .32 | 0.77 (0.46–1.29) | .75 | 1.09 (0.65–1.83) | .89 | 0.97 (0.60–1.56) | |
| rs2070744 T/C | Overall | 2159/2291 | .05 | 0.87 (0.76–1.00) | .47 | 1.10 (0.85–1.42) | .10 | 1.12 (0.98–1.29) | .06 | 0.90 (0.80–1.00) |
| Caucasian | 518/772 | .69 | 1.05 (0.83–1.32) | .71 | 0.94 (0.68–1.30) | .89 | 0.98 (0.79–1.23) | .64 | 1.04 (0.88–1.22) | |
| East Asian | 1419/1195 | .01 | 0.77 (0.63–0.94) | .16 | 1.42 (0.87–2.32) | .04 | 1.24 (1.01–1.52) | .006 | 0.78 (0.66–0.93) | |
| aSAH | 977/132 | .21 | 0.82 (0.59–1.12) | .86 | 1.03 (0.75–1.40) | .28 | 1.11 (0.92–1.33) | .27 | 0.85 (0.64–1.31) | |
| IA | 1182/968 | .09 | 0.83 (0.67–1.03) | .30 | 1.27 (0.81–2.01) | .21 | 1.15 (0.93–1.42) | .06 | 0.84 (0.70–1.01) | |
| rs869109213 VNTR | Overall | 1443/1741 | .49 | 0.90 (0.66–1.22) | .26 | 1.32 (0.81–2.14) | .65 | 1.08 (0.78–1.48) | .40 | 0.90 (0.70–1.15) |
| Caucasian | 525/777 | .62 | 0.82 (0.37–1.82) | .48 | 0.77 (0.37–1.61) | .53 | 1.34 (0.54–3.32) | .79 | 0.93 (0.53–1.63) | |
| East Asian | 696/640 | .72 | 0.95 (0.71–1.27) | .12 | 2.19 (0.82–5.86) | .83 | 0.97 (0.72–1.31) | .39 | 0.89 (0.68–1.16) | |
| aSAH | 685/1019 | .24 | 0.72 (0.41–1.25) | .21 | 1.48 (0.80–2.75) | .33 | 1.33 (0.74–2.40) | .19 | 0.75 (0.49–1.15) | |
| IA | 758/722 | .53 | 1.09 (0.84–1.42) | .81 | 1.10 (0.50–2.41) | .49 | 0.91 (0.69–1.19) | .60 | 1.07 (0.84–1.35) | |
Abbreviations: aSAH, aneurysmal subarachnoid hemorrhage; CI, confidence interval; IA, intracranial aneurysm; OR, odds ratio.
The values in bold represent there is statistically significant differences between cases and controls.
3.3. Meta‐analyses of eNOS rs2070744 polymorphism and hemorrhagic cerebral vascular diseases
Twelve studies involving 2,159 cases and 2,291 control subjects were eligible for estimation of relationship between eNOS rs2070744 polymorphism and hemorrhagic cerebral vascular diseases. eNOS rs2070744 polymorphism was found to be significantly associated with hemorrhagic cerebral vascular diseases in East Asians (dominant comparison: OR = 0.77, 95% CI 0.63–0.94; overdominant comparison: OR = 1.24, 95% CI 1.01–1.52; allele comparison: OR = 0.78, 95% CI 0.66–0.93) (see Table 2).
3.4. Meta‐analyses of eNOS rs869109213 polymorphism and hemorrhagic cerebral vascular diseases
Nine studies involving 1,443 cases and 1741 control subjects were eligible for estimation of relationship between eNOS rs869109213 polymorphism and hemorrhagic cerebral vascular diseases. The pooled meta‐analyses did not reveal any significant associations for eNOS rs869109213 polymorphism and hemorrhagic cerebral vascular diseases (see Table 2).
3.5. Sensitivity analyses
Stabilities of pooled meta‐analyses results were examined by omitting one study each time and pooling the results of the other studies. The trends of associations remained unchanged in sensitivity analyses, indicating that our pooled meta‐analyses results were statistically stable.
3.6. Publication biases
Publication biases were examined by funnel plots. Funnel plots were overall symmetrical, suggesting that our pooled meta‐analyses results were not likely to be severely influenced by publication biases (Figure S1).
4. DISCUSSION
Endothelial nitric oxide synthase, which is primarily found in the vascular endothelium, can continuously generate NO, and thus, it is essential for maintaining basal vascular tone and normal cerebral blood flow, and its dysregulation has also been found to be closely related to vascular endothelial dysfunction (Rudic et al., 1998; Faraci & Brian 1994). Since vascular endothelial dysfunction is clearly implicated in vascular diseases, eNOS gene polymorphisms were also intensively studied regarding their relationship with predisposition to various types of cerebral vascular diseases. There were already abundant meta‐analyses regarding eNOS gene polymorphisms and ischemic cerebral vascular diseases (Shyu et al., 2017; Wei et al., 2017). So in this meta‐analysis, we focused on hemorrhagic cerebral vascular diseases. The pooled meta‐analyses results demonstrated that eNOS rs2070744 polymorphism might influence susceptibility to hemorrhagic cerebral vascular diseases in East Asians, but we failed to find any significant associations for eNOS rs1799983 and rs869109213 polymorphisms. The trends of associations remained unchanged in sensitivity analyses, suggesting that our pooled meta‐analyses results were quite statistically stable.
Few points need to be considered when interpreting our findings. First, previous experimental studies demonstrated that all investigated polymorphisms were correlated with altered gene expression or protein structure of eNOS (AlFadhli, 2013; Dosenko, Zagoriy, Haytovich, Gordok, & Moibenko, 2006; Elakkad, Abou‐Aisha, Hassanein, & Gad, 2017). To be brief, the rs2070744 polymorphism is associated with a thymine to cytosine mutation at coden‐786 in the 5′‐flanking region of the eNOS gene, which can significantly reduce eNOS gene expression. The rs1799983 polymorphism is associated with a Glu‐to‐Asp change at nucleotide 298 in exon 7 that is demonstrated to be associated with a trend of reduction of eNOS enzyme activity, and rs869109213 polymorphism, which can result in a variable number of tandem repeats (27 bp VNTRs) in intron 4, is also associated with altered eNOS enzyme activity. Thus, it is likely that these eNOS variations might influence normal functioning of eNOS, lead to vascular endothelial dysfunction, and influence predisposition to hemorrhagic cerebral vascular diseases. In this meta‐analysis, only rs2070744 polymorphism was found to be significantly associated with predisposition to hemorrhagic cerebral vascular diseases. Nevertheless, we noticed that the trends of associations for rs1799983 and rs869109213 polymorphisms were actually similar to rs2070744 polymorphism. Therefore, maybe our pooled meta‐analyses were still not statistically sufficient to detect the real associations between eNOS polymorphisms and hemorrhagic cerebral vascular diseases, and future studies in larger populations are still needed so as to get a more statistically robust conclusion. Second, the etiologies and pathogenesis mechanisms of hemorrhagic cerebral vascular diseases are extremely sophisticated. In this meta‐analysis, we noticed that almost all eligible studies were about IA and its associated aSAH, whereas only a few studies were about hypertensive cerebral hemorrhage. Nevertheless, considering that the majority of hemorrhagic cerebral vascular diseases should be related to hypertensive disorders. Future studies should concentrate more on elucidating the relationship between eNOS polymorphisms and hemorrhagic cerebral vascular diseases of other etiologies, particularly hypertension. Third, we aimed to analyze all eNOS polymorphisms at the beginning. However, we did not find sufficient eligible studies to support meta‐analyses of other eNOS polymorphisms, so we have to focus on only three polymorphisms in this meta‐analysis.
Like all meta‐analyses, a few limitations of our pooled meta‐analyses have to be acknowledged. First, our pooled meta‐analyses results were derived from pooling unadjusted findings of eligible studies since the authors did not have raw data (Xie, Shi, & Liu, 2017). Second, environmental factors might also influence relationship between eNOS polymorphisms and hemorrhagic cerebral vascular diseases. However, most investigators only focused on genetic associations in their works, so genetic–environmental interactions could not be explored in this meta‐analysis (Wang et al., 2018). Third, we did not consider gray literatures (data that are not formally published in scientific books or journals, which include working papers, theses and dissertations, market reports, government documents, and conference posters). Therefore, despite that funnel plots were overall symmetrical, we still could not rule out the possibility that our pooled results might be affected by potential publication biases (Suvatha et al., 2018).
So to conclude, this meta‐analysis demonstrated that eNOS rs2070744 polymorphism might influence predisposition to hemorrhagic cerebral vascular diseases in East Asians. These results also suggested that eNOS might involve in the development of hemorrhagic cerebral vascular diseases, and it may serve as a potential therapeutic target for hemorrhagic cerebral vascular diseases.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHORS' CONTRIBUTIONS
Qiuling Wang and Minfeng Zhou designed this meta‐analysis. Qiuling Wang and Hongri Sun searched literatures. Xiaoguang Qi analyzed the data. Qiuling Wang and Minfeng Zhou wrote the manuscript. All authors approved the final manuscript as submitted.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors; thus, ethical approval is not required.
Supporting information
Wang Q, Sun H, Qi X, Zhou M. eNOS rs2070744 polymorphism might influence predisposition to hemorrhagic cerebral vascular diseases in East Asians: A meta‐analysis. Brain Behav. 2020;10:e01538 10.1002/brb3.1538
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.1538
REFERENCES
- AlFadhli, S. (2013). Influence of endothelial nitric oxide synthase gene intron‐4 27bp repeat polymorphism on its expression in autoimmune diseases. Disease Markers, 34, 349–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhaus, R. , Schlachetzki, F. , Rackl, W. , Baldaranov, D. , Leitzmann, M. , Hubert, G. J. , … Boy, S. (2015). Intracranial hemorrhage: Frequency, location, and risk factors identified in a TeleStroke network. NeuroReport, 26, 81–87. 10.1097/WNR.0000000000000304 [DOI] [PubMed] [Google Scholar]
- Besler, C. , Heinrich, K. , Rohrer, L. , Doerries, C. , Riwanto, M. , Shih, D. M. , … Landmesser U. (2011). Mechanisms underlying adverse effects of HDL on eNOS‐activating pathways in patients with coronary artery disease. Journal of Clinical Investigation, 121, 2693–2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres, J. A. , & Goldstein, J. N. (2012). Intracranial hemorrhage. Emergency Medicine Clinics of North America, 30, 771–794. 10.1016/j.emc.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter, A. M. , Singh, I. P. , Gandhi, C. D. , & Prestigiacomo, C. J. (2016). Genetic risk factors for spontaneous intracerebral haemorrhage. Nature Reviews Neurology, 12, 40–49. [DOI] [PubMed] [Google Scholar]
- Chen, Y. C. , Chang, K. H. , & Chen, C. M. (2018). Genetic polymorphisms associated with spontaneous intracerebral hemorrhage. International Journal of Molecular Sciences, 19, E3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenko, V. E. , Zagoriy, V. Y. , Haytovich, N. V. , Gordok, O. A. , & Moibenko, A. A. (2006). Allelic polymorphism of endothelial NO‐synthase gene and its functional manifestations. Acta Biochimica Polonica, 53, 299–302. 10.18388/abp.2006_3342 [DOI] [PubMed] [Google Scholar]
- Elakkad, A. M. , Abou‐Aisha, K. , Hassanein, S. I. , & Gad, M. Z. (2017). T‐786C variation in the promoter sequence of human eNOS gene markedly influences its expression level. Drug Discoveries and Therapeutics, 11, 193–197. [DOI] [PubMed] [Google Scholar]
- Faraci, F. M. , & Brian, J. E. (1994). Nitric oxide and the cerebral circulation. Stroke, 25, 692–703. 10.1161/01.STR.25.3.692 [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease Study 2013 Collaborators (2015). Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet, 386, 743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keat Wei, L. , Griffiths, L. R. , Irene, L. , & Kooi, C. W. (2019). Association of NOTCH3 gene polymorphisms with ischemic stroke and its subtypes: A meta‐analysis. Medicina (Kaunas), 55, E351 10.3390/medicina55070351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan, K. , Beishon, L. , Berge, E. , Christensen, H. , Dineen, R. A. , Ozturk, S. , … Bath, P. M. (2018). Relationship between race and outcome in Asian, Black, and Caucasian patients with spontaneous intracerebral hemorrhage: Data from the virtual international stroke trials archive and efficacy of nitric oxide in stroke trial. International Journal of Stroke, 13, 362–373. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati, A. , Tetzlaff, J. , Altman, D. G. , & PRISMA group (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA statement. Annals of Internal Medicine, 151, 264–269. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Rudic, R. D. , Shesely, E. G. , Maeda, N. , Smithies, O. , Segal, S. S. , & Sessa, W. C. (1998). Direct evidence for the importance of endothelium‐derived nitric oxide in vascular remodeling. Journal of Clinical Investigation, 101, 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu, H. Y. , Chen, M. H. , Hsieh, Y. H. , Shieh, J. C. , Yen, L. R. , Wang, H. W. , & Cheng, C. W. (2017). Association of eNOS and Cav‐1 gene polymorphisms with susceptibility risk of large artery atherosclerotic stroke. PLoS ONE, 12, e0174110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang, A. (2010). Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. European Journal of Epidemiology, 25, 603–605. 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- Suvatha, A. , Sibin, M. K. , Bhat, D. I. , Narasingarao, K. V. L. , Vazhayil, V. , Chetan, G. K. (2018). Factor XIII polymorphism and risk of aneurysmal subarachnoid haemorrhage in a south Indian population. BMC Medical Genetics, 19, 159 10.1186/s12881-018-0674-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui, M. , Shimokawa, H. , Morishita, T. , Nakashima, Y. , & Yanagihara, N. (2006). Development of genetically engineered mice lacking all three nitric oxide synthases. Journal of Pharmacological Sciences, 102, 147–154. 10.1254/jphs.CPJ06015X [DOI] [PubMed] [Google Scholar]
- Wang, T. , Fu, W. , Song, S. , Han, Y. , Yao, L. , Lu, Y. , … Wang, J. (2018). Matrix metalloproteinase‐9 gene polymorphisms and their interaction with environment on subarachnoid hemorrhage risk. Experimental Biology and Medicine (Maywood), 243, 749–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, L. K. , Au, A. , Menon, S. , Griffiths, L. R. , Kooi, C. W. , Irene, L. , … Aziz, Z. A. (2017). Polymorphisms of MTHFR, eNOS, ACE, AGT, ApoE, PON1, PDE4D, and Ischemic Stroke: Meta‐Analysis. Journal of Stroke and Cerebrovascular Diseases: The Official Journal of National Stroke Association, 26, 2482–2493. [DOI] [PubMed] [Google Scholar]
- Wei, L. K. , Griffiths, L. R. , Kooi, C. W. , & Irene, L. (2019). Meta‐analysis of factor V, factor VII, factor XII, and factor XIII‐A gene polymorphisms and ischemic stroke. Medicina (Kaunas), 55, E101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, X. , Shi, X. , & Liu, M. (2017). The roles of TLR gene polymorphisms in atherosclerosis: A systematic review and meta‐analysis of 35,317 subjects. Scandinavian Journal of Immunology, 86, 50–58. 10.1111/sji.12560 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


