Abstract
Background.
Development of collateral vasculature is key in compensating for arterial occlusions in patients with peripheral artery disease (PAD). We aimed to examine the development of collateral pathways after ligation of native vessels in a porcine model of PAD.
Methods.
Right hindlimb Ischemia was induced in domestic swine (N=11) using two versions of arterial ligation. Version 1 (N=6) consisted of ligation/division of the right external iliac, profunda femoral and superficial femoral arteries. Version 2 (N=5) consisted of the ligation of Version 1 with additional ligation/division of the right internal iliac artery. Development of collateral pathways was evaluated with standard angiography prior to arterial ligation and at termination (30 days later). Relative luminal diameter of the arteries supplying the ischemic right hindlimb were determined by 2D angiography.
Results.
The dominant collateral pathway that developed after Version 1 ligation connected the right internal iliac artery to the right profunda femoral and then to the right superficial femoral/popliteal artery. Mean luminal diameter of the right internal iliac artery at termination increased by 38% compared to baseline. Two co-dominant collateral pathways developed in Version 2 ligation: (i) from the left profunda femoral artery to the reconstituted right profunda femoral artery; and (ii) from the common internal iliac trunk and the left internal iliac artery to the reconstituted right internal iliac artery, which then supplied the right profunda femoral and then the right superficial femoral/popliteal artery. Mean diameter of the left profunda and the left internal iliac artery increased at termination by 26% and 21%, respectively (p < 0.05).
Conclusion.
Two versions of hindlimb ischemia induction (right ilio-femoral artery ligation with and without right internal iliac artery ligation) in swine produced differing collateral pathways, along with changes to the diameter of the inflow vessels (i.e., arteriogenesis).
Keywords: swine, porcine, animal models, arteriogenesis, ischemia, angiography, peripheral artery disease
INTRODUCTION
Peripheral artery disease (PAD) has a prevalence of about 12% in the general population, with an impact on functional status, quality of life, and life expectancy 1–4. The vast majority of symptomatic PAD patients present with exercise-associated leg discomfort and ambulatory disability known as intermittent claudication 1–3; only 1-2% of patients present with critical ischemia, including severe pain at rest and/or tissue loss/gangrene. Treatment options for symptomatic PAD include open and percutaneous revascularization but, after referral, only 13% of patients with claudication and 50% of patients with critical limb ischemia undergo a revascularization procedure 5,6. The presence of complex occlusive disease and/or medical comorbidities prevents some patients from receiving surgical intervention. The majority of claudicants can be managed with walking programs and medical therapy and do need surgical revascularization. The patients who will not undergo revascularization will benefit from development of collateral blood flow to improve blood flow in the ischemic limb,6,7 which makes the study of blood vessel growth in response to ischemia an important field of investigation.
The groups of Drs. Brewster and Lefer in collaboration with our group 8,9, have demonstrated that two swine strains (with and without comorbidities such as diet-induced metabolic syndrome) can recapitulate the pathophysiology of human PAD, including (i) persistently decreased hindlimb hemodynamics/perfusion, (ii) decreased treadmill performance, (iii) and ischemic myopathy (end-organ damage). The ability of swine to model human PAD prompted us to use a porcine model of PAD to determine: 1) the pathways and size of the collaterals developing over thirty days after two different versions of arterial ligation in the right hindlimb; and 2) the effect of these ligations and resulting collateral development on limb hemodynamics, oxygenation, and muscle histology. The rationale for studying this collateralization phenomenon was to develop a clinically-relevant large-animal platform in which we could test the efficacy of cell-based therapies (or other interventions) in improving perfusion of the ischemic limb.
MATERIALS AND METHODS
Animal Welfare Statement.
The animals utilized to generate the data for this report were maintained and treated in accordance with the Guide for the Care and Use of Laboratory Animals (8th ed.) from the National Research Council and the National Institutes of Health 10 and also in accordance with the Animal Welfare Act of the United States (U.S. Code 7, Sections 2131 – 2159). The animal protocol pertaining to this manuscript was approved by the Institutional Animal Care and Use Committee (IACUC) of the VA Nebraska-Western Iowa Health Care System (ID number 00950) and by the IACUC of the University of Nebraska Medical Center (ID number 15-068-07-ET). All procedures were performed in animal facilities approved by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC; www.aaalac.org) and by the Office of Laboratory Animal Welfare of the Public Health Service (grants.nih.gov/grants/olaw/olaw.htm). All surgical procedures were performed under isoflurane anesthesia, and all efforts were made to minimize suffering. Euthanasia was performed in accordance with the AVMA Guidelines 11.
Experimental Subjects.
Wild type domestic swine (N = 13; castrated males; age 11-13 wk) were purchased from the Animal Research and Development Center at the University of Nebraska Lincoln (ardc.unl.edu). At the time of purchase, only male swine were available. Swine were housed individually in pens, with multiple subjects per room, and fed ad lib with standard hog feed (Purina Nature’s Match Sow and Pig Complete Feed).
Experimental Design.
Refer to Fig. 1. The design included an ischemia-induction procedure (i.e., arterial ligation) on day 0, with endpoint measurement and necropsy 30 d later. Two methods of arterial ligation were compared in two nonrandomized groups of domestic swine; the groups were matched for age, size, and sex.
Fig. 1. Experimental Flow Diagram.
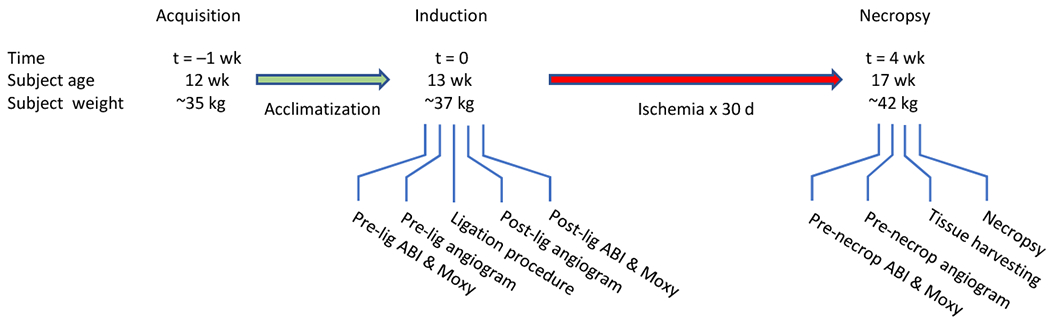
Domestic swine were 3 mo old at time of acquisition (t = −1 wk).
Set-Up and Anesthesia 12.
Swine were fasted for 24 h prior to the procedure, with free access to water and up to two 500 cc bottles of regular Gatorade™. On day zero, each subject underwent induction with ketamine (Zoetis, 2.2 mg/kg), Telazol® (Zoetis; 4.4 mg/kg) and xylazine (Zoetis; 2.2 mg/kg), given as a single IM injection. Each subject then was weighed and endotracheally intubated. EKG, pulse oximetry, rectal temperature, and lingual end-tidal CO2 monitors were placed. The subject rested on a water-circulated warming blanket that was set at 102°F. An auricular intravenous (IV) line was placed. Anesthesia was maintained with isoflurane (0.5-1%) and supplemental oxygen (3-5 L/min) using a Matrx® ventilator (midmark.com). The ventilator rate initially was set at 12-15 breaths per minute with a tidal volume of 10 mL/kg, and subsequently adjusted to maintain the EtCO2 at 40-50 mm Hg. Cotton blankets were placed over non-surgical areas to minimize any decrease in body temperature. Vital signs were continuously recorded to a laptop computer via a Bionet BM5 monitor (www.bionetus.com). A single dose of cefovecin sodium (Zoetis; 8 mg/kg IM) was given before incision.
Muscle Oximetry & Arterial Indices.
Extremity muscle oximetry measurements13 measured transcutaneously in the supine position under general anesthesia using a Near Infrared Spectroscopy probe (Moxy device, moxymonitor.com) with the Peripedal software (peripedal.com); see Fig.9S. The Moxy device has a noninvasive probe which measures muscle hemoglobin/myoglobin oxygen saturation (StO2). In the forelimb, the Moxy probe was placed over the medial biceps brachii of the forelimb (i.e., medial aspect of the flexor mass of the forelimb), proximal to the elbow joint; in the hindlimb, the probe was placed over the medial gastrocnemius muscle. The Moxy index was defined as the ratio of the hindlimb saturation over the higher of the two forelimb saturations. For determination of arterial indices, a pediatric-size sphygmomanometer cuff was applied to each extremity, about 10 cm above the hoof (see Fig.9S), and systolic pressure was measured with the aid of a Doppler device (Model 811-B, Parks Medical Doppler; parksmed.com). The hindlimb-forelimb arterial pressure index (“ABI”, meant to be the porcine analog of the clinical ankle-brachial index) was defined as the ratio of hindlimb systolic blood pressure over the higher of the two forelimb systolic pressures.
Laparotomy, Vessel Exposure, and Angiography.
With the subject under general anesthesia and in the supine position, the chest, abdomen, groins, and bilateral lower extremities were shaved with an electric clipper, washed with soap and water, and then prepped using ChloraPrep™ applicators (chlorhexidine gluconate/isopropyl alcohol; BD Medical). Vessel exposure was obtained using a retroperitoneal approach through a right paramedian incision that started to the right and just inferior to the urethral meatus and medial to the right nipple line, and then extended inferiorly across the right inguinal ligament (not divided) and onto the right inner thigh (Fig.8S), The abdominal wall layers were incised carefully down to the peritoneum, and then the dissection was performed laterally between the peritoneum and abdominal wall to develop the retroperitoneal space. The peritoneal sac, containing the intraabdominal organs, was retracted superiorly and to the left, which exposed the distal aorta and right-sided pelvic vasculature. In all subjects an arterial line (20-gauge Angiocatheter) was placed in the infrarenal aorta, 4-5 cm above the aortic trifurcation, through a site controlled with a 6-0 polypropylene U-stitch (see Fig.4, and the postmortem dissection in Fig.7S). This line was used for arterial blood pressure monitoring and angiography. A baseline aortogram with runoff was obtained with injection of 10 mL of Visipaque™ 320 (lodixanol; GE Healthcare) and C-arm fluoroscopy (GE OEC 9900 Elite).
Fig. 4. Arterial Interruption procedure (Version 2), intraoperative photos.
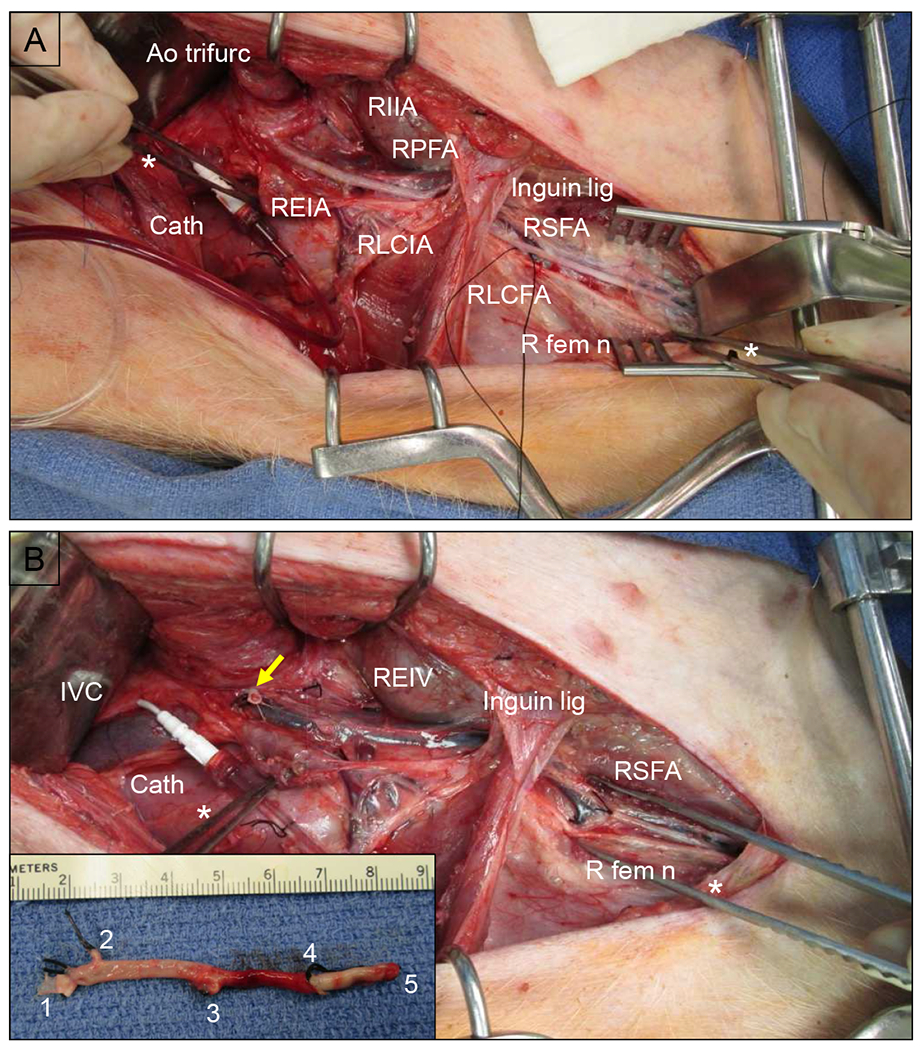
(A) Prior to ligation, showing a right retroperitoneal dissection. Large white arrow points cephalad. The peritoneal sac, containing the intraabdominal viscera, was retracted medially and cephalad. The right iliofemoral complex has been dissected out above and below the inguinal ligament (Inguin lig). The region of the aortic trifurcation (Ao trifurc) is indicated with a dashed white circle. The right internal iliac artery (RIIA) already has been ligated (only for Version 2). There is a silk loop around the right lateral circumflex femoral artery (RLCFA). Cath = angiography catheter & line; REIA = right external iliac artery; RLCIA = right lateral circumflex iliac artery; RPFA = right profunda femoris artery; RSFA = right superficial femoral artery; R fem n = right femoral nerve. (B) The right iliofemoral complex has been ligated and excised down to the distal RSFA, with ligation of the RPFA and RCIA. The right external iliac vein (REIV) is visible in the bed of the resected artery. The yellow arrow indicates the stump of the ligated REIA. DeBakey forceps indicated with asterisk (*). Inset: resected right iliofemoral complex. Vessel stumps: 1 = REIA; 2 = RLCIA; 3 = RPFA; 4 = RLCFA; 5 = RSFA.
Arterial Interruption.
Refer to Figs. 4, 2, and 7S. Once baseline angiography was obtained, the region of the aortic trifurcation was dissected. In the pig, the distal aorta trifurcates into the right and left external iliac arteries (REIA and LEIA) and the common internal iliac trunk (CIIT); the latter then bifurcates into the right and left internal iliac arteries (RIIA and LIIA). The REIA was dissected down to the bifurcation of the right superficial femoral artery (RSFA) and profunda femoris artery (RPFA). The REIA, right lateral circumflex iliac and lateral circumflex femoral artery (RLCIA and RLCFA, respectively) were suture ligated proximally with 3-0 silk. Distal dissection exposed the bifurcation of the RSFA into the right popliteal and saphena arteries (RPop and RSA). The RSFA was suture ligated distal to the takeoff of the RLCFA. A continuous segment of the REIA-RSFA was then excised (Figs. 4 and 2). This series of steps constituted the Version 1 arterial interruption procedure. Version 2 consisted of Version 1 plus ligation of the RIIA. To summarize:
Version 1 Interruption (Fig.2B): ligation of the REIA, RSFA, RPFA, RLCIA, RLCFA and excision of a continuous segment of the REIA-RSFA.
Version 2 Interruption (Fig.2C): Version 1 + RIIA ligation
A completion angiogram was performed after arterial interruption was accomplished. The aortic puncture site utilized for angiographic access was then closed by removing the Angiocatheter and tying the previously-placed U-stitch. The abdominal incision was closed anatomically in layers, with 2-0 polyglactin 910 in the peritoneum and posterior layers, 0-polydioxanone in the anterior aponeurosis, 3-0 polyglactin 910 in the panniculus carnosus, and 4-0 polyglactin 910 in the skin. Cyanoacrylate glue was applied over the skin incision; no other incisional dressing was applied. The animal’s recovery from anesthesia was monitored until the subject was awake and mobile. Subjects were given half feeds on post-ligation day 1, and were placed back on ad lib feeds on day 2.
Fig. 2. Arterial anatomy of the porcine hindlimb.
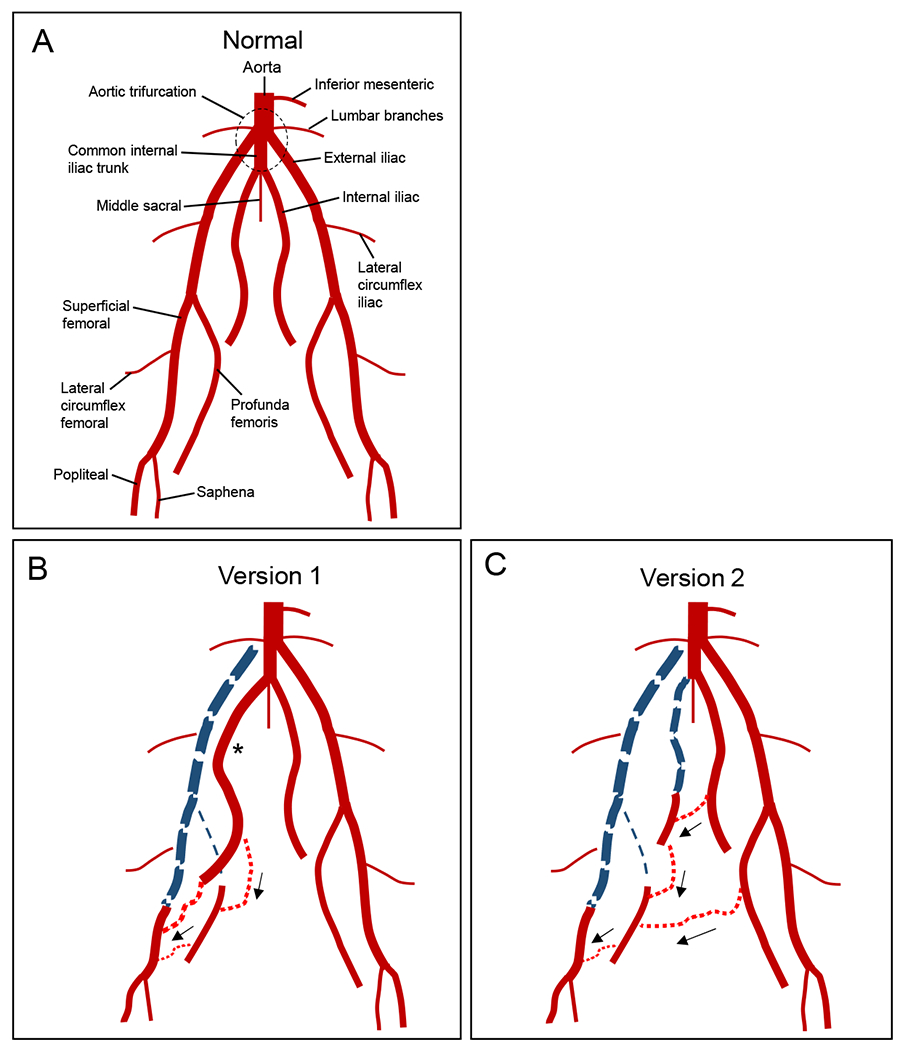
(A) Normal anatomy. (B) Ligation Version 1. The right iliofemoral segment has been ligated and excised (thick dashed blue line), along with the right profunda femoris (thin dashed blue line). One month after ligation, the right internal iliac artery has enlarged (*), and there is collateral flow from this vessel which reconstitutes both the distal superficial femoral artery and the distal right profunda femoris (black arrows). (C) Ligation Version 2. In addition to the ligations performed for Version 1, the right internal iliac vessel was ligated. One month after ligation, collaterals from the left profunda femoris reconstitute the right profunda femoris while collaterals from the left internal iliac artery reconstitute the right internal iliac, which then helps reconstitute the right profunda femoris and distal right superficial femoral artery through further collateralization (black arrows).
Terminal procedure.
Subjects underwent their terminal procedure at 30 days after arterial interruption. After induction of general endotracheal anesthesia, the right carotid artery was exposed with a cervical cut-down. Hindlimb perfusion measurements (muscle oximetry and arterial indices) were performed as described above. A catheter was then placed into the right common carotid artery and advanced into the infrarenal aorta using the Seldinger technique. An abdominal aortogram with distal runoff was performed. Bilateral hindlimb dissection was performed prior to euthanasia. Biopsies of the medial heads of the bilateral gastrocnemius were harvested prior to euthanasia and processed as described under “Bright-Field Microscopy”.
Arterial Diameter Analysis.
Each angiogram obtained during the procedure was saved into the Digital Imaging and Communications in Medicine (DICOM®) format within the GE OEC 9900 Elite C-arm instrument. Images subsequently were accessed and analyzed using the RadiANT DICOM Viewer (Version 4.0.2; www.radiantviewer.com). The cross-sectional diameter of the distal aorta was defined as 10 units. Relative diameter of each distal artery was then expressed in units of distal aortic diameter.
Bright-Field Microscopy.
Porcine muscle specimens were fixed immediately in cold methacarn fixative for 48 h, transferred into cold 50% ethanol, and then embedded in paraffin. Sections (4 μm) from these blocks were deparaffinized in xylene, rehydrated in water, and then H&E stained. Bright field images were captured with a Leica DMRXA2 microscope configured with a Leica DFC color camera (North Central Instruments; www.ncimicro.com). Sections from archived paraffin blocks of human gastrocnemius muscle from deidentified patients (a PAD patient and a control subject) were similarly processed and imaged. These human specimens were obtained through an IRB protocol utilized in a prior study 14.
Statistical analysis.
Continuous data were analyzed using ANOVA within Microsoft Excel; the level of significance was defined as p < 0.05.
RESULTS
Perioperative Events.
Mean starting weight in subjects who completed the study was 32.8 ± SD 4.0 kg (range = 26.6 – 39.0 kg, N = 6) and 37.0 ± 10.5 kg (range = 35.0 – 55.6 kg, N = 5) for Versions 1 and 2, respectively (p = 0.19, unpaired t-test). A total of 13 subjects underwent the ligation procedure. One pig developed non-reducible rectal prolapse four days after operation and was euthanized; another pig expired on postoperative day one secondary to intestinal ischemia. Necropsy of the latter subject revealed a stomach distended with feed and generalized severe ischemia (purplish discoloration) of the small and large intestine, but otherwise was inconclusive; specifically, all mesenteric vessels were patent. Of the eleven subjects which survived to the scheduled termination (N = 6 for Version 1 and N = 5 for Version 2), two subjects (one of each version) developed clinically-apparent ischemia of the right hoof, manifested by ulcer formation on the weight-bearing region. Their mobility was affected, but each animal could still ambulate and both survived until scheduled termination.
Arterial Pressure Indices at Rest.
One subject’s ABI and Moxy data (Version 1) were missing, so ten subjects underwent analysis of ABI and Moxy data. The ABI was calculated at rest (i.e., under anesthesia) in both hindlimbs on day 0 (pre-ligation and immediately post-ligation), and then again on day 30 (pre-necropsy); see Table 1. In both the ischemic (right) and non-ischemic (left) hindlimb, the mean ABI was not different between the two ligation versions at any individual time point, including at day 30 (unpaired t-tests in Table 1). Immediately post-ligation in the ischemic hindlimb, the ABI dropped to zero for both versions. At 30 d (pre-necropsy), the mean ABI in the ischemic hindlimb had recovered in both ligation versions to ~0.6. In the non-ischemic hindlimb, the ABI did not change significantly over time in either ligation version.
Table 1.
Hindlimb arterial pressure indices (“ABI”) in Version 1 vs. 2.
| Right (ischemic) hindlimb | ||||
|---|---|---|---|---|
| Day 0 | ||||
| Version (N) | ABI, pre | ABI, post | ABI, Day 30 | *ANOVA |
| 1 (N = 5) | 1.00 ± 0.22 | 0 | 0.62 ± 0.25 | <0.01 |
| 2 (N = 5) | 1.24 ± 0.30 | 0 | 0.62 ± 0.35 | <0.01 |
| **Unpaired t-test | 0.20 | 1.00 | 0.97 | |
| Left (non-ischemic) hindlimb | ||||
| Day 0 | ||||
| Version (N) | ABI, pre | ABI, post | ABI, Day 30 | *ANOVA |
| 1 (N = 5) | 1.18 ± 0.23 | 1.02 ± 0.09 | 1.15 ± 0.20 | 0.37 |
| 2 (N = 5) | 1.18 ± 0.30 | 1.09 ± 0.26 | 1.22 ± 0.24 | 0.72 |
| **Unpaired t-test | 0.97 | 0.59 | 0.61 | |
The reference limb pressure (the “brachial” pressure or denominator in the ABI ratio) was the higher of the two forelimb pressures. ABI was expressed as mean ± standard deviation. Terms “pre” and “post” refer to before and after arterial ligation. The Day 30 ABI was determined immediately prior to necropsy.
The ANOVA compares ABI of one hindlimb in one version over three time points.
The unpaired t-test compares ABI between two versions at a single time point.
The raw data for Table 1 is available in the Supplemental Material.
Muscle hemoglobin/myoglobin oxygen saturation (StO2) at Rest.
Similar to ABI, the Moxy index (i.e., ratio of hindlimb over forelimb StO2), was calculated at rest (under anesthesia) in both hindlimbs on day 0 (pre-ligation and immediately post-ligation), and then again on day 30 (pre-necropsy); see Table 2. Bar plots of absolute hindlimb StO2 values are shown in Supplemental Fig.10S. In both the ischemic (right) and non-ischemic (left) hindlimb, the mean Moxy index was not different between the two ligation versions at any individual time point (unpaired t-tests in Table 2). Immediately post-ligation in the ischemic hindlimb, the mean Moxy index dropped to ~0.4 for both versions. At 30 d (pre-necropsy), the mean Moxy index in the ischemic hindlimb had recovered in both ligation versions to 0.6 – 0.7. In the non-ischemic hindlimb, the mean Moxy index did not change significantly over time in either ligation version. Interestingly, the bar plots of the raw Moxy measurements (Fig.10S) demonstrated a decrease in StO2 immediately post-ligation in the non-ischemic limb for both ligation versions, with partial recovery by day 30. Otherwise, the bar plots of the raw Moxy measurements yielded results comparable with the Moxy index data.
Table 2.
Muscle hemoglobin/myoglobin oxygen saturation (Moxy) indices in Version 1 vs. 2.
| Right (ischemic) hindlimb | ||||
|---|---|---|---|---|
| Day 0 | ||||
| Version (N) | Pre | Post | Day 30 | *ANOVA |
| 1 (N = 5) | 1.06 ± 0.22 | 0.39 ± 0.04 | 0.63 ± 0.06 | <0.01 |
| 2 (N = 5) | 1.19 ± 0.27 | 0.39 ± 0.12 | 0.71 ± 0.17 | <0.01 |
| **Unpaired t-test | 0.42 | 0.96 | 0.36 | |
| Left (non-ischemic) hindlimb | ||||
| Day 0 | ||||
| Version (N) | Pre | Post | Day 30 | *ANOVA |
| 1 (N = 5) | 1.07 ±0.29 | 0.87 ± 0.13 | 0.94 ± 0.18 | 0.34 |
| 2 (N = 5) | 1.24 ± 0.35 | 0.87 ± 0.07 | 1.16 ± 0.15 | 0.06 |
| **Unpaired t-test | 0.43 | 0.98 | 0.09 | |
The reference limb saturation (the denominator in the Moxy index) was the higher of the two forelimb saturations. The Moxy index was expressed as mean ± standard deviation. Terms “pre” and “post” refer to before and after arterial ligation. The Day 30 index was determined immediately prior to necropsy.
The ANOVA compares Moxy index of one hindlimb in one version over three time points.
The unpaired t-test compares Moxy index between two versions at a single time point.
The raw data for Table 2 is available in the Supplemental Material.
Collateral Pathway Development.
Refer to Videos 1–12 in the Supplemental Material. Sample images (video stills) of the pre-ligation (baseline) aortography with runoff for Versions 1 and 2 are shown in Figs. 3A and 5A, respectively (see accompanying Videos 1–2 and 7–8, respectively). Immediately after ligation in Version 1, the REIA-RSFA segment was no longer present; there was delayed filling of the RFPA and remaining distal arteries from the nonligated RIIA, with some minor contribution to the distal hindlimb through collaterals involving the RCIA (Fig. 3B and Videos 3–4). After 30 days of ischemia in Version 1, pre-necropsy angiography (Fig. 3C) demonstrated that the RIIA was noticeably enlarged. Collateral vessels from the RIIA reconstituted the RPFA, which was followed by RSFA/RPop reconstitution (Video 5–6). The RSFA/RPop was reconstituted either from the RIIA through collaterals to the RLCFA, or through collaterals from the RPFA system. Immediately after ligation in Version 2, both the REIA-RSFA segment and the RIIA were no longer visible (Fig. 5B; Video 9–10). There was delayed filling of the RIIA and RPFA, but no reconstitution of RSFA or RPop. After 30 days of ischemia in Version 2, pre-necropsy angiography (Fig. 5C; Video 11–12) demonstrated the presence of two co-dominant collateral pathways supplying the ischemic right hindlimb: (i) from the LPFA to the reconstituted RPFA; and (ii) from the CIIT and LIIA to the reconstituted RIIA, subsequently supplying the reconstituted RPFA and RSFA/RPop.
Fig. 3. Aortogram with runoff angiography, Version 1.
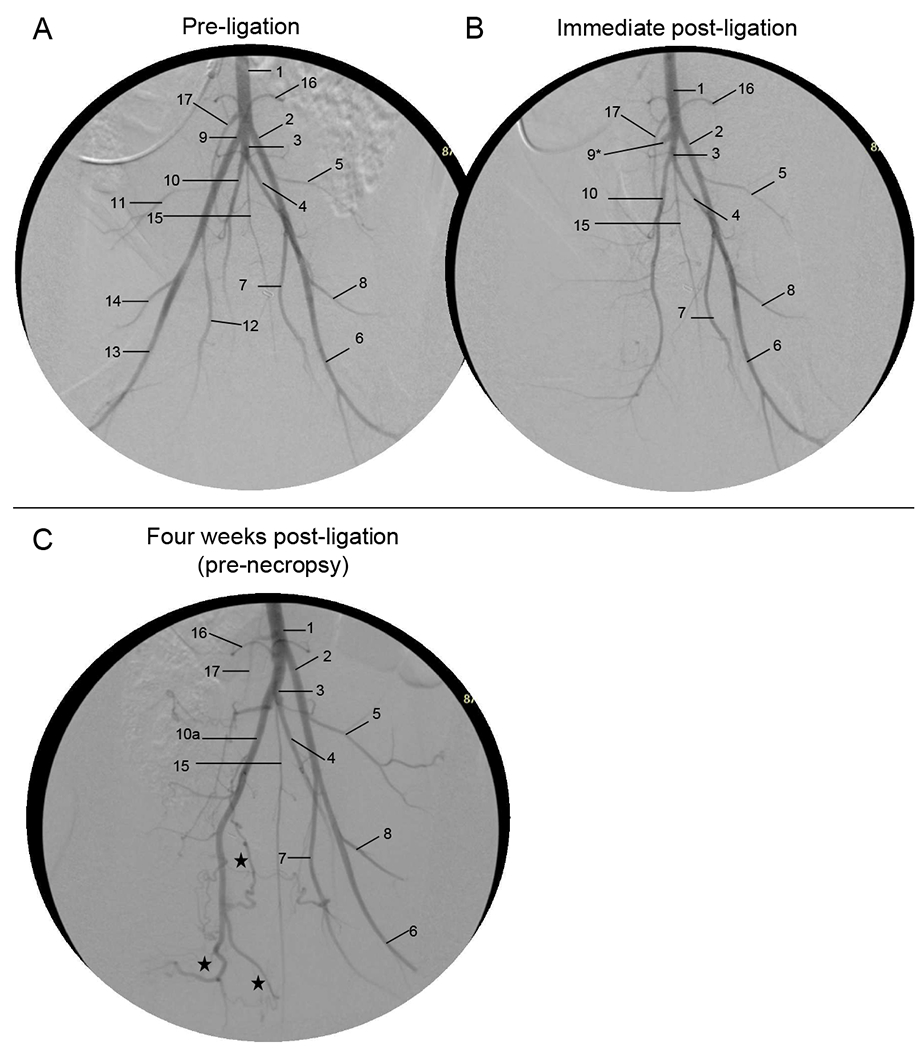
- Aorta
- Left external iliac artery
- Common internal iliac artery trunk
- Left internal iliac artery
- Left lateral circumflex iliac artery
- Left superficial femoral artery
- Left profunda femoris
- Left lateral circumflex femoral artery
- Right external iliac artery (*ligated stump)
- Right internal iliac artery
-
10a.Enlarged RIIA
-
10a.
- Right lateral circumflex iliac artery (very faint)
- Right profunda femoris;
- Right superficial femoral artery
- Right lateral circumflex femoral artery
- Middle sacral artery
- Lumbar artery
- Inferior mesenteric artery
Fig. 5. Aortogram with runoff angiography, Version 2.
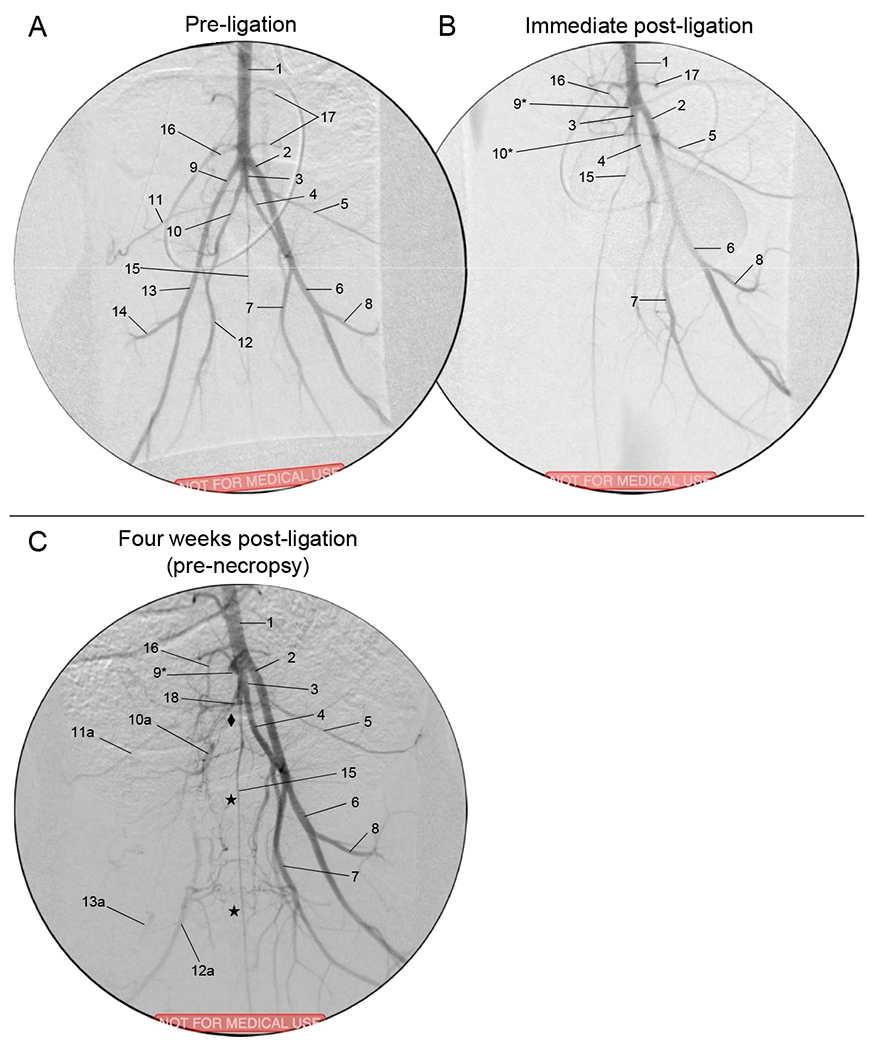
- Aorta
- Left external iliac artery
- Common internal iliac artery trunk
- Left internal iliac artery
- Left lateral circumflex iliac artery
- Left superficial femoral artery
- Left profunda femoris
- Left lateral circumflex femoral artery
- Right external iliac artery (*ligated stump)
- Right internal iliac artery (*ligated stump)
-
10a.Reconstituted RIIA
-
10a.
- Right lateral circumflex iliac artery
-
11a.Reconstituted RLCIA
-
11a.
- Right profunda femoris
-
12a.Reconstituted RPFA
-
12a.
- Right superficial femoral artery
-
13a.Reconstituted RSFA
-
13a.
- Right lateral circumflex femoral artery
- Middle sacral artery
- Inferior mesenteric artery
- Lumbar branch
- Posterior branch of CIIT
Arteriogenesis.
The relative arterial diameters of the hindlimb arteries at pre-ligation (day 0) and pre-necropsy (day 30) are shown in Table 2. Prior to ligation (i.e., day 0), the mean diameter of the LEIA and the dRPFA (distal right profunda femoral artery); were 6% and 30% larger, respectively, in the Version 2 compared to the Version 1 subjects (p< 0.05). The meaning of this difference in baseline arterial diameter between Version 1 vs. 2 subjects is not clear. After 30 days of ischemia in Version 1 subjects, the CIIT, RIIA, and dRPFA increased in diameter by 17%, 38%, and 17%, respectively (p < 0.05); the LIIA and RPop both trended toward increased diameter, but did not reach significance. The dRPFA and RPop both represented reconstituted vessels at 30 days. After 30 days of ischemia in Version 2 subjects, the CIIT, LEIA, LIIA, and LPFA all had a diameter increase in the range of 20% (23%, 18%, 21%, and 26%, respectively; p < 0.05). The dRPFA trended toward increased diameter, but this did not reach significance. The only vessel noted to have a decrease in mean diameter at 30 days was the RPop in the Version 2 subjects, which decreased by 21% (p < 0.05).
Ischemic Myopathy.
Chronically ischemic muscle from both porcine and human subjects exhibited microscopic features of ischemic myopathy (Fig.6). H&E micrographs of chronically ischemic (day 30) vs. control (contralateral) porcine gastrocnemius muscle are shown in Fig.6A & 6C, respectively, along with analogous micrographs of gastrocnemius muscle from a claudicating PAD patient and a control non-PAD patient (Fig. 6B & 6D, respectively). Control muscle from both swine (Fig.6A) and humans (Fig.6B) demonstrated polygonal myofibers having relatively similar shape and size, with barely perceptible endomysium and perimysium. However, in ischemic muscle from both swine (Fig. 6C) and humans (Fig. 6D), there was a wide range of myofiber size and shape (demonstrating significant myofiber degeneration), with thickening of the endomysium and perimysium (demonstrating significant fibrosis). The myopathic features and other characteristics of chronically ischemic muscle from PAD patients have been previously described 15–18.
Fig. 6. Ischemic myopathy in porcine vs. human subjects.
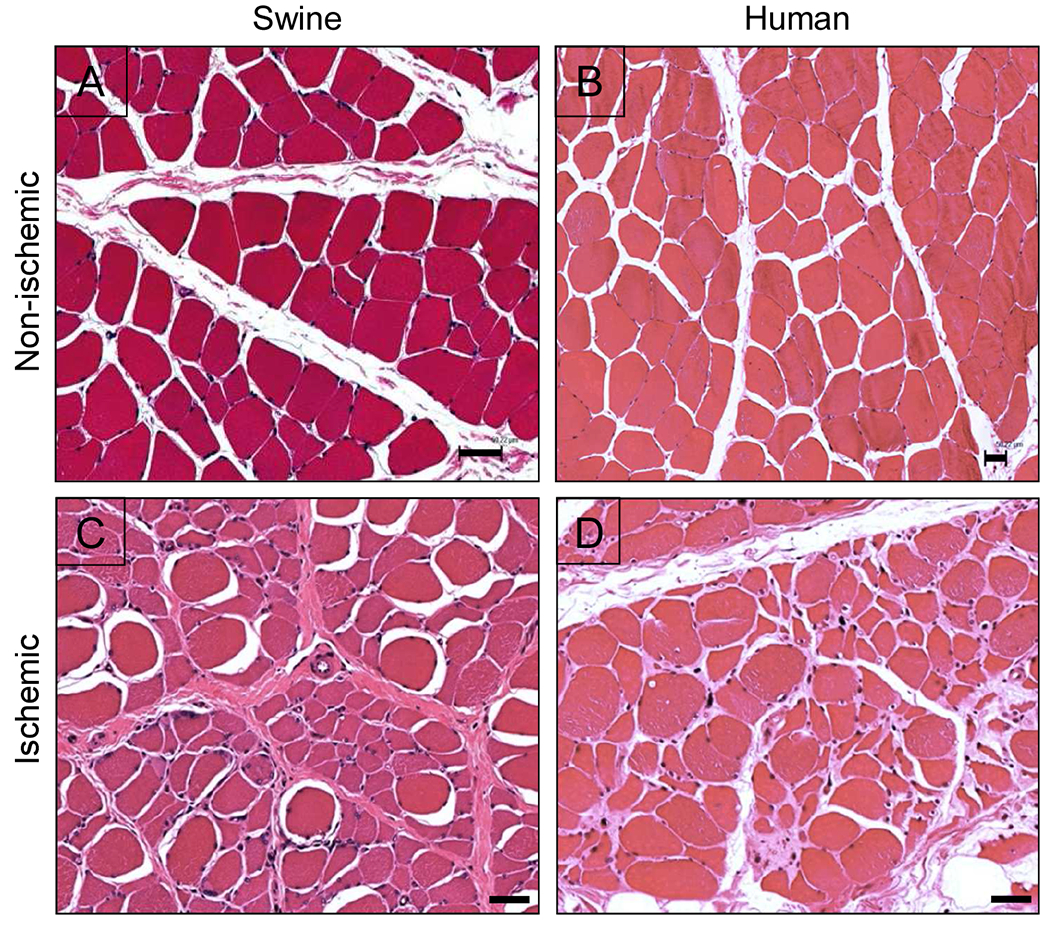
(A & B) Non-ischemic muscle, human vs. porcine, respectively. (C & D) Ischemic muscle, human vs. porcine, respectively. Samples from both human and porcine subjects were taken from the medial head of the gastrocnemius muscle. H&E; bars = 50 μm. The human images were taken from a previous quantitative study of ischemic myopathy in PAD patients17.
DISCUSSION
Herein we described the pattern and relative size of the collateral pathways that develop after ligation of the native iliofemoral pedicle in a porcine model of PAD. These data demonstrated that two versions of hindlimb ischemia induction (right iliofemoral artery ligation/excision with and without right internal iliac artery ligation) produced a similar degree of ischemia, but differing pathways of collateral development, along with outward remodeling/arteriogenesis (seen as increases of the diameter) of the major inflow vessels. After ligation and excision of the right iliofemoral artery, a dominant pathway developed which connected the patent RIIA to the reconstituted RPFA and RSFA/popliteal artery of the ischemic limb. However, after ligation and excision of the right iliofemoral artery with concomitant ligation of the RIIA, two co-dominant collateral pathways developed: (i) the first connected the left profunda artery to the reconstituted RPFA which then supplied the reconstituted RSFA/popliteal arteries: (ii) the second connected the common internal iliac trunk and left internal iliac artery to the reconstituted RIIA, which then helped supply the reconstituted RPFA and RSFA/popliteal arteries.
Of note, three strains of swine have been tested to date as models of human PAD: (i) Ossabaw mini-swine fed a high-fat diet in order to mimic metabolic syndrome 8; (ii) Yorkshire swine fed regular chow 9; and (iii) domestic swine fed regular chow (present report). Our data has confirmed previous findings from the groups of Drs. Brewster and Lefer 8,9 showing that all of these models, which have utilized both endovascular and open arterial interruption for induction of hindlimb ischemia, have demonstrated the ability to recapitulate the pathophysiologic characteristics of human PAD, including decreased hindlimb hemodynamics/perfusion and ischemic myopathy (i.e., end-organ damage). Our study also demonstrated that ligation of the iliofemoral artery, in addition to producing a persistently decreased ABI at rest, also produced a decrease in the oxygen saturation of the gastrocnemius that persisted out to 30 days. Another study looked at porcine hindlimb collateral development induced by placement of a femoral arteriovenous fistula19, but this methodology differed markedly from that in the present report.
The use of domestic swine fed regular chow along with an open arterial ligation technique (present report) allowed for a relative cost saving (a less expensive animal and diet, and a lower cost of operative and device supplies). The open ligation approach does not require specialized endovascular experience or operative setup, yet retains the ability to tailor the collateral pathway of interest. The main limitation of the open ligation approach compared to endovascular occlusion 8,9 is the requirement for a large incision for the arterial ligations. The degree of tissue trauma and pain associated with this open procedure might affect the recovery of the subjects from the induction operation, and may become a confounding factor when studying functional status (e.g., gait, treadmill performance) of the subjects.
A unique aspect of this project was the careful characterization of arteriogenesis and collateral formation. We were able to demonstrate specific locations within the porcine vascular network that experienced the greatest luminal growth after induction of ischemia. Quantification of arterial growth at specific sites was used to support the descriptive finding of reproducible collateral formation that were observed in both versions of hindlimb ischemia during angiography. Arteriogenesis is thought to be a reactive process to blood flow redistribution that occurs in occlusive disease 20–23 Changes in fluid dynamics within patent arteries nearby an occlusion cause an increase in shear forces that leads to upregulation of cell adhesion molecules and nitric oxide production in endothelial cells, with a subsequent rise in cytokine and growth factor release, which leads to endothelial and smooth cell proliferation, and a persistent increase (i.e., structural change) in arterial lumen. Given the current understanding of the pathophysiology of the vessel remodeling that occurs in PAD, we can use the swine ischemia model for quantitative testing of arteriogenic therapies, as well as to investigate and improve delivery methods and dosing regimens.
Both ligation versions induced a similar amount of ischemia initially and at day 30, per the resting arterial index and muscle StO2 data. That is, addition of internal iliac artery ligation did not produce an increase in ischemia that was detectable at rest with the methods we used. Induction of ischemia in the right hindlimb did not affect the arterial pressure index in the contralateral hindlimb. The muscle StO2 index in the contralateral control limb appeared to trend downward immediately after induction of right hindlimb ischemia, with apparent recovery by day 30; to be fair, these changes were modest (nonsignificant, p >0.05) and may not have been real. If the muscle StO2 did decrease in the control limb in the absence of change in large artery pressure, this might suggest a remote effect of limb ischemia on small vessel hemodynamics, e.g., through neuromodulation and/or systemic soluble mediators.
Regarding the two premature mortalities in this study, rectal prolapse is common in growing swine from 8-20 weeks old 24 The one occurrence of rectal prolapse in our study may have been exacerbated by increased intraabdominal pressure secondary to postoperative ileus. The subject which died from intestinal ischemia had patent mesenteric vessels; the inciting event(s) which produced the intestinal ischemia in this subject are not known. The development of hoof ulcers in two of the surviving eleven subjects demonstrated that some subjects may have had critical limb ischemia.
Limitations of this study which could impact the validity of our porcine PAD model include its acute onset of ischemia, which differs from the more progressive and chronic nature of human PAD. Another limitation concerns mimicry of clinical conditions; while PAD patients often are aged and have comorbidities such as hypertension, dyslipidemia, and diabetes, our domestic swine were juvenile, without comorbidities. Regarding hemodynamic and perfusion measurements, all data were collected at rest under anesthesia, so relevant exercise-induced phenomena could have been missed. Regarding quantification of arteriogenesis, only larger inflow arteries were evaluated, so growth in smaller, unnamed collateral arteries may also have been missed. The limited resolution of angiography in delineating collateral development was a limitation of this study. The use of CTA or MRA would have provided more image resolution; however, these imaging modalities were not available for animal use at our facility at the time this study was performed. Use of only males in this study also was a limitation, especially considering the NIH Policy on Sex as a Biological Variable25. Future work can incorporate comorbidities into the model, and/or acquire measurements during stress maneuvers (e.g., post-occlusive hyperemia or exercise). Use of equal numbers of male and female subjects also will be necessary.
The development of collateral vasculature is a key mechanism which compensates for arterial occlusion in PAD, and has the potential to be manipulated therapeutically to treat afflicted patients. In particular, the advent of different angiogenic therapies for humans, including administration of angiogenic cytokines (either as recombinant protein or with gene therapy) and, more recently, investigations of stem/progenitor cell therapy, has opened a new frontier in therapeutic opportunities for PAD 26–28 We believe that the present report can serve as a reference for future preclinical studies on novel therapeutic interventions that would enhance collateral growth. For example, a porcine model could help in the optimization of delivery methods, dosing, and efficacy testing of experimental arteriogenic treatments, including cell-based therapies 27,29–32
CONCLUSION
We documented collateral network formation and quantified arteriogenesis in our open model of porcine hindlimb ischemia model. These phenomena are important compensatory mechanisms in the ischemic lower extremity of the patient with symptomatic PAD. Using the porcine model and the measurements described herein, we intend to develop, study, and optimize therapies for the treatment of PAD, particularly for patients whose revascularization options are limited.
Supplementary Material
Fig7S-Fig10S.
1. “Vid01_v1_PreLigAngio_Proximal.mp4”. Description: Version 1 aortobifemoral angiography shot pre-ligation, proximal view.
2. “Vid02_v1_PreLigAngio_Distal.mp4”. Description: Version 1 aortobifemoral angiography shot pre-ligation, distal view.
3. “Vid03_v1_PostLigAngio_Proximal.mp4”. Description: Version 1 aortobifemoral angiography shot immediately post-ligation, proximal view.
4. “Vid04_v1_PostLigAngio_Distal.mp4”. Description: Version 1 aortobifemoral angiography shot immediately post-ligation, distal view.
5. “Vid05_v1_PreNecAngio_Proximal.mp4”. Description: Version 1 aortobifemoral angiography shot immediately pre-necropsy (30 days after ligation), proximal view.
6. “Vid06_v1_PreNecAngio_Distal.mp4”. Description: Version 1 aortobifemoral angiography shot immediately pre-necropsy (30 days after ligation), distal view.
7. “Vid07_v2_PreLigAngio_Proximal.mp4”. Description: Version 2 aortobifemoral angiography shot pre-ligation, proximal view.
8. “Vid08_v2_PreLigAngio_Distal.mp4”. Description: Version 2 aortobifemoral angiography shot pre-ligation, distal view.
9. “Vid09_v2_PostLigAngio_Proximal.mp4”. Description: Version 2 aortobifemoral angiography shot immediately post-ligation, proximal view.
10. “Vid10_v2_PostLigAngio_Distal.mp4”. Description: Version 2 aortobifemoral angiography shot immediately post-ligation, distal view.
11. “Vid11_v2_PreNecAngio_Proximal.mp4”. Description: Version 2 aortobifemoral angiography shot immediately pre-necropsy (30 days after ligation), proximal view.
12. “Vid12_v2_PreNecAngio_Distal.mp4”. Description: Version 2 aortobifemoral angiography shot immediately pre-necropsy (30 days after ligation), distal view.
Table 3.
Relative hindlimb arterial diameters, Version 1 vs. 2, at days 0 vs. 30.
| CIIT | LEIA | RIIA | LIIA | dRPFA | LPFA | RPop | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VERSION 1 | Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | †Day 30 | Day 0 | Day 30 | Day 0 | †Day 30 |
| Mean | 6.70 | 7.87 | 5.51 | 5.37 | 3.75 | 5.19 | 3.65 | 3.85 | 2.58 | 3.02 | 3.25 | 3.25 | 3.24 | 3.00 |
| SD | 0.34 | 0.44 | 0.27 | 0.26 | 0.31 | 0.22 | 0.19 | 0.24 | 0.29 | 0.25 | 0.25 | 0.39 | 0.37 | 0.32 |
| *Paired t | <0.01 | 0.19 | <0.01 | 0.07 | 0.02 | 0.49 | 0.05 | |||||||
| CIIT | LEIA | dRIIA | LIIA | dRPFA | LPFA | RPop | ||||||||
| VERSION 2 | Day 0 | Day 30 | Day 0 | Day 30 | Day 0 | †Day 30 | Day 0 | Day 30 | Day 0 | †Day 30 | Day 0 | Day 30 | Day 0 | †Day 30 |
| Mean | 6.68 | 8.24 | 5.84 | 6.92 | 3.13 | 3.44 | 3.90 | 4.72 | 3.35 | 3.67 | 3.50 | 4.42 | 3.37 | 2.65 |
| SD | 0.26 | 0.85 | 0.32 | 0.75 | 0.24 | 0.74 | 0.33 | 0.33 | 0.39 | 0.18 | 0.35 | 1.03 | 0.39 | 0.40 |
| *Paired t | 0.02 | 0.01 | 0.13 | <0.01 | 0.06 | 0.02 | 0.02 | |||||||
| **Unpaired t | 0.47 | 0.19 | <0.05 | <0.01 | NA | NA | 0.07 | <0.01 | <0.01 | <0.01 | 0.09 | 0.01 | 0.29 | 0.07 |
Relative diameter of each artery was expressed in units of distal aortic diameter (which was defined as 10 within each angiogram). Day 0 = pre-ligation measurement. Day 30 = pre-necropsy measurement. CIIT = common internal iliac trunk; LEIA = left external iliac artery; RIIA = right internal iliac artery; LIIA = left internal iliac artery; dRPFA = reconstituted distal right profunda femoral artery; LPFA = left profunda femoral artery; RPop = right popliteal artery; dRIIA = reconstituted distal right internal iliac artery.
Paired t-test compared day 0 vs. 30 for each artery (p-value).
Unpaired t-test compared Version 1 vs. Version 2 at each time point (p-value).
Reconstituted vessela.
NA = comparison not applicable (RIIA was measured in Version 1, dRIIA was measured in Version 2). The raw data for Table 3 is available in the Supplemental Material.
ACKNOWLEDGEMENTS
This work was supported by NIH grants R01 AG034995 and R01 AG049868, by the Charles and Mary Heider Fund for Excellence in Vascular Surgery, by internal seed funds from the Department of Surgery of the University of Nebraska Medical Center, and with resources and the use of facilities at the VA Nebraska-Western Iowa Health Care System. Portions of this study were presented at Vascular Discovery: From Genes to Medicine (San Francisco, CA; May, 2018). The authors would like to acknowledge the technical assistance of Chris Hansen.
Abbreviations
- AAALAC
Association for Assessment and Accreditation of Laboratory Animal Care International
- ABI
Ankle/Brachial Index
- CLI
Chronic Limb Ischemia
- CIIT
Common Internal Iliac Trunk
- DICOM
Digital Imaging and Communications in Medicine
- dRPFA
reconstituted distal Right Profunda Femoral Artery
- dRIIA
reconstituted distal Right Internal Iliac Artery
- IACUC
Institutional Animal Care and Use Committee
- LEIA
Left External Iliac Artery
- LIIA
Left Internal Iliac Artery
- LPFA
Left Profunda Femoral Artery
- REIA
Right External Iliac Artery
- RLCIA
Right Lateral Circumflex Iliac
- RLCFA
Right Lateral Circumflex Femoral Artery
- RIIA
Right Internal Iliac Artery
- RPFA
Right Profunda Femoral Artery
- RSFA
Right Superficial Femoral Artery
- RPop
Right Popliteal Artery
- PAD
Peripheral Arterial Disease
- Moxy
Muscle Oximetry
- StO2
Muscle oxygen saturation
- THb
Total Hemoglobin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
The authors declare no competing interests.
REFERENCES
- 1.Criqui MH, Fronek A, Barrett-Connor E, Klauber MR, Gabriel S, Goodman D. The prevalence of peripheral arterial disease in a defined population. Circulation. 1985;71(3):510–515. [DOI] [PubMed] [Google Scholar]
- 2.Hiatt WR, Hoag S, Hamman RF. Effect of diagnostic criteria on the prevalence of peripheral arterial disease The San Luis Valley Diabetes Study. Circulation. 1995;91(5):1472–1479. [DOI] [PubMed] [Google Scholar]
- 3.Selvin E, Erlinger TP. Prevalence of and risk factors for peripheral arterial disease in the United States: results from the National Health and Nutrition Examination Survey, 1999-2000. Circulation. 2004;110(6):738–743. [DOI] [PubMed] [Google Scholar]
- 4.Kalbaugh CA, Kucharska-Newton A, Wruck L, et al. Peripheral Artery Disease Prevalence and Incidence Estimated From Both Outpatient and Inpatient Settings Among Medicare Fee-for-Service Beneficiaries in the Atherosclerosis Risk in Communities (ARIC) Study. J Am Heart Assoc. 2017;6(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devine EB, Alfonso-Cristancho R, Yanez ND, et al. Effectiveness of a Medical vs Revascularization Intervention for Intermittent Leg Claudication Based on Patient-Reported Outcomes. JAMA Surg. 2016;151(10):e162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45 Suppl S:S5–67. [DOI] [PubMed] [Google Scholar]
- 7.Hiatt WR, Armstrong EJ, Larson CJ, Brass EP. Pathogenesis of the limb manifestations and exercise limitations in peripheral artery disease. Circ Res. 2015;116(9):1527–1539. [DOI] [PubMed] [Google Scholar]
- 8.Polhemus DJ, Bradley JM, Islam KN, et al. Therapeutic potential of sustained-release sodium nitrite for critical limb ischemia in the setting of metabolic syndrome. American journal of physiology Heart and circulatory physiology. 2015;309(1):H82–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Long CA, Timmins LH, Koutakis P, et al. An endovascular model of ischemic myopathy from peripheral arterial disease. Journal of vascular surgery. 2017;66(3):891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Washington, DC: The National Academies Press; 2011. [Google Scholar]
- 11.American Veterinary Medical Association Panel on Euthanasia. AVMA Guidelines for the Euthanasia of Animals: 2013 Edition. Schaumberg, IL: American Veterinary Medical Association; 2013. [Google Scholar]
- 12.Swindle MM, Smith AC. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. 3rd ed. Boca Raton, FL: CRC Press; 2016. [Google Scholar]
- 13.Fuglestad MA, Hernandez H, Gao Y, et al. A low-cost, wireless near-infrared spectroscopy device detects the presence of lower extremity atherosclerosis as measured by computed tomographic angiography and characterizes walking impairment in peripheral artery disease. J Vasc Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koutakis P, Myers SA, Cluff K, et al. Abnormal myofiber morphology and limb dysfunction in claudication. The Journal of surgical research. 2015;196(1):172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pipinos II, Swanson SA, Zhu Z, et al. Chronically ischemic mouse skeletal muscle exhibits myopathy in association with mitochondrial dysfunction and oxidative damage. American journal of physiology Regulatory, integrative and comparative physiology. 2008;295(1):R290–296. [Google Scholar]
- 16.Cluff K, Miserlis D, Naganathan GK, et al. Morphometric analysis of gastrocnemius muscle biopsies from patients with peripheral arterial disease: objective grading of muscle degeneration. American journal of physiology Regulatory, integrative and comparative physiology. 2013;305(3):R291–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papoutsi E CG, Koutakis P, Myers S, Thompson JR, Ha D, Swanson SA, Zhu Z, Kim K, Wurdeman S, Johanning JM, McComb RD, Pipinos II. Revascularization Improves the Myopathy, Hemodynamics and Function of the Limbs of Patients With Peripheral Arterial Disease Circulation. 2013;128:A19103. [Google Scholar]
- 18.Weiss DJ, Casale GP, Koutakis P, et al. Oxidative damage and myofiber degeneration in the gastrocnemius of patients with peripheral arterial disease. Journal of translational medicine. 2013;11:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pipp F, Boehm S, Cai WJ, et al. Elevated fluid shear stress enhances postocclusive collateral artery growth and gene expression in the pig hind limb. Arterioscler Thromb Vasc Biol. 2004;24(9):1664–1668. [DOI] [PubMed] [Google Scholar]
- 20.Carmeliet P, Eelen G, Kalucka J. Arteriogenesis versus angiogenesis In: Krams R, Back M, eds. The ESC Textbook of Vascular Biology. Oxford University Press; 2017. [Google Scholar]
- 21.Deindl E, Schaper W. The art of arteriogenesis. Cell Biochem Biophys. 2005;43(1):1–15. [DOI] [PubMed] [Google Scholar]
- 22.Rizzi A, Benagiano V, Ribatti D. Angiogenesis versus arteriogenesis. Rom J Morphol Embryol. 2017;58(1):15–19. [PubMed] [Google Scholar]
- 23.van Royen N, Piek JJ, Schaper W, Fulton WF. A critical review of clinical arteriogenesis research. Journal of the American College of Cardiology. 2009;55(1):17–25. [DOI] [PubMed] [Google Scholar]
- 24.Garden S Rectal prolapse in pigs. Vet Rec. 1988;123(25):654. [PubMed] [Google Scholar]
- 25.Miller LR, Marks C, Becker JB, et al. Considering sex as a biological variable in preclinical research. FASEB J. 2017;31(1):29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cooke JP, Losordo DW. Modulating the vascular response to limb ischemia: angiogenic and cell therapies. Circ Res. 2015;116(9):1561–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frangogiannis NG. Cell therapy for peripheral artery disease. Curr Opin Pharmacol. 2018;39:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parikh PP, Liu ZJ, Velazquez OC. A Molecular and Clinical Review of Stem Cell Therapy in Critical Limb Ischemia. Stem Cells Int. 2017;2017:3750829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aranguren XL, Verfaillie CM, Luttun A. Emerging hurdles in stem cell therapy for peripheral vascular disease. J Mol Med (Berl). 2009;87(1):3–16. [DOI] [PubMed] [Google Scholar]
- 30.Gupta NK, Armstrong EJ, Parikh SA. The current state of stem cell therapy for peripheral artery disease. Current cardiology reports. 2014;16(2):447. [DOI] [PubMed] [Google Scholar]
- 31.Lawall H, Bramlage P, Amann B. Treatment of peripheral arterial disease using stem and progenitor cell therapy. Journal of vascular surgery. 2011;53(2):445–453. [DOI] [PubMed] [Google Scholar]
- 32.Qadura M, Terenzi DC, Verma S, Al-Omran M, Hess DA. Concise Review: Cell Therapy for Critical Limb Ischemia: An Integrated Review of Preclinical and Clinical Studies. Stem cells. 2018;36(2):161–171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig7S-Fig10S.
1. “Vid01_v1_PreLigAngio_Proximal.mp4”. Description: Version 1 aortobifemoral angiography shot pre-ligation, proximal view.
2. “Vid02_v1_PreLigAngio_Distal.mp4”. Description: Version 1 aortobifemoral angiography shot pre-ligation, distal view.
3. “Vid03_v1_PostLigAngio_Proximal.mp4”. Description: Version 1 aortobifemoral angiography shot immediately post-ligation, proximal view.
4. “Vid04_v1_PostLigAngio_Distal.mp4”. Description: Version 1 aortobifemoral angiography shot immediately post-ligation, distal view.
5. “Vid05_v1_PreNecAngio_Proximal.mp4”. Description: Version 1 aortobifemoral angiography shot immediately pre-necropsy (30 days after ligation), proximal view.
6. “Vid06_v1_PreNecAngio_Distal.mp4”. Description: Version 1 aortobifemoral angiography shot immediately pre-necropsy (30 days after ligation), distal view.
7. “Vid07_v2_PreLigAngio_Proximal.mp4”. Description: Version 2 aortobifemoral angiography shot pre-ligation, proximal view.
8. “Vid08_v2_PreLigAngio_Distal.mp4”. Description: Version 2 aortobifemoral angiography shot pre-ligation, distal view.
9. “Vid09_v2_PostLigAngio_Proximal.mp4”. Description: Version 2 aortobifemoral angiography shot immediately post-ligation, proximal view.
10. “Vid10_v2_PostLigAngio_Distal.mp4”. Description: Version 2 aortobifemoral angiography shot immediately post-ligation, distal view.
11. “Vid11_v2_PreNecAngio_Proximal.mp4”. Description: Version 2 aortobifemoral angiography shot immediately pre-necropsy (30 days after ligation), proximal view.
12. “Vid12_v2_PreNecAngio_Distal.mp4”. Description: Version 2 aortobifemoral angiography shot immediately pre-necropsy (30 days after ligation), distal view.


