Highlights
-
•
Bradyrhizobium japonicum and Trichoderma harzianum coexisted in soybean rhizosphere.
-
•
Soybean inoculated with both microbes nodulated with high nitrate concentrations.
-
•
T. harzianum produced auxins in culture medium.
-
•
Auxins applied with B. japonicum alone relieved nitrate inhibition of nodulation.
Abbreviations: AG, arabinose-gluconate medium; CFU, colony-forming units; CR, Congo Red; DAI, days after inoculation; IAA, indoleacetic acid; LPCB, lactophenol cotton blue; MFS, modified Fåhræus solution; PDA, potato-dextrose agar; PGPM, plant-growth promoting microbe; YM, yeast-extract mannitol medium; YMA, YM with 1.5 % (w/v) agar
Keywords: Coinoculation, Soybean, Bradyrhizobium, Trichoderma, Nitrate
Abstract
Coinoculation of plants with mixtures of beneficial microbes sometimes produces synergistic effects. In this study, the effect of soybean coinoculation with the N2-fixing Bradyrhizobium japonicum E109 and the biocontrol fungus Trichoderma harzianum Th5cc was analyzed. Nodulation by E109 was not hampered by Th5cc, which antagonized five out of seven soybean pathogens tested. Furthermore, Th5cc relieved nitrate-inhibition of nodulation, enabling the formation of nodules containing infected cells with bacteroids in the presence of the otherwise inhibitory 10 mM KNO3. Th5cc released micromolar amounts of auxin, and addition of 11 μM indoleacetic acid to soybean plants inoculated with E109 in the absence of Th5cc also induced nodulation in the presence of 10 mM KNO3. Thus, Th5cc may release auxins into the soybean rhizosphere, which hormones might participate in overcoming the nitrate-inhibition of nodulation. Our results suggest that soybean plants coinoculated with these microorganisms might benefit from biocontrol while contributing to soil-nitrogen preservation.
1. Introduction
Food security demands sustainable-food provision for an increasing population, while expansion of cropping lands is becoming constrained. For instance, in Argentina, the third largest soybean producer in the world, the soybean cropped surface expanded at a rate of 825,000 hectares per year between 1998 and 2012 [1] but after this accelerated growth the surface remained constant in around 20 million hectares [2,3], indicating that the soybean agricultural frontier was reached in this country. Therefore, any current increase in production necessitates a greater productivity per hectare, leading to higher nutrients extraction by plants possibly harming soil fertility and stability. Among the technologies available to mitigate pressure on soil health is the inoculation of pulse crops with symbiotic and plant-growth-promoting microbes (PGPMs), which are intended to diminish the use of fertilizers and pesticides. Soybean plants are regularly inoculated with N2-fixing Bradyrhizobium spp., but the use of other PGPMs on this crop is only beginning. It was reported that coinoculation of soybean with Bradyrhizobium sp. and the arbuscular mycorrhizal fungus Glomus mosseae lead to higher plant biomass if the soil is poor in N and P [4]. However, the production of arbuscular mycorrhizal fungi at commercial scale for use in extensive crops still awaits full development. Strains of Bacillus spp. were also used for coinoculation with B. japonicum, yielding improved nodulation with respect to plants inoculated with B. japonicum alone [5,6]. In addition, increases in soybean nodulation, biomass production, and crop yield were observed after coinoculation with Bradyrhizobium spp. and Azospirillum brasilense [7] or Streptomyces griseoflavus [8]. However, there are no reports on coinoculation of soybean with B. japonicum and biocontrol fungi.
Species of the filamentous fungus Trichoderma spp. are well-characterized biocontrol agents for several crop plants [9]. This PGPM possesses the advantage that it is easy to cultivate under laboratory conditions and fermentation methods for its scale production have been proposed [[10], [11], [12]]. In particular, T. harzianum inhibited 56.3 % of growth of the soybean pathogen Sclerotinia sclerotiorum in dual culture tests and contained the disease caused by this pathogen in plants, although effects in enhancing soybean production were not consistently observed [13,14]. Moreover, T. harzianum induced resistance to Fusarium oxysporum in soybean seedlings [15]. In addition to its biocontrol properties, T. harzianum elaborates plant-growth regulators [16]. However, these works did not investigate whether T. harzianum may be compatible with Bradyrhizobium spp., the main symbiont of soybean. Therefore, in this work we aimed at testing whether B. japonicum and T. harzianum could coexist in soybean rhizospheres, and if so, whether that coexistence was beneficial.
2. Materials and methods
2.1. Strains and culture conditions
B. japonicum E109, recommended for soybean inoculants in Argentina [17], was grown in yeast-extract- mannitol (YM) [18] or arabinose-gluconate (AG) [19] at 28 °C. When grown in liquid medium, the cultures were agitated by rotary shaking at 180 rpm and the biomass was estimated by optical density at 500 nm. For growth in solid medium, YM was supplemented with 1.5 % (w/v) agar (YMA) and 3.6 μM Congo Red (CR). T. harzianum Th5cc, isolated from wheat phyllosphere [20], was grown in potato-dextrose agar (PDA) or Trichoderma-selective medium [21], in both cases with 1.5 % (w/v) agar at 28 °C in the dark. Occasionally, this fungus was grown in YM, or in YMA with CR, as indicated.
2.2. Biocontrol assays
To test T. harzianum Th5cc biocontrol against a known soybean pathogen, two 5-mm-diameter discs were placed, one with the pathogen and the other with T. harzianum Th5cc, facing each other in a PDA plate. Then, both fungi were grown for 7 days at 28 °C and the growth assessed by the test-fungal-growth inhibition effected by T. harzianum Th5cc on a semiquantitative scale previously described by Bell et al. [22]. This scale classifies the protective capacity according to the following scores. 1: indicates a complete overgrowth of the biocontrol fungus over the pathogen fungus, 2: a growth of the biocontrol fungus over at least two-thirds of the medium surface, 3: a colonization of one-half of the surface by each of the two fungi with neither one dominating the other, 4: a colonization of at least two-thirds of the surface by the pathogen, 5: a complete overgrowth of the pathogen over the biocontrol fungus. Scores ≤ 2 indicate significant antagonism of T. harzianum on the pathogen. The pathogens tested were: Alternaria spp. D18, Cercospora kikuchii D33, Phomopsis longicolla DP38, P. longicolla DP41, Rhizoctonia spp. R24, Rhizoctonia spp. RM, and Sclerotinia sclerotiorum L50, all of them obtained from the Rizobacter collection.
2.3. Plant experiments
Soybean Don Mario 4800 seeds were surface-sterilized and germinated as described [23]. To evaluate nodulation, sets of 10 soybean plants were cultivated in sterile perlite/sand (2:1) and watered with sterile modified Fåhræus solution (MFS) as described [24], with each set being inoculated as follows: 1) with B. japonicum E109 grown to the exponential phase in AG broth, 2) with T. harzianum Th5cc grown in PDA, 3) with an admixture of both microorganisms, 4) with sterile MFS. B. japonicum was diluted in the MFS directly from AG broths at the desired cell concentrations assessed by counting in a Neubauer chamber. To inoculate T. harzianum, conidia were harvested by flooding the PDA cultures with sterile distilled water and then rubbing the culture surface with a sterile glass rod followed by counting in a Neubauer chamber. Plants were cultivated for the indicated days after inoculation (DAI) in a plant-growth chamber at 30 °C/20 °C day/night temperature with a photophase of 16 h. During the cultivation, the plants were watered twice per week with sterile MFS either with or without KNO3 at the indicated concentrations. All treatments were conducted with ten plants per condition, and each entire experiment was repeated three times.
2.4. Auxin determinations
T. harzianum Th5cc hyphae were transferred to liquid YM and agitated at 180 rpm for 7 days. Then, the cultures were centrifuged at 13,000 × g for 3 min and the supernatant was used for auxin determination with Salkowski’s reagent [25]. Quantification was performed at 535 nm by comparison with a calibration curve constructed with purified indoleacetic acid (IAA, Sigma Chemical Co.) as a standard.
2.5. Microscopy
For hyphae observations, mycelium growing in YMA-CR plates either in contact with B. japonicum colonies or not, was examined under a Nikon Eclipse E200 microscope at the indicated magnifications. For hyphae staining inside roots, root segments from plants inoculated with T harzianum Th5cc were washed, embedded in 3% (w/v) KOH for 20 min to soften the tissues, and stained with lactophenol cotton blue (LPCB). Then, the roots were observed under a Nikon Eclipse E200 microscope at 40 × magnification in search for endophytic hyphae. For nodules observations, nodules were excised from plants, transversally cut into halves, and fixed in 2% (v/v) glutaraldehyde. Fixed nodules were then dehydrated, infiltrated with epoxy resin, and sectioned. For optical microscopy, glutaraldehyde-fixed 2-μm-thick nodule sections were stained with a saturated solution of toluidine blue and analyzed under a Nikon Eclipse E200 microscope (Melville, NY). For transmission electron microscopy, 70-nm ultrathin sections were stained with uranyl acetate as described [23] and viewed with a JEM 1200 EX (JEOL, Japan Electron Optics Laboratory Co., Ltd.). The electron micrographs were obtained with an ES500W Erlangshen charge-coupled camera device (Gatan Inc., Pleasanton, CA).
3. Results and discussion
3.1. Absence of interference of B. japonicum and T. harzianum with one another
We grew T. harzianum Th5cc in YMA with CR [18], a medium commonly used for B. japonicum cultivation. To check if either of these microbes was antagonistic to the other, four equidistant B. japonicum E109 macrocolonies were cultured in a YMA-CR plate for 7 days, and then a 5-mm-diameter T. harzianum Th5cc plug was laid in the center of the plate amidst the B. japonicum macrocolonies. Four days later the edge of the fungal colony reached the B. japonicum macrocolonies, and by 10 days the fungal colony had passed over the bacteria to colonize the entire plate surface (Fig. S1a). Fungal growth rate and conidia formation were not affected by the presence of B. japonicum E109 (Fig. S1b). Moreover, there was no evidence of growth inhibition or mycelia damage in the Bradyrhizobium-Trichoderma contact zone (Fig. S1c). To verify a normal rhizobial growth after contact with the fungus, the bacterial macrocolonies were scraped from under the mycelia and streaked in fresh YMA-CR supplemented with 100 mg.l−1 cycloheximide as a fungicide. Thereafter B. japonicum E109 grew normally, indicating that the biocontrol fungus did not antagonize rhizobial replication.
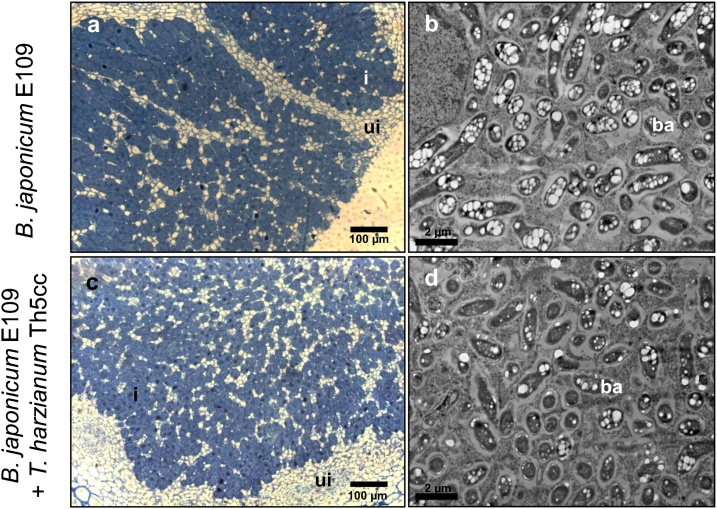
To observe the effects of coinoculation on nodulation and plant growth, sets of 10 soybean plants received the following inoculations: 1) B. japonicum E109, 2) T. harzianum Th5cc, 3) an admixture of both microorganisms, 4) sterile MFS. Twenty-one DAI, only those plants that had received B. japonicum E109 became nodulated. Those plants inoculated B. japonicum E109 alone developed 16 ± 4 nodules per plant, while those inoculated with the admixture manifested 12 ± 3 nodules per plant (average ± SD), indicating that T. harzianum Th5cc had not precluded nodulation. The nodules from the coinoculated plants had the same ultrastructure as those inoculated with only B. japonicum E109 (Fig. 1). In addition, washes from T. harzianum Th5cc-infested roots cultivated in Trichoderma-selective medium [21] developed (0.97 ± 0.24) x 105 T. harzianum colony-forming units (CFU). g−1 root fresh weight, whereas washes from roots inoculated with the admixture produced (1.24 ± 0.62) x 105 T. harzianum CFU. g−1 root fresh weight, indicating that T. harzianum Th5cc had colonized the soybean rhizosphere, staying at root surfaces, and B. japonicum had not interfered.
Fig. 1.
Structure of the soybean nodules produced by Bradyrhizobium japonicum E109 alone (a, b) or by the admixture of B. japonicum E109 and Trichoderma harzianum Th5cc (c, d). Histological cuts (a, c) and ultrastructure (b, d) of nodules formed during 21 DAI in perlite/sand pots watered with MFS. Uninfected cells (ui) are present contiguous to infected cells (i), which contain bacteroids (ba).
To ascertain whether T. harzianum Th5cc may be endophytic in soybean roots, LPCB-stained root segments from plants inoculated with T harzianum Th5cc were observed under the microscope. No hyphae with typical conidial-morphologic structure were observed below the root epidermis, indicating that Th5cc underwent no endophytic phase in soybeans under the conditions studied.
3.2. Antagonism of soybean pathogenic fungi by T. harzianum
To test the capability of T. harzianum Th5cc as biocontrol agent, dual cultures with known soybean pathogens were performed. By using the Bell’s semiquantitative scale [22] it was observed that T. harzianum Th5cc inhibited Cercospora kikuchii D33 (score 1), Alternaria sp. D18, Phomopsis longicolla DP38, and Rhizoctonia sp. RM and R24 (score 2); whereas the control of Sclerotinia sclerotiorum L50 and P. longicolla DP41 was weaker (score 3; Fig. S2). According to this semiquantitative scale, antagonism cannot be considered in those interactions with score ≥ 3 [22]. Therefore, T. harzianum Th5cc was antagonist against 5 of the 7 pathogen strains tested.
3.3. Noninhibition by T. harzianum of soybean nodulation by B. japonicum in the presence of inhibitory nitrate concentrations
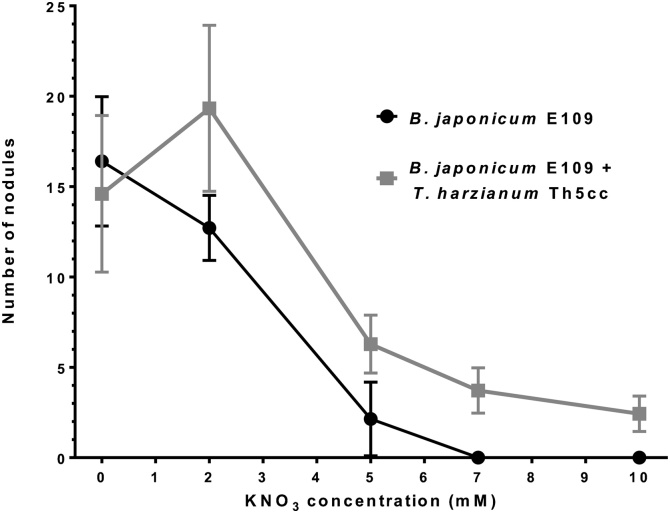
Soybean nodulation is hampered if combined nitrogen sources, and especially nitrate, are available [26]. Furthermore, plants inoculated with T. harzianum alone and cultivated without a nitrogen source may be nitrogen-limited, thus masking the fungus's effects on plant growth. Hence, in new experiments we evaluated the response of sets of soybean plants inoculated with or without combinations of B. japonicum and T. harzianum as described above and watered with MFS containing increasing KNO3 concentrations. In the absence of T. harzianum Th5cc, nodulation steadily decreased until reaching an inhibition above 5 mM KNO3. By contrast, in coinoculated plants, the fungus had a positive effect on nodulation at all KNO3 concentrations tested (Fig. 2). As expected, neither uninoculated controls nor plants inoculated with T. harzianum Th5cc alone contained nodules.
Fig. 2.
Nodulation of soybeans inoculated with Bradyrhizobium japonicum E109 in the exponential growth phase at 1.106 rhizobia. ml−1 and Trichoderma harzianum Th5cc at 1.107 conidia. ml−1. Nodules produced at 21 DAI (ordinate) by B. japonicum E109 alone (black circles) or B. japonicum E109 along with T. harzianum Th5cc (gray squares) at increasing KNO3 concentrations (abscissa). Plants inoculated without B. japonicum E109 did not nodulate. Values are averages ± standard deviations (error bars).
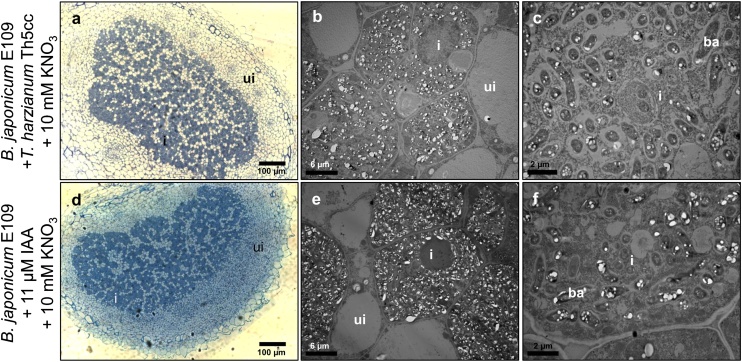
The nodules produced in coinoculated plants in the presence of 10 mM KNO3 were red inside and contained infected cells and normal bacteroids (Fig. 3a–c). Upon comparing these results to the ultrastructure of nodules produced by B. japonicum E109 in the absence of KNO3 (Fig. 1a, b), we concluded that the addition of KNO3 in the presence of T. harzianum Th5cc did not substantially affect nodule development.
Fig. 3.
Structure of the soybean nodules produced at 21 DAI in perlite/sand pots watered with MFS supplemented with 10 mM KNO3 throughout the entire experiment. Histological cuts (a,d) or ultrastructure (b, c, e, f) of nodules obtained with Bradyrhizobium japonicum E109 coinoculated with Trichoderma harzianum (a–c), or B. japonicum E109 with 11 μM indoleacetic acid (d–f). Uninfected cells (ui) are present next to bacteriod-containing (ba) infected cells (i).
Previous results had raised the question as to whether the nodules formed in the presence of KNO3 contributed to plant growth. In response, we compared shoot and root dry weights produced by soybean plants inoculated with B. japonicum with or without T. harzianum and watered with N-free MFS, or MFS supplemented with 10 mM KNO3 during 40 DAI to promote a sufficient dry-mass formation. As shown in Table 1, in the condition without KNO3 all plants inoculated with B. japonicum E109 nodulated either with or without T. harzianum Th5cc, with no significant differences in nodules number between both inoculation treatments. By contrast, in the condition with 10 mM KNO3, only the plants inoculated with the admixture contained nodules. Although shoot and root dry weights were significantly higher in plants grown with 10 mM KNO3, there were no differences within each N-condition among the different inoculation treatments (Table 1), in agreement with previous observations on T. harzianum effects on soybean growth [13].
Table 1.
Nodulation and dry weight of soybean plants inoculated with B. japonicum E109, T. harzianum Th5cc or both, in the presence or absence of combined N.
| Inoculation | Nodules per plant ± SD§ |
Shoot dry weight ± SD§ (g.plant―1) |
Root dry weight ± SD§ (g.plant―1) |
|||
|---|---|---|---|---|---|---|
| without KNO3 | with KNO3* | without KNO3 | with KNO3* | without KNO3 | with KNO3* | |
| E109 | 16 ± 4 A | 0 | 0.76 ± 0.14 A | 1.56 ± 0.24 B | 0.47 ± 0.06 A | 0.76 ± 0.10 B |
| Th5cc | 0 | 0 | 0.67 ± 0.07 A | 1.32 ± 0.29 B | 0.43 ± 0.09 A | 0.82 ± 0.14 B |
| E109 + Th5cc | 15 ± 4 A | 3 ± 1 B | 0.73 ± 0.13 A | 1.46 ± 0.15 B | 0.49 ± 0.06 A | 0.88 ± 0.10 B |
| Control | 0 | 0 | 0.69 ± 0.14 A | 1.21 ± 0.21 B | 0.41 ± 0.06 A | 0.68 ± 0.08 B |
SD: Standard deviation. Values followed by different letters within each trait were statistically different with p < 0.005 according to ANOVA followed by Tukey test. Differences among values followed by the same letter within each trait were non significant.
KNO3 concentration: 10 mM during the whole assay.
3.4. Possible induction of nodulation by auxins in the presence of KNO3
Auxin transport and accumulation may be related to the response of nodulation to combined nitrogen [[27], [28], [29]]. Since T. harzianum may produce auxins [16] we were interested in determining whether the Th5cc strain may produce this plant growth regulator. To this end, we suspended this strain in YM medium, and quantified the auxins released to the culture supernatant. In this way, we detected the presence of ca. 11 μM extracellular auxins in these supernatants, using IAA as standard. To assess whether auxins might be responsible for the results observed in Fig. 3a–c and to investigate this issue, soybean plants were inoculated with B. japonicum E109 suspended in MFS containing 10 mM KNO3 and 11 μM IAA. At twenty-one DAI all plants thus inoculated and watered with MFS supplemented with 10 mM KNO3 formed nodules (Fig. 3d–f). These nodules were histologically and ultrastructurally indistinguishable from the ones obtained from plants inoculated in parallel with B. japonicum E109 and T. harzianum Th5cc in the presence of 10 mM KNO3 without IAA (Fig. 3a–c).
The correlation observed between auxin production by T. harzianum Th5cc, nodulation by coinoculation in the presence of KNO3, or via exogenous IAA application suggested that a possible release of auxins by T. harzianum Th5cc in the soybean rhizosphere might have induced nodulation by B. japonicum E109 in the presence of high KNO3 concentrations. In contrast, the putative auxin production by T. harzianum Th5cc in the soybean rhizosphere had no effect on plant-biomass production under the conditions employed here.
4. Conclusion
Although this work was performed with only one strain from each microorganism, the symbiotic and biocontrol properties observed here were in agreement with those reported previously with other strains [[13], [14], [15],23]. However, T. harzianum Th5cc enabled the production of functional nodules in soybean by B. japonicum in the presence of inhibitory nitrate concentrations, which is a synergistic outcome that cannot be predicted from the performance of each microorganism alone. This property might be related to the production of auxins and their release by the fungus. Furthermore, nodulation in the presence of nitrate might be advantageous in preserving soil fertility because those plants coinoculated with both microorganisms might obtain part of their nutrient nitrogen from the atmosphere even in soils relatively rich in nitrogen. In addition, T. harzianum Th5cc demonstrated to be antagonist to five soybean pathogens, whereby this coinoculation method may allow benefits from the T. harzianum–biocontrol activity. Nevertheless, other strains should be analyzed to optimize the tripartite bacterium-fungus-plant relationship in order to obtain better responses for plant biomass production and crop yield.
CRediT authorship contribution statement
Esteban Tomás Iturralde: Investigation, Visualization, Formal analysis, Validation, Writing - review & editing. Marina Celeste Stocco: Investigation, Writing - review & editing. Andrés Faura: Investigation, Resources, Writing - review & editing. Cecilia Inés Mónaco: Supervision, Resources, Writing - review & editing. Cristina Cordo: Supervision, Resources, Writing - review & editing. Julieta Pérez-Giménez: Supervision, Conceptualization, Methodology, Validation, Project administration, Writing - review & editing. Aníbal Roberto Lodeiro: Conceptualization, Formal analysis, Validation, Resources, Project administration, Funding acquisition, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by Agencia Nacional de Promoción de la Investigación Científica y Tecnológica (ANPCyT) grant number PICT2017-2456, and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) grant number PIP 0386 both from Argentina. JPG and ARL are members of the Scientific Career of CONICET; ETI is fellow of CONICET. We are grateful to Paula Giménez, Silvana Tongiani, Claudio Mazo and Abel Bortolameotti for technical assistance. Dr. Donald F. Haggerty, a retired academic career investigator and native English speaker, edited the final version of the manuscript.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.btre.2020.e00461.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Calzada J., Russo C. Informativo Semanal BCR 1666. 2014. En el cultivo de soja, hay que cuidar a los productores del NOA y el NEA; pp. 5–6.https://www.bcr.com.ar/es/mercados/investigacion-y-desarrollo/informativo-semanal/noticias-informativo-semanal/en-el-cultivo Accessed on Feb. 6, 2020. [Google Scholar]
- 2.Ybran R.G., Lacelli G.A. INTA; 2016. Informe estadístico mercado de la soja.https://inta.gob.ar/documentos/informe-estadistico-del-mercado-de-la-soja Accessed on Feb. 6, 2020. [Google Scholar]
- 3.D’Angelo L.R. Ministerio de Agroindustria; 2017. Perspectivas del mercado de soja. Subsecretaría de Mercados Agropecuarios.https://www.agroindustria.gob.ar/sitio/areas/ss_mercados_agropecuarios/informes/perspectivas_de_soja_2018.pdf Accessed on Feb. 6, 2020. [Google Scholar]
- 4.Wang X., Pan Q., Chen F., Yan X., Liao H. Effects of co-inoculation with arbuscular mycorrhizal fungi and rhizobia on soybean growth as related to root architecture and availability of N and P. Mycorrhiza. 2011;21:173–181. doi: 10.1007/s00572-010-0319-1. [DOI] [PubMed] [Google Scholar]
- 5.Atieno M., Herrmann L., Okalebo R., Lesueur D. Efficiency of different formulations of Bradyrhizobium japonicum and effect of co-inoculation of Bacillus subtilis with two different strains of Bradyrhizobium japonicum. World J. Microbiol. Biotechnol. 2012;28:2541–2550. doi: 10.1007/s11274-012-1062-x. [DOI] [PubMed] [Google Scholar]
- 6.Masciarelli O., Llanes A., Luna V. A new PGPR co-inoculated with Bradyrhizobium japonicum enhances soybean nodulation. Microbiol. Res. 2014;169:609–615. doi: 10.1016/j.micres.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 7.Hungria M., Nogueira M.A., Silva-Araujo R. Soybean seed co-inoculation with Bradyrhizobium spp. and Azospirillum brasilense: a new biotechnological tool to improve yield and sustainability. Am. J. Plant Sci. 2015;6:811–817. doi: 10.4236/ajps.2015.66087. [DOI] [Google Scholar]
- 8.Htwe A.Z., Moh S.M., Moe K., Yamakawa T. Effects of co-inoculation of Bradyrhizobium japonicum SAY3-7 and Streptomyces griseoflavus P4 on plant growth, nodulation, nitrogen fixation, nutrient uptake, and yield of soybean in a field condition. Soil Sci. Plant Nutr. 2018 doi: 10.1080/00380768.2017.1421436. [DOI] [Google Scholar]
- 9.Benítez T., Rincón A.M., Limón M.C., Codón A.C. Biocontrol mechanisms of Trichoderma strains. Int. Microbiol. 2004;7:249–260. [PubMed] [Google Scholar]
- 10.Li Y.Q., Song K., Li Y.C., Chen J. Statistical culture-based strategies to enhance chlamydospore production by Trichoderma harzianum SH2303 in liquid fermentation. J. Zhejiang Univ. Sci. B. 2016;17:619–627. doi: 10.1631/jzus.B1500226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez-Ramirez N., Volke-Sepulveda T., Gaime-Perraud I., Saucedo-Castañeda G., Favela-Torres E. Effect of stirring on growth and cellulolytic enzymes production by Trichoderma harzianum in a novel bench-scale solid-state fermentation bioreactor. Bioresour. Technol. 2018;265:291–298. doi: 10.1016/j.biortech.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Rayhane H., Josiane M., Gregoria M., Yiannis K., Nathalie D., Ahmed M., Sevastianos R. From flasks to single used bioreactor: scale-up of solid state fermentation process for metabolites and conidia production by Trichoderma asperellum. J. Environ. Manage. 2019;252 doi: 10.1016/j.jenvman.2019.109496. [DOI] [PubMed] [Google Scholar]
- 13.Menendez A.B., Godeas A. Biological control of Sclerotinia sclerotiorumattacking soybean plants. Degradation of the cell walls of this pathogen by Trichoderma harzianum (BAFC 742) Mycopathologia. 1998;142:153–160. doi: 10.1023/A:1006910707804. [DOI] [PubMed] [Google Scholar]
- 14.Zhang F., Ge H., Zhang F., Guo N., Wang Y., Chen L., Ji X., Li C. Biocontrol potential of Trichoderma harzianum isolate T-aloe against Sclerotinia sclerotiorum in soybean. Plant Physiol. Biochem. 2016;100:64–74. doi: 10.1016/j.plaphy.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F., Chen C., Zhang F., Gao L., Liu J., Chen L., Fan X., Liu C., Zhang K., He Y., Chen C., Ji X. Trichoderma harzianum containing 1-aminocyclopropane-1-carboxylate deaminase and chitinase improved growth and diminished adverse effect caused by Fusarium oxysporum in soybean. J. Plant Physiol. 2017;210:84–94. doi: 10.1016/j.jplph.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 16.Contreras-Cornejo H.A., Macías-Rodríguez L., del-Val E., Larsen J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: interactions with plants. FEMS Microbiol. Ecol. 2016;92 doi: 10.1093/femsec/fiw036. fiw036. [DOI] [PubMed] [Google Scholar]
- 17.Torres D., Revale S., Obando M., Maroniche G., Paris G., Perticari A. Genome sequence of Bradyrhizobium japonicum E109, one of the most agronomically used nitrogen-fixing rhizobacteria in Argentina. Genome Announc. 2015;3:e01566–14. doi: 10.1128/genomeA.01566-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vincent J.M. IBP Handbook No. 15. Blackwell Scientific Publications; Oxford, UK: 1970. A manual for the practical study of the root nodule bacteria. [Google Scholar]
- 19.Sadowsky M.J., Tully R.E., Cregan P.B., Keyser H.H. Genetic diversity in Bradyrhizobium japonicum serogroup 123 and its relation to genotype-specific nodulation of soybean. Appl. Environ. Microbiol. 1987;53:2624–2630. doi: 10.1128/aem.53.11.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cordo C.A., Mónaco C.I., Segarra C.I., Simon M.R., Mansilla A.Y., Perelló A.E. Trichoderma spp. as elicitors of wheat plant defense responses against Septoria tritici. Biocontrol Sci. Technol. 2007;17:687–698. doi: 10.1080/09583150701527094. [DOI] [Google Scholar]
- 21.Elad Y., Chet I. Improved selective media for isolation of Trichoderma spp. or Fusarium spp. Phytoparasitica. 1983;11:55–58. doi: 10.1007/BF02980712. [DOI] [Google Scholar]
- 22.Bell D.K., Wells H.D., Markham C.R. In vitro antagonism of Trichoderma species against six fungal plant pathogens. Phytopathology. 1982;72:379–382. doi: 10.1094/Phyto-72-379. [DOI] [Google Scholar]
- 23.Quelas J.I., Mongiardini E.J., Casabuono A., López-García S.L., Althabegoiti M.J., Covelli J.M. Lack of galactose or galacturonic acid in Bradyrhizobium japonicum USDA 110 exopolysaccharide leads to different symbiotic responses in soybean. Mol. Plant Microbe Interact. 2010;23:1592–1604. doi: 10.1094/MPMI-05-10-0122. [DOI] [PubMed] [Google Scholar]
- 24.Lodeiro A.R., González P., Hernández A., Balagué L.J., Favelukes G. Comparison of drought tolerance in nitrogen-fixing and inorganic nitrogen-grown common beans. Plant Sci. 2000;154:31–41. doi: 10.1016/S0168-9452(99)00246-0. [DOI] [PubMed] [Google Scholar]
- 25.Gravel V., Antoun H., Tweddell R.J. Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA) Soil Biol. Biochem. 2007;39:1968–1977. doi: 10.1016/j.soilbio.2007.02.015. [DOI] [Google Scholar]
- 26.Nishida H., Suzaki T. Two negative regulatory systems of root nodule symbiosis - how are symbiotic benefits and costs balanced? Plant Cell Physiol. 2018;59:1733–1738. doi: 10.1093/pcp/pcy102. [DOI] [PubMed] [Google Scholar]
- 27.Caba J.M., Centeno M.L., Fernández B., Gresshoff P.M., Ligero F. Inoculation and nitrate alter phytohormone levels in soybean roots: differences between a supernodulating mutant and the wild type. Planta. 2000;211:98–104. doi: 10.1007/s004250000265. [DOI] [PubMed] [Google Scholar]
- 28.Jin J., Watt M., Mathesius U. The autoregulation gene SUNN mediates changes in root organ formation in response to nitrogen through alteration of shoot-to-root auxin transport. Plant Physiol. 2012;159:489–500. doi: 10.1104/pp.112.194993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng J.L., Hassan S., Truong T.T., Hocart C.H., Laffont C., Frugier F., Mathesius U. Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell. 2015;27:2210–2226. doi: 10.1105/tpc.15.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.