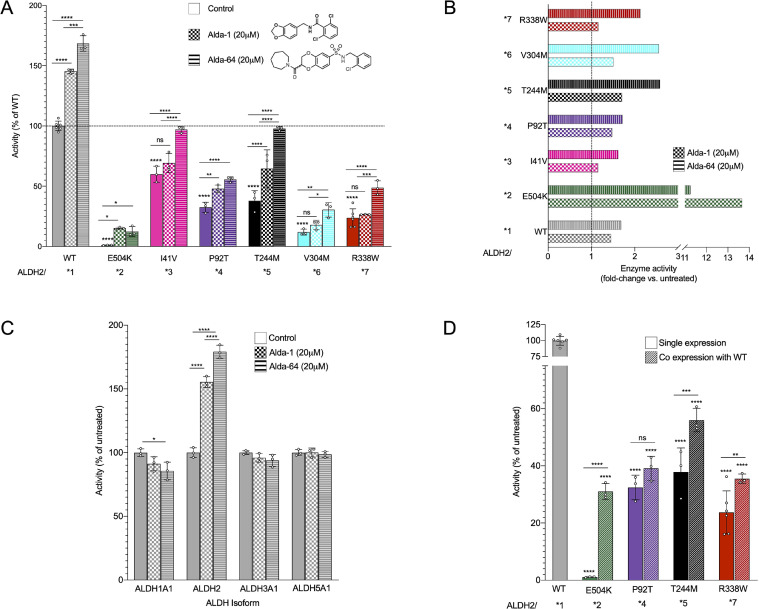
Fig. 2.
Newly characterized ALDH2 mutants show decreased activity in vitro compared to wildtype (WT) enzyme. (A)ALDH2 activity of the recombinant mutants upon acetaldehyde treatment was measured using the conversion of NAD+ to NADH, without treatment (solid bars) and with the ALDH2 activators Alda-1 and Alda-64 (checkered and striped bars, respectively). n = 3–6; Mean, standard deviation, probability by one‐way ANOVA (with Fischer's LSD post hoc test) **** p-value <0.0001; *** p-value <0.001; ** p-value <0.01; * p-value <0.05. (B) Fold change of ALDH2 activity improvement in each individual ALDH2 variants by Alda-1 and Alda-64. (C) Dehydrogenase activity measured in other ALDH isoforms after treatment with Alda-1 and Alda-64. n = 3; Mean, standard deviation, probability by one‐way ANOVA (with Fischer's LSD post hoc test) **** p-value <0.0001; * p-value <0.05. (D)ALDH2 activity measured in enzyme produced in a co-expression system of WT and ALDH2 variants to mimic heterozygous carriers. n = 3; Mean, standard deviation, probability by one‐way ANOVA (with Fischer's LSD post hoc test) **** p-value <0.0001; ** p-value <0.01; * p-value <0.05.