Abstract
Exosomes are informative microvesicles associated with intercellular communication via the transfer of many molecular constituents such as proteins, lipids, and nucleic acids; environmental changes and the cellular status around cells greatly affect exosome components. Cells of the retinal pigment epithelium (RPE) are key players in retinal homeostasis. Transforming growth factor (TGF)-β and tumour necrosis factor (TNF)-α are increased in the vitreous and retina in several retinal diseases and activate and undergo epithelial-mesenchymal transition (EMT) in RPE cells. EMT is closely associated with mechanisms of wound healing, including fibrosis and related angiogenesis; however, whether exosome components depend on the cell status, epithelium or mesenchyme and whether these exosomes have pro- or anti-angiogenic roles in the retina are unknown. We performed this study to investigate whether these EMT inducers affect the kinds of components in exosomes secreted from RPE cells and to assess their angiogenic effects. Exosomes were collected from culture media supernatants of a human RPE cell line (ARPE-19) stimulated with or without 10 ng/ml TNF-α and/or 5 ng/ml TGF-β2. NanoSight tracking analysis and immunoblot analysis using exosome markers were used to qualify harvested vesicles. Angiogenic factor microarray analysis revealed that exosomes derived from ARPE-19 cells cultured with TNF-α alone (Exo-TNF) and co-stimulated with TNF-α and TGF-β2 (Exo-CO) contained more angiogenic factors than exosomes derived from control cells (Exo-CTL) or ARPE-19 cells cultured with TGF-β2 alone (Exo-TGF). To assess the effect on angiogenesis, we performed chemotaxis, tube formation, and proliferation assays of human umbilical vein endothelial cells (HUVECs) stimulated with or without exosomes. HUVECs migrated to RPE-derived exosomes, and exosomes derived from ARPE-19 cells accelerated HUVEC tube formation. In contrast, Exo-TNF and Exo-CO reduced HUVEC proliferation. Our findings provide insight into the mechanisms underlying the relation between angiogenesis and exosomes derived from RPE cells.
Keywords: Exosome, Retinal pigment epithelial cells, Angiogenesis, Tumour necrosis factor, Transforming growth factor
Abbreviations: RPE, retinal pigment epithelium; TNF-α, tumour necrosis factor-α; TGF-β, transforming growth factor-β; HUVEC, human umbilical vein endothelial cell
Highlights
-
•
Exosomes are intercellular communication tools for informing the environment.
-
•
Exosomal angiogenic factors differ between TNF-α and TGF-β stimulation.
-
•
Exosomes derived from RPE cells are associated with angiogenic behaviour of HUVECs.
-
•
RPE-derived exosomes inhibit the proliferation of HUVECs.
-
•
RPE may contribute to angiogenesis via changes in exosomal angiogenic factors.
1. Introduction
Age-related macular degeneration (AMD) is a major cause of severe visual impairment in western countries, and wet AMD, the exudative type of AMD, is characterized by abnormal choroidal neovascularization (CNV) invading into the retinal pigment epithelium (RPE) from the choroidal layer [1,2]. Intravitreal anti-vascular endothelial growth factor (VEGF) therapy is a mainstream treatment that is dramatically effective for treating wet AMD; however, problems such as the recurrence of CNV, non-response to therapy, and fibrosis remain.
The RPE is located between the sensory retina and the choroidal layer and is essential for retinal homeostasis due to its various functions, including phagocytosis, transportation of nutrients, and regulation of the blood-retinal barrier (BRB) [3]. A recent study revealed that the expression of Snail, a transcription factor associated with epithelial-mesenchymal transition (EMT), was expressed in RPE cells in human CNV tissues [4]. When EMT occurs in epithelia, cells gain mesenchymal characteristics and overexpress extracellular matrix components, growth factors, and cytokines, including angiogenic factors, to repair injured tissues [5]. In RPE, aberrant EMT at the inflammatory site causes breakdown of the BRB, and this phenomenon in the microenvironment facilitates the migration of endothelial cells, macrophages, and fibroblasts to inflammatory regions [6]. Many kinds of EMT triggers have been reported. Among them, transforming growth factors (TGFs) such as (TGF)-β are the most well-known, and ocular fibrotic diseases feature EMT in the RPE [7]. In addition, proinflammatory cytokines such as tumour necrosis factor (TNF)-α can induce EMT in RPE cells [8]. In the retina, TNF-α mRNA was detected in membranes during proliferative vitreoretinopathy (PVR) [9]and immune cells, glial cells, and activated RPE cells are reported to be sources of TNF-α [[10], [11], [12]]. In addition, not only TGF-β but also TNF-α promotes VEGF expression in RPE cells [13,14]. These results strongly suggest that EMT in RPE cells is associated with CNV development.
Exosomes are membraned vesicles with a nanosize diameter that are released into the extracellular space by most cells. Since exosomes contain many types of informative components such as proteins, mRNA and microRNA, cells receive information from other cells localized near or far by incorporating exosomes [15]. Recent studies have reported that the contents and intermediating roles of exosomes depend on their cell origin and microenvironment. For example, exosomes from cancer cells or tumour-associated macrophages are associated with angiogenesis and tumour metastasis and invasion, and pathological conditions, such as ischaemia, hypoxia, oxidative stress or inflammation, affect exosome components even in the same cell type [[16], [17], [18]]. In addition, exosomes are found in RPE cells and in drusen, a precursor of wet CNV, in patients with AMD and some reports show that exosomes from RPE cells play part in neovascularization [[19], [20], [21], [22]].
Based on these facts, we hypothesized that there were different roles for exosomes secreted by EMT-induced (mesenchymal) RPE cells and by steady (epithelial) RPE cells. In this study, we demonstrated that RPE cells secrete exosomes and that these exosomes have different effects on the angiogenic responses of human umbilical vein endothelial cells (HUVECs).
2. Materials and methods
2.1. Cell culture
Human retinal pigment epithelial cells (ARPE-19 cells) were purchased from American Type Culture Collection (Manassas, VA, USA). The cells were cultured in 5% CO2 at 37 °C in Dulbecco's modified Eagle's medium (DMEM)/Ham's F-12 nutrient mixture (DMEM/F-12) (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10% foetal bovine serum (FBS). HUVECs were obtained from Lonza (Walkersville, MD, USA) and were cultured in 5% CO2 at 37 °C in Endothelial Cell Basal Medium (EBM)-2 basal medium supplemented with EGM-2 SingleQuots (Lonza). We used cells from the following passage numbers were used: for ARPE-19 cells, passage 5 and 10; for HUVECs, passage 3 and 6.
2.2. Antibodies and chemicals
We purchased human recombinant TNF-α and TGF-β2 from R&D Systems (Minneapolis, MN, USA). The following antibodies were obtained: anti-CD63 (Abcam, Cambridge, UK), anti-HSP70 (Cell Signaling Technology, Danvers, MA, USA), anti-GM130 (Abcam), anti-β-actin (Sigma-Aldrich), and anti-bromodeoxyuridine (BrdU; Millipore, Billerica, MA, USA).
2.3. Exosome isolation from culture media and characterization
Exosomes were collected using Total Exosome Isolation Reagent (Invitrogen, CA, USA) according to the manufacturer's protocol. Briefly, after the cells were cultured for three days with or without TNF-α (10 ng/ml) and/or TGF-β2 (5 ng/ml) in DMEM/F-12 supplemented with 10% Exosome-depleted FBS (System Biosciences, Mountain View, CA, USA), the media were collected and centrifuged at 2000×g for 30 min. The supernatant was mixed with half volumes of Total Exosome Isolation Reagent and incubated at 4 °C overnight. The mixture was centrifuged at 10,000×g for 1 h at 4 °C, and the supernatant was then discarded. The exosome pellet was resuspended in PBS for exosome quantitation and cell culture or lysed in RIPA buffer containing a phosphatase inhibitor cocktail (Nacalai Tesque, Kyoto, Japan), protease inhibitor cocktail (Thermo Fisher Scientific, MA, USA) and 5 mM ethylenediaminetetraacetic acid (EDTA) for Western blot analysis. The size and concentration of the harvested particles were analysed with the NanoSight LM10V-HS nanoparticle tracking system (Quantum Design Japan). For immunoblot analysis of exosome markers, the protein concentration in cell or exosome lysates was measured using a Pierce Bicinchoninic Acid (BCA) Protein Assay Kit (Thermo Fisher Scientific). Equal protein concentrations were loaded onto the gel and transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). The PVDF membranes were incubated with primary antibodies for 1 h at room temperature or overnight at 4 °C. Then, the membranes were washed and incubated with an appropriate horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The labelled specific proteins were visualized using an enhanced chemiluminescence system (Amersham Biosciences/GE Healthcare, Tokyo, Japan).
2.4. Human angiogenesis array
Exosome pellets were lysed in lysis buffer (1% Igepal CA-630, 20 mM Tris-HCl [pH 8.0], 137 mM NaCl, 10% glycerol, and 2 mM EDTA) containing a phosphatase inhibitor cocktail and protease inhibitor cocktail. The protein concentration was measured by BCA assay, and the same amount of protein was subjected to Human Angiogenesis Array (R&D Systems). All procedures were performed according to the manufacturer's protocol. The pixel densities of spots on the array were analysed using ImageJ software.
2.5. Labelling and uptake of exosomes
Isolated exosomes were labelled using a PKH26 Red Fluorescent Cell Linker Kit (Sigma-Aldrich) according to the manufacturer's protocol. After the exosomes were labelled, the protein concentration of each sample was measured by BCA assay. HUVECs were cultured in basal media supplemented with 1% exosome-depleted foetal calf serum (FCS) for 1 h. Then, each concentration of labelled exosomes was added, and the cells were incubated for an additional hour. Internalized labelled exosomes were visualized using a fluorescence microscope (BZ-X710, Keyence, Osaka, Japan). Based on the results of this experiment, we used 40 ng/ml exosomes in all subsequent experiments.
2.6. Transwell migration assay
Transwell migration assays were performed using a previously described protocol with modifications [23]. Briefly, 10 × 104 cells per well were seeded onto each insert (24-well cell culture insert, 3-μm pore size) (BD Falcon, Tokyo, Japan), and the basal medium containing 1% exosome-depleted FCS with or without 40 μg/ml exosomes was added to the bottom chamber. After the cells were incubated for 21 h, the cells on the upper portion of the insert were removed. The cells that migrated to the bottom were fixed, stained with crystal violet (Wako, Osaka, Japan) and counted by DAPI staining using a fluorescence microscope (BZ-X710) and BZ-X Analyzer software (Keyence). Each experiment was repeated three times.
2.7. Cell growth
Cell growth was assessed by BrdU (Sigma-Aldrich) incorporation assay as previously described with modifications [24]. Briefly, HUVECs were incubated in 1% exosome-depleted FCS containing 40 μg/ml exosomes for 24 h and then incubated in the presence of 10 μM BrdU for the last 10 h. The cells were fixed and subjected to immunofluorescence microscopy analysis. Each experiment was repeated three times.
2.8. Endothelial tube formation assay
HUVECs were seeded at 10000 cells per well of a 96-well plate onto Growth Factor-reduced Matrigel (Invitrogen) and incubated in 5% CO2 at 37 °C in EBM-2 medium supplemented 1% exosome-depleted FCS with or without exosomes. Tube formation was imaged using a phase contrast microscopy (objective, x4). The number of branching points and the total length of the tubes were quantified by counting five random fields/well under a microscope. Each experiment was repeated five times.
3. Results
3.1. RPE secretion of exosomes is increased by TNF-α stimulation
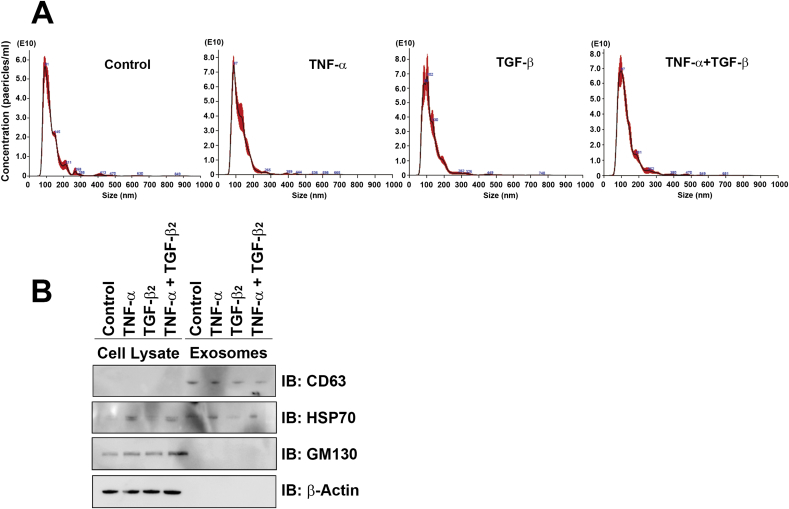
First, we examined whether TNF-α and TGF-β2 induced the release of exosomes in ARPE-19 cells. We cultured the cells in media containing 10% exosome depleted-FCS for analysis. We assessed the size and concentration of extravesicles, which were collected from culture media via Exosome Isolation Reagent, using the NanoSight system. This nanoparticle tracking analysis showed that the mean diameter, mode diameter, and the concentration of the particles were 127.3 ± 2.3, 96.8 ± 4.6 nm and 3.78 × 1012 ± 1.03 × 1011 particles/ml in the control group; 122.1 ± 2.3, 89.0 ± 1.6 nm and 4.99 × 1012 ± 2.47 × 1011 particles/ml in the TNF-α group; 122.9 ± 4.0, 91.4 ± 3.5 nm and 5.15 × 1012 ± 1.15 × 1011 in the TGF-β2 group; and 130.9 ± 3.1, 96.0 ± 5.5 nm and 5.53 × 1012 ± 1.07 × 1011 in the co-stimulated group (Fig. 1A). Next, we confirmed the expression of the exosome-associated proteins CD63 and HSP70 in particles isolated from culture media via Exosome Isolation Reagent (Fig. 1B). These exosome samples were depleted of GM130 (Golgi marker), which indicated a lack of contamination of the cellular fraction of exosome lysates (Fig. 1B). These results showed that particles harvested from culture media via Exosome Isolation Reagent had the characteristics of exosomes.
Fig. 1.
Quantification of exosomes released from ARPE-19 cells. (A) NanoSight nanoparticle tracking analysis of vesicles released form ARPE-19 cells. The histograms show the particle size distributions, and the red area ± standard error of the mean from five samples per group. (B) Immunoblot analysis of CD63, HSP70, and GM130 in cell lysates and purified exosomes. ARPE-19 cells were treated with TNF-α and/or TGF-β2 for 3 days. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.2. RPE-derived exosomes contain angiogenesis-associated factors
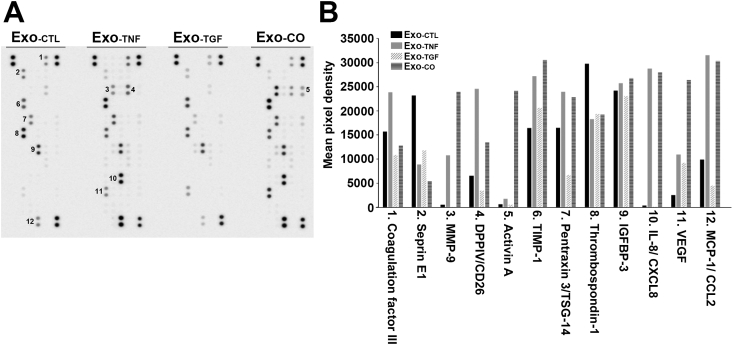
We next performed angiogenesis arrays to examine whether exosomes released from RPE cells contained angiogenetic factors (Fig. 2). We found nine types of angiogenic factors in control exosomes (Exo-CTL). The factors in exosomes of TGF-β2-stimulated cells (Exo-TGF) were identical to those of Exo-CTL. By contrast, the spots corresponding to the expression levels of DPPΙV (#4), pentraxin (#7) and MCP-1 (#12) in Exo-TGF had the lowest intensity among all exosome groups. We detected eleven types of angiogenic factors in exosomes secreted from TNF-α-stimulated cells (Exo-TNF), and the expression levels of coagulation factor ΙΙΙ (#1) and DPPΙV (#4) were higher in these exosomes than in exosomes in other groups. Exosomes from co-stimulated cells (Exo-CO) included the same types of factors as Exo-TNF. The expression levels of TIMP-1 (#6), pentraxin (#7), IL-8 (#10) and MCP-1 (#12) were higher in Exo-TNF and Exo-CO than in Exo-CTL and Exo-TGF. The expression levels of MMP-9 (#3), Activin A (#5) and VEGF (#11) were the highest in Exo-CO (Fig. 2). These results showed that exosomes released by ARPE-19 cells contained several angiogenic factors and that TNF-α alone, TGF-β2 alone, and co-treatment have different effects on the exosome components.
Fig. 2.
The profiles of angiogenesis-related proteins in RPE-derived exosomes. Each type of exosome was collected from supernatants of ARPE-19 cells that were incubated without (Ex-CTL) or with TNF-α (Ex-TNF), TGF-β2 (Ex-TGF) or co-stimulation (Ex-CO) for 3 days. Equal amounts of total protein from each sample were subjected to human angiogenesis array. Spot images of arrays at the same exposure time and the mean pixel density are shown in the graph. The numbers on the images denote 1, coagulation factor ΙΙΙ; 2, Serpin E1; 3, MMP-9; 4, DPPIV; 5, Activin A; 6, TIMP-1; 7, Pentraxin 3; 8, Thrombospondin-1; 9, IGFBP-3; 10, IL-8; 11, VEGF; and 12, MCP-1.
3.3. HUVECs uptake exosomes derived from RPE cells
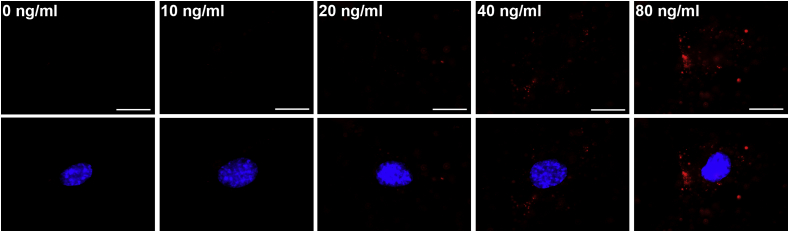
Before we assessed the effect of RPE-derived exosomes on HUVECs, we checked the uptake of exosomes labelled with PKH 26 and optimized the concentration of exosomes for HUVEC stimulation. Fluorescence microscopy analysis showed that the PKH signals increased dose-dependently in the cytosol (Fig. 3), and we used 40 ng/ml exosomes in all subsequent experiments.
Fig. 3.
HUVECs uptake exosomes released from RPE cells. HUVECs were incubated with the indicated concentration of PKH26-labelled exosomes for 1 h, and incorporated exosomes (red) were detected by fluorescence microscopy. Nuclei were stained with DAPI. Scale bar, 20 μm. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
3.4. The effects of RPE-derived exosomes on HUVEC chemotaxis, proliferation and tube formation
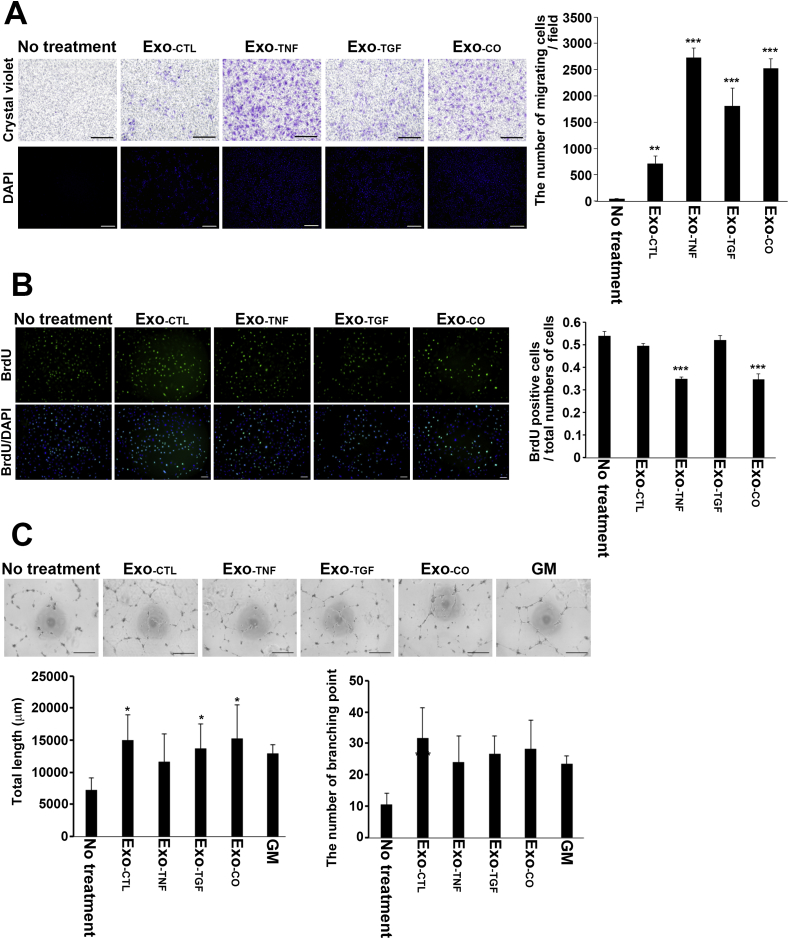
We tested the HUVEC migration activity of each exosome type using a Transwell migration assay. HUVECs scarcely migrated to the control medium without exosomes. HUVECs showed a significant migratory response to exosomes compared to their response to the control medium. The numbers of HUVECs that migrated to the different exosome groups were as follows: Exo-TNF, 2725 ± 182 (p < 0.001); Exo-TGF, 1813 ± 338 (p < 0.001); Exo-CO, 2526 ± 178 (p < 0.001); and Exo-CTL, 709 ± 143 (p = 0.00719) (Fig. 4A).
Fig. 4.
The effects of exosomes released from RPE cells on the angiogenic behaviours of HUVECs. (A) HUVECs were plated in the upper chambers, and the lower chambers contained medium supplemented with 1% FCS with or without exosomes. Exosomes were released from unstimulated (Ex-CTL), TNF-α-stimulated (Ex-TNF), TGF-β2-stimulated (Ex-TGF), and co-stimulated (Ex-CO) ARPE-19 cells. Chemotactic cells were fixed and stained with crystal violet and DAPI. Scale bar, 500 μm. The cell number was counted by nuclei staining; the graph shows the number of translocated cells per field. The data are presented as the means ± SD in four different fields in each of three independent experiments. ***P < 0.001 (Dunnett's test). (B) HUVECs were incubated in medium supplemented with 1% FCS containing each type of exosome for 24 h and then in the additional presence of 10 μM BrdU for the last 10 h. Representative fluorescence microscopy images of cells stained with an anti-BrdU antibody (green) and of nuclei stained with DAPI. Scale bar, 100 μm. The number of BrdU-positive cells and the total number of nuclei were counted. The data are presented as the means ± SD in three different fields. ***P < 0.001 (n = 3, Dunnett's test). (C) Representative images showing tube formation in HUVECs cultured on growth factor-reduced Matrigel with or without each type of exosome released from ARPE-19 cells. We used angiogenic growth factor-containing medium (GM) as the positive control. Graphs showing the quantification of the total length of the tubes and number of branching points. Each bar indicates the mean ± SD (n = 5, *P < 0.05, **P < 0.01, Dunnett's test). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To evaluate the effects of exosomes on HUVEC proliferation, we performed BrdU incorporation assays and counted the ratio of BrdU-positive cells to total cells. HUVECs treated with Exo-CTL (0.5335 ± 0.0357, p = 0.162) or Exo-TGF (0.5272 ± 0.0491, p = 0.988) showed proliferation rates similar to those of control cells. On the other hand, we found a significant decrease in the number of BrdU-positive HUVECs in the presence of Exo-TNF (0.3486 ± 0.0467, p < 0.001) and Exo-CO (0.3531 ± 0.0410, p < 0.001) (Fig. 4B). We further examined the effects of RPE-derived exosomes on capillary-like structure formation in HUVECs by quantifying the total length of the tubes and the number of branching points by tube formation assay. Representative images revealed that the number of branching points increased in the presence of all exosome types (Exo-CTL: 10.6 ± 3.58, p < 0.001; Exo-TNF: 24.57 ± 8.46, p = 0.0275; Exo-TGF: 26.6 ± 5.77, p = 0.00725; Exo-CO: 28.2 ± 9.18, p = 0.00305) compared with the control (10.6 ± 3.5777). The total tube length increased upon exosome stimulation (Exo-CTL: 7282 ± 1843, p = 0.012; Exo-TGF: 13,702 ± 3819, p = 0.0441; Exo-CO: 15,211 ± 5229, p = 0.0101), with the exception of Exo-TNF (11,607 ± 4346, p = 0.255) compared to the control (7282 ± 1843). However, the total length of the tubes and the number of branching points did not significantly differ between the exosome groups (Fig. 4C).
4. Discussion
Exosomes are intercellular transmitter substances [15]. The number of studies showing that exosomes contribute to several phenomena and pathologies has been increasing. For example, tumour cells have been shown to facilitate angiogenesis by secreting exosomes during tumour growth and metastasis [[25], [26], [27]]. In this study, we reported that RPE cells secrete exosomes containing different components, which depend on cell characteristics—epithelial or mesenchymal—and that the effects of exosomes on HUVECs depend on the type of stimuli to which RPE cells are exposed.
The development of abnormal retinal angiogenesis is associated with the infiltration of inflammatory cells such as fibroblasts and macrophages, as well as with the RPE vascular response underlying pathological conditions such as oxidative stress and inflammation [[28], [29], [30]]. Inflammation facilitates angiogenesis during tissue repair and simultaneously induces EMT in epithelial cells of the lesion. We sought to verify the mode of communication between EMT-induced RPE cells and vascular endothelial cells via exosomes to transmit information about environmental changes surrounding RPE cells. We previously reported that TNF-α and TGF-β2 are EMT-inducing factors in ARPE-19 cells [8]. Therefore, we first examined the effects of these factors on the secretion of exosomes by RPE cells and confirmed that RPE cells released nanovesicles with the characteristics of exosomes—nanosized and expressing exosomal markers (Fig. 1). These exosomes contained a rich variety of angiogenic factors (Fig. 2). Interestingly, the kinds of angiogenic factors were dependent on the stimulation—TNF-α alone, TGF-β2 alone, co-stimulation, or no treatment. Additionally, although the detailed mechanisms of exosome uptake are not completely clear, uptake generally occurs via pinocytosis and membrane fusion [31], and we confirmed via a PKH labelling system that HUVECs incorporated the RPE-derived exosomes (Fig. 3).
Angiogenesis is a phenomenon of new capillary vessel formation from existing blood vessels, and hypoxic or inflammatory conditions induce angiogenic processes, vessel instability by directing pericytes away from endothelial cells, and budding and extending by promoting endothelial cell migration and proliferation [32]. In vitro chemotaxis, tube formation, and proliferation assays were used to identify which factors or agents had stimulatory or inhibitory effects on angiogenesis.
Chemotaxis of endothelial cells is an essential step for capillary budding in angiogenesis, and tumour-derived exosomes are reported to promote endothelial cell migration [32,33]. We found that exosomes derived from RPE cells facilitated the chemotaxis of HUVECs (Fig. 4A). These findings suggested that exosomes secreted by activated RPE cells play a role as directional biochemical cues to promote the chemotaxis of endothelial cells towards inflammatory sites. Furthermore, the data in Fig. 4C suggest that exosomes derived from RPE cells contribute to endothelial cell tube formation. By contrast, exosomes from RPE cells showed no effect on the proliferative activity of HUVECs; rather, Exo-TNF and Exo-CO significantly inhibited the proliferation of HUVECs (Fig. 4B). Thus, exosomes derived from RPE cells under inflammatory stimulation promote the differentiation and maturation, not the proliferation, of endothelial cells. In the retina, several cells, including not only RPE cells but also astrocytes and glial cells, secrete exosomes, and retinal astrocyte-derived exosomes have an inhibitory effect on the development of laser-induced CNV [11,12,34]. Further studies are necessary to clarify which cells or exosomes control each step of angiogenesis.
In conclusion, we demonstrated that EMT-associated factors promote the secretion of angiogenic factor-rich exosomes by RPE cells and that RPE-derived exosomes accelerate endothelial cell migration and tube formation, which may result in the development of CNV.
Role of the funding source
The funding source had no role in experimental design and execution, data analysis, and decision to submit results.
Funding
This work was supported in part by Novartis Research Grants in 2019.
Data availability
All data during this study are included in this article.
Informed consent
N/A.
CRediT authorship contribution statement
Ayako Fukushima: Investigation, Validation, Writing - original draft. Eri Takahashi: Conceptualization, Investigation, Visualization, Writing - review & editing. Junji Saruwatari: Validation, Formal analysis. Hidenobu Tanihara: Supervision. Toshihiro Inoue: Supervision, Project administration.
Declaration of competing interest
None.
Footnotes
Transparency document related to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100760.
Transparency document
References
- 1.Klein R., Peto T., Bird A., Vannewkirk M.R. The epidemiology of age-related macular degeneration. Am. J. Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069. [DOI] [PubMed] [Google Scholar]
- 2.Wong T.Y., Chakravarthy U., Klein R., Mitchell P., Zlateva G., Buggage R., Fahrbach K., Probst C., Sledge I. The natural history and prognosis of neovascular age-related macular degeneration: a systematic review of the literature and meta-analysis. Ophthalmology. 2008;115:116–126. doi: 10.1016/j.ophtha.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 4.Hirasawa M., Noda K., Noda S., Suzuki M., Ozawa Y., Shinoda K., Inoue M., Ogawa Y., Tsubota K., Ishida S. Transcriptional factors associated with epithelial-mesenchymal transition in choroidal neovascularization. Mol. Vis. 2011;17:1222–1230. [PMC free article] [PubMed] [Google Scholar]
- 5.Thiery J.P. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Canc. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 6.Sunderkotter C., Steinbrink K., Goebeler M., Bhardwaj R., Sorg C. Macrophages and angiogenesis. J. Leukoc. Biol. 1994;55:410–422. doi: 10.1002/jlb.55.3.410. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z., Shao Y., Li X. The roles of signaling pathways in epithelial-to-mesenchymal transition of PVR. Mol. Vis. 2015;21:706–710. [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi E., Nagano O., Ishimoto T., Yae T., Suzuki Y., Shinoda T., Nakamura S., Niwa S., Ikeda S., Koga H., Tanihara H., Saya H. Tumor necrosis factor-alpha regulates transforming growth factor-beta-dependent epithelial-mesenchymal transition by promoting hyaluronan-CD44-moesin interaction. J. Biol. Chem. 2010;285:4060–4073. doi: 10.1074/jbc.M109.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Limb G.A., Earley O., Jones S.E., LeRoy F., Chignell A.H., Dumonde D.C. Expression of mRNA coding for TNF alpha, IL-1 beta and IL-6 by cells infiltrating retinal membranes. Graefes Arch. Clin. Exp. Ophthalmol. 1994;232:646–651. doi: 10.1007/bf00171378. [DOI] [PubMed] [Google Scholar]
- 10.Ooi K.G., Galatowicz G., Calder V.L., Lightman S.L. Cytokines and chemokines in uveitis: is there a correlation with clinical phenotype? Clin. Med. Res. 2006;4:294–309. doi: 10.3121/cmr.4.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tezel G., Wax M.B. Increased production of tumor necrosis factor-alpha by glial cells exposed to simulated ischemia or elevated hydrostatic pressure induces apoptosis in cocultured retinal ganglion cells. J. Neurosci. 2000;20:8693–8700. doi: 10.1523/JNEUROSCI.20-23-08693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanihara H., Yoshida M., Yoshimura N. Tumor necrosis factor-alpha gene is expressed in stimulated retinal pigment epithelial cells in culture. Biochem. Biophys. Res. Commun. 1992;187:1029–1034. doi: 10.1016/0006-291x(92)91300-f. [DOI] [PubMed] [Google Scholar]
- 13.Nagineni C.N., Samuel W., Nagineni S., Pardhasaradhi K., Wiggert B., Detrick B., Hooks J.J. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: involvement of mitogen-activated protein kinases. J. Cell. Physiol. 2003;197:453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- 14.Wang H., Han X., Wittchen E.S., Hartnett M.E. TNF-alpha mediates choroidal neovascularization by upregulating VEGF expression in RPE through ROS-dependent beta-catenin activation. Mol. Vis. 2016;22:116–128. [PMC free article] [PubMed] [Google Scholar]
- 15.Thery C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 16.Yang M., Chen J., Su F., Yu B., Su F., Lin L., Liu Y., Huang J.D., Song E. Microvesicles secreted by macrophages shuttle invasion-potentiating microRNAs into breast cancer cells, Mol. Cancer. 2011;10:117. doi: 10.1186/1476-4598-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J.E., Tan H.S., Datta A., Lai R.C., Zhang H., Meng W., Lim S.K., Sze S.K. Hypoxic tumor cell modulates its microenvironment to enhance angiogenic and metastatic potential by secretion of proteins and exosomes. Mol. Cell. Proteomics. 2010;9:1085–1099. doi: 10.1074/mcp.M900381-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Biasutto L., Chiechi A., Couch R., Liotta L.A., Espina V. Retinal pigment epithelium (RPE) exosomes contain signaling phosphoproteins affected by oxidative stress. Exp. Cell Res. 2013;319:2113–2123. doi: 10.1016/j.yexcr.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang A.L., Lukas T.J., Yuan M., Du N., Tso M.O., Neufeld A.H. Autophagy and exosomes in the aged retinal pigment epithelium: possible relevance to drusen formation and age-related macular degeneration. PloS One. 2009;4 doi: 10.1371/journal.pone.0004160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atienzar-Aroca S., Fluores-Bellver M., Srrano-Heras G., Martinez-Gil N., Barcia J.M., Aparicio S., Perez-Cremades D., Garcia-Verdugo J.M., Diaz-Llopis M., Romero F.J., Sncho- Pelluz J. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell. Mol. Med. 2016;20:1457–1466. doi: 10.1111/jcmm.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atienzar-Aroca S., Srrano-Heras G., Valls A.F., Ruis de Almodovar C., Muriach M., Barcia J.M., Garcia-Verdugo J.M., Romero F.J., Sncho-Pelluz J. Role of retinal pigment epithelium-derived exosomes and autophagy in new blood vessel formation. J. Cell. Mol. Med. 2018;22:5244–5256. doi: 10.1111/jcmm.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maisto R., Oltra M., Vidal-Gil L., Martínes-Gil N., Sancho-Pellúz J., Di Filippo C., Rossi S., D’Amico M., Barcia J.M., Romero F.J. ARPE-19-derived VEGF-containing exosomes promote neovascularization in HUVEC: the role of the melanocortin receptor 5. Cell Cycle. 2019;18:413–424. doi: 10.1080/15384101.2019.1568745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takahashi E., Inoue T., Fujimoto T., Kojima S., Tanihara H. Epithelial mesenchymal transition-like phenomenon in trabecular meshwork cells. Exp. Eye Res. 2014;118:72–79. doi: 10.1016/j.exer.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Takahashi E., Haga A., Tanihara H. Merlin regulates epithelial-to-mesenchymal transition of ARPE-19 cells via TAK1-p38MAPK-mediated activation. Invest. Ophthalmol. Vis. Sci. 2015;56:2449–2458. doi: 10.1167/iovs.14-16300. [DOI] [PubMed] [Google Scholar]
- 25.Skog J., Wurdinger T., van Rijn S., Meijer D.H., Gainche L., Sena-Esteves M., Curry W.T., Jr., Carter B.S., Krichevsky A.M., Breakefield X.O. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat. Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grange C., Tapparo M., Collino F., Vitillo L., Damasco C., Deregibus M.C., Tetta C., Bussolati B., Camussi G. Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Canc. Res. 2011;71:5346–5356. doi: 10.1158/0008-5472.can-11-0241. [DOI] [PubMed] [Google Scholar]
- 27.Svensson K.J., Kucharzewska P., Christianson H.C., Skold S., Lofstedt T., Johansson M.C., Morgelin M., Bengzon J., Ruf W., Belting M. Hypoxia triggers a proangiogenic pathway involving cancer cell microvesicles and PAR-2-mediated heparin-binding EGF signaling in endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 2011;108:13147–13152. doi: 10.1073/pnas.1104261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong A., Xie B., Shen J., Yoshida T., Yokoi K., Hackett S.F., Campochiaro P.A. Oxidative stress promotes ocular neovascularization. J. Cell. Physiol. 2009;219:544–552. doi: 10.1002/jcp.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cherepanoff S., McMenamin P., Gillies M.C., Kettle E., Sarks S.H. Bruch's membrane and choroidal macrophages in early and advanced age-related macular degeneration. Br. J. Ophthalmol. 2010;94:918–925. doi: 10.1136/bjo.2009.165563. [DOI] [PubMed] [Google Scholar]
- 30.Ambati J., Fowler B.J. Mechanisms of age-related macular degeneration. Neuron. 2012;75:26–39. doi: 10.1016/j.neuron.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fan Q., Yang L., Zhang X., Peng X., Wei S., Su D., Zhai Z., Hua X., Li H. The emerging role of exosome-derived non-coding RNAs in cancer biology. Canc. Lett. 2018;414:107–115. doi: 10.1016/j.canlet.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 32.Adams R.H., Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 33.Lamalice L., Le Boeuf F., Huot J. Endothelial cell migration during angiogenesis. Circ. Res. 2007;100:782–794. doi: 10.1161/01.RES.0000259593.07661.1e. [DOI] [PubMed] [Google Scholar]
- 34.Hajrasouliha A.R., Jiang G., Lu Q., Lu H., Kaplan H.J., Zhang H.G., Shao H. Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J. Biol. Chem. 2013;288:28058–28067. doi: 10.1074/jbc.M113.470765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data during this study are included in this article.