Abstract
Deproteinization of crude polysaccharides in the residue from Lycium barbarum berries (LBBs) was conducted using the Sevag method. A Box-Behnken design based on single-factor experiments was employed to optimize the deproteinization technology. The results showed that the deproteinization conditions had significant effects on the extraction yield of polysaccharides and the residual protein content in Lycium barbarum polysaccharides (LBP). The experimental data were fitted to a second-order polynomial equation, using multiple regression analysis with a high coefficient of determination (R2) value. The optimal conditions were as follows: a ratio of raw material to water extract concentrate from the residual LBBs of 0.15 g/mL; a ratio of chloroform to n-butyl alcohol of 2.17 mL/mL; and a ratio of water extract concentrate from residual LBBs to Sevag reagent of 0.50 mL/mL; with a maximum polysaccharide yield of 0.49% and minimum residual protein content of 0.087%. The results were confirmed through validation experiments. GPC analysis indicated that deproteinized LBP molecules became much more homogeneous. X-ray diffraction indicated that the hydrogen bonding of deproteinized LBP was weakened. This optimization of LBP should be a useful method for purification of crude LBP.
Keywords: Food science, Lycium barbarum berry residue, LBP, Box-Behnken design, Deproteinization
Food Science, Lycium barbarum berry residue; LBP; Box-Behnken design; Deproteinization.
1. Introduction
Lycium barbarum berries (LBBs), also named wolfberries and Goji berries, have been used as a traditional Chinese medicine for over 2000 years (Cheng et al., 2015) and can be also used as a food under Chinese food and drug administration. There has been great progress in analyzing the bioactive substances in Lycium barbarum L., such as polysaccharides, carotenoids (e.g., Zeaxanthin dipalmitate (Chang et al., 2012), β-cryptoxanthin monopalmitate (Hsu et al., 2017), and various small molecules, including betaine, cerebroside, β-sitosterol, p-coumaric acid, vitamins, and minerals. Lycium barbarum polysaccharides (LBPs) have received much attention due to their anti-oxidative activity, hypoglycemic effects (Zhu et al., 2013), and potential application to reversing osteoporosis (Jing and Jia, 2018). The residual of LBBs is a by-product of the extraction of LBB oil by supercritical carbon dioxide extraction, and is often used as animal feed or directly discarded as a drug residue.
Crude LBPs account for 10.65% of the residue from LBBs (Long et al., 2017). LBPs are a complex mixture of highly branched and partly characterized polysaccharides and proteoglycans, which are considered the most important functional constituents in LBBs (Cheng et al., 2015). The material basis of its bioactive components is the L. barbarum polysaccharide-protein complex (LBP-P). The effect of LBP-P on bioactivity was demonstrated over twenty years ago (Geng et al., 1989). Furthermore, Gan et al. (Gan et al., 2003) showed that LBP-P could induce immune responses. More recently, LBP-P was found to enhance host immunity (Chen et al., 2009) and inhibit the proliferation and migration of BIU87 cells as a therapeutic strategy for bladder cancer treatment. Many deproteinization methods have been developed to try to remove protein impurities of polysaccharides (Cheng et al., 2019; Li et al., 2019; Shi et al., 2019; Song et al., 2019; Duan et al., 2020) (Table 1). Among these methods, the Sevag method was a typical and traditional deproteinization technology for the purification of polysaccharides in Lycium barbarum berries (Chen et al., 2008; Yang and Zhang, 2009; Zhu et al., 2010). However, there has been little attention given to using design and process optimization based on the Sevag method for the deproteinization of polysaccharides in LBB residue. The design and optimization of deproteinization based on the Sevag method presents many factors that influence the LBP-P. A Box-Behnken response surface design would prove an effective way of acquiring the necessary factors and responses. Hence, the main objective of this paper is to study and optimize the effect of the deproteinization process variables, such as the concentration of LBPs, the volume fraction of chloroform, and the volume fraction of the LBP aqueous solution on the yield of polysaccharide and the residual protein content (RPC) in the deproteinized LBP.
Table 1.
Deproteinization methods comparisons of polysaccharides.
| Deproteinization method | Materials |
|---|---|
| Functional adsorbent | adsorbent |
| Protease method | Protease |
| TCA method | TCA |
| Salt method | Salt |
| Sevag method | organic agent |
2. Materials and methods
2.1. Materials
Residual LBB, prepared by oil extraction using supercritical carbon dioxide extraction, was obtained from Qinghai General Health Bio-science Co., Qinghai Province, China. The LBBs were from the Qaidam Basin, at an elevation of over 3000 m. The residual LBB (moisture content 10–12%) was stored in aluminum foil packing and kept in a dry environment prior to the experiment. A BCA Protein Assay Kit was obtained from Sangon Biotech (Shanghai, China). All chemicals used were analytical grade and obtained from the China National Pharmaceutical Group Corporation.
2.2. Extraction and deproteinization of crude LBP
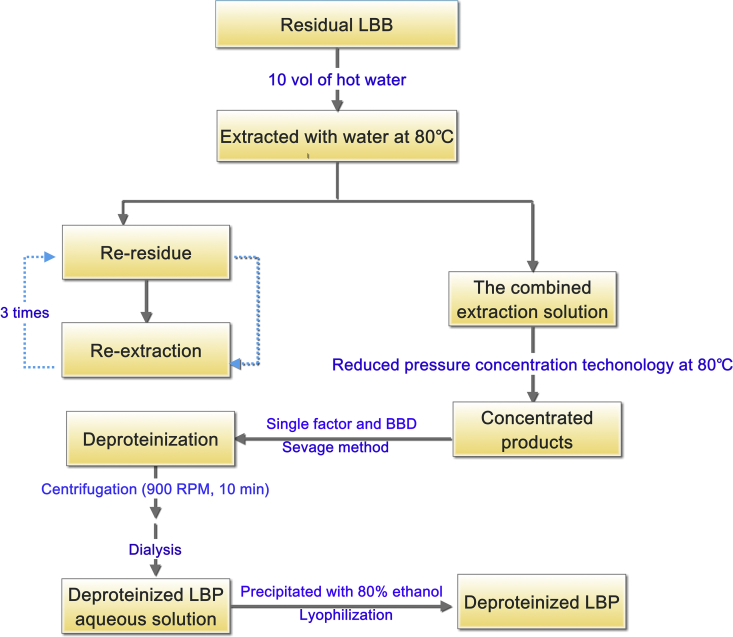
The procedure for extraction and purification of LBP from residual LBB, as shown in Figure 1, was carried out following the methods of previous studies on this subject (Yin and Dang, 2008; Potterat, 2010). Briefly, residual LBB was extracted 3× with 10 vol of distilled water at 80 °C for 80, 60 and 40 min. Insoluble material was removed by filtration, and the combined supernatant was concentrated to the desired volume with a rotary evaporator at a temperature below 80 °C. The concentrated products were then deproteinated using the Sevag method. The resulting solution was dialyzed against deionized water, using a dialysis bag with a MWCO of 3500 Da, giving rise to a non-dialyzable sample inside the dialysis bag. A 3-fold volume of absolute ethanol was added to the non-dialyzable sample solution for precipitation at 4 °C for 8 h. The precipitate was collected and freeze-dried under vacuum using a FreeZone 12 freeze-drier (LABCONCO, USA), giving the desired polysaccharides.
Figure 1.
The process of extraction and deproteinization of crude LBP.
The polysaccharide content of products was determined based on the phenol-sulfuric acid method using D-glucose as a standard ( = 0.9993) (Chua et al., 2012; Qu et al., 2016). Briefly, 1 mL of dilute polysaccharide sample solution was mixed with 1.0 mL 5% phenol and 5 mL 98% H2SO4, and allowed to stand for 10 min at room temperature, prior to incubation at 40 °C for 15 min. Absorbance was then measured at 490 nm using a U-3900H spectrophotometer (HITACHI, Japan) and LBP yield (%) was calculated using Eq. (1) (State Administration of Quality Supervision, Inspection and Quarantine of PR China (2014)):
| (1) |
where ρ is the concentration of polysaccharides as calculated from the calibrated regression equation (mg/mL); is the dilution factor; is the total volume of extraction solution (mL); and is the weight of the sample (g); 3.19 is the conversion factor from glucose to polysaccharides.
The remains of protein content (RPC) in the LBP was measured using a bicinchoninic acid (BCA) protein assay reagent kit at an absorbance of 562 nm in a microplate reader (ThermoFisher, Finland), according to the kit specifications. Protein content was then calculated according to the standard curve ( = 0.9996). RPC (%) was calculated using Eq. (2):
| (2) |
where is the concentration of proteins as calculated from the calibrated regression equation (μg/mL); is the dilution factor; is the total volume of extraction solution (mL); and is the weight of the sample (g).
2.3. Single-factor design for the deproteinization of LBP
A single-factor design was used to determine the preliminary range of purification factors, including A (ratio of raw material to water extract concentrate from residual LBBs) equal to 0.25–0.10 g/mL, B (ratio of chloroform to n-butyl alcohol) equal to 1.0–5.0 mL/mL, and C (ratio of water extract concentrate from residual LBBs to Sevag reagent) equal to 0.3–1.5 mL/mL). The extraction yield of LBP and RPC were the dependent variables.
2.4. Box- Behnken design for the deproteinization of LBP
Based on the single-factor experiment, the software Design Expert (Trial Version 8.0.6.1) was employed for the experimental design, data analysis and model building. The effects of the three independent variables (A = 0.13–0.17 g/mL, B = 1.5–3 mL/mL and C = 0.33–0.50 mL/mL) on the responses (LBP yield and RPC in LBP) were investigated, and the optimal conditions to maximize the percent yield of LBP and to minimize RPC in LBP from residual LBBs were determined. The symbols and levels used are shown in Table 2.
Table 2.
BBD with the experimental values and predicted values for deproteinization.
| Run order | Independent variables |
: LBP yield (%) |
: RPC in LBP (%) |
||||
|---|---|---|---|---|---|---|---|
| Ratio of raw material to water extract concentrate from residual LBBs (A, X1) | Ratio of chloroform to n-butyl alcohol (B,X2) | Ratio of water extract concentrate from residual LBBs to Sevag reagent (C,X3) | Observed | Predicted | Observed | Predicted | |
| 1 | 0.13 (-1) | 1.5 (-1) | 0.42 (0) | 0.428 | 0.426 | 0.168 | 0.170 |
| 2 | 0.15 (0) | 2.25 (0) | 0.42 (0) | 0.504 | 0.502 | 0.087 | 0.087 |
| 3 | 0.15 (0) | 3.00 (1) | 0.50 (1) | 0.445 | 0.445 | 0.122 | 0.120 |
| 4 | 0.17 (1) | 1.50 (-1) | 0.42 (0) | 0.409 | 0.415 | 0.100 | 0.097 |
| 5 | 0.15 (0) | 3.00 (1) | 0.33 (-1) | 0.424 | 0.429 | 0.090 | 0.089 |
| 6 | 0.15 (0) | 1.50 (-1) | 0.33 (-1) | 0.398 | 0.399 | 0.112 | 0.110 |
| 7 | 0.17 (1) | 3.00 (1) | 0.42 (0) | 0.490 | 0.491 | 0.121 | 0.120 |
| 8 | 0.15 (0) | 2.25 (0) | 0.42 (0) | 0.495 | 0.502 | 0.088 | 0.087 |
| 9 | 0.15 (0) | 2.25 (0) | 0.42 (0) | 0.494 | 0.502 | 0.089 | 0.087 |
| 10 | 0.17 (1) | 2.25 (0) | 0.50 (1) | 0.450 | 0.449 | 0.083 | 0.084 |
| 11 | 0.13 (-1) | 2.25 (0) | 0.33 (-1) | 0.353 | 0.354 | 0.112 | 0.110 |
| 12 | 0.15 (0) | 2.25 (0) | 0.42 (0) | 0.505 | 0.502 | 0.084 | 0.087 |
| 13 | 0.15 (0) | 2.25 (0) | 0.42 (0) | 0.504 | 0.502 | 0.088 | 0.087 |
| 14 | 0.15 (0) | 1.50 (-1) | 0.50 (1) | 0.463 | 0.458 | 0.105 | 0.110 |
| 15 | 0.13 (-1) | 2.25 (0) | 0.50 (1) | 0.481 | 0.487 | 0.159 | 0.160 |
| 16 | 0.17 (1) | 2.25 (0) | 0.33 (-1) | 0.512 | 0.506 | 0.109 | 0.110 |
| 17 | 0.13 (-1) | 3.00 (1) | 0.42 (0) | 0.372 | 0.366 | 0.126 | 0.130 |
2.5. Assessment of homogeneity and molecular weight of LBP
A quantity of deproteinated LBP, prepared under the above optimal conditions, and crude LBP (25 mg) was added to 10 mL of eluent (a mixture of 0.01% NaNO3 and 0.02% NaN3) with vigorous agitation, then the polysaccharide solution was filtered with a 0.45 μm filter (micro PES). The homogeneity and average molecular weight of the polysaccharide fractions were determined by gel permeation chromatography (GPC, Agilent G1310 A) coupled with a multi-angle laser light scattering photometer (MALLS; λ = 609.0 nm, T = 35 °C, Wyatt Technology Co., USA) and refractive index detector (RID). The flow rate was 0.5 mL/min. The chromatographic column was an aqueous SEC Start Up Kit 300 mm × 7.5 mm (Agilent). The refractive index increment (dn/dc) of the polysaccharides was 0.135.
2.6. X-ray diffraction analysis of LBP
The X-ray diffraction of the deproteinized LBP (prepared under the above optimal conditions) and crude LBP was obtained using an X-ray diffractometer (PANalytical, Holland). The X-ray diffraction patterns with CuKα at 40 kV and 40 mA were recorded from 2θ of 4.00–80.00°.
3. Results and discussion
3.1. Single-factor experiments relating to LBP deproteinization
3.1.1. Effect of the ratio of raw material to water extract concentrate from residual LBBs on LBP deproteinization
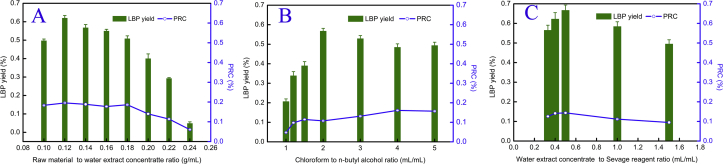
Figure 2A shows that the LBP yield increased when the ratio of raw material to water extract concentrate from residual LBBs was between 0.10 and 0.14 g/mL. The maximum LBP yield was 0.628%, when the ratio was 0.14 g/mL; it decreased thereafter. The RPC in LBP increased when the ratio of raw material to water extract concentrate from residual LBBs was between 0.10 and 0.13 g/mL, and decreased thereafter. Interestingly, when the ratio was 0.14 g/mL, the RPC in LBP was significantly lower (0.179%) than when the ratio was 0.17 and 0.10 g/mL. A possible explanation is that the decrease in the ratio of raw material to water extract concentrate may increase the diffusivity of the Sevag reagent into LBP molecules and enhance desorption of free proteins from the water extract concentrate from residual LBBs. The results indicated that, although there was no deviation in RPC in LBP between the ratios of 0.13 and 0.14, there was significant deviation in LBP yield. Therefore, a ratio of raw material to water extract concentrate from residual LBBs of 0.13–0.17 g/mL was selected for the Box-Behnken design (BBD) experiments.
Figure 2.
LBP deproteinization efficiency mediated by the ratio of raw material to water extract concentrate (A), Chloroform to n-butyl alcohol ratio (B) and Water extract concentrate to Sevage reagent ratio (C).
3.1.2. Effect of the ratio of chloroform to n-butyl alcohol on LBP deproteinization
There is variation in the literature in the ratio of chloroform to n-butyl alcohol in Sevag reagents (Ping, Mao-ping et al., 2013; Xu et al., 2017). In order to find the optimal ratio of chloroform to n-butanol in Sevag reagents for the removal of free protein in water extract concentrate from residual LBBs, the samples were treated with different ratios of chloroform to n-butyl alcohol (Figure 2B). The results showed that the yield of LBP increased from 1.0 to 2.0 mL/mL. The maximum LBP yield was 0.568% when the ratio was 2.0; it decreased thereafter. The RPC in LBP increased when the ratio was between 1.0 to 1.5, decreased to its minimum value (0.107%) when the ratio was 2.0, and increased thereafter. These results indicate that significant deviation was found in both LBP yield and RPC in LBP, between the ratios of 0.12 and 0.15. Therefore, a ratio of chloroform to n-butanol in Sevag reagents for the removal of free protein in water extract concentrate from residual LBBs of 1.5–3.0 mL/mL was selected for the BBD experiments.
3.1.3. Effect of the ratio of water extract concentrate from residual LBBs to Sevag reagent
The ratio of water extract concentrate from residual LBBs to Sevag reagent is also an important factor that influences deproteinization (Zhao et al., 2017). Figure 2C illustrates LBP yield and RPC in LBP as this ratio changed from 0.33 to 1.50 mL/mL. LBP yield and RPC in LBP increased initially, reached their maximums (LBP 0.667% and RPC in LBP 0.144%) at the ratio 0.50 mL/mL, and decreased thereafter. The results show that, although a higher ratio of water extract concentrate from residual LBBs to Sevag reagent could lead to protein decrease in LBP, the LBP yield also decreased. Consequently, a ratio of water extract concentrate from residual LBBs to Sevag reagent of 0.33–0.50 mL/mL was selected for BBD experiments.
3.2. Model fitting and optimization of LBP deproteinization
3.2.1. Statistical analysis and model fitting
In order to study the combined effect of independent variables (ratio of water extract concentrate from residual LBBs to raw material, ratio of chloroform to n-butyl alcohol, and ratio of Sevag reagent to water extract concentrate from residual LBBs) on LBP deproteinization, experiments were conducted for different combinations of the physical parameters. The results are shown in Table 2. Model adequacy checking was used on the experimental data to determine whether the approximating model gave poor or misleading results. The quadratic models for maximum LBP yield and for minimum RPC in LBP were found to have maximum R2, adjusted R2 and predicted R2, and also exhibited low -values. The mathematical relationships of the polynomial equations for % LBP yield () and % RPC in LBP (), in terms of actual factors, are shown as Eq. (3) () and Eq. (4) ().
| (3) |
| (4) |
A positive value indicates an effect that favors the optimization, whereas a negative value indicates an antagonistic effect. The observed data were analyzed using Pareto analysis of variance (ANOVA); the results are in Table 3. As indicated by a low -value and a high -value, an insignificant “lack of fit” reveals that the two models can be applied to fit the observed data. The regressions of the observed versus predicted values for both (R = 0.9907) and (R = 0.9942) showed a straight line with satisfactory correlation, implying that the models can be effectively applied for the optimization of LBP deproteinization (Wang et al., 2018). The goodness of fit of the models was evaluated by the determination coefficient () and adjusted determination coefficient (); these are listed in Table 3, and show that only ~0.01% of the total variation was not explained by the model (Maran et al., 2013). The adjusted determination coefficients of (0.9787) and (0.9868) were found to be very close to , which confirmed that the models were significant, with low coefficients of variation () (1.62 for and 2.66 for ). Adequate precisions of and were found to be 48.70 and 31.29 respectively, which are greater than 4, indicating an adequate signal and confirming that the two models are significant for LBP deproteinization. In this case, LBP yield was significantly affected by the three linear terms and all the interaction terms; RPC in LBP was significantly affected by the three linear terms, three interaction terms and two quadratic terms ( and ).
Table 3.
ANOVA of the regression model for prediction of LBP yield and RPC in LBP.
| Source | LBP yield |
RPC in LBP |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SSa | dfb | MSc | -value | -value | SSa | dfb | MSc | -value | -value | |
| Model | 0.04 | 9 | 4.48×10−3 | 82.77∗∗ | <0.0001 | 0.010 | 9 | 1.12×10−3 | 134.13∗∗ | <0.0001 |
| 6.64×10−3 | 1 | 6.64×10−3 | 118.90∗∗ | <0.0001 | 2.87×10−3 | 1 | 2.87×10−3 | 344.59∗∗ | <0.0001 | |
| 1.39×10−4 | 1 | 1.39×10−4 | 2.55 | 0.155 | 8.52×10−5 | 1 | 8.52×10−5 | 10.24∗∗ | 0.0151 | |
| 2.88×10−3 | 1 | 2.88×10−3 | 53.29∗∗ | <0.0001 | 2.68×10−4 | 1 | 2.68×10−4 | 32.23∗∗ | 0.0008 | |
| 4.69×10−3 | 1 | 4.69×10−3 | 86.69∗∗ | <0.0001 | 1.01×10−3 | 1 | 1.01×10−3 | 121.24∗∗ | <0.0001 | |
| 9.00×10−3 | 1 | 9.00×10−3 | 166.21∗∗ | <0.0001 | 1.35×10−3 | 1 | 1.35×10−3 | 162.43∗∗ | <0.0001 | |
| 4.71×10−4 | 1 | 4.71×10−4 | 8.70∗∗ | <0.0001 | 3.69×10−5 | 1 | 3.69×10−4 | 44.33∗∗ | 0.0003 | |
| 3.82×10−3 | 1 | 3.82×10−3 | 70.66∗∗ | <0.0001 | 2.59×10−3 | 1 | 2.59×10−3 | 311.95∗∗ | <0.0001 | |
| 9.23×10−3 | 1 | 9.23×10−3 | 170.47∗∗ | <0.0001 | 1.16×10−3 | 1 | 1.16×10−3 | 139.45∗∗ | <0.0001 | |
| 2.09×10−3 | 1 | 2.09×10−3 | 38.64∗∗ | 0.0004 | 5.29×10−5 | 1 | 4.50×10−5 | 6.36 | 0.0397 | |
| Residual | 3.79×10−4 | 7 | 5.41×10−5 | 5.82×10−5 | 7 | 8.32×10−6 | ||||
| Lack of fit | 1.90×10−4 | 3 | 6.32×10−5 | 1.34 | 0.3804 | 4.16×10−5 | 3 | 1.39×10−5 | 3.34 | 0.1370 |
| Pure error | 1.89×10−4 | 4 | 4.73×10−5 | 1.66×10−5 | 4 | 4.15×10−6 | ||||
| Correlation Total | 0.041 | 16 | 0.010 | 16 | ||||||
| 0.9907 | 0.9787 | 0.9942 | 0.9868 | |||||||
| 1.62 | 2.66 | |||||||||
∗∗ Significance (significance level 0.05).
Sum of Squares.
Degree of Freedom.
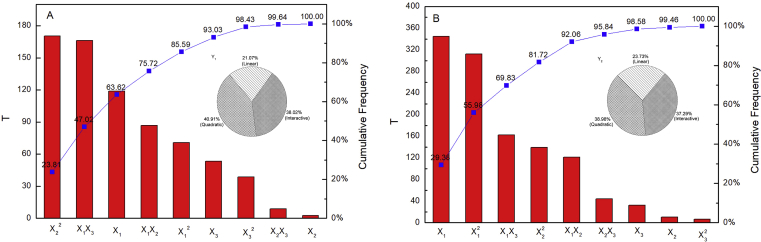
Based on the sum of squares obtained from the ANOVA, the Pareto chart of main and interaction effects and the percentage contributions for each individual process variable were calculated and detailed schematic figure showing the percentage contributions of process variables onand were shown in Figure 3. The Pareto Diagram shown that only X2 has no significant the influence on , all the others items have significant the influence on and . The quadratic terms showed highest percentage (40.91% and 38.98%) contributions on the percent yield of LBP and RPC in LBP compared with the other terms and this was followed by the interactive terms (38.02% and 37.29%) and the linear terms (21.07% and 23.73%). The linear terms showed the highest level of contribution (38.95%) on the minimizing RPC in LBP compared with the other terms and this was followed by the quadratic terms (32.54%) and the interactive terms (28.51%). Hence, percentage contribution values proved that quadratic and interactive independent variables have a direct relationship on and .
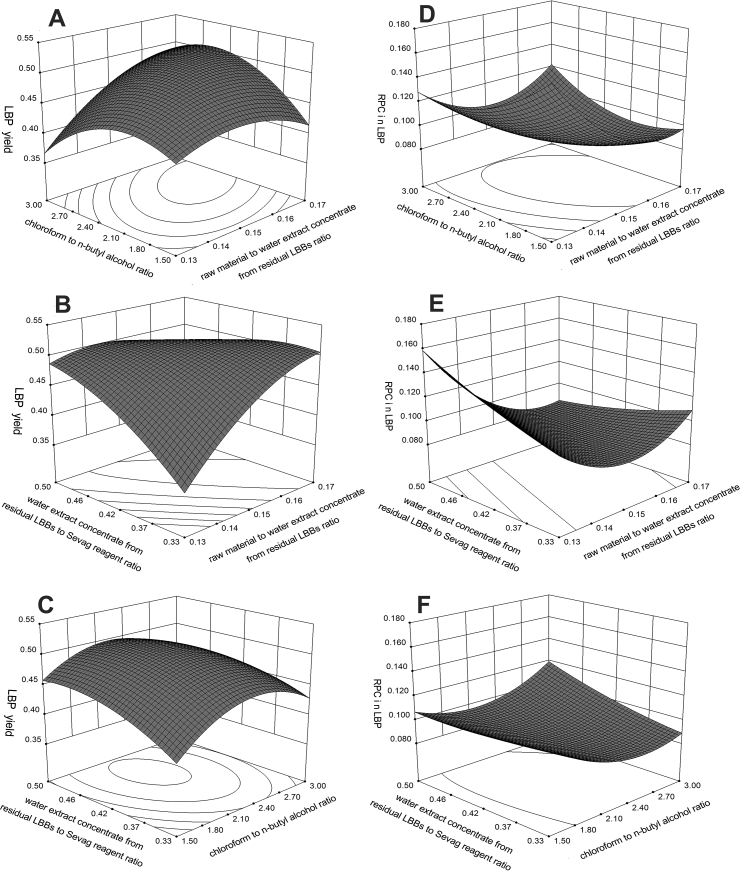
Figure 3.
Response surface plots showing the effects on deproteinization of crude polysaccharides in lycium barbarum berry residue: the ratio of raw material to water extract concentrate from residual LBBs vs. the ratio of chloroform to n-butyl alcohol (A), the ratio of raw material to water extract concentrate from residual LBBs vs. the ratio of water extract concentrate from residual LBBs to Sevag reagent (B), the ratio of chloroform to n-butyl alcohol vs. the ratio of water extract concentrate from residual LBBs to Sevag reagent (C), the ratio of raw material to water extract concentrate from residual LBBs vs. the ratio of chloroform to n-butyl alcohol (D), the ratio of raw material to water extract concentrate from residual LBBs vs. the ratio of chloroform to n-butyl alcohol (E), the ratio of chloroform to n-butyl alcohol vs. the ratio of water extract concentrate from residual LBBs to Sevag reagent (F).
3.2.2. Effect of process variables on deproteinization
Three factors at three levels of BBD were used in this paper to investigate the effect on deproteinization of the ratio of raw material to water extract concentrate from residual LBBs, the ratio of chloroform to n-butyl alcohol, and the ratio of water extract concentrate from residual LBBs to Sevag reagent. From the developed models, the three dimensional response surfaces and contour plots were constructed to illustrate the main and interactive effects of the independent variables (Maran et al., 2013). To determine the effect of independent variables and their mutual interaction on deproteinization, the response surface plots were established according to Eq. (3) and Eq. (4). The shape of the contour plots is elliptical rather than circular (Figure 4), indicating that the mutual interactions between variables are significant (Wang et al., 2018). Generally, higher variables result in higher deproteinization; this includes the ratio of raw material to water extract concentrate from residual LBBs, the ratio of chloroform to n-butyl alcohol, and a lower ratio of water extract concentrate from residual LBBs to Sevag reagent. There are differences in the contribution to deproteinization, and it can be seen that there are optimum conditions for a maximum response.
Figure 4.
Pareto chart of influencing factor standardization. A: LBP yield; B: RPC in LBP.
3.3. Determination of optimal extraction conditions
The objective of this method was to determine the levels of the trial parameters that result in maximization of the percent yield of LBP and minimum RPC in LBP from residual LBBs. The optimal deproteinization parameters were as follows: a ratio of raw material to water extract concentrate from residual LBBs of 0.15, a ratio of chloroform to n-butyl alcohol of 2.17, and a ratio of water extract concentrate from residual LBBs to Sevag reagent ratio of 0.50. Under these optimal conditions, the experimental values of the yield of LBP and RPC in LBP from residual LBBs were 0.48 ± 0.02% and 0.089 ± 0.002%, respectively; these are in agreement with the predicted values of 0.49% and 0.087%, respectively. The results indicate that a BBD incorporated with a desirability function could be effectively used to optimize the deproteinization parameters for the yield of LBP and RPC in LBP from residual LBBs.
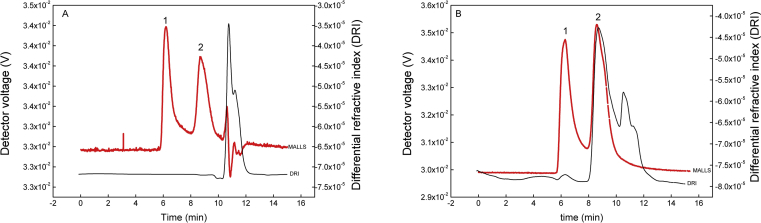
3.4. GPC analysis
The deproteinized LBP and un-deproteinized LBP were analyzed by GPC and monitored with MALLS and DRI, as described in Figure 5. The shape and amplitude were affected after the LBP was treated by deproteinization. Un-deproteinized LBP showed three peaks on GPC-MALLS and only one peak on both GPC-MALLS and GPC-RID, indicating that there was only one fraction in the un-deproteinized LBP solutions. The molecular weight was 6.587 × 103 g/mol and the molecular weight distribution was 1.176. Deproteinized LBP showed two peaks on GPC-MALLS and GPC-RID, which were in close agreement with the LBP reported by (Zhang et al., 2015). The molecular weights were 9.300 × 107 g/mol (mass fraction of 0.65%) and 1.354 × 105 g/mol (mass fraction of 99.35%), with molecular weight distributions 1.088 and 1.206. There was a leftwards shift of peak 3 (merging to peak 2) in the molecular mass distribution of LBP treated by deproteinization, indicating that the molecular weight of the peak 3 fraction was increased after the deproteinization treatment. This indicates that LBP molecules were made much more homogeneous by the deproteinization.
Figure 5.
GPC profiles of crude LBP (A) and deproteinized LBP (B) by MALLS and DRI.
3.5. XRD analysis
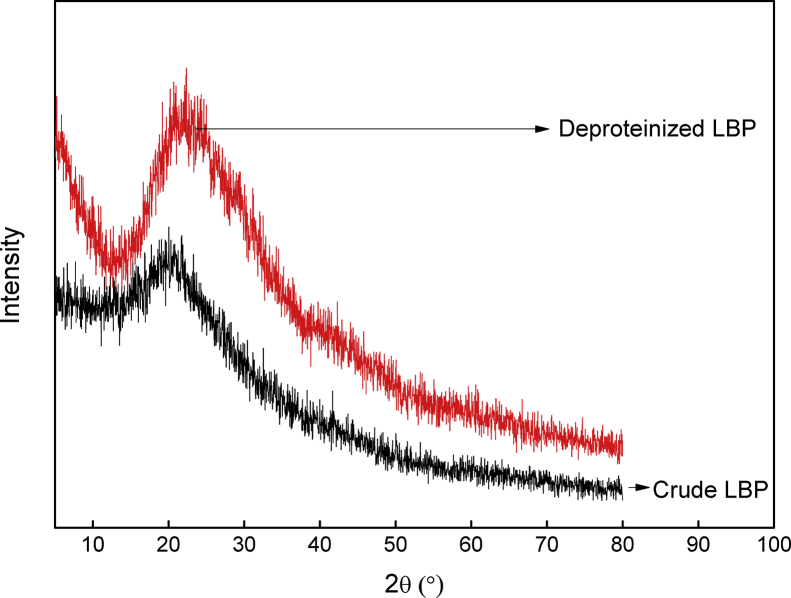
X-ray diffraction measurements were used to assess whether the deproteinization process altered the aggregation structures of LBP. The XRD patterns are in Figure 6. There is a broad peak of crude LBP at 2θ of around 19.9°. Comparing the pattern of deproteinized LBP to that of crude LBP, a relatively sharp and strong diffraction peak is apparent at 2θ of around 22.2°. The crude LBP XRD pattern had a peak with higher intensity than the crude LBP. Both the crude and deproteinized LBP showed a non-crystalline state, which are similar to other polysaccharides (Lin et al., 2010). The X-ray diffraction indicated that the aggregation structures of LBP were modified with deproteinization, and that the hydrogen bonding of deproteinized LBP was weakened.
Figure 6.
XRD curves of deproteinized LBP and crude LBP.
4. Conclusion
In this study, deproteinization of polysaccharides from Lycium barbarum berry residue was optimized, using a single-factor and a BBD with Sevag method; a predicting model was also obtained by fitting experimental data. Analysis of variance showed a high coefficient of determination value (R2), ensuring a satisfactory fit of the developed second-order polynomial regression model with the experimental data. The optimal conditions are a ratio of raw material to water extract concentrate from residual LBBs of 0.15 g/mL, a ratio of chloroform to n-butyl alcohol of 2.17 mL/mL, and a ratio of water extract concentrate from residual LBBs to Sevag reagent of 0.50 mL/mL. Under these optimized conditions, the experimental deproteinization agreed closely with the predicted deproteinization. GPC analysis indicated that LBP molecules became much more homogeneous. X-ray diffraction indicated that, with deproteinization, the aggregation structures of LBP were modified and the hydrogen bonding of deproteinized LBP was weakened.
Declarations
Author contribution statement
Xiaoyan Long: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Quan Yan: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Linjun Cai, Guangying Li: Performed the experiments.
Xuegang Luo: Contributed reagents, materials, analysis tools or data.
Funding statement
Xiaoyan Long was supported by Longshan Academic Talent Research Supporting Program of SWUST (18LZX537).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Chang L.-P., Cheng J.-H., Hsu S.-L., Fu Y.-C., Lin K.-L., Shieh C.-J., Zhou X.-Q., Chang C.-M.J. Supercritical carbon dioxide anti-solvent purification of anti-oxidative compounds from Lycium barbarum fruits by using response surface methodology. Separ. Purif. Technol. 2012;100:66–73. [Google Scholar]
- Chen Z., Tan B.K.H., Chan S.H. Activation of T lymphocytes by polysaccharide-protein complex from Lycium barbarum L. Int. Immunopharm. 2008;8(12):1663–1671. doi: 10.1016/j.intimp.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Chen Z., Lu J., Srinivasan N., Tan B.K.H., Chan S.H. Polysaccharide-protein complex from lycium barbarum L. Is a novel stimulus of dendritic cell immunogenicity. J. Immunol. 2009;182(6):3503–3509. doi: 10.4049/jimmunol.0802567. [DOI] [PubMed] [Google Scholar]
- Cheng J., Zhou Z.-W., Sheng H.-P., He L.-J., Fan X.-W., He Z.-X., Sun T., Zhang X., Zhao R.J., Gu L., Cao C., Zhou S.-F. An evidence-based update on the pharmacological activities and possible molecular targets of Lycium barbarum polysaccharides. Drug Des. Dev. Ther. 2015;9:33–78. doi: 10.2147/DDDT.S72892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng R., Wang L., Li J., Fu R., Wang S., Zhang J. In vitro and in vivo anti-inflammatory activity of a succinoglycan Riclin from Agrobacterium sp. ZCC3656. J. Appl. Microbiol. 2019;127(6):1716–1726. doi: 10.1111/jam.14447. [DOI] [PubMed] [Google Scholar]
- Chua M., Chan K., Hocking T.J., Williams P.A., Perry C.J., Baldwin T.C. Methodologies for the extraction and analysis of konjac glucomannan from corms of Amorphophallus konjac K. Koch. Carbohydr. Polym. 2012;87(3):2202–2210. [Google Scholar]
- Duan S.Z., Huang Q., Shen X.Q., Hu J., Yi X.Z., Li Z.S., Ding B.M. Deproteinization of four macroporous resins for rapeseed meal polysaccharides. Food Sci. Nutr. 2020;8(1):322–331. doi: 10.1002/fsn3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan L., Zhang S.-H., Liu Q., Xu H.-B. A polysaccharide-protein complex from Lycium barbarum upregulates cytokine expression in human peripheral blood mononuclear cells. Eur. J. Pharmacol. 2003;471(3):217–222. doi: 10.1016/s0014-2999(03)01827-2. [DOI] [PubMed] [Google Scholar]
- Geng C.S., Xing S.T., Zhou J.H., Chu B.M. Enhancing effect of Lycium barbarum polysaccharides on the interleukin-2 activity in mice. Chin. J. Pharmacol. Toxicol. 1989;3(3):175–179. [Google Scholar]
- Hsu H.J., Huang R.F., Kao T.H., Inbaraj B.S., Chen B.H. Preparation of carotenoid extracts and nanoemulsions from Lycium barbarum L. and their effects on growth of HT-29 colon cancer cells. Nanotechnology. 2017;28(13) doi: 10.1088/1361-6528/aa5e86. [DOI] [PubMed] [Google Scholar]
- Jing L., Jia X.-W. Lycium barbarum polysaccharide arbitrates palmitate-induced apoptosis in MC3T3-E1 cells through decreasing the activation of ERS-mediated apoptosis pathway. Mol. Med. Rep. 2018;17(2):2415–2421. doi: 10.3892/mmr.2017.8128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., Wang A., Liu L., Tian G., Xu F. Effect of deproteinization methods on the antioxidant activity of polysaccharides extracted from Lentinus edodes stipe. J. Food Meas. Char. 2019;13(2):1382–1389. [Google Scholar]
- Lin X., Wu Q., Luo X., Liu F., Luo X., He P. Effect of degree of acetylation on thermoplastic and melt rheological properties of acetylated konjac glucomannan. Carbohydr. Polym. 2010;82(1):167–172. [Google Scholar]
- Long X., Ye L., Cai L., Liu Y. Extraction of polysaccharide in Lycium barbarum residue. Sci. Tech. Food Industry. 2017;38(9):248–251. [Google Scholar]
- Maran J.P., Manikandan S., Thirugnanasambandham K., Nivetha C.V., Dinesh R. Box-Behnken design based statistical modeling for ultrasound-assisted extraction of corn silk polysaccharide. Carbohydr. Polym. 2013;92(1):604–611. doi: 10.1016/j.carbpol.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Ping Z., Mao-ping H., Li Y., Qiu-ping G. Deproteinization of the crude polysaccharide from pomegranate peel with Sevage method. Food Sci. Technol. 2013;38(12):219–231. [Google Scholar]
- Potterat O. Goji (lycium barbarum and L-chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76(1):7–19. doi: 10.1055/s-0029-1186218. [DOI] [PubMed] [Google Scholar]
- Qu Y., Li C., Zhang C., Zeng R., Fu C. Optimization of infrared-assisted extraction of Bletilla striata polysaccharides based on response surface methodology and their antioxidant activities. Carbohydr. Polym. 2016;148:345–353. doi: 10.1016/j.carbpol.2016.04.081. [DOI] [PubMed] [Google Scholar]
- Shi S., Zhang W., Ren X., Li M., Sun J., Li G., Wang Y., Yue T., Wang J. An advanced and universal method to high-efficiently deproteinize plant polysaccharides by dual-functional tannic acid-fe(III) complex. Carbohydr. Polym. 2019;226 doi: 10.1016/j.carbpol.2019.115283. [DOI] [PubMed] [Google Scholar]
- Song Q.-y., Zhu Z.-Y., Wang X.-T., Chen L.-T., Wang D.-Y. Effects of solution behavior on polysaccharide structure and inhibitory of alpha-glucosidase activity from Cordyceps militaris. J. Mol. Struct. 2019;1178:630–638. [Google Scholar]
- State Administration of Quality Supervision, Inspection and Quarantine of PR China . National Standards of PR China: Wolfberry. GB/ T; 2014. pp. 18672-2014. [Google Scholar]
- Wang C., Wang H., Gu G. Ultrasound-assisted xanthation of cellulose from lignocellulosic biomass optimized by response surface methodology for Pb(II) sorption. Carbohydr. Polym. 2018;182:21–28. doi: 10.1016/j.carbpol.2017.11.004. [DOI] [PubMed] [Google Scholar]
- Xu S., Tan A., Jin C., Hu Y. Optimization of deproteinization technology on chondroitin sulfate with Sevag method. Food Machinery. 2017;33(7):184–188. [Google Scholar]
- Yang L., Zhang L.-M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009;76(3):349–361. [Google Scholar]
- Yin G., Dang Y. Optimization of extraction technology of the Lycium barbarum polysaccharides by Box–Behnken statistical design. Carbohydr. Polym. 2008;74(3):603–610. [Google Scholar]
- Zhang Q., Lv X., Wu T., Ma Q., Teng A., Zhang Y., Zhang M. Composition of Lycium barbarum polysaccharides and their apoptosis-inducing effect on human hepatoma SMMC-7721 cells. Food Nutr. Res. 2015;59 doi: 10.3402/fnr.v59.28696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Liu C., Qiu M., Liu Y., Yan M., Shao S. Comparison of two methods to optimize the purification process of the glycoprotein from schisandra chinensis fructus. The Food Industry. 2017;38(9):57–62. [Google Scholar]
- Zhu M., He C., Xie H., Ma N., Wang C. Extraction, characterization of polysaccharides from lycium barbarum and its effect on bone gene expression in rats. Carbohydr. Polym. 2010;80(3):672–676. [Google Scholar]
- Zhu J., Liu W., Yu J., Zou S., Wang J., Yao W., Gao X. Characterization and hypoglycemic effect of a polysaccharide extracted from the fruit of Lycium barbarum L. Carbohydr. Polym. 2013;98(1):8–16. doi: 10.1016/j.carbpol.2013.04.057. [DOI] [PubMed] [Google Scholar]