Abstract
Over 200 million people are exposed to arsenic worldwide in their daily lives. Arsenic is a toxic ubiquitous metalloid distributed in the ground water. From the last few decades it is obtaining considerable attention for its severe neurotoxic properties. In this study the neuroprotective efficacy of devil's claw (DCW), a potent antioxidant has been investigated against arsenic induced neurotoxicity in female rats. Neurotoxicity was established by oral administration of 13 mg/kg sodium arsenite. The animals were divided into five groups (n = 6) including normal control, disease/arsenic control, standard treatment (Apocynin, 10 mg/kg), DCW treatment I (DCW, 200 mg/kg) and DCW treatment II (DCW, 400 mg/kg). Exploratory, anxiety and motor coordination related behavior of the animals was assessed using hole-board, forced swimming, beam walk and elevated plus maze tests. Findings revealed that DCW treatment ameliorated anxiety and motor in-coordination in the rats compared to the arsenic control group. In addition, arsenic induced a significant oxidative stress in arsenic only treated group, whereas co-administration with DCW the oxidative stress was reduced prominently. Arsenic control group produced gliosis and nuclear pyknosis of the brain cells which were prominently suppressed with the treatment of DCW for 21 days. The activity of DCW was in correlation with the concentration of harpagoside in the serum estimated by the HPLC method, supports that harpagoside was the active constituent responsible for neuroprotective effect. Further findings are required to understand the molecular mechanisms involved in neuroprotective effect of harpagoside and DCW.
Keywords: Neuroscience, Arsenic, Devil's claw, Harpagophytum procumbens, Harpagoside, Neurobehavior and neurotoxicity
Neuroscience; Arsenic; Devil's claw; Harpagophytum procumbens; Harpagoside; Neurobehavior and neurotoxicity
1. Introduction
Arsenic is profoundly distributed in ground water as a pollutant across the world from its natural and anthropogenic sources and its exposure is a distressing problem, leading to various disorders and diseases in millions of people worldwide [1, 2, 3]. A plethora of dermatological and non-dermatological health complications were reported upon chronic arsenic exposure. The non-dermatological effects of arsenic include multi-organ cancer, developmental abnormalities, cardiovascular, metabolic and neuronal disorders [4]. Arsenic is predominantly ingested in the form of inorganic pentavalent arsenate (iAsV) and upon redox reaction it converts to trivalent arsenite (iAsIII) which is more toxic and later metabolizes to methylated arsenicals [5].
All forms of arsenic were reported to accumulate easily in different regions of the brain due to good Blood Brain Barrier (BBB) permeability [6]. An increased accumulation of arsenic was found in the cortex, striatum, hypothalamus, cerebellum and hippocampus regions of rat brain upon administration with 100 ppm sodium arsenite for 60 days [7, 8]. Earlier studies delineated the role of arsenic in diminishing the ability of learning and memory in animals and further more it is able to bring about the structural, morphological and pathological changes in the developmental neuronal cells indicating the toxic effects of it on nervous system [9, 10]. Investigations revealed that, generation of Reactive Oxygen Species (ROS) by activating diverse molecular pathways is the typical underlying mechanism of arsenic toxicity [11, 12, 13]. Due to rich polyunsaturated fatty acids and high oxygen demand, brain tissue is more vulnerable to oxidative damage and additionally decrease in antioxidant enzyme levels was also reported. Research on arsenic neurotoxic effects has been extensive, but the focus of the neurobehavioral changes caused by arsenic is less, which attributes to the need of extensive exploration of associated neurobehavioral and toxicity consequences. Moreover, evaluating the pharmacological interventions in treating arsenic induced neuronal damage is attaining gravity due to lack of potential therapeutic options.
Natural medicine is popularized these days as an account of improvement in its analytical techniques, quality control and advancements in clinical research. Devil's claw is a South African traditional medicine primarily used to alleviate pain and arthritis. It is a secondary root of Harpagophytum procumbens of Pedaliaceae family, with harpagoside an iridoid glycoside as a major constituent [14]. According to European medicines agency's (EMEA) report DCW root extract was claimed to relieve minor joint pain and mild digestive disorders [15]. Anti-inflammatory effect of DCW was illustrated in many reports and proved to act by inhibiting interleukins initiated production of metalloproteinases and release of cytokines and prostaglandin E2, as in further DCW was also validated for its antioxidant potential [16]. Arsenic mediated neurotoxicity is majorly due to proliferation of oxidative stress and inflammatory marker, in this context DCW may subdue these toxicity mechanisms by its strong antioxidant and anti-inflammatory properties. Accordingly, this piece of work was outlined to probe the therapeutic potential of DCW as a neuroprotective agent in case of arsenic produced neurobehavioral modifications and neurotoxicity by measuring various parameters in female Wistar rats.
2. Materials and methods
DCW capsules were procured from Nature's Way Brands, LLC Green Bay, WI, USA. All other chemicals were of analytical grade, purchased from Sigma Aldrich, Hi-media and Merck India Ltd.
2.1. Experimental animals
Female rats are more susceptible to neurotoxicity; hence they were selected for arsenic induced neurotoxicity study [17]. Adult healthy female Wistar albino rats weighing 180–200g were obtained from a Control and Supervision of Experiments on Animals (CPCSEA) approved breeder and housed in individual polypropylene cages in a well ventilated room under hygienic conditions throughout the study and the animals were maintained according to the CPCSEA guidelines. All experimental protocols were approved by the Institutional Animal Ethics Committee [Regd. No. 1677/PO/Re/2012/CPCSEA/13].
2.2. Dose selection and preparation
Doses of 200 and 400 mg/kg, p.o. of DCW, were selected to evaluate its efficacy against arsenic induced neurotoxicity as per the reports of EMEA and doses were prepared daily by suspending DCW powder in 1% CMC [15]. Arsenic dose of 13 mg/kg was selected based on the previous reports and given for 21 days by oral rout [18].
2.3. Study design
Animals were randomly distributed to five groups (n = 6). All the dosing protocols of the respective groups were carried out for 21 days by oral route. The normal control group received vehicle (1% CMC), the disease/arsenic control group received sodium arsenite, standard treatment group received sodium arsenite and apocynin (10 mg/kg), DCW treatment group I received sodium arsenite and DCW (200 mg/kg) and DCW treatment group II received sodium arsenite and DCW (400 mg/kg). Sodium arsenite was given at a dose of 13 mg/kg, p.o.
2.4. Behavioral parameters
The behavioral observations were carried out on every 7th day during the entire study and the parameters were as mentioned below.
2.4.1. Hole-board test
Anxiety levels were evaluated by using a hole-board apparatus (L × W × H: 35 × 35 × 15 cm), which consists of a square floor plate furnished with 16 holes, 3 cm in diameter symmetrically distributed in four rows. The test was performed by placing the animal on one corner of the board and allowed to move freely. Then the number of head dips by the animal was noted for 5 min [19].
2.4.2. Forced swim test (FST)
FST is the most widely used pharmacological model for assessing depressive behavior of animals. The animals were placed in a plexiglass cylinder (H × W: 50 × 20 cm) filled with water to a depth of 30 cm and maintained at 23 ± 1 °C. Rats were forced to swim in a condition from which they cannot escape and they rapidly become immobile, floating in an upright and making only small movements to keep their heads above water. The development of immobility reflects the cessation of persistent escape directed behavior or learned helplessness. The animal was allowed to acclimatize for 5 min, then swimming and immobility time periods were recorded in the next 5 min [20, 21].
2.4.3. Walking function test
The motor balance and locomotor coordination in rats was analysed by beam walk test [22]. The apparatus used for beam walk test constituted a beam of 1–2 cm wide, 1 m long and elevated 30 cm from the ground. The animal was placed at one end of the beam and allowed to move to the other end and the number of foot slips from the beam was counted. The test was repeated thrice.
2.4.4. Elevated plus maze (EPM) test
The EPM test has been widely standardised to measure anxiety in rodents [23]. Each animal was placed in the central square of the plus maze facing an enclosed arm and allowed to explore the apparatus for 5 min. During the test period, the total number of entries in both open and closed arms, time spent in each arm were noted and data was used to calculate % time spent in open field and % open field entries. The EPM was cleaned with a wet tissue paper dipped in 10% ethanol between every two observations.
2.5. Biochemical parameters
On 21st day, blood samples were collected by retro-orbital puncture and serum was separated by centrifugation at 3,000 rpm for 10 min at 4 °C, followed by immediate sacrifice of animals by cervical decapitation. The brain tissues were homogenized (Remi RQT-127 homogeniser) in lysis buffer (pH 7.4) and supernatant was employed to estimate biochemical parameters including total protein, malondialdehyde (MDA) [24], reduced glutathione (GSH) [25], catalase (CAT) [26], superoxide dismutase (SOD) [27], nitric oxide (NO) [28] and protein carbonyl content.
2.5.1. Estimation of CRP levels in serum
CRP levels were estimated in the serum, to determine the arsenic induced aggravation of inflammatory mediators. The assay was done as per the protocol given by manufacturer using CRP XL systems pack kit (XSYS0047) from ERBA.
2.5.2. Estimation of harpagoside concentration in the serum by HPLC method
The serum concentration of harpagoside an active constituent of DCW was estimated using a HPLC method. Serum proteins were precipitated with methanol by adding at a ratio of 1:4 to the serum and centrifuged at 10,000 rpm at 4 °C and the supernatant was used for analysis. The HPLC system (Shimadzu, Kyoto, Japan) with a photodiode array detector and reverse phase C18 column (250 mm × 4.6 mm, 5 μm) which was set at a wavelength of 272 nm was employed. Mobile phase A (methanol: acetonitrile at 45:40) and mobile phase B (0.6% acetic acid aqueous solution) were mixed at a proportion of 40:60 and filtered through a 0.45 μm membrane filter. It was pumped at a flow rate of 0.8 ml/min for the run time of 10 min with an injection volume of 30 μL of the sample solution. The standard calibration curve was constructed with 0.05, 0.1, 0.5 and 1.5 ppm concentrations of harpagoside (Sigma Aldrich, India Ltd).
2.5.3. Estimation of total protein content in brain homogenate
Total protein content was measured calorimetrically using Bradford assay method [29] and bovine serum albumin (BSA) 1 mg/ml was employed as the standard.
2.5.4. Estimation of protein carbonyl content in brain homogenate
The samples were treated with 10% streptomycin sulfate for 5 min and centrifuged at 4,000 rpm to remove nucleic acids. The protein content of the homogenate was determined according to Bradford method and the sample concentration was adjusted to 1 mg/ml. The protein carbonyl content was measured by forming protein hydrazone derivatives using 2, 4-dinitrophenylhydrazide (DNPH). The protein hydrazone derivatives were precipitated with 10% trichloroacetic acid (TCA) and 1:1 ethanol/ethyl acetate and centrifuged. The pellet was dissolved in 6 M guanidine hydrochloride and absorbance was measured at 370 nm. The spectral difference of DNPH-protein in guanidine hydrochloride and a guanidine hydrochloride-protein blank was used to calculate protein carbonyl content [30].
2.5.5. Histopathological studies
For microscopic evaluation, brain tissues were fixed in 10% neutral formalin solution, cleared in xylene and embedded in paraffin. Tissue sections of 5μm were stained with hematoxylin and eosin. A minimum of 10 fields for each brain section was examined for possible histopathological changes. Tissue section images were taken using optical microscope at 10X magnification (Olympus BX 51 fluorescent microscope) [31].
2.6. Statistical analysis
All values were expressed as mean ± standard deviation (SD) and the statistical analysis were done using one or two way ANOVA followed by Bonferroni's post test. The statistical significance was set at p < 0.05 and prism graph pad software (version 5.01) was used for the above mentioned analysis.
3. Results
3.1. Effect of DCW on hole-board test
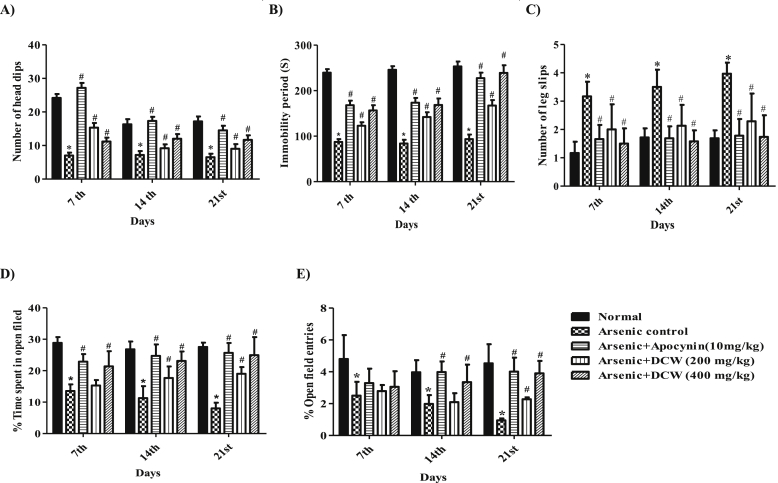
The arsenic control animals have shown a significant (p < 0.05) decrease in the number of head dips when compared to normal animals, evidencing anxiety induced with arsenic. The overall effects of DCW on the number of head dips in hole-board were summarized in Figure 1A. Statistical analysis revealed that DCW (200 and 400 mg/kg) and apocynin treatment has a significant increase in head dipping activity in a dose dependent way compared to disease control on 7th, 14th and 21st days. An improvement in the exploratory behavior of animals upon DCW treatment has indicated its positive action on suppressing arsenic induced anxiety.
Figure 1.
Effect of DCW on hole-board, forced swimming, beam walk and EPM tests A) Hole-board test B) Forced swim test C) Beam walk test D) % Time spent in Open field of EPM E) % Open field entries of EPM [Data was expressed as mean ± SD (n = 6); analysed by two way ANOVA followed by Bonferroni's post test.∗ = p < 0.05, considered statistically significant when compared to the normal and # = p < 0.05 when compared to the Disease control].
3.2. Effect of DCW on FST
In the FST, immobility period of the animals was recorded and the effect of DCW on it was shown in Figure 1B. In arsenic control group significant (p < 0.05) reduction in the immobility time period of animals was noted, compared to the normal group. The immobility time has significantly improved in the DCW (200 and 400 mg/kg) and apocynin experimental groups, in comparison to arsenic control animals. The effect of DCW was in a dose dependent manner.
3.3. Effect of DCW on walking function test
The walking function test was accomplished to evaluate the changes in fine motor coordination and the results were outlined in the Figure 1C. The number of leg slips from the beam was recorded in all the groups and in arsenic control group the number of leg slips were significantly (p < 0.05) more compared to the normal group. It's interesting to note that, co-treatment with DCW (200 and 400 mg/kg) reduced the number of leg slips in a significant and dose dependent manner in comparison with arsenic control group, representing DCW ability in restoring the fine motor coordination and balance in the animals.
3.4. Effect of DCW on EPM test
Figure 1(D&E) is representing the effect of DCW on % open field time spent and entries which is linked to the exploratory behaviour of the animals. The % open field time spent by the arsenic control animals is significantly less than the normal and DCW 400 mg/kg treatment effects a significant raise in % open field time spent from the 7th day onwards and subsequently with DCW 200 mg/kg it was observed from the 14th day. Arsenic treatment even reduced the % open field entries and DCW 400 mg/kg treated group exhibited the significant improvement in % open field entries from the 14th day of the study and DCW 200 mg/kg on the 21st day when compared to disease control.
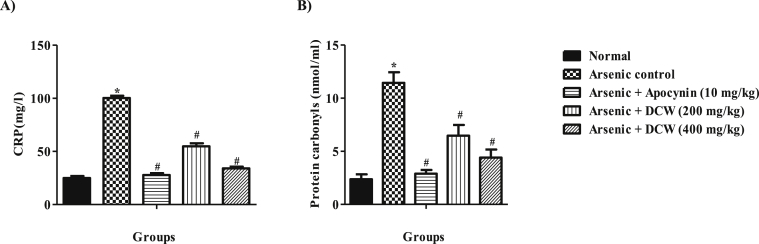
3.5. Effect of DCW on CRP levels
A significant (p < 0.05) elevation of CRP levels in the arsenic control group was noted compared to normal. At both the doses DCW, has significantly suppressed the levels of CRP in the serum compared to the arsenic control group dose dependently and was comparable to apocynin (Figure 3).
Figure 3.
Effect of DCW on CRP level and protein carbonyl levels A) CRP (mg/ml) in serum sample B) Protein carbonyl content (nmol/ml) in brain homogenate. [Data was expressed as mean ± SD (n = 6); analyzed by one way ANOVA followed by Bonferroni's post test.∗ = p < 0.05, considered statistically significant when compared to the normal and # = p < 0.05 when compared to the disease control].
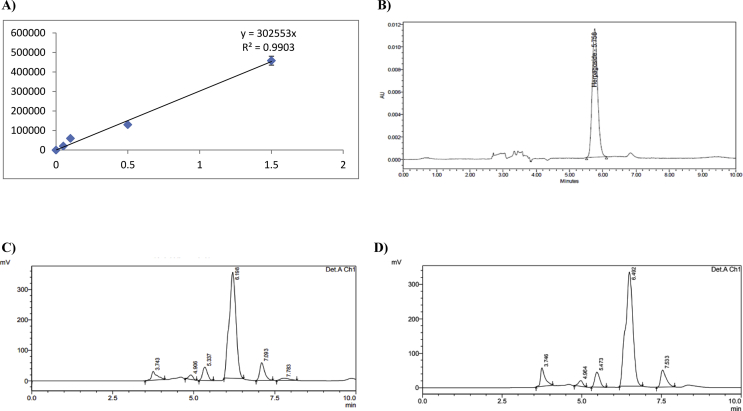
3.6. Estimation of harpagoside levels in serum by HPLC
HPLC chromatogram of treated groups and standard curve of harpagoside has been represented in Figure 2. The serum harpagoside concentrations in the DCW treated 200 and 400 mg/kg groups, were found as 0.19 mg/ml and 0.44 mg/ml respectively using the standard calibration curve. This specifies a dose dependent reach of harpagoside in the blood, which can be correlated to the therapeutic benefits noticed.
Figure 2.
Estimation of harpagoside levels in serum by HPLC A) Standard plot of harpagoside with different concentrations B) Typical chromatogram of harpagoside C) Chromatogram of DCW 200 mg/kg serum sample D) Chromatogram of DCW 400 mg/kg Serum Sample.
3.7. Effect of DCW on total protein content
The total protein content in the arsenic control animals was significantly (p < 0.05) less than the normal and co-treatment with DCW restored the total protein content at both the doses back to normal revealing the DCW protective activity against protein damage. The results were represented in Table 1.
Table 1.
Effect of DCW on arsenic induced alteration in total protein, MDA, GSH, SOD, CAT and NO levels.
| Parameter (n = 6) | Normal | Arsenic control | Arsenic + Apocynin (10 mg/kg) | Arsenic + DCW (200 mg/kg) | Arsenic + DCW (400 mg/kg) |
|---|---|---|---|---|---|
| Total protein (mg/ml) | 0.78 ± 0.01 | 0.56 ± 0.05∗ | 0.77 ± 0.02# | 0.71 ± 0.05# | 0.72 ± 0.01# |
| MDA (nM/mg of protein) | 10.83 ± 0.26 | 19.85 ± 1.60∗ | 11.02 ± 0.43# | 15.68 ± 0.73# | 12.29 ± 0.81# |
| GSH (μM/mg of protein) | 26.75 ± 1.12 | 9.54 ± 0.69∗ | 25.71 ± 0.62# | 19.89 ± 0.79# | 23.80 ± 0.83# |
| SOD (U/mg of protein) | 20.04 ± 0.75 | 2.11 ± 0.19∗ | 12.19 ± 0.75# | 8.68 ± 0.62# | 9.80 ± 0.26# |
| Catalase (μM of H2O2 consumed/min/mg) | 768.04 ± 9.41 | 167.7 ± 6.6∗ | 764.6 ± 5.77# | 372.71 ± 5.12# | 570.93 ± 1.95# |
| NO (μM/mg of protein) | 14.50 ± 0.24 | 32.4 ± 0.32∗ | 17.26 ± 0.41# | 24.18 ± 0.32# | 19.29 ± 0.29# |
Data were expressed as mean ± SD (n = 6); analysed by one way ANOVA followed by Bonferroni's post test.∗ = p < 0.05, considered statistically significant when compared to the normal and # = p < 0.05 when compared to the disease control.
3.8. Effect of DCW on arsenic induced alteration in MDA, GSH, SOD, CAT and NO levels
The antioxidant profile of the study was compiled in Table 1. The arsenic control animals indicated a significant (p < 0.05) suppression of protective antioxidant enzymes GSH, SOD and CAT level. In addition MDA and nitric oxide quantities were prominently enhanced compared to normal which represents the induction of oxidative stress in the disease model. DCW (200 and 400 mg/kg) treatments were significantly able to restore GSH, SOD and CAT level and on other hand MDA and NO content was also alleviated compared to arsenic control group supporting the antioxidant properties of DCW. The effect of DCW on antioxidant profile was found to be dose dependent and comparable to apocynin.
3.9. Effect of DCW on protein carbonyl content
Protein carbonyl content estimation in the brain homogenate served as an index to assess oxidative damage of proteins in the brain by arsenic. Significantly (p < 0.05) high concentration of protein carbonyl was recorded with the arsenic control compared to normal and the treatment groups of DCW (200 and 400 mg/kg) and apocynin has significantly and dose dependently diminished the formation of protein carbonyl, compared to the arsenic control animals (Figure 3).
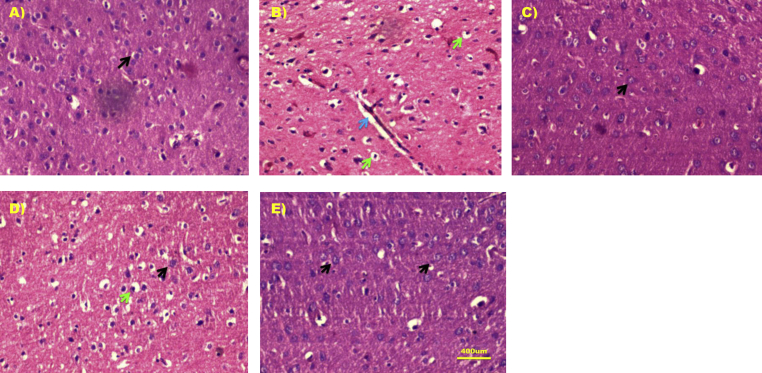
3.10. Effect of DCW on brain histopathological alterations
Normal control group animals have retained the intact brain histomorphology with normal neuronal and glial cell arrangement (Figure 4A). Degenerated neurons, hyperemic vessels and vacuolization were the first evidentiary findings of the arsenic control group. The arsenic treatment in addition exhibited the different necrotic stages of the neuronal cells, including karyolysis, pyknosis and karyorrhexis. Moderate to severe gliosis, chromatolysis, infiltration of inflammatory cells and hemorrhage was also noticed in the disease control sections (Figure 4B). In DCW 200 mg/kg experimental group, mild to moderate number of pyknotic cells and vacuolization were observed (Figure 4D) and in DCW 400 mg/kg treated groups, necrotic changes were suppressed and normal neuronal and glial cell arrangement was retained in several layers (Figure 4E&C). The neuroprotection offered by DCW 400 mg/kg in terms of histopathology was comparable to the effect of apocynin (Figure 4E&C).
Figure 4.
Effect of DCW on brain histopathological alterations A) Normal control with normal brain histology and black arrow indicates the normal brain cells B) Arsenic control group showing pyknotic cells (green arrow) and hyperemic blood vessels (blue arrow) C) Arsenic + Apocynin 10 mg/kg D) Arsenic + DCW 200 mg/kg pyknotic cells (green arrow) and normal neuronal cells (black arrow) and E) Arsenic + DCW 400 mg/kg.
4. Discussion
Arsenic is ranked first in the toxicity profile of metalloids and its exposure pose risk to multiple neurotoxic effects. GLUT 1 receptors substantially located in the endothelial cells of BBB facilitate the translocation of inorganic arsenic (iAs) and aquaglycoporins 7 & 9 as well has a key importance in uptake of arsenicals into the brain [32]. iAsV reduces to iAsIII which in turn forms monomethyl and dimethyl metabolites which are less toxic to iAs [33]. Chandravanshi et al. and Tolins et al. delineated the developmental neurotoxicity of arsenic owing to induction of behavioral and cognitive consequences, oxidative stress and mitochondrial dysfunction [34, 35]. Multiple studies previously established the toxic effects of arsenic and this work was planned to investigate the therapeutic benefits of DCW against neurotoxicity caused by arsenic.
DCW, a traditional South African medicine has been subjected to assorted clinical studies for its reduction in pain sensation in cases of rheumatoid arthritis and improves mobility of patients with in few weeks due to its potent analgesic and anti-inflammatory properties [36]. In fact, DCW was thoroughly studied for the constituents responsible for anti-inflammatory properties. Earlier findings supports harpagoside as the most effective therapeutic component of DCW [14]. A study reviewed by Gagnier et al. determined that DCW equivalent to 60 mg of harpagoside concentration is moderately efficacious in treating osteoarthritis [37]. DCW is an established anti-inflammatory agent, but research on the antioxidant potential of DCW is still at its primitive level and the scope of exploring unrevealed therapeutic benefits of DCW is more. Other than that, its additional medicinal values need to be investigated to widen its therapeutic applications; in this regard DCW was selected in the current study to evaluate its neuroprotective efficacy against arsenic neurotoxicity.
Numerous studies were performed at different dose levels and time periods to assess the toxicity mechanisms of arsenic. Chattopadhyay et al. in his study has given sodium arsenite in drinking water to female rats during gestation, which gives rise to necrotic and apoptotic changes in the developing brain cells as a result of oxidative stress induction [38]. Arsenic at a dose of 20 mg/kg was able to produce oxidative stress, depletion of biogenic amines and brings about the neurobehavioral changes in female rats, whereas exposure at 100 ppm for 60 days reported to cause endothelial dysfunction by setting up the levels of serum nitrite/nitrates to high [39]. In the current investigation, sodium arsenite a source of trivalent arsenic was employed to induce arsenic neurotoxicity at a dose of 13 mg/kg for 21 days, which has resulted the neurobehavioral changes and oxidative damage. The criterion behind the selection of this female rat model was, females are more susceptible to the neurotoxicity than the male animals due to the differences in gonadal hormones [17]. The neuroprotective activity of DCW was examined at two selected doses of 200 and 400 mg/kg as a therapeutic intervention for arsenic induced neurotoxicity.
Epidemiological research evidenced the neurocognitive and behavioral changes put in by arsenic in children and adults, including poor learning & memory, visual & executive skills, global cognition and mood disorders [40]. Arsenic trioxide exposed mice for 14 days had shown a biphasic locomotor activity and altered metabolism and function of CNS [41]. In the current study, the neurotoxicity progression which sets up the neurobehavioral changes in the rats was monitored by different behavioral parameters at regular intervals of time. Arsenic treatment has shown a gradual suppression of exploratory behavior of the animals assessed by number of head dips in the hole-board, % open field entries and % open field time spent in EPM tests. Interestingly, DCW complementation restored these experimental parameters nearly to the normal. DCW treatment also enhanced the immobility period of the animals in FST which was noted less in the arsenic treatment group animals. These constructive outcomes of DCW treatment hinted its anxiolytic properties.
Yadav et al. reported an impaired locomotion and muscle coordination with arsenic treatment [42]. Nishimura et al. correlated the disturbances in neurotransmitter levels to behavioral and learning changes in a study and the results concluded that the impaired secretion of neurotransmitters induces behavior and learning deficits in mice. Chronic arsenic exposure subsides the mRNA expression of the dopamine D2 receptors in the striatal pathway and at 4 ppm for 60 days also alters mRNA expressions of precursor enzymes for biogenic amine synthesis including tyrosine, tryptophan and dopamine beta hydroxylases [43]. Our findings also support that arsenic is able to disturb the muscular coordination and balance assessed by beam walk test and along with DCW treatment these parameters were retrieved to normal. Even though, the neurotransmitter assays were not carried out in this study the observed behavioral disturbances might be because of imbalance in neurotransmitter levels and destruction of dopaminergic receptors by arsenic, based on the earlier reports. Further findings are required to determine the mechanism of DCW by which it suppresses the anxiety and restores the balance.
Arsenic induces oxidative stress in the biological system by increasing the free radicals and in turn increases the peroxidation of membrane lipids and causes loss of integrity and functioning of the membranes [13]. Enzymes with sulfhydryl group are the major target for the arsenic species and a study by Shila et al. in rat administered with arsenic also resulted in alteration of GSH and glutathione peroxidase levels as they contain sulfhydryl groups [44].
Arsenic in addition activates few enzyme systems accountable for the generation of ROS which comprises NADPH Oxidase, thioredoxin reductase and haemoxygenase reductase enzymes [45, 46]. Based on this, a potent NADPH Oxidase inhibitor apocynin, which has an established antioxidant profile, was selected as the standard control for the present study. Few investigations also noticed a decrease in activity of SOD and CAT in rat brain exposed to arsenic and present study results coincide with the previously reported ones by decrease in GSH, SOD and CAT enzyme levels. Additionally, MDA and protein carbonyl contents were elevated, indicates the induction of oxidative stress with arsenic. According to Schaffer et al. Harpagophytum procumbens (DCW) is able to diminish lipid peroxidation and enhance CAT activity and cell viability in brain cortical sections of rats [47]. Few more reports by Georgiev et al. also supported the dynamic antioxidant properties of DCW [16, 48]. The current investigation also showed promising antioxidant activity of DCW, by reducing the lipid peroxidation, protein carbonyl content and improving GSH, SOD and CAT enzyme concentrations in the animals receiving arsenic, corroborating with the previous results.
In a rat model, animals administered with a 10 mg/kg of arsenic for 8 days, up regulated the expression of inflammatory markers like IL-1β, TNF-α and INF-γ responsible for neuroinflammation [49]. Druwe et al. probed with FvB female mice treated with 100 ppb arsenic for 6 months found to cause activation of NF-κB by enhancing the expression of CRP [50]. Chronic exposure to the permissive limit of arsenic 10–50 μg/L evidences systemic inflammation by up streaming pro-inflammatory mediators, including IL-6, IL-8, IL-12, TNF-α and CRP in the sera of women from the Indo-Gangetic basin [51]. These investigations suggest CRP as an end inflammatory marker; thus in this study CRP levels were estimated and found to increase in arsenic control group indicating the activation of inflammatory mediators by arsenic.
Anti-inflammatory potential of DCW was validated by many studies, Gyurkovska et al. outlined that DCW exhibited robust anti-inflammatory activity in murine macrophages in relation to NO, TNF-α, IL-6, COX-1 and 2 expressions and at doses of 400 and 800 mg/kg suppressed edema and inflammation [52]. DCW in the current research suppressed elevated CRP and NO levels upon arsenic treatment.
Besides of behavioral and biochemical findings, pathological alterations brought by arsenic like degenerative changes, gliosis, vacuolization of matrix and nuclear pyknosis were subsided prominently with DCW at 400 mg/kg co-administration and improved the histo-architecture of brain. As already discussed earlier iridoid glycosides are the active constituents of DCW, hence harpagoside concentration was measured in the serum samples. The obtained results of neuroprotective DCW treatment was in concurrence with the serum concentrations of harpagoside, strongly supporting it as an active constituent exhibiting neuroprotective property.
The study results demonstrated protective effect of DCW which sets up the new horizons in the treatment of arsenic poisoning. Our results clearly suggested the anti-inflammatory activity and antioxidant property of DCW might have contributed for the neuroprotective effect of DCW and were able to reduce anxiety behavior caused by arsenic. However the study highlighted the therapeutic importance of harpagoside, additional investigations are suggested to explore the molecular mechanisms of DCW mainly on dopamine receptor expression and molecular inflammatory and oxidative stress markers.
Declarations
Author contribution statement
Rupasree Peruru: Performed the experiments; Wrote the paper.
Usha Rani R: Performed the experiments.
Jhansyrani Thatiparthi: Analyzed and interpreted the data.
Sunitha Sampathi: Contributed reagents, materials, analysis tools or data.
Sujatha Dodoala: Conceived and designed the experiments.
Prasad KVSRG: Conceived and designed the experiments; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors acknowledge UGC-SAP and DST-FIST of Institute of Pharmaceutical Technology and DST-CURIE of Sri Padmavati Mahila Visvavidyalayam for providing the infrastructural facilities to carry out the study.
References
- 1.Shankar S., Shanker U. Arsenic contamination of groundwater: a review of sources, prevalence, health risks, and strategies for mitigation. Sci. World J. 2014;2014:1–18. doi: 10.1155/2014/304524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazumder D.N.G., Ghosh A., Majumdar K.K., Ghosh N., Saha C., Mazumder R.N.G. Arsenic contamination of ground water and its health impact on population of district of Nadia, West Bengal, India. Indian J. Commun. Med. – Offic. Publ. Indian Assoc. Prev. Soc. Med. 2010;35:331. doi: 10.4103/0970-0218.66897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mukherjee A., Sengupta M.K., Hossain M.A., Ahamed S., Das B., Nayak B. Arsenic contamination in groundwater: a global perspective with emphasis on the Asian scenario. J. Health Popul. Nutr. 2006;24(2):142–163. [PubMed] [Google Scholar]
- 4.Ratnaike R.N. Acute and chronic arsenic toxicity. Postgrad. Med. 2003;79:391–396. doi: 10.1136/pmj.79.933.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes M.F. Arsenic toxicity and potential mechanisms of action. Toxicol. Lett. 2002;133:1–16. doi: 10.1016/s0378-4274(02)00084-x. [DOI] [PubMed] [Google Scholar]
- 6.Su C.-K., Yang C.-H., Lin C.-H., Sun Y.-C. In-vivo evaluation of the permeability of the blood–brain barrier to arsenicals, molybdate, and methylmercury by use of online microdialysis–packed minicolumn–inductively coupled plasma mass spectrometry. Anal. Bioanal. Chem. 2014;406:239–247. doi: 10.1007/s00216-013-7429-5. [DOI] [PubMed] [Google Scholar]
- 7.Shila S., Kokilavani V., Subathra M., Panneerselvam C. Brain regional responses in antioxidant system to alpha-lipoic acid in arsenic intoxicated rat. Toxicology. 2005;210:25–36. doi: 10.1016/j.tox.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Shila S., Subathra M., Devi M.A., Panneerselvam C. Arsenic intoxication-induced reduction of glutathione level and of the activity of related enzymes in rat brain regions: reversal by dl-α-lipoic acid. Arch. Toxicol. 2005;79:140–146. doi: 10.1007/s00204-004-0614-8. [DOI] [PubMed] [Google Scholar]
- 9.Rios R., Zarazua S., Santoyo M.E., Sepulveda-Saavedra J., Romero-Diaz V., Jimenez V. Decreased nitric oxide markers and morphological changes in the brain of arsenic-exposed rats. Toxicology. 2009;261:68–75. doi: 10.1016/j.tox.2009.04.055. [DOI] [PubMed] [Google Scholar]
- 10.Kaler S., Dhar P., Bhattacharya A., Mehra R.D. Preliminary morphological and immunohistochemical changes in rat hippocampus following postnatal exposure to sodium arsenite. Toxicol. Int. 2013;20:160. doi: 10.4103/0971-6580.117259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das J., Ghosh J., Manna P., Sinha M., Sil P.C. Arsenic-induced oxidative cerebral disorders: protection by taurine. Drug Chem. Toxicol. 2009;32:93–102. doi: 10.1080/01480540802564171. [DOI] [PubMed] [Google Scholar]
- 12.Singh N., Ma L.Q., Srivastava M., Rathinasabapathi B. Metabolic adaptations to arsenic-induced oxidative stress in Pterisvittata L and Pterisensiformis L. Plant Sci. 2006;170:274–282. [Google Scholar]
- 13.Flora S.J.S. Arsenic-induced oxidative stress and its reversibility. Free Radic. Biol. Med. 2011;51:257–281. doi: 10.1016/j.freeradbiomed.2011.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Qi J., Chen J.-J., Cheng Z.-H., Zhou J.-H., Yu B.-Y., Qiu S.X. Iridoid glycosides from Harpagophytum procumbens DC (devil's claw) Phytochemistry. 2006;67:1372–1377. doi: 10.1016/j.phytochem.2006.05.029. [DOI] [PubMed] [Google Scholar]
- 15.(London) EMA . 2009. Assessment Report on Harpagophytum Procumbens Dc. And/Or Harpagophytum Zeyheri Decne, Radix. [Google Scholar]
- 16.Georgiev M.I., Alipieva K.I., Denev P. Antioxidant activity and bioactive constituents of the aerial parts of Harpagophytum procumbens plants. Biotechnol. Biotechnol. Equip. 2010;24:438–443. [Google Scholar]
- 17.Held H.E., Pilla R., Ciarlone G.E., Landon C.S., Dean J.B. Female rats are more susceptible to central nervous system oxygen toxicity than male rats. Physiol. Rep. 2014;2 doi: 10.14814/phy2.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh A., Mandal A.K., Sarkar S., Panda S., Das N. Nanoencapsulation of quercetin enhances its dietary efficacy in combating arsenic-induced oxidative damage in liver and brain of rats. Life Sci. 2009;84:75–80. doi: 10.1016/j.lfs.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 19.File S.E., Pellow S. The effects of triazolobenzodiazepines in two animal tests of anxiety and in the holeboard. Br. J. Pharmacol. 1985;86:729–735. doi: 10.1111/j.1476-5381.1985.tb08952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Detke M.J., Rickels M., Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology. 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 21.Porsolt R.D. Animal model of depression. Biomedicine [publiee pour l'AAICIG] 1979;30:139–140. [PubMed] [Google Scholar]
- 22.Goldstein L.B., Davis J.N. Beam-walking in rats: studies towards developing an animal model of functional recovery after brain injury. J. Neurosci. Methods. 1990;31:101–107. doi: 10.1016/0165-0270(90)90154-8. [DOI] [PubMed] [Google Scholar]
- 23.Lister R.G. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 24.Niehaus W.G., Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. FEBS J. 1968;6:126–130. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 25.Moron M.S., Depierre J.W., Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochimica et Biophysica Acta (BBA)-General Subjects. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 26.Bonaventura J., Schroeder W.A., Fang S. Human erythrocyte catalase: an improved method of isolation and a reevaluation of reported properties. Arch. Biochem. Biophys. 1972;150:606–617. doi: 10.1016/0003-9861(72)90080-x. [DOI] [PubMed] [Google Scholar]
- 27.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 28.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal. Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 29.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 30.Dalle-Donne I., Rossi R., Giustarini D., Milzani A., Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin. Chim. Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J., Xiong H. Current Laboratory Methods in Neuroscience Research. Springer; 2014. Brain tissue preparation, sectioning, and staining; pp. 3–30. [Google Scholar]
- 32.Zijuan Liu J.S., Carbrey Jennifer M., Mukhopadhyay Rita, Peter Agre, Rosen Barry P. Arsenite transport by mammalian aquaglyceroporins AQP7 and AQP9. PNAS. 2002;99:6053–6058. doi: 10.1073/pnas.092131899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vahter M., Concha G. Role of metabolism in arsenic toxicity. Pharmacol. Toxicol. 2001;89:1–5. doi: 10.1034/j.1600-0773.2001.d01-128.x. [DOI] [PubMed] [Google Scholar]
- 34.Tolins M., Ruchirawat M., Landrigan P. The developmental neurotoxicity of arsenic: cognitive and behavioral consequences of early life exposure. Ann. Global Health. 2014;80:303–314. doi: 10.1016/j.aogh.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 35.Chandravanshi L.P., Gupta R., Shukla R.K. Developmental neurotoxicity of arsenic: involvement of oxidative stress and mitochondrial functions. Biol. Trace Elem. Res. 2018;186:185–198. doi: 10.1007/s12011-018-1286-1. [DOI] [PubMed] [Google Scholar]
- 36.Wegener T., Lupke N.P. Treatment of patients with arthrosis of hip or knee with an aqueous extract of devil's claw (Harpagophytum procumbens DC.) Phytother Res. – Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003;17:1165–1172. doi: 10.1002/ptr.1322. [DOI] [PubMed] [Google Scholar]
- 37.Gagnier J.J., Chrubasik S., Manheimer E. Harpgophytum procumbens for osteoarthritis and low back pain: a systematic review. BMC Compl. Alternative Med. 2004;4:13. doi: 10.1186/1472-6882-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chattopadhyay S., Bhaumik S., Chaudhury A.N., Gupta S.D. Arsenic induced changes in growth development and apoptosis in neonatal and adult brain cells in vivo and in tissue culture. Toxicol. Lett. 2002;128:73–84. doi: 10.1016/s0378-4274(01)00535-5. [DOI] [PubMed] [Google Scholar]
- 39.Sharma B., Sharma P.M. Arsenic toxicity induced endothelial dysfunction and dementia: pharmacological interdiction by histone deacetylase and inducible nitric oxide synthase inhibitors. Toxicol. Appl. Pharmacol. 2013;273:180–188. doi: 10.1016/j.taap.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 40.O'Bryant S.E., Edwards M., Menon C., Gong G., Barber R. Long-term low-level arsenic exposure is associated with poorer neuropsychological functioning: a Project FRONTIER study. Int. J. Environ. Res. Publ. Health. 2011;8:861–874. doi: 10.3390/ijerph8030861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tadanobu I., Zhang Y.F., Shigeo M., Hiroko S., Hiromichi N., Hiroki M. The effect of arsenic trioxide on brain monoamine metabolism and locomotor activity of mice. Toxicol. Lett. 1990;54:345–353. doi: 10.1016/0378-4274(90)90202-w. [DOI] [PubMed] [Google Scholar]
- 42.Yadav R.S., Sankhwar M.L., Shukla R.K., Chandra R., Pant A.B., Islam F. Attenuation of arsenic neurotoxicity by curcumin in rats. Toxicol. Appl. Pharmacol. 2009;240:367–376. doi: 10.1016/j.taap.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J., Liu X., Zhao L., Hu S., Li S., Piao F. Subchronic exposure to arsenic disturbed the biogenic amine neurotransmitter level and the mRNA expression of synthetase in mice brains. Neuroscience. 2013;241:52–58. doi: 10.1016/j.neuroscience.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 44.Samuel S., Kathirvel R., Jayavelu T., Chinnakkannu P. Protein oxidative damage in arsenic induced rat brain: influence of DL-α±-lipoic acid. Toxicol. Lett. 2005;155:27–34. doi: 10.1016/j.toxlet.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Kitchin K.T., Conolly R. Arsenic-induced carcinogenesis— oxidative stress as a possible mode of action and future research needs for more biologically based risk assessment. Chem. Res. Toxicol. 2009;23:327–335. doi: 10.1021/tx900343d. [DOI] [PubMed] [Google Scholar]
- 46.Shi H., Shi X., Liu K.J. Oxidative mechanism of arsenic toxicity and carcinogenesis. Mol. Cell. Biochem. 2004;255:67–78. doi: 10.1023/b:mcbi.0000007262.26044.e8. [DOI] [PubMed] [Google Scholar]
- 47.Schaffer L.F., Peroza L.R., Boligon A.A., Athayde M.L., Alves S.H., Fachinetto R. Harpagophytum procumbens prevents oxidative stress and loss of cell viability in vitro. Neurochem. Res. 2013;38:2256–2267. doi: 10.1007/s11064-013-1133-x. [DOI] [PubMed] [Google Scholar]
- 48.Georgiev M., Alipieva K., Pashova S., Denev P., Angelova M., Kerns G. Antioxidant activity of devil's claw cell biomass and its active constituents. Food Chem. 2010;121:967–972. [Google Scholar]
- 49.Firdaus F., Zafeer M.F., Ahmad M., Afzal M. Anxiolytic and anti-inflammatory role of thymoquinone in arsenic-induced hippocampal toxicity in Wistar rats. Heliyon. 2018;4 doi: 10.1016/j.heliyon.2018.e00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Druwe I.L., Sollome J.J., Sanchez-Soria P., Hardwick R.N., Camenisch T.D., Vaillancourt R.R. Arsenite activates NFκB through induction of C-reactive protein. Toxicol. Appl. Pharmacol. 2012;261:263–270. doi: 10.1016/j.taap.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Prasad P., Sinha D. Low-level arsenic causes chronic inflammation and suppresses expression of phagocytic receptors. Environ. Sci. Pollut. Control Ser. 2017;24:11708–11721. doi: 10.1007/s11356-017-8744-8. [DOI] [PubMed] [Google Scholar]
- 52.Soulimani R., Younos C., Mortier F., Derrieu C. The role of stomachal digestion on the pharmacological activity of plant extracts, using as an example extracts of Harpagophytum procumbens. Can. J. Physiol. Pharmacol. 1994;72:1532–1536. doi: 10.1139/y94-220. [DOI] [PubMed] [Google Scholar]