Abstract
Human adenovirus type 19 (HAdV-19) is a major cause of the epidemic keratoconjunctivitis. Outbreaks of keratoconjunctivitis are problematic to human health, especially for infants, the elderly, and immunocompromised individuals. However, the development of anti-HAdV drugs has been hampered by inconvenient screening systems; therefore, development of a simple screening method is highly desirable. In this study, we identified that HAdV-19 can infect a human lymphoid cell line transformed with human T-cell leukemia virus (MT-2 cells). MT-2 cells supported HAdV-19 replication and showed apparent cytopathic effects within five days post-infection. Using a thiazolyl blue tetrazolium bromide (MTT)-based colorimetric assay on MT-2 cells, we were able to detect the anti-HAdV-19 activities of previously reported nucleoside/tide compounds, including (S)-1–(3-hydroxy-2-phosphonylmethoxypropyl)cytosine (cidofovir), 2′,3′-dideoxycytidine (zalcitabine) and 3′-deoxy-3′-fluorothymidine (trifluridine). Compared with previous methods, this system represents a more simple and rapid method to screen anti-HAdV-19 agents.
Keywords: Adenovirus, screening, antiviral, pandemic, disaster
Introduction
Human adenoviruses (HAdV) cause various mucosal infections, including respiratory infections, gastroenteritis, and hemorrhagic cystitis.1 While most infections are acute and cause severe symptoms, most patients have a good prognosis. However, HAdV infections can induce severe and lethal disseminated diseases in immunocompromised individuals. Additionally, ocular HAdV infection causes epidemic keratoconjunctivitis (EKC). HAdV type 19 (HAdV-19) is a major etiological agent of EKC, a severe and contagious infection associated with blurred vision and irritation. EKC outbreaks are problematic when they emerge in hospitals, schools, and other communities. A number of compounds, such as cidofovir (CDV), zalcitabine (ddC), and ribavirin (RBV), reportedly show anti-HAdV activity in vitro or in vivo.2 A newly developed compound, brincidofovir, a promising lipid-linked derivative of CDV, has been used in clinical trials.3 However, no anti-HAdV agents have been clinically approved.
The anti-HAdV activity of compounds has been examined in vitro using adherent cells, such as A549, HEp-2, and human embryonic lung (HEL) fibroblast cells.4–6 These antiviral assays can require considerable effort to change culture media. Additionally, the trypsin treatments or digestions, cell loss, and other procedures can create variation in assay results. In this study, we developed an anti-adenoviral assay using non-adherent cells to avoid these problems. We demonstrated that MT-2 cells, which are a human lymphocytic cell line transformed with human T-cell leukemia virus (HTLV-1), support the infection and propagation of HAdV-19. Cytopathic effects (CPE) of HAdV-19 infection on MT-2 cells were also evaluated by testing the infected cells for viability using a thiazolyl blue tetrazolium bromide (MTT) assay. We tested several classes of nucleoside/tide analogues such as CDV, ddC, RBV, ganciclovir (GCV), and 3′-deoxy-3′-fluorothymidine (FdT) that are known to inhibit HAdV.2,6–10 Except FdT, our MTT assay results agreed with previous reports on the anti-HAdV activities of these compounds. These results suggest that screening HAdV inhibitors using an MTT assay on MT-2 cells may be a useful tool for the development of novel HAdV inhibitors.
Materials and methods
Cell cultures and virus
A human lymphoid cell line, MT-2 (a kind gift from Dr. Shiro Shigeta, Fukushima Medical University, Fukushima, Japan), was grown in RPMI 1640 medium (Sigma-Aldrich Japan, Tokyo, Japan) supplemented with 10% fetal calf serum (FCS; Gibco, Thermo Fisher Scientific, Tokyo, Japan), 2 mM L-glutamine, 100 units/ml of penicillin, and 100 µg/ml of streptomycin (Meiji Seika Pharma Co. Ltd, Tokyo, Japan). A549 cells (procured from the RIKEN BRC through the National BioResource Project of the MEXT/AMED, Japan) were grown in DMEM (Sigma) supplemented with 10% FCS, 2 mM L-glutamine, 100 units/ml of penicillin, and 100 µg/ml of streptomycin. Cells were incubated at 37°C in a humidified atmosphere with 5% CO2. HAdV-19 (ATCC, Manassas, VA) was propagated in A549 cells and stored at −80°C. Mean titers were calculated and expressed as median tissue culture infectious dose per ml (TCID50/ml).
MTT-based antiviral and cytotoxic assay
To evaluate anti-HAdV activity, the RPMI 1640- or DMEM-based assay medium, for MT-2 or A549 cells, respectively, with (S)-1–(3-hydroxy-2-phosphonylmethoxypropyl) cytosine (HPMPC), ddC, FdT, RBV, and GCV (Sigma-Aldrich Japan), was added in duplicate to wells on flat-bottom 96-well plates. MT-2 cells and A549 cells were mixed with HAdV-19, 2× 105 cells/mL with 104 TCID50/mL and 1× 105 cells/mL with 3 × 103 TCID50/mL, respectively, in plate wells in the presence or absence of various concentrations of the compounds (MOI: 0.035 for MT-2 cells and 0.021 for A549). The plates were incubated for five days. At the end of the incubation period, CPE was determined using the MTT assay, as described previously.11,12 Thus, 25 µl of the MTT solution (7.5 mg/ml) in phosphate-buffered saline (Wako, Japan) was added to each well of the plate. Then, the plate was incubated at 37°C for 2 h. After incubation, 150 µl of medium was removed with care in order not to draw cells. To solubilize the formazan crystals and neutralize the viral infectivity, 100 μl of acidified isopropanol (4 ml concentrated HCl per 1 l of isopropanol) containing 10% (v/v) Triton X-100 was added to each well. Formazan crystals were completely solubilized by pipetting up and down, the absorbance at 560 nm of the wells was read by microplate reader (GloMax, Promega, Japan). The 50% antiviral effective concentration (EC50) was defined as the drug concentration that protects 50% of virus-infected cells from virus-induced cell damage and/or death.12 The cytotoxicity of each compound was measured in parallel using the MTT assay. The 50% cytotoxicity concentration (CC50) was defined as the drug concentration that reduces cell viability by 50%. Data shown represent mean EC50 and CC50values (±1 standard deviation) derived from the results of two to four independent experiments conducted in duplicate.
Quantitative PCR for detecting viral DNA from HAdV-19
A set of primers synthesized by Echavarria et al.13 that amplify a 139-bp region of the hexon gene (set II) was used, as previously described. Thermal Cycler Dice Real Time System Lite (Takara Bio, Kusatsu, Japan) was used for DNA amplification.
Results
Cytopathic effects of HAdV-19 infection against MT-2 cells
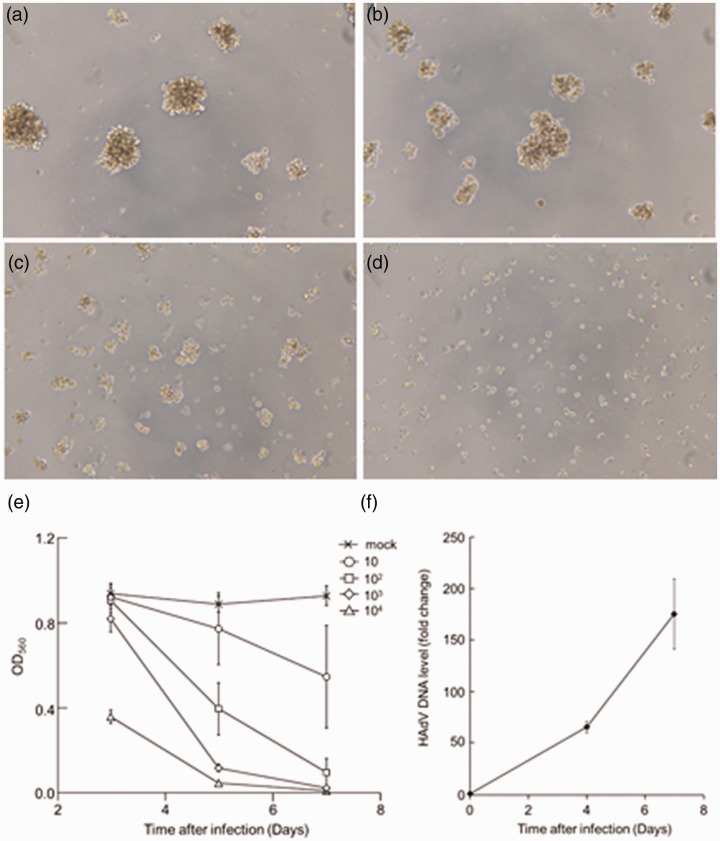
To examine the infectivity and CPE of HAdV-19, we microscopically observed HAdV-19-infected MT-2 cells. CPE was observed in a dose-dependent manner after five days of HAdV-19 infection at 102, 103, and 104 TCID50. A 100% CPE was observed at 103 and 104 TCID50 (Figure 1(c) and (d)). To determine the time course of HAdV-19-induced CPE, we examined the inhibition of formazan formation in MT-2 cells on days 3, 5, and 7 post-infection. The optical density at 560 nm (OD560) values decreased in a time-dependent manner (Figure 1(e)). On day 5, the OD560 values had decreased by approximately 80%, 40%, 20%, and 10% compared with the control, upon infection with HAdV-19 at 104, 103, 102, and 10 TCID50 per well, respectively (Figure 1(e)). The Z′ factor was 0.94 when the cells were infected with 103 TCID50 of HAdV-19, indicating robustness for screening. Therefore, we incubated 103 TCID50 of HAdV-19 with cells for five days prior to the MTT antiviral assay.
Figure 1.
Cell viability and viral replication in HAdV-infected MT-2 cells. (a to d) MT-2 cells five days post infection with HAdV-19. Cell death was observed in a dose-dependent manner. (a) mock-infected, (b) 102 TCID50, (c) 103 TCID50, (d) 104 TCID50. (e) inhibition of formazan formation in virus-infected and mock-infected MT-2 cells. Formazan formation by mock-infected, and HAdV-19-infected MT-2 cells was monitored for seven days after infection. (f) The qPCR detection of HAdV-19 in culture medium. MT-2 cells were infected with HAdV-19 for four or seven days before qPCR detection of HAdV-19 DNA in culture supernatant. HAdV DNA levels are shown as fold change for each day after infection compared with the amount at 0 days post-infection. After four and seven days of infection, the amount of viral DNA increased by more than 50 and 150 times compared with the initial amount of viral DNA, respectively.
Detection of HAdV-19 by qPCR
To confirm HAdV-19 replication in MT-2 cells, we determined the amount of viral DNA in the culture supernatant of infected cells using qPCR methods.4 MT-2 cells were infected with 103 TCID50 of HAdV-19 for four or seven days. As shown in Figure 1(f), the amount of detected viral DNA increased in a time-dependent manner. Seven days post-infection, viral DNA increased more than 150 times compared with the initial amount of viral DNA. Thus, HAdV-19 efficiently replicated in MT-2 cells.
Effect of antiviral compounds against HAdV-19
The antiviral activities of test compounds (CDV, ddC, FdT, RBV, and GCV) were evaluated against HAdV-19 using an MTT assay with MT-2 cells (Table 1). FdT and ddC inhibited HAdV-19 replication with EC50 values of 1.8 and 2.6 µM, respectively. The EC50 values of CDV and GCV were comparable at 62 and 60 µM, respectively. In contrast, RBV showed little antiviral activity against HAdV-19. For comparison, we also evaluated the EC50 and CC50 of the compounds using A549 cells by MTT assay.2,14 The results showed a similar tendency. Thus, FdT and ddC exerted more potent activity than CDV and GCV. In our assay, CDV, ddC, and GCV were tolerated by MT-2 cells at a concentration of 100 µM as was the case with A549 cells. CC50 values of FdT and RBV against MT-2 cells (36 and 18 µM, respectively) were similar values to A549 cells (7.6 and 53 µM, respectively).
Table 1.
Antiviral and cytotoxic effects of tested compounds against HAdV-19 determined by MTT assay.
|
EC50 (µM) |
CC50 (µM) |
SI |
||||
|---|---|---|---|---|---|---|
| Compounds | MT-2 | A549 | MT-2 | A549 | MT-2 | A549 |
| CDV | 62 ± 26 | 4.2 ± 1.1 | >100 | >1.6 | >24 | |
| ddC | 2.6 ± 0.4 | 0.35 ± 0.04 | >100 | >38 | >286 | |
| FdT | 1.8 ± 0.2 | 0.03 ± 0.01 | 36 ± 5 | 7.6 ± 4.2 | 20 | >253 |
| RBV | >18 | >53 | 18 ± 1 | 53 ± 21 | NA | |
| GCV | 60 ± 7 | 54 ± 27 | >100 | >1.7 | >1.9 | |
Note: All assays to determine EC50 were conducted two to four times independently. The results are shown as means ± 1 standard deviation. The selectivity index (SI) is the ratio of CC50 and EC50 values.
CDV: cidofivir; ddC: zalcitabine; FdT: 3′-deoxy-3′-fluorothymidine; RBV: ribavirin; GCV: ganciclovir.
Discussion
Most HAdV infections are severe but self-limited, even without a specific therapy. However, HAdV infection in immunosuppressed patients can cause life-threating illnesses. Additionally, ocular HAdV infection is important because it can cause EKC. Despite this, there are no approved anti-HAdV agents.
The development of anti-HAdV agents has been hampered by problematic antiviral assay methods. Plaque assays have been conventionally used for detecting HAdV infectivity and the anti-HAdV activity of compounds.13,15,16 Additionally, qPCR methods for detecting HAdV were developed and have been useful in analyzing HAdV infection, spread or replication, and time dependence.4 However, plaque assays and qPCR methods are not convenient for large-scale screening of antiviral agents. In contrast, the development of anti-HIV-1 and anticancer drugs has proven that MTT assays are well suited for drug screening. To date, several MTT methods for screening anti-HAdV agents have been developed.12,17–20 However, these assays use adherent cells such as A549, HEp-2, and HEL fibroblast cells, and thus require more than one week to complete and significant labor to change culture medium.21 In this report, we have demonstrated that HAdV-19, which was a representative pathogen causing EKC, could infect the human lymphoid cell line, MT-2, although the relationships between lymphoid cells and HAdV-19 pathogenesis have not been clarified, yet. Additionally, we established an anti-HAdV screening method with MT-2 cells using an MTT assay. MT-2 cells could be completed in five days without medium exchange. The anti-HAdV activities of compounds measured by the MTT assay on MT-2 cells showed a similar tendency to those on A549 cells. However, it is well known that the anti-HAdV activities of several compounds are serotype- and cell line dependent.2,3,8,11 Indeed, our method detected an EC50 value of FdT (1.8 µM) that is 10 to 100 times higher than the previously reported EC50 values determined in HAdV-2 and -3-infected Fogh and Lund (FL) cells,22 which were originally obtained from normal amnion but were subsequently believed to be HeLa cell contamination. Thus, the discrepancies between the reported EC50 values for FdT might result from the different HAdV serotypes and/or cell lines used in each study. These findings suggest that an MTT assay with MT-2 cells could be a useful tool for high-throughput screening of anti-HAdV agents. This simple, rapid, and convenient method can accelerate and enhance the development of anti-HAdV agents.
Acknowledgments
We are grateful to Emiko Usui for editorial help. We thank Lara Wilson, PhD, and Gillian Campbell, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this article.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: K.N. is an employee of Yamasa Corporation. The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by research grants from the Japan Society of the Promotion for Science (JSPS No. 16H05346) and by the Joint Usage/Research Center, Research Center for Zoonosis Control, Hokkaido University.
ORCID iD
Eiichi N Kodama https://orcid.org/0000-0002-6622-2752
References
- 1.Ghebremedhin B. Human adenovirus: viral pathogen with increasing importance. Eur J Microbiol Immunol (Bp) 2014; 4: 26–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenaerts L, De Clercq E, Naesens L. Clinical features and treatment of adenovirus infections. Rev Med Virol 2008; 18: 357–374. [DOI] [PubMed] [Google Scholar]
- 3.Hartline CB, Gustin KM, Wan WB, et al. Ether lipid-ester prodrugs of acyclic nucleoside phosphonates: activity against adenovirus replication in vitro. J Infect Dis 2005; 191: 396–399. [DOI] [PubMed] [Google Scholar]
- 4.Stock R, Harste G, Madisch I, et al. A rapid quantitative PCR-based assay for testing antiviral agents against human adenoviruses demonstrates type specific differences in ribavirin activity. Antiviral Res 2006; 72: 34–41. [DOI] [PubMed] [Google Scholar]
- 5.Kaneko H, Kato K, Mori S, et al. Antiviral activity of NMSO3 against adenovirus in vitro. Antiviral Res 2001; 52: 281–288. [DOI] [PubMed] [Google Scholar]
- 6.Naesens L, Lenaerts L, Andrei G, et al. Antiadenovirus activities of several classes of nucleoside and nucleotide analogues. Antimicrob Agents Chemother 2005; 49: 1010–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trousdale MD, Goldschmidt PL, Nóbrega R. Activity of ganciclovir against human adenovirus type-5 infection in cell culture and cotton rat eyes. Cornea 1994; 13: 435. [DOI] [PubMed] [Google Scholar]
- 8.Gordon Y, Romanowski E, Araullo-Cruz T, et al. Inhibitory effect of (S)-HPMPC,(S)-HPMPA, and 2′-nor-cyclic GMP on clinical ocular adenoviral isolates is serotype-dependent in vitro. Antiviral Res 1991; 16: 11–16. [DOI] [PubMed] [Google Scholar]
- 9.Morfin F, Dupuis-Girod S, Mundweiler S, et al. In vitro susceptibility of adenovirus to antiviral drugs is species-dependent. Antivir Ther (Lond) 2005; 10: 225–229. [PubMed] [Google Scholar]
- 10.Mentel R, Kinder M, Wegner U, et al. Inhibitory activity of 3′-fluoro-2′ deoxythymidine and related nucleoside analogues against adenoviruses in vitro1. Antiviral Res 1997; 34: 113–119. [DOI] [PubMed] [Google Scholar]
- 11.Kodama E, Shigeta S, Suzuki T, et al. Application of a gastric cancer cell line (MKN-28) for anti-adenovirus screening using the MTT method. Antiviral Res 1996; 31: 159–164. [DOI] [PubMed] [Google Scholar]
- 12.Pauwels R, Balzarini J, Baba M, et al. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods 1988; 20: 309–321. [DOI] [PubMed] [Google Scholar]
- 13.Echavarria M, Forman M, Ticehurst J, et al. PCR method for detection of adenovirus in urine of healthy and human immunodeficiency virus-infected individuals. J Clin Microbiol 1998; 36: 3323–3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romanowski EG, Yates KA, Gordon YJ. The in vitro and in vivo evaluation of ddC as a topical antiviral for ocular adenovirus infections. Invest Ophthalmol Vis Sci 2009; 50: 5295–5299. [DOI] [PubMed] [Google Scholar]
- 15.Bonifas V, Schlesinger R. Nutritional requirements for plaque production by adenovirus. In: Federation proceedings Bethesda, MD: Federation Proc Amer Soc Exp Biol, 1959, p.560–590.
- 16.Kjellén L. A study of adenovirus-host cell system by the plaque technique. Virology 1961; 14: 234–239. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh AK, Ramu Sridhar P, Kumaragurubaran N, et al. Bis-tetrahydrofuran: a privileged ligand for darunavir and a new generation of HIV protease inhibitors that combat drug resistance. ChemMedChem: Chemistry Enabling Drug Discovery 2006; 1: 939–950. [DOI] [PubMed] [Google Scholar]
- 18.Maeda K, Desai DV, Aoki M, et al. Delayed emergence of HIV-1 variants resistant to 4′-ethynyl-2-fluoro-2′-deoxyadenosine: comparative sequential passage study with lamivudine, tenofovir, emtricitabine and BMS-986001. Antivir Ther (Lond) 2014; 19: 179–189. [DOI] [PubMed] [Google Scholar]
- 19.Takamatsu Y, Das D, Kohgo S, et al. The high genetic barrier of EFdA/MK-8591 stems from strong interactions with the active site of drug-resistant HIV-1 reverse transcriptase. Cell Chem Biol 2018; 25: 1268–1278. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hattori S-I, Hayashi H, Bulut H, et al. Halogen bond interactions of novel HIV-1 protease inhibitors (PI)(GRL-001-15 and GRL-003-15) with the flap of protease are critical for their potent activity against wild-type HIV-1 and multi-PI-resistant variants. Antimicrob Agents Chemother 2019; 63: e02635–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith C, Craft D, Shiromoto R, et al. Alternative cell line for virus isolation. J Clin Microbiol 1986; 24: 265–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogura H, Yoshinouchi M, Kudo T, et al. Human papillomavirus type 18 DNA in so-called HEP-2, KB and FL cells – further evidence that these cells are HeLa cell derivatives. Cellular and Molecular Biology (Noisy-le-Grand, France.) 1993; 39: 463–467. [PubMed] [Google Scholar]