Abstract
Background
Phenylketonuria (PKU) treatment consists of life-long protein restriction and Phe-free medical foods for adequate nutritional intake and growth. A relationship between body composition and blood phenylalanine (Phe) concentrations in subjects with PKU has been proposed but this has not been consistently reported.
Methods
Dietary intake, lean body mass (LBM) and fat mass (FM) were measured in 30 pediatric subjects with PKU compared to 30 age, and sex matched controls. The relationship between body composition and blood Phe was analyzed within the PKU cohort from clinically collected dried blood spot Phe concentrations.
Results
Male subjects with PKU had less LBM% and more FM% than controls (p = .024). There was no difference in LBM% and FM% among female subjects. Age (p = .02) and FM% (p = .02) were positively correlated to dried blood spot Phe. Synthetic protein intake (g/kg body weight) was negatively correlated with dried blood spot Phe (p = .04). Natural protein intake was not related to blood spot Phe.
Conclusions
Children with PKU face additional dietary challenges maintaining healthy growth and body composition while keeping Phe levels low. We observed higher FM% and lower LBM% in male subjects with PKU. Correlations do not prove cause and effect but suggest a relationship between increased blood Phe, lower synthetic protein intake and increased FM%. Future studies may explore if lower blood Phe concentrations is associated with a lower FM% and higher LBM%; particularly among adult patients now managed on pegvaliase (Palynziq®) who consume normal amounts of natural protein or among younger patients who consume glycomacropeptide (GMP).
Keywords: Phenylketonuria, Body composition, Nutrient intake, Phe-free medical foods, Dietary protein
1. Introduction
Phenylketonuria (PKU; OMIM 261600) is a rare autosomal recessive inborn error of metabolism characterized by the enzymatic deficiency of phenylalanine hydroxylase (PAH; OMIM 612349) which limits the conversion of the essential amino acid phenylalanine (Phe) into tyrosine (Tyr) [[1], [2], [3]]. If left untreated, high concentrations of Phe accumulate in the blood and brain causing severe and irreversible neurological damage [3]. Newborn screening for PKU, along with early dietary intervention have successfully ameliorated and even prevented these complications. Life-long treatment consists of dietary restriction of Phe, severely limiting natural protein intake, and providing semi-synthetic protein based medical food as a Phe-free, essential amino acid supplement or a low-Phe glycomacropeptide (GMP) supplement [2,4]. The current goal of dietary therapy aims to maintain blood Phe between 120 and 360 μmol/L (2–6 mg/dL) throughout the life span while maintaining Tyr within normal limits [4,5].
Medical food may contribute up to 85% of an individual's required protein intake, which shifts protein intake from natural sources to semi-synthetic sources [3,6]. Medical food is Phe-free and supplemented with Tyr, essential amino acids, vitamins, minerals, and trace nutrients meeting the nutritional requirements for a specific age population. Medical food is essential for PKU patients to prevent protein deficiency, optimize metabolic control, improve growth, and prevent micronutrient deficiency, specifically zinc, iron, carnitine, selenium, and vitamin B12 [7]. Special low-protein foods (SLPFs) are now manufactured into specialty items such as breads, pastas, cereals, pizza, and hot dogs, which greatly expands food choices, satisfies appetite, and meets energy requirements to promote normal growth [5]. However, along with greater food choices comes a remarkable increase in carbohydrate, fat, and energy content in this diet [6,7]. It is important that SLPF manufacturers ensure high quality food in respect to taste, acceptability, appearance, without sacrificing adequate nutritional value to improve patient adherence and avoid obesity and other comorbidities.
Currently, a total protein/amino acid intake exceeding the recommended dietary allowance (RDA) is advised but diet related changes in growth and body composition are still being reported [8]. The first reports of overweight and obese individuals with PKU emerged in the 1970's and 80's, [6,9,10] which coincide with an increase in more readily available low-protein foods believed to have led to a significant increase in energy and fat intake among individuals with PKU [11]. Recent studies found significantly higher body fat percentage among girls and women (11 years-old and older) with PKU when compared to healthy controls [8,12]. Current research provides no conclusive evidence regarding the prevalence of overweight and obesity in individuals with PKU; however, numerous studies have suggested that the incidence of overweight and obesity is increasing within this population [8,13,14]. This trend can be potentially associated with poor blood Phe control, poor dietary adherence, or perhaps due to the PKU diet composition in itself [14]. In this study, we propose that body composition in PKU subjects will differ from their matched controls. Specifically, we hypothesize that PKU subjects will have a higher fat mass percent (FM%) and a lower lean body mass percent (LBM%) due to their different diet composition. We analyzed body composition in children with PKU when compared to healthy controls, as well as explored the relationship between blood Phe concentration, body composition and dietary factors specific to the population with PKU including natural and synthetic protein intake.
2. Methods
This is an observational, cross-sectional study carried out at the OHSU Doernbecher Children's Hospital Metabolic Clinic and PKU Family Camp in Antelope, Oregon. All subjects or their guardians gave written informed consent after the study had been explained to them. Subjects younger than 18 years of age also gave assent to participate. The study was approved by the OHSU institutional review board (IRB #10819).
We compared body composition in children between the ages of 5 to 18 years with PKU (n = 30) and age-sex-matched controls in the general pediatric population (n = 30), as well as explored the potential relationship between body composition, dietary intake, and blood Phe control. Criteria for inclusion for the subjects included a diagnosis of classic PKU treated by dietary Phe restriction since diagnosis, followed at the Doernbecher Children's Hospital Metabolic Clinic or attended the PKU Family Camp in Antelope, Oregon. Matched controls were recruited from the Doernbecher Children's Hospital Pediatric Clinic aged 5 to 18 years in overall good general health. Subjects and controls were excluded if ill or dehydrated; were pregnant or lactating; had one or more missing limbs; had failure to thrive, an eating disorder or abnormal weight gain; and had an implanted defibrillator because the bioimpedance analyzer has not been tested in people with this cardiac device.
2.1. Body composition
Anthropometrics were obtained and recorded in standard measurements. Measurements to assess body composition included LBM% and FM% were measured using a bioimpedance analyzer (Biodynamics, model 450). For this test, the subjects remained barefoot with light clothing and removed any additional jewelry on their right arm or right foot. Subjects lay in a supine position on the exam table, with arms by their sides, and arms and thighs not touching. The wrist, hand, ankle and foot were gently cleaned with alcohol wipes. The electrodes were attached to the subject's right wrist and ankle. Resistance was recorder in ohms, LBM and FM in pounds (lbs) and as a percent. BMR was recorded in calories, BMI was recorded in kg/m2. Outcome measures such as height, weight, and body mass index (BMI) were normalized as a z-score and as a percentile. BMI percentile was categorized as underweight, normal weight, overweight, and obese.
2.2. Dietary intake
A 24-h dietary recall was obtained using the multi-pass technique and intake was analyzed in Food Processor (10.12.0 ESHA Research, Inc) or Metabolic Pro (Genetic Metabolic Dietitians International) which is a specialized database designed specifically to analyze special low-protein medical food. For this analysis, the study subject or the subject's parent or guardian provided a list of foods eaten in the prior 24 h, provided the time and occasion of dietary intake, provided the details of the food consumed (brand, preparation, frozen/raw/canned, and how much was eaten), and confirmed the 24-h dietary intake. Dietary intake obtained from children with PKU was analyzed in Metabolic Pro and intake obtained from children in the general pediatric population was analyzed in Food Processor. Outcome measures such as milligrams of Phe, grams of protein, fat and carbohydrates were normalized per kilogram and as a percentage of total dietary intake. Medical foods (GMP based or amino acid based) consumed were totaled and described as grams of synthetic protein, then subtracted from total grams of protein to determine grams of natural protein.
2.3. Blood Phe
The average dried blood spot Phe over the previous 12 months was obtained from the electronic medical record system at OHSU or acquired from the individual's metabolic clinic. The average Phe was calculated from a median of 10 different dried blood spot samples for each subject with PKU (range 1–50 dried blood spot measures). All sample measurements were made by standardized tandem mass spectrometry.
2.4. Data analysis
Data are reported as the mean and standard deviation (SD). Differences in body composition between subjects with PKU and age-sex-matched controls were determined with a one-tailed paired t-test. BMI categories among subjects with PKU and controls were compared by Chi-square analysis. Diet composition measures were available for 29 subjects with PKU and 29 control subjects and were compared by two-tailed unpaired t-test by sex. Diet recall data from two female control subjects was excluded due to underreporting of energy less than 75% of the estimated basal metabolic rate. Diet parameters were compared between subjects with PKU and normal controls with a two-tailed unpaired t-test. We used Pearson correlation analysis to examine the relationships between our variables including body composition, dietary intake and dried blood Phe, within the PKU cohort. For all analysis, a p < .05 was considered statistically significant. Statistical analysis and graphics were completed using Prism 8.0 GraphPad Software Inc. (La Jolla, California).
3. Results
Thirty subjects with PKU enrolled in this study ranged in age from 5 to 16 years of age with a mean of 11.6 years. Twelve were female (40%) and 18 were male (60%). Their BMIs ranged from 13.8 to 34.2 kg/m2 with a mean of 20.86 kg/m2 (±4.43). Thirty age and sex matched healthy controls were also enrolled in this study. All participants were white Caucasian from a similar demographic background. Anthropometrics were compared between subjects with PKU and normal controls by sex; female subjects with PKU were compared to female control subjects and male subjects with PKU were compared to male control subjects (Table 1).
Table 1.
Subject characteristics and anthropometrics.
| Group |
PKU n = 30 |
Control n = 30 |
||
|---|---|---|---|---|
| Gender | Female n = 12 (40%) | Male n = 18 (60%) | Female n = 12 (40%) | Male n = 18 (60%) |
| Age | 11.75 ± 3.41 | 11.5 ± 3.27 | 11.75 ± 3.49 | 11.3 ± 3.27 |
| Weight (kg) | 48.84 ± 23.18 | 44 ± 15.10 | 47.27 ± 23.38 | 48.62 ± 23.38 |
| Height (m) | 1.45 ± 0.18 | 1.45 ± 0.18* | 1.50 ± 0.20 | 1.52 ± 0.20* |
| BMI | 21.91 ± 5.88 | 20.16 ± 3.12 | 20.03 ± 5.55 | 19.82 ± 5.28 |
| Lean body mass (%) | 77.87 ± 9.0 | 85.38 ± 4.9* | 76.50 ± 9.87 | 89.09 ± 5.94 |
| Fat mass (%) | 22.12 ± 9.0 | 14.61 ± 4.9* | 20.60 ± 6.77 | 10.9 ± 5.94 |
Data are presented as mean ± standard deviation. Female subjects with PKU were compared to female control subjects and male subjects with PKU were compared to male control subjects by paired students t-test. *p < .05.
Normalized height was significantly lower in the male PKU group when compared to their matched control group (z-scores: −0.741 ± 1.002 vs. 0.546 ± 1.108; p = .0023) as shown in Table 1. This difference was not observed in females (p = .40). There was no difference in LBM% and FM% in females (p = .34 and p = .34 respectively, Fig. 1A and C), but males with PKU had significantly lower LBM% (p = .02) and a significantly higher FM% (p = .02) than male controls (Fig. 1B and D).
Fig. 1.
Lean Body Mass (LBM%) and Fat Mass Percentages (FM%).
Data are presented as dot plots where each subject is one dot. The middle vertical bar is the mean and the whisker bars above and below represent 1 standard deviation around the mean. A) There was no difference in LBM% (p = .34) between females with PKU (n = 12, LBM% of 77.87 ± 9.0%) and the control group (LBM% of 76.5 ± 9.87%). B) LBM% was significantly lower (p = .026) in males with PKU (n = 18, 85.38 ± 4.9%) when compared with their matched control group (n = 18, 89.09 ± 5.94%). C) There was no significant difference in FM% (p = .34) between female subjects with PKU (n = 12, FM% of 22.12 ± 9.0%) and the control group (FM% of 20.6 ± 6.77%). D) FM% was significantly higher (p = .026) in males with PKU (n = 18, 14.61 ± 4.9%) than their matched control group (10.9 ± 5.9%).
Fifty-six percent of the subjects with PKU and 76.6% of the controls were normal weight (BMI 5-84th percentile, n = 17 vs. n = 23); 30% of the subjects with PKU and 6.67% of the controls were overweight (BMI 85-95th percentile, n = 9 vs. n = 2); and 10% of the subjects with PKU and 13.33% of the controls were obese (BMI > 95th percentile, n = 3 vs. n = 4). Only 1 (3%) subject on each group was underweight (BMI <5%). Although there was a trend for more overweight among the subjects with PKU compared to the control group, this difference was not significant (p = .07; OR = 2.7, CI 95% = 0.85–9.2, Fig. 2).
Fig. 2.

BMI percentiles in subjects with PKU compared to control subjects.
Data are presented as # of subjects in that BMI percentile category. Black bars = subjects with PKU; white bars = control subjects. Most PKU subjects where within a normal weight (black bars; n = 17) just as most control subjects where within a normal weight (white bars, n = 23). There was a trend for more PKU patients to be in the overweight and obesity category but the difference was not significant (p = .07; OR = 2.7, CI 95% = 0.85–9.2).
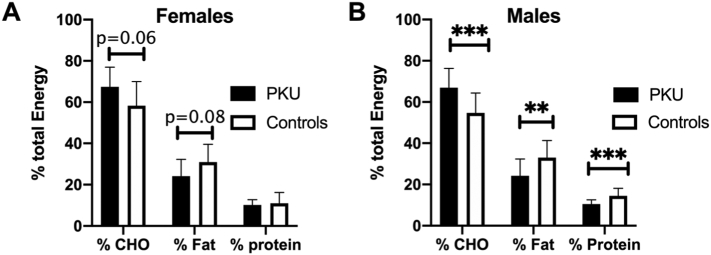
Twenty-four hour dietary recalls were completed in 29 of the 30 study participants with PKU, and 29 controls. Two female control subjects were excluded from the analysis due to a reported energy intake less than 75% of their estimated BMR and were considered to represent significant underreporting of nutrient intake. Only one female subject and 3 male subjects were on a form of BH4 or sapropterin dihydrochloride (Kuvan®). There were no significant differences between subjects with PKU and controls in regards to total energy, energy per kg of body weight, and total grams of protein per kg of body weight (Table 2). The percent of total energy of protein among male subjects with PKU was lower than male control subjects (10.4 ± 2.1% vs. 14.5 ± 3.7; p = .003), but % energy from protein was similar between female subjects with PKU and control female subjects (10.1 ± 2.6% vs. 11.0 ± 5.2%; p = .63). The percent energy from carbohydrate was significantly higher among the male subjects with PKU compared to the male control subjects (67 ± 9.3% vs. 54.6 ± 9.7%; p = .0005), and trended higher among the female subjects with PKU compared to female control subjects (67.5 ± 9.5% vs. 58.3 ± 11.7%; p = .06). The percent energy from fat was significantly lower among the male subjects with PKU compared to male control subjects (24.2 ± 8.2% vs. 33 ± 8.26; p = .0033), and trended lower among the female subjects with PKU compared to female control subjects (24.1 ± 8.2% vs. 31 ± 8.6%; p = .078) (Fig. 3A and B).
Table 2.
Subject dietary intake.
| Group |
PKU n = 29 |
Control n = 27 |
||
|---|---|---|---|---|
| Gender | Female n = 11 | Male n = 18 | Female n = 10 | Male n = 17 |
| Kcal | 2313 ± 742 | 2382 ± 534 | 2282 ± 531 | 2339 ± 623 |
| Kcal/kg | 53 ± 22 | 58 ± 16 | 62 ± 20 | 56 ± 19 |
| % CHO | 67.5 ± 9.5 | 67.3 ± 9.6*** | 58.3 ± 11.7 | 54.6 ± 9.7 |
| % FAT | 24.1 ± 8.2 | 24.0 ± 8.3** | 31.0 ± 8.60 | 33.0 ± 8.26 |
| % PRO | 10.1 ± 2.6 | 10.0 ± 2.3*** | 11.0 ± 5.2 | 14.5 ± 3.7 |
| Pro/kg | 1.3 ± 0.56 | 1.44 ± 0.48 | 1.9 ± 0.96 | 2.0 ± 0.77 |
| P:E ratio | 2.53 ± 0.64 | 2.51 ± 0.58*** | 3.09 ± 0.91 | 3.62 ± 0.92 |
| Synthetic Pro/kg | 0.95 ± 0.65 | 1.03 ± 0.37 | NA | NA |
| Natural Pro/kg | 0.36 ± 0.31 | 0.41 ± 0.32 | NA | NA |
Data are presented as mean ± standard deviation. Female subjects with PKU were compared to female control subjects and male subjects with PKU were compared to male control subjects by paired students t-test. NA = not applicable. ***p < .0005.
Fig. 3.
Macronutrient distribution between subjects with PKU and matched controls.
Data presented as mean ± the standard deviation of the mean. Black bars = subjects with PKU and white bars = control subjects. A) Percent energy from carbohydrate trended higher and % energy from fat trended lower in female subjects with PKU compared to controls but there was no significant difference in % of energy protein. B) Percent energy from carbohydrate is significantly higher while % of energy from fat and protein was significantly lower among male subjects with PKU compared to control subjects. **p < .005; ***p < .0005.
There was no difference in the total grams of protein per kg of body weight in subjects with PKU compared to controls. However, the protein to energy ratio was significantly lower among male subjects with PKU compared to male control subjects (2.51 ± 0.58 vs. 3.62 ± 0.92; p = .0003). This difference was not observed between female subjects with PKU and female control subjects (Table 2).
Dried blood spot Phe concentration was recorded in 28 of the 30 subjects with PKU, two subjects declined to release their medical records. Mean dried blood spot Phe concentration was 391.74 ± 183.65 μmol/L (treatment range 120–360 μmol/L), with a range of 173.97 to 915.5 μmol/L. We used Pearson correlation to explore the relationships between dried blood spot Phe, age, body composition (FM%), and dietary parameters. The correlation matrix is presented in Fig. 4. The relevant relationships between dried blood Phe are also shown in Fig. 5. Age and FM% were positively correlated with dried blood spot Phe (Fig. 5A and B). Medical food protein intake was negatively correlated with dried blood spot Phe suggesting increased intake of medical foods was associated with lower blood spot Phe (Fig. 5C). However, natural protein intake was not correlated with blood spot Phe (Fig. 5D).
Fig. 4.

Correlation Matrix between blood spot Phe, body composition and dietary intake variables.
Our initial analysis used a correlation matrix between blood Phe concentration, measures of body composition and diet intake parameters to determine if these factors were related. The matrix gives the strength of the correlation by color in each box between the 2 variables that intersect at that box. Blue is a positive association with darker blue indicating a stronger relationship. Red is negative association with darker red indicating a stronger relationship.
Fig. 5.
Correlation between dried blood spot Phe, age, FM%, synthetic and natural protein intake among subjects with PKU.
Scatter plots show the relationship between mean dried blood spot Phe (x-axis) and A) age; B) fat mass %; C) synthetic protein intake (g/kg body weight); D) natural protein intake (g/kg body weight). There was a positive correlation between blood spot Phe, age and fat mass % and a negative correlation with synthetic protein intake. Natural protein intake was not related to blood spot Phe in this cohort of subjects with PKU.
4. Discussion and conclusion
An early diagnosis of PKU, as well as the establishment and adherence to the PKU specific therapeutic diet, prevent the most serious neurological manifestations of this metabolic disease. Even with adequate Phe control, a variety of subtle physical, neuropsychological and behavioral impairments have been recognized [7]. Care of individuals with PKU is a multidisciplinary effort and lifetime support is required to maintain a good outcome. Even if Phe levels are on average within the treatment range, body composition may be altered [15]. Difference in FM% and body composition between individuals with PKU and controls has been inconsistently reported [8,15,16]. Using a variety of body composition methods, five studies found no difference in FM% between males and females with PKU when compared to controls and one study found higher FM% between individuals with PKU when compared to controls, with a more pronounced difference within females [6,8,10,13,14]. In this study, we analyzed body composition in pediatric individuals with PKU and found a significant increase of FM% in males (p = .02) which differs from previous reports observing differences in females [8]. Larger cohorts may be needed to confirm these findings.
We are aware that difference in outcomes stem from a complex array of factors, including the genetic cause of PKU and other complex environmental factors like; full access to medical foods, access to medical follow-ups, dietitian, psychological support to patients and caregivers, as well as genetic counseling. Other factors include adherence to diet, use and response to additional medications like Kuvan® and the use of novel drugs that reduce Phe blood levels like pegvaliase (Palynziq®), which was recently approved by the FDA in adults with PKU significantly lowering their Phe blood levels and frequently allowing normal amounts of natural protein intake [17].
Despite a trend for increased FM% in the subjects with PKU, we found no differences in weight and BMIs between the groups. The literature is also inconsistent regarding weight and BMI for age among subjects with PKU. Three studies found weight and BMI z-scores of subjects with PKU to be similar to controls, three studies found children with PKU to weigh more than controls, while one study found that children with PKU weighed less than controls [13,14,[18], [19], [20], [21]]. These differences could be due to small cohorts with limited power, the year when the study was conducted as the trend appears to change over time, the amount of low-protein, energy dense foods available for consumption in the cohort, and various countries around the globe whose diet and lifestyle customs may ultimately impact weight [22]. The PKU formulas vary widely across different countries and can have very different nutritional compositions. For example, many European formulas do not contain added fat or fiber; GMP containing formulas may have higher carbohydrate and energy content; some amino acid mixtures contained added oligosaccharides; and formulas designed for the first year of life have more carbohydrate, and fat than formulas designed for later childhood [23]. These differences should be considered as they could potentially explain some of the variability in the reported literature regarding body composition among patients with PKU.
Interestingly, we found that male subjects with PKU have a significantly lower height z-scores compared to controls. Some studies report no negative effects on growth in the pediatric population, while other studies report poor growth outcomes including similar reduced height z-scores [10,14,20,24]. Relationships to height and weight have been proposed previously in correlation to dried blood spot Phe [20] however we did not observe these correlations. The differences observed in height z-scores between subjects with PKU and healthy controls could be related to pubertal maturation and/or genetic potential and not to the metabolic disease or its treatment. However, we did not include Tanner staging for sexual maturity and we were unable to determine if genetic potential contributed to the differences in height as we did not collect parental height. This finding is still worth studying further and we plan to monitor height in our patients with PKU.
As expected, the type of energy consumed differed between subjects and controls; in all subjects with PKU the percent of energy from carbohydrates is higher than control subjects. Even if this difference was significant only in the male subjects, female subjects also trended in that direction (p = .0005 and p = .06, respectively). Also, the percent of energy from fat and protein is lower when compared with controls. As subjects must restrict their protein intake, this also lowers fat intake and energy requirements are met by overconsumption of carbohydrates. The differences found in macronutrient distribution among patients with PKU may contribute to the differences in body composition. The type of carbohydrate in the diet of subjects with PKU such as simple vs complex, or low vs high glycemic index warrants further investigation.
We hypothesized that a higher Phe on dried blood spot could have an impact on FM% and we found a positive correlation, which differs from some reports in the literature where no correlation was found between mean blood Phe and FM% [8]. However, we found that as age and FM% increase, so does Phe levels on dried blood spot (p = .02 for both parameters). We also suspected that the type of protein intake would also correlate with blood Phe levels and while synthetic protein intake (g/kg body weight) was negatively correlated with dried blood spot Phe (p = .04), natural protein intake was not correlated to blood spot Phe (p = .06). This particular finding could be explained by selection bias since this cohort included PKU subjects actively invested in their own health and diet who agreed to participate in this study. With a larger range of protein intake or blood spot Phe concentrations, a relationship, if one exists, might be observable. It will be interesting to study this finding further as well as understanding how the amount of natural protein intake with adjuvant therapies such as BH4 and pegvaliase may influence body composition in patients with PKU as expanded use of these therapies will have a substantial effect on the dietary intake of patients with PKU in the future.
BMI has also been extensively studied in patients with PKU. Previous studies observed a correlation between increasing BMI and higher Phe concentrations in males and females aged 16 and older [25]. However using BMI as a screening tool for overweight and obesity is not a preferred measurement for children since these individuals have a different amount of FM% and LBM% as body composition undergoes dramatic changes throughout the lifecycle [26]. In westernized societies, adolescents may continue to gain weight throughout the lifecycle, resulting in an increased BMI secondary to an increase in FM. In a healthy Turkish pediatric population without PKU, plasma Phe has been shown to increase with age in males, but not females; this could be due to the rapid gain of LBM observed in males [27]. Other studies report rapid LBM increments without an increase in blood Phe levels [25,28]. Therefore, age is a covariant since both blood Phe and FM% increase with puberty as a function of normal growth [26,27], but when we controlled for age, increased blood Phe was still associated with increased FM%. Further studies on patients with PKU undergoing puberty are needed to observe the relationship between blood Phe and FM% and other anthropometric measurements.
Based on our results, there may be some differences in body composition among subjects with PKU compared to controls, even among those with a normal BMI. Multiple factors contribute to body composition including genetics, activity level, and diet. Future studies could examine the role of diet in body composition among subjects with PKU by looking at changes in body composition in subjects who either; 1) consume normal amounts of natural protein because of the addition of Kuvan® and Palynziq® to their management, 2) consume GMP-based medical food that is a low Phe intact protein source, 3) consume less carbohydrate through processed low protein, low Phe food alternatives, and consume more fruits and vegetables, or 4) improve their overall metabolic control with diet and activity.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Zimmermann M., Jacobs P., Fingerhut R., Torresani T., Thony B., Blau N., Baumgartner M.R., Rohrbach M. Positive effect of a simplified diet on blood phenylalanine control in different phenylketonuria variants, characterized by newborn BH4 loading test and PAH analysis. Mol. Genet. Metab. 2012;106:264–268. doi: 10.1016/j.ymgme.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Prochazkova D., Jarkovsky J., Vinohradska H., Konecna P., Machacova L., Dolezel Z. Controlled diet in phenylketonuria and hyperphenylalaninemia may cause serum selenium deficiency in adult patients: the Czech experience. Biol. Trace Elem. Res. 2013;154:178–184. doi: 10.1007/s12011-013-9724-6. [DOI] [PubMed] [Google Scholar]
- 3.Miras A., Dolores Boveda M., Leis M.R., Mera A., Aldamiz-Echevarria L., Fernandez-Lorenzo J.R., Fraga J.M., Couce M.L. Corrigendum to "Risk factors for developing mineral bone disease in phenylketonuric patients". Mol. Genet. Metab. 2013;108:149–154. doi: 10.1016/j.ymgme.2012.12.008. Mol Genet Metab 114 (2015) 483. [DOI] [PubMed] [Google Scholar]
- 4.Singh R.H., Rohr F., Frazier D., Cunningham A., Mofidi S., Ogata B., Splett P.L., Moseley K., Huntington K., Acosta P.B., Vockley J., Van Calcar S.C. Recommendations for the nutrition management of phenylalanine hydroxylase deficiency. Genet. Med. 2014;16:121–131. doi: 10.1038/gim.2013.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Baulny H.O., Abadie V., Feillet F., de Parscau L. Management of phenylketonuria and hyperphenylalaninemia. J. Nutr. 2007;137:1561S–1563S. doi: 10.1093/jn/137.6.1561S. discussion 1573S–1575S. [DOI] [PubMed] [Google Scholar]
- 6.Huemer M., Huemer C., Moslinger D., Huter D., Stockler-Ipsiroglu S. Growth and body composition in children with classical phenylketonuria: results in 34 patients and review of the literature. J. Inherit. Metab. Dis. 2007;30:694–699. doi: 10.1007/s10545-007-0549-3. [DOI] [PubMed] [Google Scholar]
- 7.Manta-Vogli P.D., Dotsikas Y., Loukas Y.L., Schulpis K.H. The phenylketonuria patient: a recent dietetic therapeutic approach. Nutr. Neurosci. 2018:1–12. doi: 10.1080/1028415X.2018.1538196. [DOI] [PubMed] [Google Scholar]
- 8.Albersen M., Bonthuis M., de Roos N.M., van den Hurk D.A., Carbasius Weber E., Hendriks M.M., de Sain-van der Velden M.G., de Koning T.J., Visser G. Whole body composition analysis by the BodPod air-displacement plethysmography method in children with phenylketonuria shows a higher body fat percentage. J. Inherit. Metab. Dis. 2010;33(Suppl. 3):S283–S288. doi: 10.1007/s10545-010-9149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rocha J.C., van Spronsen F.J., Almeida M.F., Soares G., Quelhas D., Ramos E., Guimaraes J.T., Borges N. Dietary treatment in phenylketonuria does not lead to increased risk of obesity or metabolic syndrome. Mol. Genet. Metab. 2012;107:659–663. doi: 10.1016/j.ymgme.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Rocha J.C., van Spronsen F.J., Almeida M.F., Ramos E., Guimaraes J.T., Borges N. Early dietary treated patients with phenylketonuria can achieve normal growth and body composition. Mol. Genet. Metab. 2013;110(Suppl):S40–S43. doi: 10.1016/j.ymgme.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Lavoie S.M., Harding C.O., Gillingham M.B. Normal fatty acid concentrations in young children with Phenylketonuria (Pku) Top. Clin. Nutr. 2009;24:333–340. doi: 10.1097/TIN.0b013e3181c621fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burrage L.C., McConnell J., Haesler R., O'Riordan M.A., Sutton V.R., Kerr D.S., McCandless S.E. High prevalence of overweight and obesity in females with phenylketonuria. Mol. Genet. Metab. 2012;107:43–48. doi: 10.1016/j.ymgme.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Allen J.R., McCauley J.C., Waters D.L., O'Connor J., Roberts D.C., Gaskin K.J. Resting energy expenditure in children with phenylketonuria. Am. J. Clin. Nutr. 1995;62:797–801. doi: 10.1093/ajcn/62.4.797. [DOI] [PubMed] [Google Scholar]
- 14.Dobbelaere D., Michaud L., Debrabander A., Vanderbecken S., Gottrand F., Turck D., Farriaux J.P. Evaluation of nutritional status and pathophysiology of growth retardation in patients with phenylketonuria. J. Inherit. Metab. Dis. 2003;26:1–11. doi: 10.1023/a:1024063726046. [DOI] [PubMed] [Google Scholar]
- 15.MacLeod E.L., Gleason S.T., van Calcar S.C., Ney D.M. Reassessment of phenylalanine tolerance in adults with phenylketonuria is needed as body mass changes. Mol. Genet. Metab. 2009;98:331–337. doi: 10.1016/j.ymgme.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doulgeraki A., Skarpalezou A., Theodosiadou A., Monopolis I., Schulpis K. Body composition profile of young patients with phenylketonuria and mild hyperphenylalaninemia Int J. Endocrinol. Metab. 2014;12 doi: 10.5812/ijem.16061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta S., Lau K., Harding C.O., Shepherd G., Boyer R., Atkinson J.P., Knight V., Olbertz J., Larimore K., Gu Z., Li M., Rosen O., Zoog S.J., Weng H.H., Schweighardt B. Association of immune response with efficacy and safety outcomes in adults with phenylketonuria administered pegvaliase in phase 3 clinical trials. EBioMedicine. 2018;37:366–373. doi: 10.1016/j.ebiom.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Belanger-Quintana A., Martinez-Pardo M. Physical development in patients with phenylketonuria on dietary treatment: a retrospective study. Mol. Genet. Metab. 2011;104:480–484. doi: 10.1016/j.ymgme.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Scaglioni S., Verduci E., Fiori L., Lammardo A.M., Rossi S., Radaelli G., Riva E., Giovannini M. Body mass index rebound and overweight at 8 years of age in hyperphenylalaninaemic children. Acta Paediatr. 2004;93:1596–1600. [PubMed] [Google Scholar]
- 20.Aldamiz-Echevarria L., Bueno M.A., Couce M.L., Lage S., Dalmau J., Vitoria I., Andrade F., Blasco J., Alcalde C., Gil D., Garcia M.C., Gonzalez-Lamuno D., Ruiz M., Pena-Quintana L., Ruiz M.A., Gonzalez D., Sanchez-Valverde F. Anthropometric characteristics and nutrition in a cohort of PAH-deficient patients. Clin. Nutr. 2014;33:702–717. doi: 10.1016/j.clnu.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 21.White J.E., Kronmal R.A., Acosta P.B. Excess weight among children with phenylketonuria. J. Am. Coll. Nutr. 1982;1:293–303. doi: 10.1080/07315724.1982.10718998. [DOI] [PubMed] [Google Scholar]
- 22.Pinto A., Adams S., Ahring K., Allen H., Almeida M.F., Garcia-Arenas D., Arslan N., Assoun M., Altinok Y. Atik, Barrio-Carreras D., Quintana A. Belanger, Bernabei S.M., Bontemps C., Boyle F., Bruni G., Bueno-Delgado M., Caine G., Carvalho R., Chrobot A., Chyz K., Cochrane B., Correia C., Corthouts K., Daly A., De Leo S., Desloovere A., De Meyer A., De Theux A., Didycz B., Dijsselhof M.E., Dokoupil K., Drabik J., Dunlop C., Eberle-Pelloth W., Eftring K., Ekengren J., Errekalde I., Evans S., Foucart A., Fokkema L., Francois L., French M., Forssell E., Gingell C., Goncalves C., Ozel H. Gokmen, Grimsley A., Gugelmo G., Gyure E., Heller C., Hensler R., Jardim I., Joost C., Jorg-Streller M., Jouault C., Jung A., Kanthe M., Koc N., Kok I.L., Kozanoglu T., Kumru B., Lang F., Lang K., Liegeois I., Liguori A., Lilje R., Lubina O., Manta-Vogli P., Mayr D., Meneses C., Newby C., Meyer U., Mexia S., Nicol C., Och U., Olivas S.M., Pedron-Giner C., Pereira R., Plutowska-Hoffmann K., Purves J., Dionigi A. Re, Reinson K., Robert M., Robertson L., Rocha J.C., Rohde C., Rosenbaum-Fabian S., Rossi A., Ruiz M., Saligova J., Gutierrez-Sanchez A., Schlune A., Schulpis K., Serrano-Nieto J., Skarpalezou A., Skeath R., Slabbert A., Straczek K., Gizewska M., Terry A., Thom R., Tooke A., Tuokkola J., van Dam E., van den Hurk T.A.M., van der Ploeg E.M.C., Kerckhove K. Vande, Van Driessche M., van Wegberg A.M.J., van Wyk K., Vasconcelos C., Garcia V. Velez, Wildgoose J., Winkler T., Zolkowska J., Zuvadelli J., MacDonald A. Early feeding practices in infants with phenylketonuria across Europe. Mol. Genet. Metab. Rep. 2018;16:82–89. doi: 10.1016/j.ymgmr.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pena M.J., de Almeida M.F., van Dam E., Ahring K., Belanger-Quintana A., Dokoupil K., Gokmen-Ozel H., Lammardo A.M., MacDonald A., Robert M., Rocha J.C. Protein substitutes for phenylketonuria in Europe: access and nutritional composition. Eur. J. Clin. Nutr. 2016;70:785–789. doi: 10.1038/ejcn.2016.54. [DOI] [PubMed] [Google Scholar]
- 24.Allen J.R., Baur L.A., Waters D.L., Humphries I.R., Allen B.J., Roberts D.C., Gaskin K.J. Body protein in prepubertal children with phenylketonuria. Eur. J. Clin. Nutr. 1996;50:178–186. [PubMed] [Google Scholar]
- 25.Robertson L.V., McStravick N., Ripley S., Weetch E., Donald S., Adam S., Micciche A., Boocock S., MacDonald A. Body mass index in adult patients with diet-treated phenylketonuria. J. Hum. Nutr. Diet. 2013;26(Suppl. 1):1–6. doi: 10.1111/jhn.12054. [DOI] [PubMed] [Google Scholar]
- 26.Weber D.R., Leonard M.B., Zemel B.S. Body composition analysis in the pediatric population. Pediatr. Endocrinol. Rev. 2012;10:130–139. [PMC free article] [PubMed] [Google Scholar]
- 27.Macit E., Kizilgun M., Cakir E., Karaoglu A., Akgul E.O., Oztosun M., Aydin I., Aydin F.N., Agilli M., Turker T., Ogur R., Gulcan Kurt Y., Gul H., Cayci T., Ozkan E. Pediatric reference intervals for plasma and urine essential amino acids in a Turkish population. Turk. J. Med. Sci. 2014;44:323–329. doi: 10.3906/sag-1302-30. [DOI] [PubMed] [Google Scholar]
- 28.Giovannini M., Verduci E., Salvatici E., Fiori L., Riva E. Phenylketonuria: dietary and therapeutic challenges. J. Inherit. Metab. Dis. 2007;30:145–152. doi: 10.1007/s10545-007-0552-8. [DOI] [PubMed] [Google Scholar]