Summary
Proteins of all living cells undergo a myriad of post-translational modifications (PTMs) that are critical to multifarious life processes. In this study, we describe the first comprehensive multiple PTM-omics atlas in parallel with quantitative proteome analyses of two representative species of African trypanosomes, Trypanosoma brucei and Trypanosoma evansi. Ten PTM types with approximately 40,000 modified sites and 150 histone marks with a fine map on each protein of the two African trypanosomes were accomplished. The two biologically different trypanosomal species displayed distinct PTM-omic features, regulation pathways, and networks. Modifications in the proteins involved in the redox system were mainly upregulated in T. brucei, whereas proteins associated with motility were predominantly modified in T. evansi. The establishment of a database of multiple PTMs in the two parasites provides us with a deep insight into the biological mechanisms that underpin life processes in trypanosomes with different life cycles.
Subject Areas: Proteomics, Metabolomics
Graphical Abstract
Highlights
-
•
The first multi-proteomic profiles of T. brucei and T. evansi were generated
-
•
Histones are modified heavily and are highly conserved in trypanosomes
-
•
The two biologically different trypanosomes displayed distinct PTM-omic features
-
•
Crosstalk between different PTMs involved in critical biological processes
Proteomics; Metabolomics
Introduction
Trypanosomes are vector-borne protozoan parasites that cause substantial mortality and morbidity in mammalian hosts worldwide, and there is no vaccine available for trypanosomiasis (Buscher et al., 2017, Cayla et al., 2019). A subspecies of Trypanosoma brucei, T. b. brucei, is responsible for the domestic animal disease known as nagana and has been used as a model for various studies due to its close resemblance with the etiologic agents of human sleeping sickness, T. b. rhodesiense and T. b. gambiense. Trypanosoma evansi, the causative agent of the disease surra, is thought to be a mutant of T. brucei that has adapted to the gradual loss of kinetoplast DNA and is normally sensitive to human serum (Lai et al., 2008). However, it is now known to be capable of causing infections in humans (Uzureau et al., 2013, Van Vinh Chau et al., 2016, Vanhollebeke et al., 2006), and T. evansi-infected monkeys exhibit symptoms similar to those associated with sleeping sickness (Misra et al., 2016), further highlighting its zoonotic potential. Both T. brucei and T. evansi proliferate predominantly extracellularly within mammalian hosts as bloodstream form (BSF) parasites. Periodic switching of the variant surface glycoprotein coat and concomitant immune evasion results in the generation of waves of parasitemia and long-term survival (Mugnier et al., 2015). However, for polymorphic T. brucei, this phenomenon is accompanied by a developmental switch from a slender to a stumpy form; this switch is regulated by quorum sensing (QS) signaling pathways (Mony et al., 2014, Rojas et al., 2019). When the stumpy form parasites are ingested by tsetse flies, the parasites initiate development into the procyclic form (PCF). In contrast, T. evansi, which is normally monomorphic, is locked in the slender BSF and incapable of cycling through the insect due to dyskinetoplasty (Carnes et al., 2015, Lai et al., 2008). The mechanical transmission of T. evansi, however, enables its infection of a variety of mammalian hosts and transmission beyond Africa, where the obligatory vector of T. brucei resides (Jensen et al., 2008).
These two African trypanosomes harbor very similar genomes RNA polymerase II (RNAP II) promoters, and genes are present in polycistronic units (Berriman et al., 2005, Carnes et al., 2015, Zheng et al., 2019). Trypanosomes, therefore, rely almost exclusively on post-transcriptional mechanisms to regulate the output of gene products, highlighting the critical function of RNA-binding proteins (RBPs) (Kolev et al., 2012). Furthermore, the major differences between these two closely related parasites are ultimately caused by dynamic changes in protein homeostasis and post-translational modifications (PTMs). Epigenetic regulation, predominantly through histone modification, is considered as a secondary vehicle for the transmission of heritable messages (Figueiredo et al., 2009). The PTM status of histones and associated variants determines whether chromatin is repressed or activated in relation to transcription and, concomitantly, antigenic variation (Martinez-Calvillo et al., 2018, Muller et al., 2018). Proteomic analyses have already been performed on trypanosomes (Dejung et al., 2016, Roy et al., 2010), and advances in phosphoproteome and acetylome analyses of T. brucei have highlighted the role of PTMs in trypanosomes (Moretti et al., 2018, Nett et al., 2009a, Nett et al., 2009b, Urbaniak et al., 2013). However, our knowledge of the molecular biology of T. evansi lags way behind that of T. brucei. In addition, a global quantitative understanding of associated proteomes and PTM networks is still lacking, and the overall dynamic regulatory mechanisms that facilitate PTMs in trypanosomes remain to be elucidated.
In an effort to better understand the biology of African trypanosomes and unravel the molecular mechanisms that drive developmental differences, we report the first global analysis of multiproteomics for T. brucei and T. evansi using label-free quantitative techniques. The study also resulted in the generation of a modification-specific proteomic profile that contains the most PTM types in all organisms to date. We further analyzed acylations (acetylation, crotonylation, 2-hydroxyisobutyrylation, malonylation, and succinylation), phosphorylation, trimethylation, ubiquitination, N-glycosylation, and O-GlcNAcylation on each protein identified, including functional and pathway enrichment and interactive networks. Proteins that are species-specifically expressed and modified were deeply investigated. This study provides a proteome and PTM-ome resource for Trypanosoma parasites, which serves as a valuable model to study evolutionarily distinct eukaryotes (Cayla et al., 2019), laying a foundation for the development of new drugs.
Results
Proteomic Profiling and Targeted Verification Reveal Significant Differences of T. brucei and T. evansi
The proteins of BSF T. brucei and T. evansi were identified using sophisticated mass spectrometry-based quantitative analysis in three eligible biological replicates; the replicates of the same species cluster tightly in principal-component analysis (PCA) (Figures 1A and 1B). The density distribution of the quantitative values after logarithmic transformation showed a significant normal distribution between −10 and 10, which is in line with the expectation of the whole protein quantitative theory (Figure 1C). In this scenario, 3,957 and 3,450 proteins were identified, respectively, in T. brucei and T. evansi, and 2,123 proteins were shared between the two species (Figure 1D and Table S1). Differentially expressed proteins were further categorized into groups presented by Tb-exclusive, Te-exclusive, Common and Tb-high, and Common and Te-high (corresponding to Tb-e, Te-e, Tb-h, and Te-h in the figures and figure legends, respectively). Species-exclusive proteins were identified in three replicates of one species and in none in the other species; the latter two groups were defined by fold change >2 and p value <0.05 (Table S2). The numbers of the proteins categorized in the four groups were 555, 272, 516, and 369 (Table S2), respectively. These four groups of proteins were consistently the most abundant in the nucleus; the next most prevalent localization for these proteins was the cytoplasm (Figure 1E). More than 80% of the differentially expressed proteins that regulate cell motility such as the dynein complexes and that are associated with flagellum (synonymous to cilium) organization and movement (Langousis and Hill, 2014) were up-regulated in T. evansi, whereas proteins with oxidoreductase activity and the zinc finger-type RBPs were more prevalent in T. brucei than in T. evansi (Figure 1D and Table S3). Tb-exclusive and Tb-high expressed proteins were predominantly enriched in the citrate cycle (TCA cycle) pathway (Figures 1D and 1F and Table S3).
Figure 1.
Proteomic Profiling and Targeted Verification Reveal Significant Differences of T. brucei and T. evansi
(A) Flowchart illustrating the proteomic procedures for proteins and modified site identification. Parasite proteins were extracted (step 1) and trypsinized (step 2). One aliquot of peptides was separated by high-performance liquid chromatography (HPLC) (step 3) and analyzed by tandem mass spectrometry (MS/MS) (step 4). A separate aliquot was subjected to affinity enrichment (step 4∗) and PTM identification (steps 5∗ and 6∗). The steps marked with asterisks are for PTM analysis. Phosphopeptides were identified by immobilized metal-affinity chromatography (IMAC) (Ficarro et al., 2002), and N-glycopeptides were enriched using a hydrophilic interaction liquid chromatography (HILIC) strategy (Zhang et al., 2016). The other peptides were all enriched using appropriate monoclonal pan-antibodies. Finally, data from all omics sources were combined for bioinformatic analysis (steps 6 and 7∗). Parallel reaction monitoring was used to quantify individual unique peptides for targeted validation of the differentially expressed proteins in the two parasites (step 7).
(B) Principal-component analysis (PCA) of the proteome resulted in a clear separation of two groups, representing T. evansi and T. brucei. The three replicates of the same species clustered tightly.
(C) The quantitative ratio distributions of homologous proteins between T. brucei and T. evansi.
(D) Venn diagrams illustrating homologous proteins between T. brucei (red circle) and T. evansi (blue circle). Volcano plot shows the distributions of quantified proteins commonly identified in the two parasites. Highly expressed proteins in T. brucei and T. evansi are shown in red and blue, respectively, whereas proteins whose expressions were unchanged are shown in pink. Zinc finger proteins (ZFPs) and proteins associated with cell motility, the redox system, and tricarboxylic acid (TCA) cycles are represented by dots of different colors.
(E) Prediction of subcellular localization and classification statistics of the four protein groups.
(F) KEGG pathway-based enrichment of proteins with different expression intensities in T. brucei and T. evansi. Heatmap colors represent log2-fold changes in expression levels.
To validate the differential expression of proteins in these two parasites, parallel reaction monitoring was applied to quantify individual unique peptides. Targeted verifications were performed on 13 differentially expressed proteins, and the regulatory trends of these proteins in both parasites were consistent with the proteome results (Figure S1 and Table S2).
In-Depth Global Identification Highlights Species-Specific PTMs
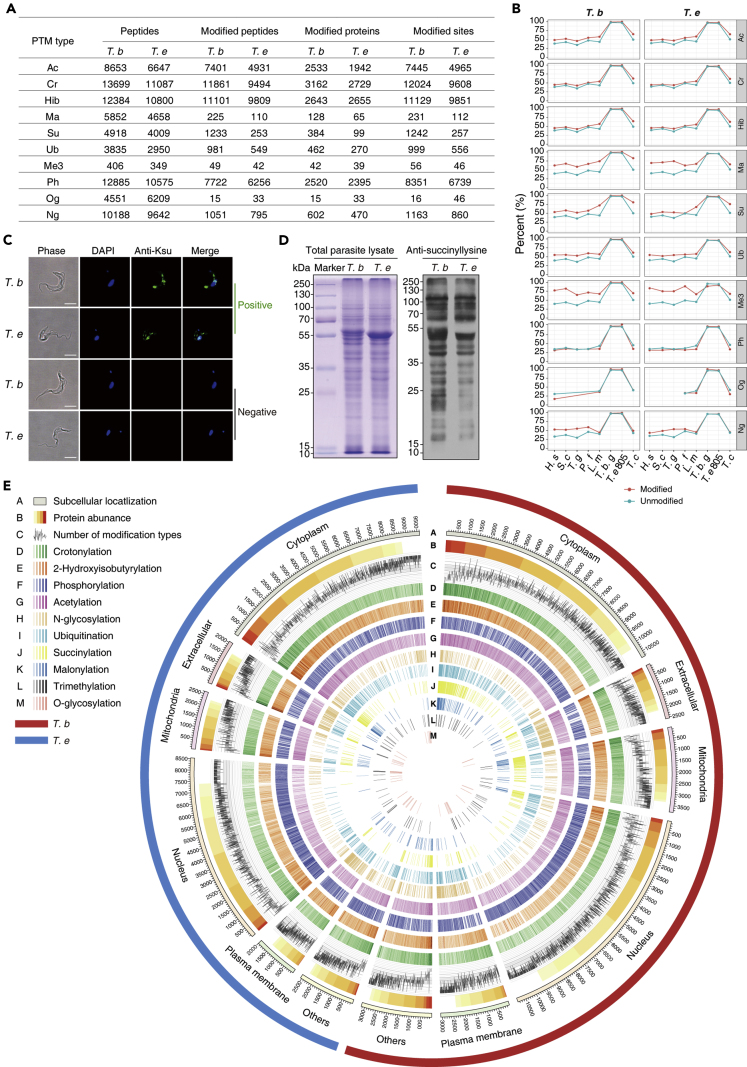
A proteomic repertoire of 10 types of PTMs was generated following PTM-specific affinity-enrichment techniques that facilitate the detection of transient and substoichiometric PTMs (Figures 1A, 2A, and S2A–S2C and Table S1 and Data S1). The replicates of the same experiments clustered tightly in PCA (Figure S2A), and the distribution of the quantitative values after logarithmic transformation was reasonable (Figures S2B and S2C). Specific masses of the modified peptides were matched to the corresponding proteins to count the modification sites. There were 42,656 and 33,040 modified sites identified in 10 PTM-omics of T. brucei and T. evansi, respectively (Figure 2A and Table S1). Respective PTM map and regulatory networks of the proteins identified from the two trypanosome species were accomplished. In general, modified sites were more conserved when aligned with their respective orthologs in species of trypanosomatid parasites, highlighting the functional significance of these sites (Figure 2B). The most significant difference in PTMs between the two species was succinylation, which predominantly occurred in T. brucei (Figures 2A, 2C and 2D). A circular proteome map was constructed to further depict the similarities and differences between the proteins and PTMs of the two parasites; the modified proteins were relatively broadly distributed in multiple cellular compartments, and the distribution of modified proteins was much prevalent across the cytoplasm (Figures 2E and S2D). There is no obvious linear relationship between the level of phosphorylation and protein abundance, and the phosphosites were not highly conserved, indicating that protein phosphorylation is more extensive than other PTM types (Figures 2B and 2E).
Figure 2.
In-Depth Global Identification Highlights Species-Specific PTMs
(A) Summary of the identified proteins and PTM sites.
(B) Analysis of the modified sites in T. brucei and T. evansi with their respective orthologs in Homo sapiens, S. cerevisiae, Toxoplasma gondii, Plasmodium falciparum, and other species of trypanosomatid parasites. Sites were deemed to be phylogenetically conserved when the amino acids of the homologous proteins were identical following alignment. Percentages of conserved modified and unmodified sites are shown in red and blue, respectively.
(C) Immunofluorescent staining of methanol-fixed T. brucei and T. evansi with the anti-succinyl lysine antibody (green). Nuclei are stained with DAPI (blue). Pan anti-succinyl lysine antibody (positive) and normal mouse IgG (negative control) were added as the primary antibody. Scale bar, 10 μm.
(D) Coomassie blue staining of 20 μg BSF trypanosome lysates showed equal loading amounts. Western blot probed with a monoclonal anti-succinyl lysine antibody.
(E) Protein abundance and PTMs in each subcellular structure. The outermost colors represent different subcellular structures, and the color gradient indicates the abundance of each protein. The number of PTM types that can occur on the proteins is represented by the line chart, whereas the numbers of modified sites of different types are illustrated by various color gradients. Ph, phosphorylation; Ac, acetylation; Cr, crotonylation; Hib, 2-hydroxyisobutyrylation; Ma, malonylation; Su, succinylation; Ub, ubiquitination; Me3, trimethylation.
Functionally, PTMs were significantly involved in pervasive biological processes including signal transduction, translation, RNA processing, transport, and protein turnover in the parasites (Figure S2E and Table S3). In both T. brucei and T. evansi, a large number of thioredoxins and the 70-kDa heat shock proteins (HSP70s) were significantly succinylated, but it was malonylated exclusively in T. brucei (Table S3). The involvement of PTMs in oxidation-reduction seems to be more critical to T. brucei (Table S3).
Furthermore, similar to the aforementioned proteomic analysis, all differentially modified proteins were classified into four groups, and the modification site numbers of these four groups were 1547, 809, 2201, and 2024, respectively (Table S2). We observed that the differentially modified proteins were predominantly involved in carbon metabolism and translation, and the non-overlap proteins for malonylation in T. brucei and T. evansi still share some significant pathways, especially in relation to carbohydrate metabolism (Figure S3A). In addition, an interactive network of all differentially modified proteins was constructed (Figure S3B). These proteins were predominantly involved in cellular processes such as translation, glycolysis, and ubiquitin-mediated protein degradation. An inosine-5′-monophosphate dehydrogenase (IMPDH1, Tb927.10.16120), which catalyzes the conversion of inosine-5′-monophosphate to xanthosine-5'-phosphate, the major pathway in purine metabolism (Bessho et al., 2013), exhibited extremely strong interactions with other proteins.
Histone Modifications Are Highly Conserved in African Trypanosomes
A large number of histone marks were observed (Figure 3A and Table S4). A total of 162 histone marks were identified in T. brucei, 135 of which were novel, whereas all the 134 marks in T. evansi were reported for the first time. Furthermore, 119 histone marks were shared in the two parasites.
Figure 3.
Histone Modifications Are Highly Conserved in African Trypanosomes
(A) PTMs of canonical histones of both T. brucei and T. evansi. Cylinders indicate the globular domain of histones. Sequences of mature histones are shown with the modified residues in bold (the numbers represent the amino acid position following removal of the initial methionine).
(B) Heatmap displaying the significance (log2 T. b/T. e ratio) of all modified sites on histones and their variants.
An extensive range of PTMs occurred on the tails of histones compared with the globular domains. In general, variant histones are less conserved than canonical histones (Croken et al., 2012). However, the modified sites of the variant histones were extensively similar between the two parasites (Figure S4). Remarkably, several hyperacetylated regions that have been postulated as being important in the establishment of euchromatins and the regulation of particular gene clusters under particular circumstances were identified. H2A.Z was predominantly regulated by hyperacetylation, with K49 in the N terminus exhibiting greater levels of acetylation in TbH2A.Z. Conversely, K164, K168, and K169 in the C terminus exhibited greater levels of acetylation in TeH2A.Z (Figure 3B and Table S4). Hyperacetylated regions at the C terminus of histone H2A and the N terminus of histone H4 were predominant in T. evansi. Furthermore, many residues underwent multiple PTMs and had similar or opposite effects on chromatin structure at different times; modification on H2BK96, H3K32, H3K61, H3K76, and H4K57 exhibited the most prominent effects in this regard.
RNA-Binding Proteins, the Regulatory Elements of Gene Expression in Trypanosomes, Undergo Extensive PTMs
The absence of RNAP II promoters in trypanosomes greatly simplifies the task of elucidating the contribution of RBPs to global gene regulation. A regulatory network of differentially expressed or modified RBPs and associated interacting proteins was constructed (Figure S5). The identified RBPs were predominantly involved in RNA processing, splicing, and ribosome biogenesis. Pyruvate kinase 1 (PYK1, TritrypDB: Tb927.10.14140), a rate-limiting enzyme involved in the glycolysis pathway, also exhibited RNA-binding activity (Lueong et al., 2016), was differentially regulated by six PTMs in the two trypanosomes. Pumilio homology domain family member 8 (PUF8, TritrypDB: Tb927.3.2470), which has been implicated in various aspects of ribosomal RNA metabolism (Droll et al., 2010), was specifically acetylated in T. brucei and closely related to RNA helicase. Furthermore, the double RNA-binding domain protein 9 (DRBD9, TritrypDB: Tb927.9.13280) with K144hib, which is predominantly present in T. evansi, appears to be highly correlated with splicing proteins.
Kinase-Phosphosite Regulatory Networks Highlighted Key Factors in Differential Phosphorylation
The sequence contexts that underwent phosphorylation in both T. brucei and T. evansi were analyzed. The flanking sequences of phosphosites exhibited different trends in the two trypanosomal species, suggesting that protein kinases act specifically (Figures 5A and 5B and Table S5). Most of the motifs in which the arginine (R) was present at the −2 or −3 position of the modified serine (S) were enriched in CAMK and AGC kinases. Notably, most CMGC kinases, chiefly CDK, MAPK, DYRK, and GSK with proline (P) at the +1 position, always had higher levels of phosphorylation in T. brucei. In T. evansi, acidic amino acids (aspartic acid [D] or glutamic acid [E]) occurred more frequently at the +1 position of phosphosites; most of the TAF1 and CMGC/CK2 kinases contained such phosphorylation motifs.
Figure 5.
Kinase-Phosphosite Regulatory Networks Highlighted Key Factors in Differential Phosphorylation
(A) Violin plot analysis comparing the phosphorylation levels and distributions of different sequence motifs in the two parasites. The vertical position of each histogram represents the relative modified level of T. brucei and T. evansi.
(B) Heatmap displaying the significance of the correlation of kinase types and motifs. The gradient color represents the degree of enrichment (log10 p value) of the motif in the corresponding kinase.
(C and D) The regulatory relationship between differentially expressed (C) and autophosphorylated (D) kinases with their substrate phosphosites. The arrows represent differentially expressed kinases, and circles represent phosphorylated sites. The size of each node reflects the degree within the network. The colors of the nodes reflect the types of regulation.
(E) Differential phosphorylated sites detected in CRK3 are represented by the three-dimensional structure of protein. The phosphosite is shown in red, and the ATP-binding pocket and active sites are marked in purple and yellow, respectively (PDB: 4GCJ).
The potential regulatory relationships between the differential expression and autophosphorylation of kinases with their substrate phosphosites were also predicted in this study; both factors were closely related to the phosphorylation level of whole proteins (Figures 5C and 5D). The elevated expression of cdc2-related kinase 6 (CRK6, TritrypDB: Tb927.11.1180) and the elevated autophosphorylation of tyrosine 34 (Y34) of CRK3 (TritrypDB: Tb927.10.4990) in T. brucei affected the phosphorylation level of other proteins, and Y34 was located at the ATP-binding sites (Figure 5E and Video S1); these were key factors leading to the differential phosphorylation of T. brucei and T. evansi.
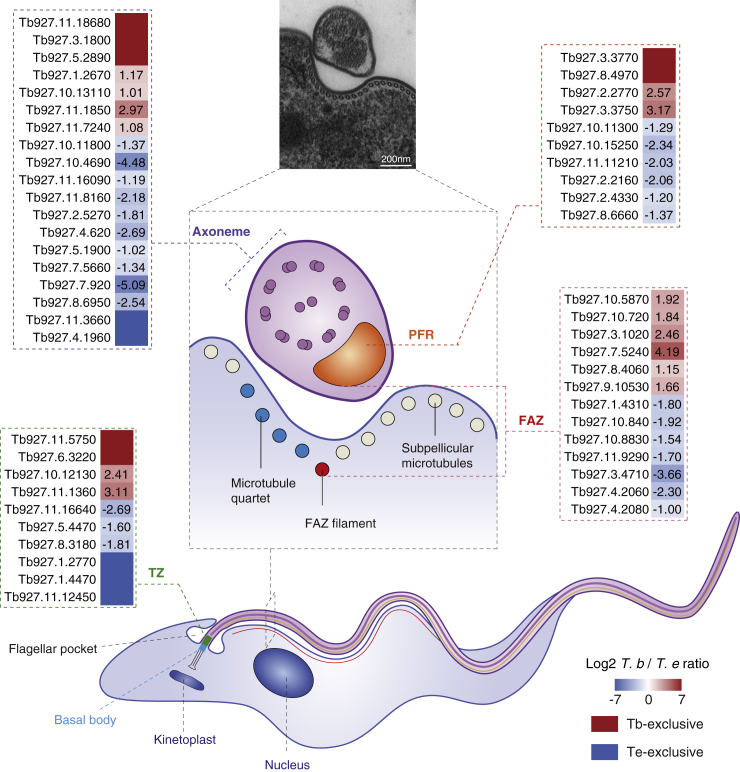
Variation in the PTM-omes of Flagellar Proteins Reflected the Differences in Parasite Motility
The trypanosome flagellum contains a canonical 9 + 2 microtubular axoneme alongside which the paraflagellar rod (PFR) runs (Bastin et al., 1998). The axoneme emanates from the basal body through the transition zone and is laterally connected to the cell membrane via the flagellum attachment zone (FAZ) (Figure 6). In each part of the flagellum, a majority of the proteins were highly or exclusively modified in T. evansi (Figure 6 and Table S6). Moreover, the differential PTMs associated with these proteins further explains the more flexible appearance of T. evansi (Bargul et al., 2016) (Tables S3 and S6). The inner-arm dynein (IAD5-1, TritrypDB: Tb927.7.920), knockdown of which causes cell motility defects, was highly expressed in T. evansi (Wei et al., 2014). K1,134 of IAD5-1 was acetylated exclusively in T. brucei and 2-hydroxyisobutyrylated in T. evansi. The flagellum attachment zone protein 2 (FAZ2, TritrypDB: Tb927.1.4310) was heavily phosphorylated, and a similar observation was reported in T. brucei (Nett et al., 2009b). However, the phosphorylation on this protein was more prevalent in T. evansi. N-glycosylated sites were mainly identified in two proteins, PFR component (PFC16, TritrypDB: Tb927.10.11300) and flagellum adhesion protein 2 (FLA2, TritrypDB: Tb927.8.4060), and the modifications occurred more frequently in T. brucei.
Figure 6.
Differences in the PTM-omes of Flagellar Proteins Associated with Parasite Motility
A simplified schematic diagram shows the architecture of the trypanosome flagellum, and the transmission electron microscopic image shows a transverse section of the flagellum. Scale bar, 200 nm. Heatmaps displaying the differential expression levels of proteins in each part of the two trypanosomes. See also Table S6.
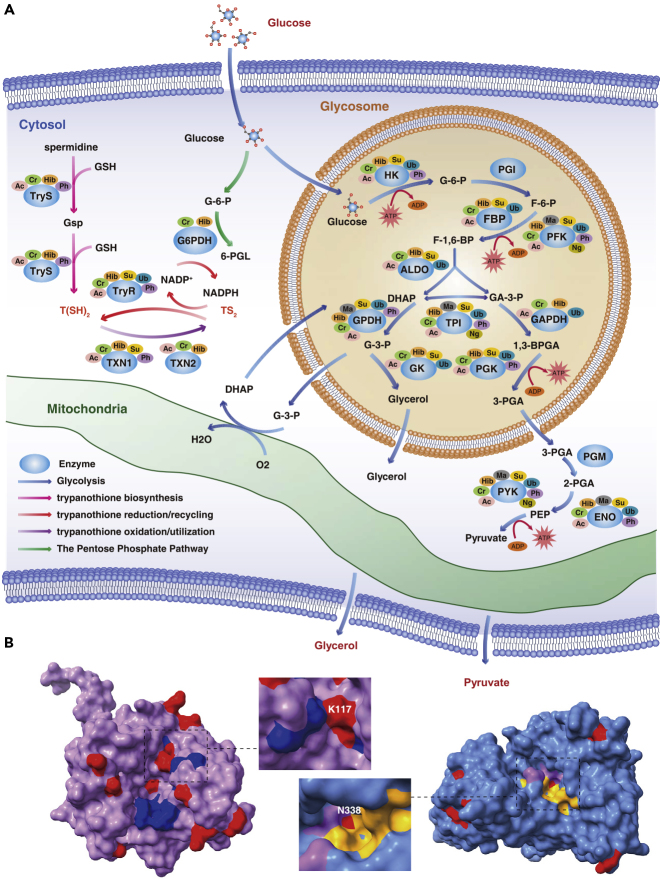
PTMs Regulated Energy Metabolism and Trypanosomatid-Specific Redox System
Eight PTMs were significantly enriched in the glycolysis pathway (Figure S3), which is the main energy metabolism pathway in BSF trypanosomes (Haanstra et al., 2016). The subcellular localization profiles of glycolytic enzymes of both T. evansi and T. brucei were similar (Moreno and Nava, 2015), and most enzymes had multiple modifications (Figure 7A). Notably, the acetylation and 2-hydroxyisobutyrylation that occurred on the three rate-limiting enzymes were highly prevalent in T. evansi (Figure S5). Furthermore, it was observed that many PTMs occurred at key residues of the enzymes. For example, the K117 of fructose-1,6-bisphosphate aldolase (ALDO), which could be modified by six types of PTMs, is precisely positioned at the active site (Figure 7B, Video S2), and N338 of enolase (ENO), which was N-glycosylated, is located near the substrate-binding pocket and metal-binding sites (Figure 7B, Video S3).
Figure 7.
PTMs Regulate Energy Metabolism and Trypanosomatid-Specific Redox System
(A) Schematic diagram of the glycolysis pathway and trypanothione-based thiol-redox system in trypanosomes. Glycerol 3-phosphate (G3P) is reoxidized by the mitochondrial glycerol phosphate complex and returned to the glycosome for further cycling. The consumption of NADPH by trypanothione reductase results in the maintenance of trypanothione in a reduced state; the latter protein is generated by the pentose phosphate pathway. G-6-P, glucose 6-phosphate; F-6-P, fructose 6-phosphate; F-1,6-BP, fructose 1,6-bisphosphate; DHAP, dihydroxyacetone phosphate; GA-3-P, glyceraldehyde 3-phosphate; G-3-P, glycerol-3-phosphate; 1,3-BPGA, 1,3-bisphosphoglycerate; 3-PGA, 3-phosphoglycerate; 2-PGA, 2-phosphoglycerate; PEP, phosphoenolpyruvate; 6-PGL, 6-phosphogluconolactone; GSH, reduced glutathione; Gsp, mono-glutathionylspermidine; TS2, trypanothione disulfide; T(SH)2, dihydro-trypanothione; HK, hexokinase; PGI, glucose phosphate isomerase; PFK, phosphofructokinase; TPI, triosephosphate isomerase; GPDH, glycerol-3-phosphate dehydrogenase; GK, glycerol kinase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase; PYK, pyruvate kinase; G6PDH, glucose-6-phosphate dehydrogenase; TryS, trypanothione synthetase; TryR, trypanothione reductase; TXN, tryparedoxin.
(B) Differentially modified sites detected in ALDO (left panel) and ENO (right panel) are represented by the three-dimensional structure of proteins. The modified sites are shown in red, and the active sites of ALDO are marked in blue. The substrate-binding pocket and the metal-binding sites of ENO are marked in yellow and purple, respectively. Protein structures were obtained from the Protein DataBank (PDB, ALDO: 1F2J, ENO: 1OEP).
Trypanosomes, as early branching eukaryotes, have evolved in a manner that has resulted in a unique trypanothione-based thiol-redox system (Manta et al., 2013). Trypanothione reductase (TryR, TritrypDB: Tb927.10.10390), which has been considered as a target for antitrypanosomatid drugs, recycles trypanothione disulfide (TS2) back to dihydro-trypanothione (T(SH)2), and tryparedoxin (TXN) catalyzes the electron transferred from T(SH)2 to different protein targets that are normally involved in multiple functions such as cell proliferation and antioxidant defense (De Gasparo et al., 2019). We observed multiple PTMs pertaining to TryS, TryR, and TXN, and both TXN1 and TXN2 were expressed more prevalently in T. brucei than T. evansi (Figure 7 and Table S7).
Crosstalk between Different Types of PTMs Involved in Critical Biological Processes
Correlation and Functional Overlaps
Proteins and sites modified by different PTMs were compared to assess the significance of the overlaps (Figure S6A and Table S8). A large amount of overlap was observed among acetylated, crotonylated, and 2-hydroxyisobutyrylated proteins in both T. brucei and T. evansi. The succinylated sites in T. brucei had higher correlations with other lysine (Lys)-modified sites. However, in T. evansi, these sites had a more significant correlation with malonylated sites. Functionally, most of these PTMs were positively associated with carbohydrate metabolism (Figure S6B). For the disulfide isomerases, N-glycosylation was significantly enriched and negatively correlated with other PTMs.
Lysine Acylations
Lys can be modified by various PTMs, especially acylations (Sabari et al., 2017). In this study, five types of Lys-acylated proteins were quantified and the structural differences among their chains were observed. Quantitative correlations between different PTMs occurring on Lys were predicted based on the Pearson correlation coefficient (Figure S6C). In addition to succinylation, Lys malonylation was negatively correlated with other acylations.
Discussion
PTMs significantly increase the diversity and complexity of the proteome by altering protein function, localization, and protein-protein interactions. Previous research indicated that the expression and function of the surface-variant glycoproteins of T. brucei were controlled by several ubiquitylation pathways (Zoltner et al., 2015). Nett et al. used sequential strong-cation exchange and TiO2 enrichment of BSF T. brucei phosphopeptides, identified 1,204 phosphorylation sites on 491 proteins (Nett et al., 2009b), and employed anti-phosphotyrosine antibodies to identify 34 phosphotyrosine sites in PCF T. brucei (Nett et al., 2009a). Using stable isotope labeling by amino acids in cell culture, another study reported stage-specific phosphorylation changes in T. brucei and showed that differential phosphorylation is widespread between the PCF and BSF (Urbaniak et al., 2013). Recent works detected 288 Lys acetylation sites in 210 proteins of PCF and 380 sites in 285 proteins of BSF; notably, most K-ac proteins are enriched in metabolic processes, suggesting that they are essential for parasite adaptation in the hosts (Moretti et al., 2018). However, so far, these studies are not comprehensive with focus on a few PTMs, and such study on T. evansi has not been reported.
In this study, we systematically investigated and compared the features of PTMs in the BSF of T. brucei and T. evansi. We observed that succinylation occurred most differentially between the two trypanosomes (Figures 1A–1C). Many studies have shown that succinylation played an important role in the metabolic regulation, especially in the mitochondrial pathway (Yang and Gibson, 2019). The higher level of protein succinylation in T. brucei may explain the metabolic differences between T. brucei and T. evansi. The most abundant PTMs in the two trypanosome species were crotonylation, 2-hydroxyisobutyrylation, acetylation, and phosphorylation, and fewer O-GlcNAcylated and trimethylated proteins were identified (Figure 1A). The number of O-GlcNAcylated proteins in the two species is similar to that identified in the Plasmodium and Toxoplasma parasites (Aquino-Gil et al., 2018, Kupferschmid et al., 2017).
Proteins exhibiting species-specific expression and modification were revealed. Among the protein activities that were analyzed, oxidoreduction, which is functionally linked to environmental adaptation, was highly expressed and modified in T. brucei (Figure 1D and Table S7). This result correlates well with findings in Plasmodium, where antioxidant proteins were preferentially expressed in the mosquito stage of the parasite (Wang et al., 2018). Obviously, the modifications are essential in promoting the function of the antioxidant proteins in the insect vectors. In addition, the dynein complex and proteins associated with flagellar movement that supported the flexible appearance of T. evansi were predominantly expressed and modified (Figure 1D and Table S6). Furthermore, N-glycosylation occurred more frequently in flagellar proteins of T. brucei, suggesting that this PTM may be detrimental to parasitic motility. These findings might explain why T. evansi has a more flexible appearance with greater adaptability in relation to movement in restricted environments compared with T. brucei (Bargul et al., 2016, Broadhead et al., 2006, Langousis and Hill, 2014). Notably, IMPDH1, which exhibited extremely strong interactions with other proteins (Figure S3B), has been shown to be essential for the survival of T. brucei. It differs in structure from its mammalian counterpart and therefore may represent a novel anti-trypanosomal drug target (Bessho et al., 2013).
The enrichment of Tb-exclusive and Tb-high expressed (Figure 1F) and modified (Figure S3A) proteins in the citrate cycle may indicate that, after the parasitemia has reached a certain point, the parasite began to switch from slender form to stumpy form, a process that is regulated by QS signaling pathways (Mony et al., 2014); it is likely that proteins participating in the tricarboxylic acid cycle are expressed to prepare for development in the insect vector and for the maintenance of well-developed mitochondria with abundant cristae (Haanstra et al., 2016).
Although unusually divergent from other eukaryotes, trypanosomal parasites have kept a conserved feature in their histones (Picchi et al., 2017). The protein sequences of histones of Trypanosoma parasites and other eukaryotes differ greatly but are compacted in a similar chromatin structure and organization (Croken et al., 2012, Picchi et al., 2017). The histones and their PTMs were well conserved in trypanosomes, and 88.8% of the histone marks in T. evansi are also present in T. brucei (Figure 3 and Figure S4). Most modified sites on histones of T. brucei have been characterized in previous studies, such as H4K4ac, which is cell cycle regulated and mediated by histone acetyltransferase 3 in T. brucei (Siegel et al., 2008), and H4K10 is likely acetylated in all nucleosomes at transcription start sites (Kawahara et al., 2008, Siegel et al., 2009). Previous study found that deacetylation of H4K14ac and replacing H4K10ac by arginines delayed DNA replication and reduced transcription in Trypanosoma cruzi (Ramos et al., 2015), and both H4K10ac and H4K14ac were also identified in our study (Croken et al., 2012, Picchi et al., 2017). Dimethylation and trimethylation of H3K76, activities that are regulated by DOT1A and DOT1B, were observed in both parasites (Picchi et al., 2017). Hyperacetylation occurred in different histone regions of T. brucei and T. evansi, which suggested that the acetylated sites in each hyperacetylated region may always be co-regulated and that each hyperacetylated region has distinct functions depending on its position. In addition, a large number of novel histone modifications were revealed for the first time, laying the foundation for further research. Many PTMs on histones are substoichiometric, reflecting the dynamic properties of chromatin. The diversity of histone PTMs in trypanosomes reveals a dynamic role in the regulation of histone function.
The regulation of RNA metabolism is another important basis for determining cell function and fate. At the protein expression level, the higher expression of zinc finger-type RBPs in T. brucei compared with that of T. evansi is in accordance with the finding that there are a greater number of genes in T. brucei than in T. evansi (Figure 1D) (Zheng et al., 2019). Furthermore, dynamic modifications of RBPs add another layer of gene regulation. RBPs in trypanosomes execute important role in regulating mRNA abundance and translational repression in combination with precursor mRNA or a portion of mature mRNA. Although there is no significant difference in the expression level of the rate-limiting enzyme in the glycolysis pathway, a variation in PTMs in PYK1, a protein that was also an RBP, is likely to affect the expression of other proteins in various metabolic processes (Figure 4). Specific expression and modification of PUF8 in T. brucei might indicate different mechanisms of rRNA maturation in the two trypanosomal parasites (Figure 4).
Figure 4.
PTMs and Their Association with Networks of RNA-Binding Proteins (RBPs)
A map of protein-protein interaction networks for RBPs (square) and their substrate proteins (circle). The interactive proteins were connected by lines; different border colors represent the regulation types of protein expression level, and the filling colors represent the trends of PTMs that occur on this protein.
Furthermore, both CRK3 and CRK6 are essential in regulating the cell cycle and differentiation in T. brucei (Jones et al., 2014), and Y34 of CRK3 was located at the ATP-binding sites, which are the targets of most kinase inhibitor-based drugs (Figure 5). This provides support for the concept that trypanosome-specific inhibitors can be developed. Furthermore, a notable observation is that most glycolytic enzymes that regulate the main energy-providing pathway of BSF trypanosomes were modified, and some of the modified sites were in close proximity to the catalytic sites of these enzymes, suggesting that PTMs critically modulate the activity of key enzymes. Critical enzymes with “Erasers” and “Writers” function in the modification processes are targets for drug development and some inhibitors have already entered the market in the treatment of cancer and neurological diseases (Arrowsmith et al., 2012). The existing drugs for trypanosomiasis lead to side effects, and drug-resistant trypanosomes are emerging, emphasizing the need for action to discover novel effective trypanocidal drugs to address unmet pharmaceutical needs (Field et al., 2017). How to quickly discover the drugs and develop them effectively has always been a difficult point in the prevention and treatment of trypanosomiasis. We present evidence that sirtuins can be potential targets for the therapy for trypanosomiasis, as these enzymes are important factors controlling growth and differentiation during infection.
In conclusion, our study has revealed the most comprehensive PTMomics in the two African trypanosomal parasites, T. brucei and T. evansi. The discoveries of parasite-specific modifications, especially the fine locations of the modified residues in the metabolic enzymes of both T. brucei and T. evansi, lay the foundation for the development of new drugs.
Limitations of the Study
The values of PTM intensity were not normalized by protein intensity. We have compared the PTM data with or without normalization to that of the proteomic data. There was no significant correlation between the PTM level and the protein expression level before normalization. After normalization, however, the PTM values were negatively affected by the changes in protein levels, but should not be interpreted as real modification changes.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We appreciate very much the kind assistance from PTM BioLab scientists in the omic analysis. This study was supported by the Distinguished Scientist grant from Shenyang Agricultural University and Liaoning Province [8804-880416076], and CAMS Innovation Fund for Medical Sciences (CIFMS) [2019-I2M-5-042].
Author Contributions
N.Z. performed most of the experiments, analyzed the data, and wrote the first draft of the manuscript. N.J. mentored the experiments, K.Z., L.Z., D.Z., and X.S. assisted parasite purification, Y.F. and R.C. performed the immunofluorescent experiment, N.Y. and Z.C. assisted bioinformatics analysis, X.S. provided T. evansi isolates, Z.L. provided T. brucei strains, Q.C. conceived the study, analyzed the data, and finalized the manuscript.
Declaration of Interests
The authors declared no competing interests.
Published: May 22, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101074.
Data and Code Availability
All data is available in the main text or the Supplemental Information. The MS spectrometry measurement files have been deposited at the ProteomeXchange consortium (http://proteomecentral.proteomexchange.org) via PRIDE Archive (PXD016245).
Supplemental Information
References
- Aquino-Gil M.O., Kupferschmid M., Shams-Eldin H., Schmidt J., Yamakawa N., Mortuaire M., Krzewinski F., Hardiville S., Zenteno E., Rolando C. Apart from rhoptries, identification of Toxoplasma gondii's O-GlcNAcylated proteins reinforces the universality of the O-GlcNAcome. Front. Endocrinol. 2018;9:450. doi: 10.3389/fendo.2018.00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrowsmith C.H., Bountra C., Fish P.V., Lee K., Schapira M. Epigenetic protein families: a new frontier for drug discovery. Nat. Rev. Drug Discov. 2012;11:384–400. doi: 10.1038/nrd3674. [DOI] [PubMed] [Google Scholar]
- Bargul J.L., Jung J., McOdimba F.A., Omogo C.O., Adung'a V.O., Kruger T., Masiga D.K., Engstler M. Species-specific adaptations of trypanosome morphology and motility to the mammalian host. PLoS Pathog. 2016;12:e1005448. doi: 10.1371/journal.ppat.1005448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastin P., Sherwin T., Gull K. Paraflagellar rod is vital for trypanosome motility. Nature. 1998;391:548. doi: 10.1038/35300. [DOI] [PubMed] [Google Scholar]
- Berriman M., Ghedin E., Hertz-Fowler C., Blandin G., Renauld H., Bartholomeu D.C., Lennard N.J., Caler E., Hamlin N.E., Haas B. The genome of the African trypanosome Trypanosoma brucei. Science. 2005;309:416–422. doi: 10.1126/science.1112642. [DOI] [PubMed] [Google Scholar]
- Bessho T., Morii S., Kusumoto T., Shinohara T., Noda M., Uchiyama S., Shuto S., Nishimura S., Djikeng A., Duszenko M. Characterization of the novel Trypanosoma brucei inosine 5'-monophosphate dehydrogenase. Parasitology. 2013;140:735–745. doi: 10.1017/S0031182012002090. [DOI] [PubMed] [Google Scholar]
- Broadhead R., Dawe H.R., Farr H., Griffiths S., Hart S.R., Portman N., Shaw M.K., Ginger M.L., Gaskell S.J., McKean P.G. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–227. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- Buscher P., Cecchi G., Jamonneau V., Priotto G. Human African trypanosomiasis. Lancet. 2017;390:2397–2409. doi: 10.1016/S0140-6736(17)31510-6. [DOI] [PubMed] [Google Scholar]
- Carnes J., Anupama A., Balmer O., Jackson A., Lewis M., Brown R., Cestari I., Desquesnes M., Gendrin C., Hertz-Fowler C. Genome and phylogenetic analyses of Trypanosoma evansi reveal extensive similarity to T. brucei and multiple independent origins for dyskinetoplasty. PLoS Negl. Trop. Dis. 2015;9:e3404. doi: 10.1371/journal.pntd.0003404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayla M., Rojas F., Silvester E., Venter F., Matthews K.R. African trypanosomes. Parasit. Vectors. 2019;12:190. doi: 10.1186/s13071-019-3355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croken M.M., Nardelli S.C., Kim K. Chromatin modifications, epigenetics, and how protozoan parasites regulate their lives. Trends Parasitol. 2012;28:202–213. doi: 10.1016/j.pt.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gasparo R., Halgas O., Harangozo D., Kaiser M., Pai E.F., Krauth-Siegel R.L., Diederich F. Targeting a large active site: structure-based design of nanomolar inhibitors of Trypanosoma brucei trypanothione reductase. Chemistry. 2019;25:11416–11421. doi: 10.1002/chem.201901664. [DOI] [PubMed] [Google Scholar]
- Dejung M., Subota I., Bucerius F., Dindar G., Freiwald A., Engstler M., Boshart M., Butter F., Janzen C.J. Quantitative proteomics uncovers novel factors involved in developmental differentiation of Trypanosoma brucei. PLoS Pathog. 2016;12:e1005439. doi: 10.1371/journal.ppat.1005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droll D., Archer S., Fenn K., Delhi P., Matthews K., Clayton C. The trypanosome Pumilio-domain protein PUF7 associates with a nuclear cyclophilin and is involved in ribosomal RNA maturation. FEBS Lett. 2010;584:1156–1162. doi: 10.1016/j.febslet.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficarro S.B., McCleland M.L., Stukenberg P.T., Burke D.J., Ross M.M., Shabanowitz J., Hunt D.F., White F.M. Phosphoproteome analysis by mass spectrometry and its application to Saccharomyces cerevisiae. Nat. Biotechnol. 2002;20:301–305. doi: 10.1038/nbt0302-301. [DOI] [PubMed] [Google Scholar]
- Field M.C., Horn D., Fairlamb A.H., Ferguson M.A., Gray D.W., Read K.D., De Rycker M., Torrie L.S., Wyatt P.G., Wyllie S. Anti-trypanosomatid drug discovery: an ongoing challenge and a continuing need. Nat. Rev. Microbiol. 2017;15:217–231. doi: 10.1038/nrmicro.2016.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueiredo L.M., Cross G.A., Janzen C.J. Epigenetic regulation in African trypanosomes: a new kid on the block. Nat. Rev. Microbiol. 2009;7:504–513. doi: 10.1038/nrmicro2149. [DOI] [PubMed] [Google Scholar]
- Haanstra J.R., Gonzalez-Marcano E.B., Gualdron-Lopez M., Michels P.A. Biogenesis, maintenance and dynamics of glycosomes in trypanosomatid parasites. Biochim. Biophys. Acta. 2016;1863:1038–1048. doi: 10.1016/j.bbamcr.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Jensen R.E., Simpson L., Englund P.T. What happens when Trypanosoma brucei leaves Africa. Trends Parasitol. 2008;24:428–431. doi: 10.1016/j.pt.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones N.G., Thomas E.B., Brown E., Dickens N.J., Hammarton T.C., Mottram J.C. Regulators of Trypanosoma brucei cell cycle progression and differentiation identified using a kinome-wide RNAi screen. PLoS Pathog. 2014;10:e1003886. doi: 10.1371/journal.ppat.1003886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara T., Siegel T.N., Ingram A.K., Alsford S., Cross G.A., Horn D. Two essential MYST-family proteins display distinct roles in histone H4K10 acetylation and telomeric silencing in trypanosomes. Mol. Microbiol. 2008;69:1054–1068. doi: 10.1111/j.1365-2958.2008.06346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolev N.G., Ramey-Butler K., Cross G.A., Ullu E., Tschudi C. Developmental progression to infectivity in Trypanosoma brucei triggered by an RNA-binding protein. Science. 2012;338:1352–1353. doi: 10.1126/science.1229641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmid M., Aquino-Gil M.O., Shams-Eldin H., Schmidt J., Yamakawa N., Krzewinski F., Schwarz R.T., Lefebvre T. Identification of O-GlcNAcylated proteins in Plasmodium falciparum. Malar. J. 2017;16:485. doi: 10.1186/s12936-017-2131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai D.H., Hashimi H., Lun Z.R., Ayala F.J., Lukes J. Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansi are petite mutants of T. brucei. Proc. Natl. Acad. Sci. U.S.A. 2008;105:1999–2004. doi: 10.1073/pnas.0711799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langousis G., Hill K.L. Motility and more: the flagellum of Trypanosoma brucei. Nat. Rev. Microbiol. 2014;12:505–518. doi: 10.1038/nrmicro3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueong S., Merce C., Fischer B., Hoheisel J.D., Erben E.D. Gene expression regulatory networks in Trypanosoma brucei: insights into the role of the mRNA-binding proteome. Mol. Microbiol. 2016;100:457–471. doi: 10.1111/mmi.13328. [DOI] [PubMed] [Google Scholar]
- Manta B., Comini M., Medeiros A., Hugo M., Trujillo M., Radi R. Trypanothione: a unique bis-glutathionyl derivative in trypanosomatids. Biochim. Biophys. Acta. 2013;1830:3199–3216. doi: 10.1016/j.bbagen.2013.01.013. [DOI] [PubMed] [Google Scholar]
- Martinez-Calvillo S., Romero-Meza G., Vizuet-de-Rueda J.C., Florencio-Martinez L.E., Manning-Cela R., Nepomuceno-Mejia T. Epigenetic regulation of transcription in trypanosomatid protozoa. Curr. Genomics. 2018;19:140–149. doi: 10.2174/1389202918666170911163517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra K.K., Roy S., Choudhury A. Biology of Trypanosoma (Trypanozoon) evansi in experimental heterologous mammalian hosts. J. Parasit. Dis. 2016;40:1047–1061. doi: 10.1007/s12639-014-0633-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mony B.M., MacGregor P., Ivens A., Rojas F., Cowton A., Young J., Horn D., Matthews K. Genome-wide dissection of the quorum sensing signalling pathway in Trypanosoma brucei. Nature. 2014;505:681–685. doi: 10.1038/nature12864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S.A., Nava M. Trypanosoma evansi is alike to Trypanosoma brucei brucei in the subcellular localisation of glycolytic enzymes. Mem. Inst. Oswaldo. Cruz. 2015;110:468–475. doi: 10.1590/0074-02760150024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti N.S., Cestari I., Anupama A., Stuart K., Schenkman S. Comparative proteomic analysis of lysine acetylation in trypanosomes. J. Proteome Res. 2018;17:374–385. doi: 10.1021/acs.jproteome.7b00603. [DOI] [PubMed] [Google Scholar]
- Mugnier M.R., Cross G.A., Papavasiliou F.N. The in vivo dynamics of antigenic variation in Trypanosoma brucei. Science. 2015;347:1470–1473. doi: 10.1126/science.aaa4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller L.S.M., Cosentino R.O., Forstner K.U., Guizetti J., Wedel C., Kaplan N., Janzen C.J., Arampatzi P., Vogel J., Steinbiss S. Genome organization and DNA accessibility control antigenic variation in trypanosomes. Nature. 2018;563:121–125. doi: 10.1038/s41586-018-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett I.R., Davidson L., Lamont D., Ferguson M.A. Identification and specific localization of tyrosine-phosphorylated proteins in Trypanosoma brucei. Eukaryot. Cell. 2009;8:617–626. doi: 10.1128/EC.00366-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett I.R., Martin D.M., Miranda-Saavedra D., Lamont D., Barber J.D., Mehlert A., Ferguson M.A. The phosphoproteome of bloodstream form Trypanosoma brucei, causative agent of African sleeping sickness. Mol. Cell. Proteomics. 2009;8:1527–1538. doi: 10.1074/mcp.M800556-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picchi G.F., Zulkievicz V., Krieger M.A., Zanchin N.T., Goldenberg S., de Godoy L.M. Post-translational modifications of Trypanosoma cruzi canonical and variant histones. J. Proteome Res. 2017;16:1167–1179. doi: 10.1021/acs.jproteome.6b00655. [DOI] [PubMed] [Google Scholar]
- Ramos T.C., Nunes V.S., Nardelli S.C., dos Santos Pascoalino B., Moretti N.S., Rocha A.A., da Silva Augusto L., Schenkman S. Expression of non-acetylatable lysines 10 and 14 of histone H4 impairs transcription and replication in Trypanosoma cruzi. Mol. Biochem. Parasitol. 2015;204:1–10. doi: 10.1016/j.molbiopara.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Rojas F., Silvester E., Young J., Milne R., Tettey M., Houston D.R., Walkinshaw M.D., Perez-Pi I., Auer M., Denton H. Oligopeptide signaling through TbGPR89 drives trypanosome quorum sensing. Cell. 2019;176:306–317.e16. doi: 10.1016/j.cell.2018.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy N., Nageshan R.K., Pallavi R., Chakravarthy H., Chandran S., Kumar R., Gupta A.K., Singh R.K., Yadav S.C., Tatu U. Proteomics of Trypanosoma evansi infection in rodents. PLoS One. 2010;5:e9796. doi: 10.1371/journal.pone.0009796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabari B.R., Zhang D., Allis C.D., Zhao Y. Metabolic regulation of gene expression through histone acylations. Nat. Rev. Mol. Cell Biol. 2017;18:90–101. doi: 10.1038/nrm.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel T.N., Hekstra D.R., Kemp L.E., Figueiredo L.M., Lowell J.E., Fenyo D., Wang X., Dewell S., Cross G.A. Four histone variants mark the boundaries of polycistronic transcription units in Trypanosoma brucei. Genes Dev. 2009;23:1063–1076. doi: 10.1101/gad.1790409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel T.N., Kawahara T., Degrasse J.A., Janzen C.J., Horn D., Cross G.A. Acetylation of histone H4K4 is cell cycle regulated and mediated by HAT3 in Trypanosoma brucei. Mol. Microbiol. 2008;67:762–771. doi: 10.1111/j.1365-2958.2007.06079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbaniak M.D., Martin D.M., Ferguson M.A. Global quantitative SILAC phosphoproteomics reveals differential phosphorylation is widespread between the procyclic and bloodstream form lifecycle stages of Trypanosoma brucei. J. Proteome Res. 2013;12:2233–2244. doi: 10.1021/pr400086y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzureau P., Uzureau S., Lecordier L., Fontaine F., Tebabi P., Homble F., Grelard A., Zhendre V., Nolan D.P., Lins L. Mechanism of Trypanosoma brucei gambiense resistance to human serum. Nature. 2013;501:430–434. doi: 10.1038/nature12516. [DOI] [PubMed] [Google Scholar]
- Van Vinh Chau N., Buu Chau L., Desquesnes M., Herder S., Phu Huong Lan N., Campbell J.I., Van Cuong N., Yimming B., Chalermwong P., Jittapalapong S. A clinical and epidemiological investigation of the first reported human infection with the zoonotic parasite Trypanosoma evansi in Southeast Asia. Clin. Infect. Dis. 2016;62:1002–1008. doi: 10.1093/cid/ciw052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhollebeke B., Truc P., Poelvoorde P., Pays A., Joshi P.P., Katti R., Jannin J.G., Pays E. Human Trypanosoma evansi infection linked to a lack of apolipoprotein L-I. N. Engl. J. Med. 2006;355:2752–2756. doi: 10.1056/NEJMoa063265. [DOI] [PubMed] [Google Scholar]
- Wang W., Liu F., Jiang N., Lu H., Yang N., Feng Y., Sang X., Cao Y., Chen Q. Plasmodium TatD-like DNase antibodies blocked parasite development in the mosquito gut. Front. Microbiol. 2018;9:1023. doi: 10.3389/fmicb.2018.01023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y., Hu H., Lun Z.R., Li Z. Centrin3 in trypanosomes maintains the stability of a flagellar inner-arm dynein for cell motility. Nat. Commun. 2014;5:4060. doi: 10.1038/ncomms5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Gibson G.E. Succinylation links metabolism to protein functions. Neurochem. Res. 2019;44:2346–2359. doi: 10.1007/s11064-019-02780-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang X., Cui D., Zhu J. Proteomic and N-glycoproteomic quantification reveal aberrant changes in the human saliva of oral ulcer patients. Proteomics. 2016;16:3173–3182. doi: 10.1002/pmic.201600127. [DOI] [PubMed] [Google Scholar]
- Zheng L., Jiang N., Sang X., Zhang N., Zhang K., Chen H., Yang N., Feng Y., Chen R., Suo X. In-depth analysis of the genome of Trypanosoma evansi, an etiologic agent of surra. Sci. China Life Sci. 2019;62:406–419. doi: 10.1007/s11427-018-9473-8. [DOI] [PubMed] [Google Scholar]
- Zoltner M., Leung K.F., Alsford S., Horn D., Field M.C. Modulation of the surface proteome through multiple ubiquitylation pathways in African trypanosomes. PLoS Pathog. 2015;11:e1005291. doi: 10.1371/journal.ppat.1005236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available in the main text or the Supplemental Information. The MS spectrometry measurement files have been deposited at the ProteomeXchange consortium (http://proteomecentral.proteomexchange.org) via PRIDE Archive (PXD016245).