Abstract
Background:
Computed tomography (CT) is used worldwide; however, recent studies suggest that CT radiation exposure during childhood may be a risk factor for cancer, although the data are inconsistent.
Methods:
A comprehensive search of electronic databases including PubMed, SpringerLink, Embase, Cochrane Library, Elsevier/ScienceDirect, Medline, Orbis, and Web of Science databases from January 1990 to November 2018 for observational epidemiologic studies reporting associations between radiation exposure from CT in childhood and the subsequent risk of cancer was conducted. A linear model was used to explore the dose–response relationship.
Results:
Seven studies with 1180 987 children enrolled were included. The risk of later cancer was 1.32-fold higher for children exposed to CT than those without exposure. Compared to those not exposed to pediatric CT, the relative risk (RRs) were larger for the higher doses but with wider CIs (RR for 5-10 mGy: 0.90, 95% CI: 0.69-1.12; RR for 10-15 mGy: 1.02, 95% CI: 0.86-1.18; RR for >15 mGy: 1.13, 95% CI: 0.97-1.30), the leukemia risk was higher in exposed children (RR: 1.23, 95% CI: 1.10-1.36), and brain cancer risk was higher in exposed children (RR: 1.54, 95% CI: 0.84-2.45).
Conclusions:
Our analysis suggested that radiation exposure from CT during childhood is associated with a subsequently elevated risk of cancer. However, caution is needed when interpreting these results because of the heterogeneity among the studies. The findings should be confirmed in further studies with longer follow-up periods.
Keywords: computed tomography, child, cancer, risk, radiation
Introduction
Computed tomography (CT) has become increasingly used worldwide since its introduction in the 1970s.1 Currently, the number of annual CT scans performed in the United States is 10-fold higher than in the early 1990s, numbering over 80 million in 2005.2 Computed tomography enables early diagnosis of cardiovascular and chest diseases and optimal management of injuries, surgeries, and cancers.3 Computed tomography utilization has increased over the last 2 decades. In 2011, 85 million CT examinations were performed in the USA, of which 5% to 11% were performed in children.4 Moreover, among children under 5 years of age, the rate of CT use doubled from 11/1000 in 1996 to 20/1000 between 2005 and 2007, while for children aged 5 to 14 years, the rate of CT use almost tripled, from 10.5/1000 in 1996 to 27.0/1000 between 2005 and 2007.4 The head was the most commonly imaged region in these pediatric CT examinations, followed by the chest region. However, the risk of radiation-related cancer is not negligible.5 In 2001, Brenner et al showed that the number of pediatric CT scans was quantitatively associated with the lifetime cancer risk6; the story immediately hit the front page of USA Today. The public perception of pediatric CT became negative, and some parents even refused to allow their children to undergo CT.7
An appropriate balance between the use of CT and child protection is required. Many epidemiological studies have measured radiation exposure caused by childhood CT and the subsequent cancer risks8-11; for example, Pearce et al reported a positive association between radiation dose from CT scans and leukemia (excess relative risk [ERR] per mGy: 0.036, 95% CI: 0.005-0.120) and brain tumors (ERR: 0.023, 95% CI: 0.010-0.049).12 It remains unclear whether children with cancer are more likely to have had a prior CT scan, and whether the risk is increased by subsequent CT scans. It is also not clear whether pediatric CT increases the cancer risk to a level greater than that of nonexposed children. Epidemiological studies disagree as some reported an increased cancer risk10 while others did not.13 The discrepancies may be attributable to among-study differences in CT dose, gender, age at first CT, scan frequency, and follow-up duration. Also, radiation-associated cancer risk may vary by cancer type. Therefore, we performed a systematic review and meta-analysis and calculated the pooled cancer RR by pediatric CT status. We also performed subgroup analyses by CT dose, years to cancer development, age at first CT exposure, gender, and CT frequency. We additionally performed a dose–response analysis.
Methods
We evaluated children aged < 18 years who did and did not undergo CT in terms of radiation dose, years to development of cancer, age at first CT, gender, and CT frequency. We retrieved all papers published from January 1990 to November 2018. Cancer incidence (the RR) was compared between children who did and did not undergo CT. Doses were divided into < 30, 30 to 50, and > 50 mGy. Years to cancer development were divided into 2, 5, and 10 years. Age at first CT was divided into 0 to 5, 6 to 15, and >15 years. We considered the doses absorbed into red bone marrow (RBM, mGy)14 and combined the doses for all organ types, as shown in Table 1.
Table 1.
Characteristics of Included Retrospective Studies.
| Study | Data source | Total participants | Duration | Dose | Relative risk of “cancer” ± RT |
|---|---|---|---|---|---|
| Meulepas et al14 | Dutch Pediatric CT Study | 168 394 | 1979-2014 | Mean cumulative RMB = 9.5 mGy; brain dose = 38.5 mGy | RR: 1.47; 95% CI: 1.34-1.61 Brain cancer—RR: 2.05; 95% CI: 1.48-2.83 Leukemia—RR: 1.39; 95% CI: 1.13-1.70 |
| Gonzalez et al13 | National Health Service Central Register | 178 601 | 1980-2008 | Mean cumulative RMB = 12 mGy; HL = 8 mGy | RR: 0.92; 95% CI: 0.38-2.22 |
| Nordenskjold et al15 | Karolinska University Hospital | 26 370 | 1973-1992 | Brain: 7.3-25.7 mGy | RR: 2.28; 95% CI: 1.56-3.33 |
| Krille et al16 | German Childhood Cancer Registry | 44 584 | 1966-2008 | NA | RR: 1.82; 95% CI: 1.29-2.50 Brain cancer—RR: 1.35; 95% CI: 0.54-2.78 Leukemia—RR: 1.72; 95% CI: 0.89-3.01 |
| Journy et al17 | One of 23 radiology departments | 58 620 | 2000-2010 | NA | RR: 1.05; 95% CI: 0.95-1.09 Brain cancer—RR: 0.9; 95% CI: 0.70-1.16 Leukemia—RR: 1.12; 95% CI: 0.84-1.52 |
| Huang et al18 | National Health Insurance Research database | 24 418 | NA | NA | RR: 1.29; 95% CI: 0.90-1.85 Brain cancer—RR: 2.67; 95% CI: 0.75-9.45 Leukemia—RR: 1.33; 95% CI: 0.14-12.8 |
| Mathews et al19 | Electronic Medicare Records | 680 000 | Average 9.5 years | RR: 1.24; 95% CI: 1.20-1.29 Brain cancer—RR: 2.13; 95% CI: 1.88-2.41 Leukemia—RR: 1.19; 95% CI: 1.03-1.37 |
Abbreviations: CT, computed tomography; HL, Hodgkin Lymphoma; RMB, red bone marrow dose; RR, relative risk; RT, radiotherapy. Bold values indicates p<0.01.
Study Selection
Inclusion Criteria
We evaluated studies meeting the following criteria: (1) evaluation of cancer incidence according to the degree of exposure to any form of CT (examination, diagnosis, or treatment) performed on subjects aged <18 years (based on the United Nations Convention on the Rights of the Child,20 ie, infants [1-2 years], toddlers [2-5 years], school-aged children [6-12 years], and adolescents/teenagers [13-18 years]); (2) the availability of effect estimates as RRs (the risk of an event in the experimental group relative to that the control group), hazard ratios (HRs; the ratio between the chance of an event occurring in the treatment arm and occurring in the control arm), or odds ratios (ORs; the probability of an event occurring in the experimental group relative to that in the control group) with 95% CIs that were either already calculated or calculable using published data21; (3) prospective or retrospective cohort, or case–control, studies; (4) utility of data in terms of dose–response analysis; and (5) English-language studies.
Exclusion Criteria
The exclusion criteria were (1) case reports, letters, editorial comments, conference abstracts, reviews lacking RRs, HRs, or ORs, and retracted reports; and (2) non-English-language studies. Our meta-analysis was conducted with reference to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; http://www.prisma-statement.org/).22
Search Strategy and Data Collection
Searching proceeded in 2 steps. The search string was (“tumour”[All Fields] OR “neoplasms”[MeSH Terms] OR “neoplasms” [All Fields] OR “tumor”[All Fields]) AND (“child”[MeSH Terms] OR “child”[All Fields]) AND (“tomography, X-ray computed”[MeSH Terms] OR (“tomography”[All Fields] AND “X-ray” [All Fields] AND “computed”[All Fields]) OR “X-ray computed tomography” [All Fields] OR (“computed”[All Fields] AND “tomography”[All Fields]) OR “computed tomography” [All Fields]) AND (“radionuclide imaging” [MeSH Terms] OR (“radionuclide”[All Fields] AND “imaging” [All Fields]) OR “radionuclide imaging”[All Fields] OR “scan”[All Fields]). Two team members independently interrogated the PubMed, SpringerLink, Embase, Cochrane Library, Elsevier/ScienceDirect, Medline, Orbis, and Web of Science databases from January 1990 to November 2018. Reference lists were also checked and citations retrieved if necessary. The same team evaluated the methodological strengths and weaknesses of each paper by reference to the Cochrane Handbook for Systematic Reviews of Interventions (http://handbook.cochrane.org). During quality assessment, we explored whether the measurements were accurate, and whether statistical evaluation was rigorous. We recorded the first author’s name, year of publication, study region, follow-up duration, number of participants, baseline age, cumulative, mean or estimated RBM radiation dose associated with CT, adjusted and unadjusted RRs (95% CI; or HRs) of cancer, and confounders. Adjusted RRs were preferred; unadjusted RRs or HRs were used if adjusted RRs were not available. We considered ORs and HRs to be proxies for RR, given the low incidences of cancer. Study eligibility was decided by consensus. We used snowball approach to review all the references (backward search) and the articles that cited the included papers (forward search).23 If the required information was lacking, the authors were contacted by email. Ethical approval was not required because the data were collected from published studies.
Statistical Analysis
STATA software (version 12.0) was used to perform all meta-analyses. Heterogeneity and inconsistency were assessed using the I2 statistic. Generally, an I2 ≥50% was considered to indicate high-level heterogeneity, and a random effects model was used for meta-analysis. An I2 < 50% was considered to reflect low-level heterogeneity, and a fixed effects model was used for analysis.24 The results are presented as forest plots; the publication bias toward positive results was analyzed using funnel plots and the Begg test. A 2-tailed P value <.05 was considered to reflect statistical significance. Subgroup analyses were stratified by dose, years to cancer development, age, gender, and frequency. However, if the dose range was not given, the maximum dose was used. We employed the 2-stage, linear dose–response model of STATA (version 12.0) to pool the RRs of CT exposure.
Results
Literature Search and Characteristics of Included Studies
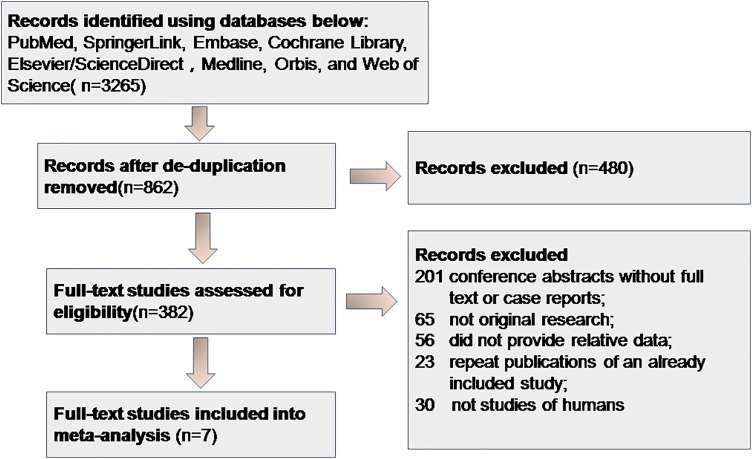
We initially retrieved 3265 records. After excluding duplications, 862 remained, of which 480 were removed after reviewing the titles or abstracts. Of the remaining 382 records, 7 full-text studies met all of the inclusion criteria.13,14,15-19 Of the studies excluded from the 382 records, 201 were conference abstracts lacking full text, or case reports, 65 did not report original research, 51 did not provide comparative data, 25 were repeat publications, and 30 were nonhuman studies (Figure 1). All 7 included studies were retrospective cohort studies. The extracted information is listed in Table 1. The 7 studies were performed in varied regions, with 1180 987 children enrolled from 1973 to 2014. Five studies were performed in Europe, and 1 in Asia and 1 in Australia. The CT radiation doses were calculated as mean RBM (red bone marrow). Eleven cancer types were reported: head-and-neck, colon, skin, breast, testis, kidney, central nervous system, thyroid, and pelvic cancer, and leukemia and Hodgkin lymphoma. Most cancer types (except leukemia and brain cancer) were not subjected to subgroup analysis because they were reported in only 1 or 2 studies. All studies used medical records or national health system registry data to confirm CT histories and cancer diagnoses. In terms of quality assessment, we explored study design, conduct, and analysis. All studies clearly described the participants, source populations, and cancer diagnoses. However, the studies exhibited various limitations that may reduce the representativeness and validity of our review. First, the age at first CT varied. Also, selection bias was in play; different studies employed distinct databases that may not have controlled for child socioeconomic status, which may have introduced heterogeneity. However, despite these limitations, the information provided by all studies was robust and reliable.
Figure 1.
Flowchart of study identification and selection process. Initially retrieved 3265 records. After excluding duplications, 862 remained, of which 480 were removed after reviewing the titles or abstracts. Of the remaining 382 records, 7 full-text studies met all of the inclusion criteria.
Pediatric CT Radiation Exposure, Radiation Dose, and Subsequent Cancer Risk
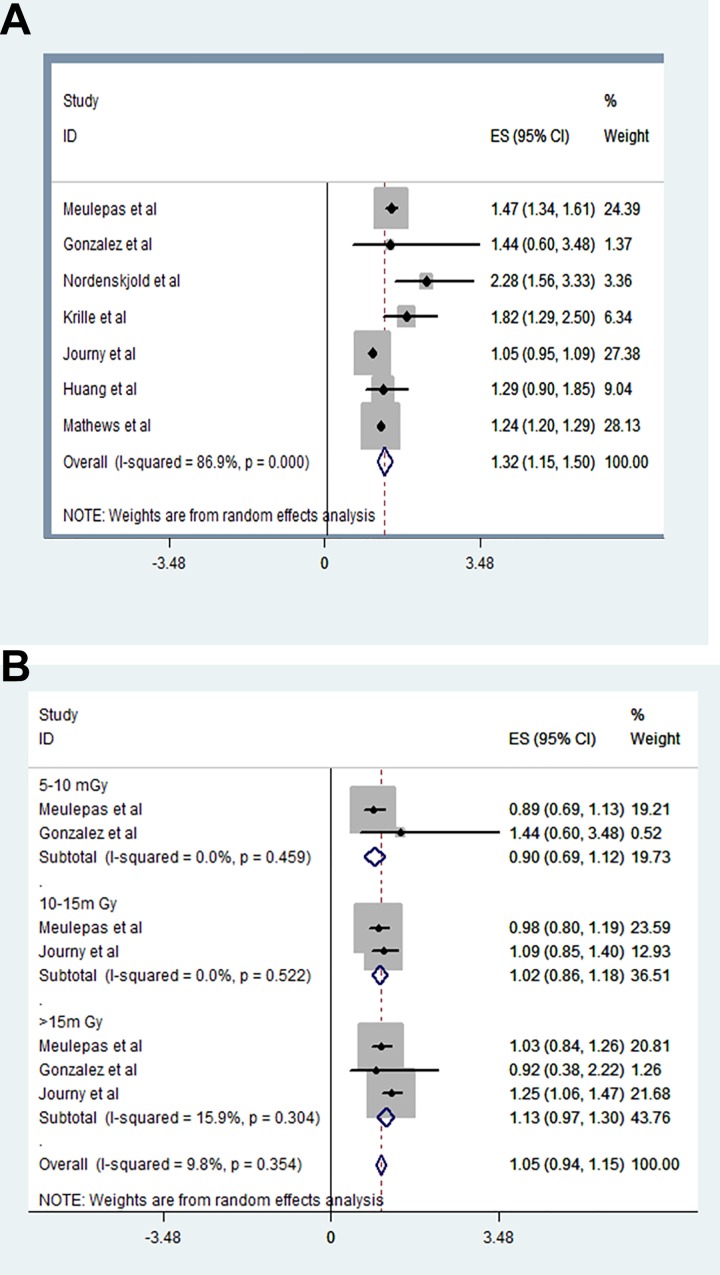
We extracted the RRs of 7 studies with 1180 987 children and used a random effects model in our meta-analysis (I2 = 86.9%, P = .000). The risk of later cancer was 1.32-fold greater in children who underwent CT compared to those who did not (RR: 1.32, 95% CI: 1.15-1.50; Figure 2A).
Figure 2.
Relationship between radiation exposure from CT scanning, radiation dose, and subsequent cancer risk. A, The risk of later cancer was 1.32-fold greater in children who underwent CT compared to those who did not (RR: 1.32, 95% CI: 1.15-1.50). B, The RRs were larger for the higher doses but with wider CIs (RR for 5-10 mGy: 0.90, 95% CI: 0.69-1.12; RR for 10-15 mGy: 1.02, 95% CI: 0.86-1.18; RR for >15 mGy: 1.13, 95% CI: 0.97-1.30). CT indicates computed tomography; RR, relative risk.
To explore CT dose-dependency, we extracted the RRs of 3 studies delivering doses 5 to 10 mGy, 10 to 15 mGy, and >15 mGy and analyzed them using a random effects model (I2 = 89.8%, P = .354). The RRs were larger for the higher doses but with wider CIs (RR for 5-10 mGy: 0.90, 95% CI: 0.69-1.12; RR for 10-15 mGy: 1.02, 95% CI: 0.86-1.18; RR for >15 mGy: 1.13, 95% CI: 0.97-1.30; Figure 2B).
Radiation-Associated Cancer Type, Elapsed Time, Age at the First Exposure, and Subsequent Risk of Various Cancers
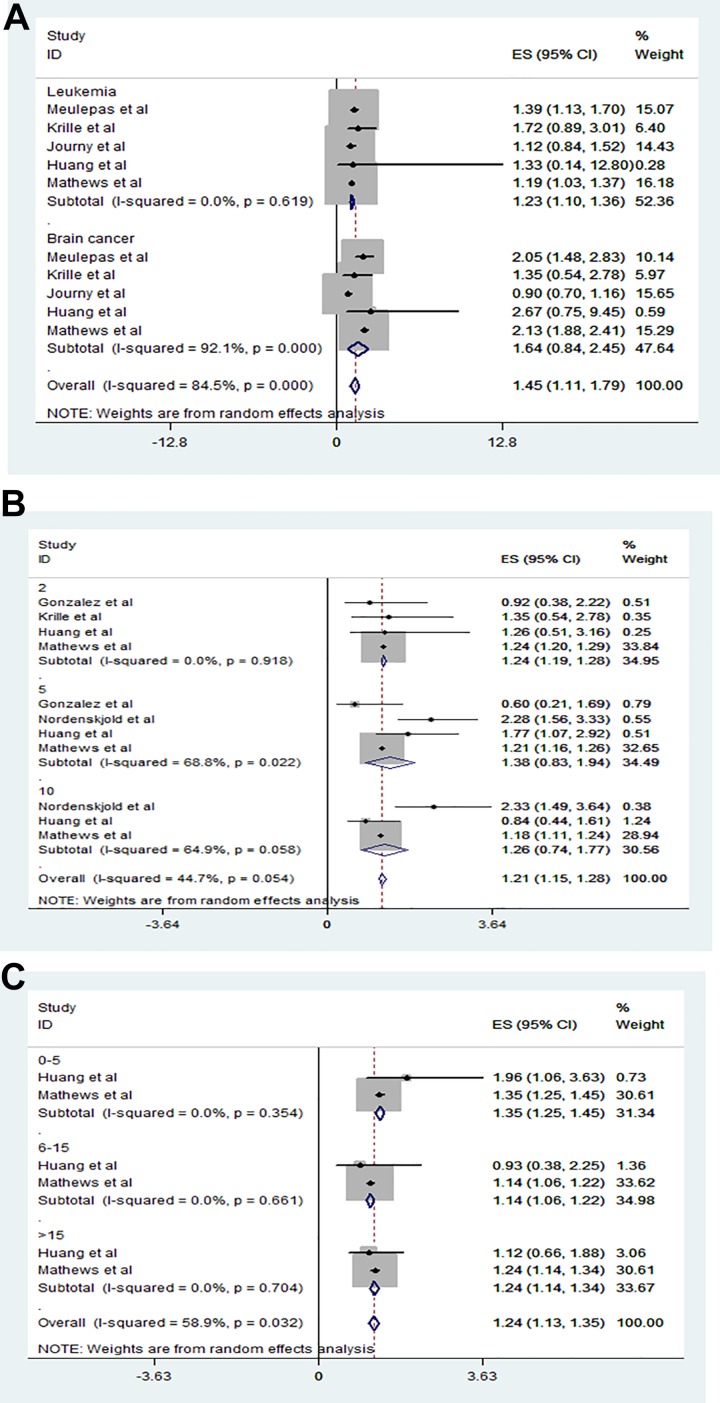
To explore whether CT radiation increased the risk of later cancers, we extracted the RRs of leukemia and brain cancer from 5 studies and analyzed them using a random effects model (I2 = 84.5%, P = .000). Exposure to CT radiation did significantly increase the risk of later leukemia (RR: 1.23, 95% CI: 1.10-1.36; Figure 3A), and the risk of brain cancer was 1.54-fold higher in exposed than nonexposed children (RR: 1.54, 95% CI: 0.84-2.45; Figure 3A).
Figure 3.
Relationship between radiation-associated cancer type, elapse time, age at the first exposure from CT scanning and subsequent cancer risk. A, Exposure to CT radiation did significantly increase the risk of later leukemia (RR: 1.23, 95% CI: 1.10-1.36). Exposure to CT radiation did significantly increase the risk of later brain cancer (RR: 1.54, 95% CI: 0.84-2.45). B, Those exposed to CT were at a 1.24-fold higher risk of cancer by 2 years after CT (RR: 1.24, 95% CI: 1.19-1.28), at a 1.38-fold higher risk after 5 years (RR: 1.38, 95% CI: 0.83-1.94), and at a 1.26-fold risk after 10 years (RR: 1.26, 95% CI: 0.74-1.77) than nonexposed children. C, Compared to those not exposed to pediatric CT, the cancer risk was 1.35-fold greater for children exposed to CT when 0 to 5 years of age (RR: 1.35, 95% CI: 1.25-1.45), 1.14-fold for children 6 to 15 years of age (RR: 1.14, 95% CI: 1.06-1.22), and 1.24-fold for children >15 years of age (RR: 1.14, 95% CI: 1.14-1.34). CT indicates computed tomography; RR, relative risk.
To explore the time elapsed between pediatric CT and subsequent cancer, we extracted the RRs of 3 studies with elapsed times of 2, 5, and 10 years and analyzed them using a random effects model (I2 = 44.7%, P = .054). Those exposed to CT were at a 1.24-fold higher risk of cancer by 2 years after CT (RR: 1.24, 95% CI: 1.19-1.28), at a 1.38-fold higher risk after 5 years (RR: 1.38, 95% CI: 0.83-1.94), and at a 1.26-fold risk after 10 years (RR: 1.26, 95% CI: 0.74-1.77) than nonexposed children (Figure 3B).
To explore if age at first CT affected the later risk of cancer, we extracted the RRs of 2 studies on children of different ages (0-5, 6-15, and >15 years) and analyzed them using a random effects model (I2 = 58.9%, P = .032). Compared to those not exposed to pediatric CT, the cancer risk was 1.35-fold greater for children exposed to CT when 0 to 5 years of age (RR: 1.35, 95% CI: 1.25-1.45), 1.14-fold for children 6 to 15 years of age (RR: 1.14, 95% CI: 1.06-1.22), and 1.24-fold for children >15 years of age (RR: 1.24, 95% CI: 1.14-1.34, Figure 3C).
Publication Bias
The Begg test P value was significant at 0.153, thereby indicating that publication bias was unlikely.
Discussion
Overall, the risk of later cancer was 1.32-fold higher in children exposed to CT than in those not so exposed. We also performed subgroup meta-analyses. Compared to those not exposed to pediatric CT, the RRs were larger for the higher doses but with wider CIs (RR for 5-10 mGy: 0.90, 95% CI: 0.69-1.12; RR for 10-15 mGy: 1.02, 95% CI: 0.86-1.18; RR for >15 mGy: 1.13, 95% CI: 0.97-1.30). Subgroup analysis showed that the leukemia risk and brain cancer risk were higher in exposed children. These data are consistent with previous findings.18,25 The reports that we analyzed had certain limitations; thus, our findings should be interpreted with caution. However, our study is the most comprehensive analysis performed to date. A systematic and detailed understanding of the association between pediatric CT and later cancer risk is required, but we suggest that medical care providers need to weigh the risks and benefits of diagnostic studies. Future randomized trials and prospective cohort studies are necessary. Our subgroup meta-analyses must be interpreted cautiously due to heterogeneity. However, the link between the brain cancer risk of children aged <5 years with time elapsed after CT requires urgent attention. Chen et al raised similar concerns,26 reporting a significant increase in all cancer risk after facial CT (RR = 1.14; 95% CI: 1.01-1.28). However, neither subgroup nor dose–response analyses were performed, and attributable risk (AR), lifetime risk (LR), and RR were calculated, perhaps weakening the conclusions. We performed subgroup and dose–response analyses to explore the effects of CT radiation exposure and dosage on the later cancer risk; CT doses should be minimized in children.
The child-specific impact of CT radiation has received attention from both the scientific community and the media. Solth et al considered it necessary to reduce pediatric exposure to CT radiation; a nationwide survey indicated that children were sensitive to even the low-dose ionizing radiation associated with CT.27 Last year, a New York Times headline read: “We Are Giving Ourselves Cancer,” creating major public concern. It was estimated that, in the United States, 29 000 tumors per annum were caused by CT radiation2; in United Kingdom in 2006, CT was thought to have caused 750 fatal cancers.28 Children may be more sensitive to radiation than adults because growth and development are still ongoing. Most experts believe that the safe CT radiation dose for children is very low. Children under 5 years of age are at particular risk; we found that they were at higher risk of later cancer than children of other ages. Brenner and Hall found that children aged < 5 years were at the highest risk of later cancer, consistent with our observation.29 The effects of radiation may be more pronounced in small bodies, and there may also be inherent age-specific risks.30 If CT could be postponed, the risk of later cancer might fall. Specifically, caution should be exercised with respect to ordering CT for children under 5 years of age.
The RR of brain cancer was higher in those who underwent pediatric CT than in those who did not, consistent with previous epidemiological findings. In 2001, Brenner et al reported radiation-induced brain tumors in Japanese atomic bomb survivors6; in 2012, Pearce et al published the first epidemiological estimates of brain tumors caused by pediatric CT.12 Brain cancer was the most common cancer developing after CT; the pediatric brain may be particularly sensitive to radiation and should be carefully protected. The need for 2 or more CT sessions was associated with a higher RR of later cancer than one-off CT. Multiple CT sessions obviously increase the absorbed radiation dose, triggering both cumulative and stochastic effects.31 Cumulative effects are often associated with radiotherapy, whereas stochastic effects are evident after X-ray or CT; radiation doses vary widely, the threshold doses remain unclear, and effects may develop many years later.32 Rajaraman et al performed a case–control study exploring early exposure to diagnostic radiation and the associated risk of childhood cancer.33 Even at radiation doses less than those of CT, a cancer risk was evident. Children aged 0 to 100 days who received diagnostic radiation were at an increased risk of cancer by 14 years of age (OR: 1.16, 95% CI: 0.83-1.62). The estimated CT radiation dose per examination, usually 3.6 to 5.8 mGy, was approximately 1- to 2-fold that of background radiation.34 Raelson et al estimated the stochastic effect of pediatric CT at an average dose of 35.3 mGy and found that the lifetime attributable cancer risk increased by 0.35%,35 supporting our finding of a dose–response relationship between CT exposure and later cancer risk. The cited authors mentioned that high radiation doses should be avoided in children. Computed tomography must be avoided altogether if possible, and, if not, tailored CT techniques are required.36 Justification is much more important than optimization; the benefits and risks must be quantitated. Second, web-based radiation exposure thresholds for children must be established by reference to dose reports and the outcomes of cohort studies evaluating exposure risks in this population. Furthermore, the reporting standards of health care services, which should evaluate the association between CT and later cancer risk, must be improved. At a minimum, all studies must be scientifically sound, reproducible, generally applicable, include clinical definitions of treated conditions, and describe outcomes. The dose calculation methods differed among our 7 included studies, seriously compromising comparisons and assessments of clinical cancers as later complications of CT. In addition, the dose per child, dose spacing, body parts targeted, and nature of the radiation delivered should be reported. Also, the cancer types explored varied; all common cancers should be included in analyses. Radiologists, physicians, technologists, and the public should be educated in terms of the radiation-related risks of CT to ensure that, when CT is deemed essential for diagnosis and treatment, it is safe. A better understanding of the cancer risks associated with CT is essential; animal or cell models could be used to explore such risks. Globally, childhood CT has increased dramatically, thereby increasing the later cancer risk. Reduction of the time spent by children in accelerator rooms, the use of personal protective equipment, better ventilation of acceleration rooms, and CT minimization in children <5 years of age are essential.
A few limitations of this study should be discussed. First, although the number of participants was relatively large, data on conditions such as neurofibromatosis, enlarged head, or headache could not be obtained. Second, whether the follow-up period was same for all children, with or without CT scans, was not known, so there was a possibility of selection or lead time bias. Third, as we lacked access to the original data, it was difficult to control for any preexisting differences in cancer incidence between the children with and without CTs. Additional main limitation of this study is that we didn’t consider the effects of environmental pollutants on the increasing risk of cancer at childhood.
Conclusions
Overall, the cancer risk was 1.32 times higher in children undergoing CT compared to individuals that had not undergone CT. The risks of certain cancer types following CT increased in younger individuals, as a function of age and dose. Although our data must be interpreted with caution, given the limitations of the papers that we reviewed, this is the most comprehensive analysis yet performed. At the least, our data should raise concerns regarding the possible impact of CT-related radiation exposure on the subsequent risk of cancer among the pediatric population. However, caution is needed when interpreting our findings because of the heterogeneity among the studies. The findings should be confirmed in further studies with longer follow-up periods.
Footnotes
Authors’ Note: Data sharing not applicable to this article as no data sets were generated or analyzed during the current study. PK Z conceived and designed the study; RX H, performed eligibility screening and data extraction; RX H, XD L, L H, analyzed the data and performed the statistical analysis. RX H drafted the initial manuscript. PK Z critically reviewed and revised the manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (Grant No. U1803124, 31370843, 31500681), the Natural Science Foundation of Hunan Province (Grant No. 2019JJ40396), and the Fundamental Research Funds for the Central Universities of Central South University (Grant No.2019zzts748).
ORCID iD: Ruixue Huang  https://orcid.org/0000-0001-9280-6551
https://orcid.org/0000-0001-9280-6551
Ping-Kun Zhou  https://orcid.org/0000-0002-5088-9608
https://orcid.org/0000-0002-5088-9608
References
- 1. Vaughan CL, Mayosi BM. Origins of computed tomography. Lancet 2007;369(9568):1168. [DOI] [PubMed] [Google Scholar]
- 2. Berrington de Gonzalez A, Mahesh M, Kim KP, et al. Projected cancer risks from computed tomographic scans performed in the United States in 2007. Arch Int Med. 2009;169(22):2071–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pickhardt PJ. CT colonography provides new insights into interval cancers. Lancet Gastroenterol Hepatol. 2018;3(5):292–294. [DOI] [PubMed] [Google Scholar]
- 4. Miglioretti DL, Johnson E, Williams A, et al. The use of computed tomography in pediatrics and the associated radiation exposure and estimated cancer risk. JAMA Pediatrics. 2013;167(8):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bagherzadeh S, Jabbari N, Khalkhali HR. Estimation of lifetime attributable risks (LARs) of cancer associated with abdominopelvic radiotherapy treatment planning computed tomography (CT) simulations. Int J Radiat Biol. 2018;94(5):454–461. [DOI] [PubMed] [Google Scholar]
- 6. Brenner D, Elliston C, Hall E, Berdon W. Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR. 2001;176(2):289–296. [DOI] [PubMed] [Google Scholar]
- 7. Larson DB, Rader SB, Forman HP, Fenton LZ. Informing parents about CT radiation exposure in children: it’s ok to tell them. AJR. 2007;189(2):271–275. [DOI] [PubMed] [Google Scholar]
- 8. Frush DP, Goske MJ. Image gently: toward optimizing the practice of pediatric CT through resources and dialogue. Pediatric Radiol. 2015;45(4):471–475. [DOI] [PubMed] [Google Scholar]
- 9. Feng ST, Law MW, Huang B, et al. Radiation dose and cancer risk from pediatric CT examinations on 64-slice CT: a phantom study. Europ J Radiol. 2010;76(2):e19–e23. [DOI] [PubMed] [Google Scholar]
- 10. Meulepas JM, Ronckers CM, Smets A, et al. Radiation exposure from pediatric CT scans and subsequent cancer risk in the Netherlands. J Natl Cancer Inst. 2018;110(10):1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou PK, Huang RX. Targeting of the respiratory chain by toxicants: beyond the toxicities to mitochondrial morphology. Toxicol Res-UK. 2018;7(6):1008–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pearce MS, Salotti JA, Little MP, et al. Radiation exposure from CT scans in childhood and subsequent risk of leukaemia and brain tumours: a retrospective cohort study. Lancet. 2012;380(9840):499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Berrington de Gonzalez A, Journy N, Lee C, et al. No association between radiation dose from pediatric CT scans and risk of subsequent Hodgkin lymphoma. Cancer Epidemiol Biomarkers Prev. 2017;26(5):804–806. [DOI] [PubMed] [Google Scholar]
- 14. Meulepas JM, Ronckers CM, Smets A, et al. Radiation exposure from pediatric CT scans and subsequent cancer risk in the Netherlands. J National Cancer Institute. 2019;111(3):256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nordenskjold AC, Bujila R, Aspelin P, Flodmark O, Kaijser M. Risk of meningioma after CT of the head. Radiology. 2017;285(2):568–575. [DOI] [PubMed] [Google Scholar]
- 16. Krille L, Dreger S, Schindel R, et al. Erratum to: risk of cancer incidence before the age of 15 years after exposure to ionising radiation from computed tomography: results from a German cohort study. Radiat Envio Biophys. 2017;56(3):293–297. [DOI] [PubMed] [Google Scholar]
- 17. Journy N, Roue T, Cardis E, et al. Childhood CT scans and cancer risk: impact of predisposing factors for cancer on the risk estimates. J Radiol Prot. 2016;36(1):N1–N7. [DOI] [PubMed] [Google Scholar]
- 18. Huang WY, Muo CH, Lin CY, et al. Paediatric head CT scan and subsequent risk of malignancy and benign brain tumour: a nation-wide population-based cohort study. British J Cancer. 2014;110(9):2354–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mathews JD, Forsythe AV, Brady Z, et al. Cancer risk in 680,000 people exposed to computed tomography scans in childhood or adolescence: data linkage study of 11 million Australians. BMJ. 2013;346:f2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He J, Ning H, Huang R. Low blood lead levels and attention-deficit hyperactivity disorder in children: a systematic review and meta-analysis. Environ Sci Pollut Res Int. 2019,26(18):17875–17884. [DOI] [PubMed] [Google Scholar]
- 21. Andrade C. Understanding relative risk, odds ratio, and related terms: as simple as it can get. J Clin Psych. 2015;76(7):e857–e861. [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Altman DG, Liberati A, Tetzlaff J. PRISMA statement. Epidemiology. 2011;22(1):128; author reply 128. [DOI] [PubMed] [Google Scholar]
- 23. Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep 2006;29(1):85–93. [DOI] [PubMed] [Google Scholar]
- 24. Huang RX, Wang K, Hu JA. Effect of probiotics on depression: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(8):E483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Doss M. Pediatric computed tomography scans and cancer risk. JAMA Pediat. 2018;172(11):1099–1100. [DOI] [PubMed] [Google Scholar]
- 26. Chen JX, Kachniarz B, Gilani S, Shin JJ. Risk of malignancy associated with head and neck CT in children: a systematic review. Otolaryngol Head Neck Surg. 2014;151(4):554–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Solth A, Mukerji N, Strachan R. Reducing the radiation exposure from CT scanning in children with shunts: a nationwide survey and a departmental CT protocol. British J Neurosurg. 2018:1–5. [DOI] [PubMed] [Google Scholar]
- 28. Hall EJ, Brenner DJ. Cancer risks from diagnostic radiology. British J Radiol. 2008;81(965):362–378. [DOI] [PubMed] [Google Scholar]
- 29. Brenner DJ, Hall EJ. Computed tomography—an increasing source of radiation exposure. New England J Med. 2007;357(22):2277–2284. [DOI] [PubMed] [Google Scholar]
- 30. Pierce DA, Shimizu Y, Preston DL, Vaeth M, Mabuchi K. Studies of the mortality of atomic bomb survivors. Report 12, part I. cancer: 1950-1990. 1996. Radiation Res. 2012;178(2):AV61–AV87. [DOI] [PubMed] [Google Scholar]
- 31. Tsapaki V, Tsalafoutas IA, Triantopoulou C, Kolliakou E, Maniatis P, Papailiou J. Radiation dose in repeated CT guided radiofrequency ablations. Phys Med. 2014;30(1):128–131. [DOI] [PubMed] [Google Scholar]
- 32. Jacobs R, Pauwels R, Scarfe WC, et al. Pediatric cleft palate patients show a 3- to 5-fold increase in cumulative radiation exposure from dental radiology compared with an age- and gender-matched population: a retrospective cohort study. Clin Oral Invest. 2018, 22(4):1783–1793. [DOI] [PubMed] [Google Scholar]
- 33. Rajaraman P, Simpson J, Neta G, et al. Early life exposure to diagnostic radiation and ultrasound scans and risk of childhood cancer: case-control study. Bmj. 2011;342(2):d472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kuhns LR, Oliver WJ, Christodoulou E, Goodsitt MM. The predicted increased cancer risk associated with a single computed tomography examination for calculus detection in pediatric patients compared with the natural cancer incidence. Pediat Emerg Care. 2011;27(4):345–350. [DOI] [PubMed] [Google Scholar]
- 35. Raelson CA, Kanal KM, Vavilala MS, et al. Radiation dose and excess risk of cancer in children undergoing neuroangiography. AJR. 2009;193(6):1621–1628. [DOI] [PubMed] [Google Scholar]
- 36. Sathya C, Alali AS, Wales PW, et al. Computed tomography rates and estimated radiation-associated cancer risk among injured children treated at different trauma center types. Injury. 2018;50(1):142–148. [DOI] [PubMed] [Google Scholar]