Abstract
Allostatic load, or the physiological dysregulation accumulated due to senescence and stress, is an established predictor of human morbidity and mortality and has been proposed as a tool for monitoring health and welfare in captive wildlife. It is estimated by combining biomarkers from multiple somatic systems into allostatic load indices (ALIs), providing a score representing overall physiological dysregulation. Such ALIs have been shown to predict disease and mortality risk in western lowland gorillas. In these prior analyses, we were unable to include lipid markers, a potential limitation as they are key biomarkers in human models. Recently, we were able to assay serum cholesterol and triglycerides and add them to our previous ALI. We then re-examined associations with health outcomes using binomial generalized linear models. We constructed ALIs using 2 pooling strategies and 2 methods. By itself, a 1-unit increase in allostatic load was associated with higher odds of all-cause morbidity and mortality, but results were mixed for cardiac disease. However, the best fit models for all-cause morbidity and cardiac disease included only age and sex. Allostatic load was retained alongside age in the best fit models for mortality, with a 1-unit increase associated with 23% to 45% higher odds of death. Compared with previous results, ALIs containing cholesterol and triglycerides better predict disease risk in zoo-housed western lowland gorillas, as evidenced by larger effect sizes for some models and better goodness of fit for all ALIs. Based on these results, we address methodology for future allostatic load research on wildlife.
Keywords: Chronic conditions, cardiac disease, nonhuman primates, animal welfare, lipid markers
Introduction
Negative effects of stress are many and wide-ranging, including anxiety, depression, the development of chronic degenerative conditions (eg, cardiac disease, diabetes), substance abuse, personality and conduct disorders, psychosomatic conditions, metabolic syndrome, and immunosuppression.1-4 Given the myriad problems associated with stress, both human and animal researchers have long focused on identifying physiological biomarkers which consistently predict risk of future health declines. Although glucocorticoids often are employed for this purpose, they may not be the best physiological measure for studying long-term effects of stress. Assumed to increase with exposure to stressors, glucocorticoid responses actually vary widely between individuals and sometimes even within the same individual at different points in time.5-15 Moreover, glucocorticoids have many functions beyond their involvement in stress responses14-16 and circulating titers vary for a number of reasons across and within species, such as seasonal variation in food availability, social status and relationships, and predation pressure.7,8,13 Although some alternatives to glucocorticoids as biomarkers of stress have been proposed (eg, glucose, immune response, reproductive hormones), few have been explored,12 and because most biomarkers have multiple physiological roles, their interpretation would likely face similar difficulties. In fact, no single biomarker is likely to be the best measure of long-term stress.
Allostatic load indices (ALIs; for reviews, see earlier works17-19) are an alternative to single biomarkers for predicting risk of poor future health and shortened lifespan. Theoretically, allostatic load represents the accumulation of physiological impairment and dysregulation, or “wear and tear,” induced over time by both senescence and stressful experiences.1 As a latent variable, allostatic load cannot be measured directly, but it can be estimated using ALIs, which combine biomarkers from multiple somatic systems (eg, cardiovascular, metabolic, neuroendocrine, immune) into a score that provides a snapshot of allostatic load at a single point in an individual’s lifespan. First implemented in humans more than 2 decades ago,20 researchers have shown higher allostatic load predicts risk of negative health outcomes, such as arthritis,21 cardiac disease,20-26 diabetes,21 higher prevalence of pain,27 frailty,28,29 hypertension,21,30 obesity and overweight,21,31 periodontal disease,26,32 and sleep disorders.33 Furthermore, higher allostatic load is associated with increased mortality risk.20,34-45 Importantly, ALIs have been consistently shown to better predict health outcomes than individual biomarkers.23,29,34-38,41,42,44-46
ALIs have been constructed with a wide variety of biomarkers and estimated using different methods.17,19 Although the lack of a “gold standard” for allostatic load estimation has been criticized,17,18,47-52 consistent predictions of risk despite varied methodologies demonstrate the robusticity of ALIs as a clinical tool.19,53,54 Indeed, this flexibility also may be advantageous for early efforts to use ALIs as tools for animal health, welfare, and conservation, as it allows researchers with existing data sets to begin by using biomarkers originally measured for other purposes. However, although this flexibility may aid some, the lack of standardized criteria and a plethora of options may leave others at a loss for how to begin. Wildlife researchers also face constraints that are less likely to be an issue for those studying human populations. For example, human research on allostatic load often is conducted using large, population-level data sets complete with physiological measures (eg, MacArthur Studies of Successful Aging and National Health and Nutrition Examination Survey in the United States, National Child Development Study in Great Britain, Copenhagen Perinatal Cohort and follow-up studies in Denmark), which are unavailable for most animal species. In addition, banked tissue samples for retrospective study are of limited quantity and new samples, especially of serum and whole blood, can be difficult to obtain from some animals and may require anesthesia. For many species, already high assay costs may be further increased by the need for assay validation and development. Because of these challenges, research on allostatic load in wildlife would benefit from methods to determine optimal ALIs for species of interest. Although we agree with the suggestion that a single ALI may not be best for predicting risk of all health outcomes,51 wildlife researchers may find it better to compromise on an ALI well-suited for use in a wide variety of contexts, rather than developing multiple outcome-specific models. These ALIs can then be tailored for specific disease processes if needed. For example, interest in developing an ALI that is particularly sensitive to cardiac disease, the leading cause of death among captive great apes,55-64 likely will follow development of a generalized model based on verified predictors that assesses welfare and well-being while predicting overall risk of health declines. Efforts to determine optimal ALIs for each species among zoo- and laboratory-housed collections also are critical for future implementations of ALIs as conservation tools in the field.
Despite the occasional criticism sometimes directed toward applications of allostatic load for their inconsistent methodology,17,18,47-52 efforts to determine how to best estimate allostatic load have not moved to a consensus on which biomarkers to include or how best to amalgamate them into an ALI. Previously, we explored one possible method, using a combination of stepwise regression, tolerance statistics, and individual biomarker associations to try and refine a list of biomarkers to include in an ALI for zoo-housed western lowland gorillas (Gorilla gorilla gorilla).65 However, when compared with our original ALI, these new ALIs were not better at predicting all-cause morbidity or mortality (cardiac disease had not been examined in our initial work). One limitation when generating these previous ALIs was an incomplete data set for total cholesterol and triglycerides that prevented their inclusion in the index while also maintaining our full sample size. Lipid markers are frequently included in ALIs in humans,17,19 and it is standard veterinary practice to measure these markers as potential indicators of poor health, suggesting they may be important components of an optimal ALI for western lowland gorillas. We have since been able to assay total cholesterol and triglycerides from the same serum samples used to measure the biomarkers included in our previous ALIs. Herein, we explore whether updating our original ALIs to include cholesterol and triglycerides better predicts risk of all-cause morbidity, cardiac disease, and mortality in gorillas. We hope this report will build on the foundation of our previous research65-68 to help guide those interested in pioneering allostatic load methodology across taxa. As the ability to measure physiological markers from noninvasive tissue samples like urine and feces increases, this research also may extend to studying and monitoring populations in the wild.
Methods
Subjects
Data are from individual serum samples from western lowland gorillas previously or currently housed at the Columbus Zoo and Aquarium, Louisville Zoo, and Omaha’s Henry Doorly Zoo (n = 63); 3 gorillas were excluded due to insufficient serum for assaying lipid markers (n = 60). Males ranged in age from 6 to 52 years (n = 29, mean = 22.0, SD = 10.90) and females ranged in age from 7 to 52 years (n = 31, mean = 24.0, SD = 14.13). Gorillas were pooled into a single sample, as age did not vary significantly between males and females overall, between any zoos, or between males and females at each location (data not shown). Data on morbid conditions and age at death, when applicable, were obtained from medical records provided by each zoo. Conditions included as chronic diseases from medical records included osteoarthritis, hypothyroidism, neoplasia, and obesity. Conditions included as cardiac disease were diagnosed antemortem echocardiographically by a human specialist (atherosclerosis and congestive heart failure), by routine blood pressure monitoring (hypertension), or postmortem (aortic aneurysm). This study was approved by The Ohio State University Animal Care and Use Committee (IACUC) and each participating institution, and also adhered to the American Society of Primatologists’ Principles for the Ethical Treatment of Primates.
Biomarker assays
The ALI herein includes albumin, cortisol, corticotropin-releasing hormone (CRH), dehydroepiandrosterone-sulfate (DHEA-S), homeostatic model assessment of insulin resistance (HOMA-IR), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), total cholesterol, and triglycerides. Albumin and glucose (for estimating insulin resistance) are routinely measured by veterinarians during immobilizations and, as such, were obtained from medical records. Remaining biomarkers were assayed from serum samples collected during the same immobilization and cryopreserved at −80°C. Samples were collected between 1991 and 2015. Although sample degradation can be an issue with long-term storage, studies indicate cortisol,69,70 CRH,71,72 and inflammatory cytokines73 maintain their integrity when cryopreserved at ultralow temperatures. Unfortunately, no data are available on the integrity of insulin in long-term storage at ultralow temperatures. Although sample degradation over the long-term can affect lipid measurements,74 these serum samples were cryopreserved at the recommended temperature for long-term storage75 and it has been demonstrated that, even when stored for 10 or more years, there is minimal effect on studying disease risk.76
CRH, cortisol, DHEA-S, insulin, IL-6, and TNF-α were assayed by The Ohio State University Clinical and Translation Science: Clinical Research Center between 2014 and 2016 using commercially available kits. CRH was measured by radioimmunoassay (RK-019-06; Phoenix Pharmaceuticals, Burlingame, CA, USA). As previously described,77,78 CRH was extracted prior to assay using ice-cold methanol to remove interfering binding proteins, then reconstituted to its original volume for assay. The intra-assay variation was <3.2% and the inter-assay variation was <14.2%. CRH measures were log-transformed due to positive skew, which normalized the data and stabilized the variance. Cortisol, DHEA-S, and insulin were measured using solid phase, competitive chemiluminescent enzyme immunoassay with an Immulite 1000 (Siemens Healthcare Diagnostics Inc., Hoffman Estates, IL, USA). Intra-assay variation was 1.5% for the low pool and 4.4% for the high pool, and inter-assay variation was <7.9%. For DHEA-S, intra-assay variation was 5.1% and inter-assay variation was <7.9%. The intra-assay variation for insulin was 5.7% and the inter-assay variation was 6.7%. Finally, solid-phase enzyme-linked immunosorbent assay was used for IL-6 (HS600B; R and D Systems, Minneapolis, MN, USA) and TNF-α (HSTA00D; R and D Systems). IL-6 intra-assay variation was <7.4% and inter-assay variation was <7.8%. Intra-assay variation for TNF-α was <5.4% and inter-assay variation was <8.3%. All samples analyzed were above the lower limit of sensitivity for each assay, and CVs are within the 10% and 15% acceptable thresholds for intra- and inter-assay variability, respectively.
Triglycerides and total cholesterol were assayed at the Smithsonian Conservation Biology Institute using an RX Daytona automated clinical chemistry analyzer (Randox Industries-US Ltd, Kearneysville, WV, USA) in 2018. Commercially available reagents (triglycerides: TR3823, cholesterol: CH3810), calibrators (CAL2351), and 2-level controls (HN1530 and HE1532) were all purchased from Randox Industries-US Ltd. The technical ranges were 0 to 12.8 mmol/L for triglycerides and 0 to 17.0 mmol/L for cholesterol. Serum was generally run neat, or diluted 1:5 with saline (SA3854) where necessary. The analyzer was subject to routine quality control measurements throughout the study, with normal and elevated controls for each analyte maintained within 2 SDs of the respective target value.
Allostatic load index
We estimated allostatic load scores using both the original 1-tailed quartile method20 as well as a multimethod approach we piloted in gorillas65-68 that incorporates the philosophy behind using 2-tailed deciles (for a review of allostatic load methodology, see19). With the quartile method, the distribution of each biomarker is divided into quartiles and 1 quartile is designated as high risk; as physiological dysregulation is reflected in low levels of albumin and DHEA-S, their first quartile was designated as high risk, and vice versa for all other biomarkers. Allostatic load scores are determined by counting the number of biomarkers an individual has within the high-risk quartile. This ALI contains 9 biomarkers, meaning scores could range from 0 (ie, no biomarkers within the high-risk quartile) to 9 (ie, all biomarkers within the high-risk quartile). We use the abbreviation “ALQ” for ALIs estimated using the traditional 1-tailed quartile method.
Proponents of using 2-tailed deciles rather than 1-tailed quartiles suggest physiological dysregulation is bidirectional, resulting in high levels in some individuals but low levels in others. Physiological evidence supports this for some biomarkers, such as cortisol,79-82 but those using 2-tailed deciles apply them to all biomarkers in the index. Our multimethod approach merges these methods by maintaining 1-tailed quartiles for most biomarkers but using a 2-tailed split quartile (top and bottom 12.5% of the distribution) for others when appropriate. As there is evidence for bidirectional dysregulation for both cortisol79-82 and total cholesterol,83-93 we used a 2-tailed split quartile for these biomarkers. We also tested ALIs with a 1-tailed quartile for total cholesterol, as this is the traditional approach in human clinical research; overall, these ALIs comparably predicted health outcomes (see Supplemental Material). As before, allostatic load scores are estimated by summing the number of biomarkers each individual has within high-risk quartiles. These ALIs are abbreviated using “ALM.”
Quantitative analyses
Data from the 3 zoos were pooled in 2 different ways, as our prior research indicates the best pooling strategy may depend on the health outcome of interest.65 For pooled sample 1 (PS1), we assume each zoo is an independent sample and preserve variation between zoos by estimating allostatic load at each zoo individually, then pooling the scores. Alternatively, pooled sample 2 (PS2) assumes all zoo-housed gorillas, or at least those in North America, are part of one population and combines biomarker data from each zoo prior to estimating allostatic load. Before determining allostatic load scores, missing values for albumin, creatinine, CRH, and HOMA-IR were replaced with their mean values. We also used t tests to analyze each biomarker for potential differences between males and females using a conservative threshold of α = 0.10; when significant differences were observed, we used sex-specific quartiles (Table 1). Means were determined and sex differences were analyzed independently at each zoo for PS1 and using the pooled sample for PS2.
Table 1.
High-risk quartile boundaries for biomarkers included in allostatic load indices for western lowland gorillas (n = 60) combined using a 1-tailed quartile approach and a multimethod, split quartile approach (top and bottom 12.5%).
| n | Albumin, g/dL | CRH, pg/mL | Cortisol, µg/dL |
DHEA-S, µg/dL | HOMA-IR | IL-6, pg/mL | TNF-α, pg/mL | TC, mmol/L |
TRIG, mmol/L | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| One-tailed quartile | Two-tailed split quartile | One-tailed quartile | Two-tailed split quartile | |||||||||
| Pooled sample 1 | ||||||||||||
| CZA | 25 | M ⩽ 3.70 F ⩽ 3.05 |
⩾4.38 | M ⩾ 10.80 F ⩾ 20.70 |
M ⩽ 8.98, ⩾ 11.78 F ⩽ 9.15, ⩾ 25.94 |
⩽17.00 | ⩾2.18 | M ⩾ 3.65 F ⩾ 15.14 |
⩾0.98 | M ⩾ 8.25 F ⩾ 6.50 |
M ⩽ 6.17, ⩾ 8.85 F ⩽ 4.53, ⩾ 7.28 |
M ⩾ 1.21 F ⩾ 3.08 |
| LZ | 21 | ⩽3.30 | M ⩾ 6.10 F ⩾ 5.64 |
M ⩾ 14.50 F ⩾ 20.88 |
M ⩽ 5.83, ⩾ 15.85 F ⩽ 7.56, ⩾ 29.23 |
⩽15.00 | ⩾1.25 | M ⩾ 13.42 F ⩾ 7.08 |
⩾0.53 | ⩾5.67 | ⩽4.23, ⩾6.09 | ⩾1.49 |
| OMA | 14 | M ⩽ 4.20 F ⩽ 3.45 |
⩾5.61 | M ⩾ 10.95 F ⩾ 19.68 |
M ⩽ 7.96, ⩾ 11.22 F ⩽ 10.31, ⩾ 20.09 |
⩽15.55 | ⩾0.55 | ⩾5.24 | M ⩾ 0.26 F ⩾ 0.62 |
⩾7.56 | ⩽5.65, ⩾7.89 | ⩾1.38 |
| Pooled sample 2 | ||||||||||||
| 60 | M ⩽ 3.60 F ⩽ 3.20 |
⩾5.71 | M ⩾ 11.25 F ⩾ 20.20 |
M ⩽ 7.26, ⩾ 14.31 F ⩽ 8.60, ⩾ 27.05 |
⩽15.00 | M ⩾ 1.14 F ⩾ 1.85 |
⩾7.9 | ⩾0.55 | ⩾6.9 | ⩽4.77, ⩾7.97 | M ⩾ 1.35 F ⩾ 2.04 |
|
Abbreviations: CRH, corticotropin-releasing hormone; CZA, Columbus Zoo and Aquarium; DHEA-S, dehydroepiandrosterone-sulfate; HOMA-IR, homeostatic model assessment of insulin resistance; IL-6, interleukin-6; LZ, Louisville Zoo; OMA, Omaha’s Henry Doorly Zoo; TC, total cholesterol; TNF-α, tumor necrosis factor-α; TRIG, triglycerides.
Sex-specific quartiles were used for biomarkers with significant differences between males and females (t tests, α = 0.10). Pooled sample 1 estimated allostatic load at each zoo independently prior to combining scores into a single sample, pooled sample 2 combined biomarker values prior to estimating allostatic load.
Because lipid markers are routinely measured by physicians and veterinarians for diagnostic purposes and may reflect poor health, we previously analyzed associations of allostatic load scores with total cholesterol and triglycerides in gorillas for which the data were available. Therefore, we first analyzed associations between our original 7-biomarker ALIs and the newly assayed values for cholesterol and triglycerides using linear regression. Then, using the new allostatic load scores that include total cholesterol and triglycerides, we analyzed associations with age and sex using linear regression and t tests, respectively. We also used t tests to examine differences between gorillas based on presence or absence of any chronic conditions (eg, arthritis, cardiac disease) in general as well as cardiac disease specifically, and between living and deceased individuals. Finally, binomial generalized linear models (GLMs) with logit links were analyzed to assess whether higher allostatic load is associated with higher odds of all-cause morbidity, cardiac disease, and mortality. First, as disease and mortality risk often are predicted by older age and may vary by sex, we analyzed baseline models containing only these 2 variables. As this work is exploratory, we wanted to independently assess the relationship between allostatic load and each health outcome, so we then analyzed models containing only allostatic load. Finally, if age and/or sex had or neared (α ≤ 0.10) a statistically significant contribution to a particular health outcome, they were added into a full model alongside allostatic load. Therefore, full models for all-cause morbidity and cardiac disease included age, sex, and allostatic load, whereas the full model for mortality included only age and allostatic load. As allostatic load increases with age and the 2 variables often are significantly associated, we assessed multicollinearity by estimating the variance inflation factor (VIF) before analyzing models; multicollinearity was not observed between age and allostatic load for any model constructed (VIF = 1.13-1.22). Goodness of fit was determined by comparing Akaike’s information criterion (AIC or AICc, corrected for small sample sizes) between models. Including zoo ID as a random effect reduced model fit for all models tested (data not shown), so it is not included in any models herein. Because our sample size is small for conducting these types of analyses, which can result in high P values and wide confidence intervals despite large effect sizes, and given that statistical significance does not equate to clinical importance,94 we describe factors as significant at P ⩽ .05 but nonsignificant factors are still considered and we discuss the effect sizes of each variable using odds ratios (ORs). We also analyzed mortality risk using Cox proportional hazards models, for which we observed similar results (data not shown). R (v3.5.0)95 was used for all quantitative analyses and GLMs were analyzed using the “lme4” package.96
Results
Sample characteristics
More than half of the gorillas in this sample were diagnosed with at least 1 chronic condition (58.3%, n = 35) and 32.8% have died since sample collection (n = 20). Cardiac disease was the most prevalent chronic condition, affecting almost half of the sample (45%, n = 27). Other diagnosed conditions included arthritis (18.0%, n = 11), hypothyroidism (4.9%, n = 3), obesity (4.9%, n = 3), and cancer (3.3%, n = 2). Among males, the majority had at least 1 chronic condition (75.9%, n = 22), more than two-thirds had cardiac disease (69.0%, n = 20), and 31% were deceased (n = 9). Females were less likely to have chronic conditions (41.9%, n = 13) and cardiac disease (22.6%, n = 7), and 35.5% were deceased (n = 11). Means, SDs, and ranges for each ALI, as well as associations of allostatic load with age and sex, are presented in Table 2.
Table 2.
Means, SDs, and ranges for each allostatic load index tested in western lowland gorillas (n = 60), as well as associations of allostatic load with age (linear regression) and sex (t test).
| ALI | Mean | SD | Range | Age |
Sex |
|||
|---|---|---|---|---|---|---|---|---|
| P | R 2 | t | df | P | ||||
| PS1 ALQ | 2.43 | 1.81 | 0-7 | .008 | 0.116 | −0.650 | 58 | .519 |
| PS2 ALQ | 3.05 | 1.71 | 1-7 | .008 | 0.115 | −0.067 | 58 | .947 |
| PS1 ALM | 2.38 | 1.56 | 0-6 | .001 | 0.182 | −0.347 | 58 | .730 |
| PS2 ALM | 2.48 | 1.82 | 0-7 | .002 | 0.151 | −0.426 | 58 | .672 |
Abbreviations: ALI, allostatic load index; ALM, multimethod approach, allostatic load estimated using primarily 1-tailed quartiles but also some 2-tailed split quartiles (top and bottom 12.5%), depending on the biomarker (eg, cortisol); ALQ, allostatic load estimated using traditional 1-tailed quartiles for each biomarker; PS1, pooled sample 1, allostatic load estimated at each zoo independently prior to pooling the sample; PS2, pooled sample 2, allostatic load estimated after pooling biomarkers from all locations.
Reference group for t tests: males.
Associations with cholesterol and triglycerides
Cholesterol and triglycerides ranged from 3.20 to 12.61 mmol/L (mean = 6.26, SD = 1.70) and 0.48 to 11.50 mmol/L (mean = 1.58, SD = 1.52), respectively. Consistent with our previous research, significant associations between allostatic load and cholesterol were not observed for any model (PS1 ALQ: P = .724, R2 = 0.003; PS2 ALQ: P = .306, R2 = 0.024; PS1 ALM: P = .691, R2 = 0.004; PS2 ALM: P = .093, R2 = 0.063). However, PS2 ALM had P ⩽ .10, indicating a possible relationship between higher allostatic load and lower cholesterol. Also consistent with our prior research, higher allostatic load was significantly associated with higher levels of circulating triglycerides for all models (PS1 ALQ: P = .005, R2 = 0.228; PS2 ALQ: P = .004, R2 = 0.240; PS1 ALM: P = .020, R2 = 0.163; PS2 ALM: P = .008, R2 = 0.204).
All-cause morbidity
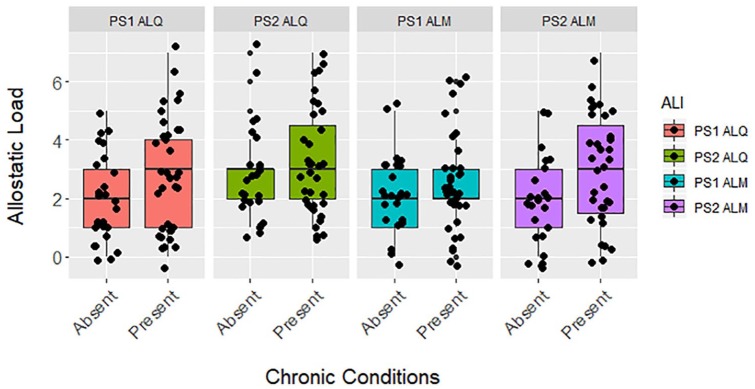
Gorillas with at least 1 chronic condition had significantly higher allostatic load than healthier conspecifics for PS1 ALQ and PS2 ALM (Figure 1). Similarly, when it was the only predictor in the models, higher allostatic load had or trended toward a significant association with higher odds of all-cause morbidity for PS1 ALQ and PS2 ALM ALIs (Table 3), predicting 34% and 38% higher odds of developing a chronic condition, respectively. However, the baseline model with age and sex only had the lowest AICc value and allostatic load was not a significant predictor in the full models (Table 3). Older gorillas had significantly higher odds of all-cause morbidity across all models, with a 5-year increase in age associated with 75% greater likelihood of disease according to the baseline model. Also according to the baseline model, males were more than 9× more likely than females to develop at least 1 chronic condition, although this estimate had wide confidence intervals.
Figure 1.
Differences between allostatic load based on the presence or absence of chronic conditions in zoo-housed western lowland gorillas (n = 60) for PS1 ALQ (t test, t = −1.900, df = 58, P = .062), PS2 ALQ (t test, t = −0.801, df = 58, P = .427), PS1 ALM (t test, t = −1.105, df = 58, P = .274), and PS2 ALM (t test, t = −2.085, df = 58, P = .041). ALI indicates allostatic load index; ALM, multimethod approach, allostatic load estimated using primarily 1-tailed quartiles but also some 2-tailed split quartiles (top and bottom 12.5%), depending on the biomarker (eg, cortisol); ALQ, allostatic load estimated using traditional 1-tailed quartiles for each biomarker; PS1, pooled sample 1, allostatic load estimated at each zoo independently prior to pooling the sample; PS2, pooled sample 2, allostatic load estimated after pooling biomarkers from all locations.
Table 3.
Risk of all-cause morbidity in western lowland gorillas (n = 60) was analyzed using binomial generalized linear models (GLMs) with logit links for a baseline model with age and sex only, a model with each allostatic load index only, and a full model with allostatic load and significant variables from the baseline model.
| ALI | Allostatic load |
Age |
Sex |
AICc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | ||
| Baseline | .0004 | 1.15 | 1.08-1.26 | .002 | 9.75 | 2.48-48.95 | 58.1 | |||
| PS1 ALQ | .067 | 1.34 | 0.99-1.87 | 82.1 | ||||||
| .270 | 1.26 | 0.85-2.00 | .0008 | 1.15 | 1.07-1.26 | .002 | 11.16 | 2.72-60.27 | 59.1 | |
| PS2 ALQ | .420 | 1.14 | 0.84-1.58 | 85.1 | ||||||
| .712 | 0.92 | 0.60-1.41 | .0003 | 1.16 | 1.08-1.27 | .002 | 9.73 | 2.47-48.83 | 60.2 | |
| PS1 ALM | .272 | 1.22 | 0.87-1.76 | 84.4 | ||||||
| .778 | 0.93 | 0.58-1.52 | .0004 | 1.16 | 1.08-1.27 | .002 | 9.65 | 2.45-48.54 | 60.3 | |
| PS2 ALM | .047 | 1.38 | 1.02-1.92 | 81.4 | ||||||
| .285 | 1.25 | 0.84-1.95 | .0009 | 1.15 | 1.07-1.26 | .002 | 10.88 | 2.67-58.07 | 59.2 | |
Abbreviations: AICc, Akaike’s information criterion, adjusted for small sample size; ALI, allostatic load index; ALM, multimethod approach, allostatic load estimated using primarily 1-tailed quartiles but also some 2-tailed split quartiles (top and bottom 12.5%), depending on the biomarker (eg, cortisol); ALQ, allostatic load estimated using traditional 1-tailed quartiles for each biomarker; CI, confidence intervals; OR, odds ratio; PS1, pooled sample 1, allostatic load estimated at each zoo independently prior to pooling the sample; PS2, pooled sample 2, allostatic load estimated after pooling biomarkers from all locations.
Reference groups: all-cause morbidity, 1 = had chronic condition; sex, male. The best fit model, as determined by the lowest AICc value, is the baseline model and is in bold.
Cardiac disease
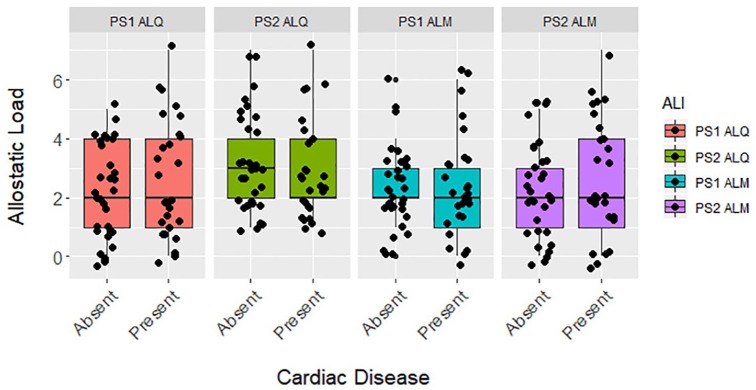
Allostatic load was not significantly higher in gorillas with cardiac disease for any ALI tested (Figure 2), nor was it significantly associated with odds of developing cardiac disease when it was the only variable included in the models (Table 4). As with all-cause morbidity, the baseline model with age and sex had the lowest AICc and allostatic load was not a significant predictor in any full models (Table 4). By itself and in the full models, allostatic load was associated with increased likelihood of cardiac disease for PS1 ALQ and PS2 ALM. However, higher allostatic load was associated with reduced odds of developing cardiac disease in PS2 ALQ and PS1 ALM. According to the baseline model, each 5-year increase in age was associated with 25% higher odds of cardiac disease and males were 10× more likely than females to develop this condition, but again this estimate had wide confidence intervals.
Figure 2.
Differences between allostatic load based on the presence or absence of cardiac disease in zoo-housed western lowland gorillas (n = 60) for PS1 ALQ (t test, t = −1.196, df = 58, P = .237), PS2 ALQ (t test, t = 0.505, df = 58, P = .616), PS1 ALM (t test, t = 0.058, df = 58, P = .954), or PS2 ALM (t test, t = −0.704, df = 58, P = .485). ALI indicates allostatic load index; ALM, multimethod approach, allostatic load estimated using primarily 1-tailed quartiles but also some 2-tailed split quartiles (top and bottom 12.5%), depending on the biomarker (eg, cortisol); ALQ, allostatic load estimated using traditional 1-tailed quartiles for each biomarker; PS1, pooled sample 1, allostatic load estimated at each zoo independently prior to pooling the sample; PS2, pooled sample 2, allostatic load estimated after pooling biomarkers from all locations.
Table 4.
Risk of cardiac disease in western lowland gorillas (n = 60) was analyzed using binomial generalized linear models (GLMs) with logit links for a baseline model with age and sex only, a model with each allostatic load index only, and a full model with allostatic load and significant variables from the baseline model.
| ALI | Allostatic load |
Age |
Sex |
AICc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | ||
| Baseline | .052 | 1.05 | 1.00-1.11 | .0004 | 10.25 | 3.06-41.98 | 71.3 | |||
| PS1 ALQ | .234 | 1.19 | 0.90-1.61 | 85.3 | ||||||
| .246 | 1.24 | 0.87-1.81 | .119 | 1.04 | 0.99-1.10 | .0004 | 11.57 | 3.32-51.33 | 72.2 | |
| PS2 ALQ | .609 | 0.92 | 0.68-1.25 | 86.6 | ||||||
| .215 | 0.78 | 0.52-1.14 | .026 | 1.06 | 1.01-1.13 | .0004 | 10.94 | 3.20-46.07 | 72.0 | |
| PS1 ALM | .953 | 0.99 | 0.71-1.38 | 86.8 | ||||||
| .455 | 0.85 | 0.55-1.29 | .037 | 1.06 | 1.01-1.12 | .0004 | 10.31 | 3.07-42.36 | 73.0 | |
| PS2 ALM | .478 | 1.11 | 0.84-1.48 | 86.3 | ||||||
| .733 | 1.06 | 0.75-1.52 | .083 | 1.05 | 1.00-1.11 | .0004 | 10.40 | 3.09-43.11 | 73.5 | |
Abbreviations: AICc, Akaike’s information criterion, adjusted for small sample size; ALI, allostatic load index; ALM, multimethod approach, allostatic load estimated using primarily 1-tailed quartiles but also some 2-tailed split quartiles (top and bottom 12.5%), depending on the biomarker (eg, cortisol); ALQ, allostatic load estimated using traditional 1-tailed quartiles for each biomarker; CI, confidence intervals; OR, odds ratio; PS1, pooled sample 1, allostatic load estimated at each zoo independently prior to pooling the sample; PS2, pooled sample 2, allostatic load estimated after pooling biomarkers from all locations.
Reference groups: cardiac disease, 1 = had condition; sex, male. The best fit model, as determined by the lowest AICc score, is the baseline model and is in bold.
Mortality
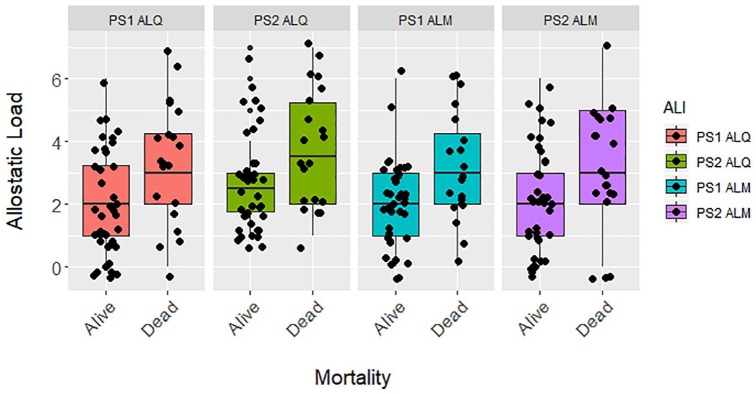
Allostatic load was significantly higher in deceased gorillas compared with those still living at time of submission (Figure 3). Allostatic load was a consistently strong predictor of mortality across all 4 ALIs. When it was the only predictor in each models, allostatic load was a significant predictor, with a 1-unit increase in allostatic load predicting a 23% to 67% increase in odds of mortality (Table 5). Unlike all-cause morbidity and cardiac disease, the best fit models for mortality contained allostatic load alongside age, with these models having lower AICc values than the baseline model. Allostatic load trended toward being a significant factor in the full models for PS1 ALQ and PS1 ALM ALIs. A 1-unit increase in allostatic load predicted 23% to 45% higher odds of mortality across the best fit models (Table 5). Age was positively associated with mortality as well, with a 25% to 30% increased likelihood of death every 5 years.
Figure 3.
Differences in allostatic load living and dead zoo-housed western lowland gorillas (n = 60) for PS1 ALQ (t test, t = −2.592, df = 58, P = .012), PS2 ALQ (t test, t = −2.505, df = 58, P = .015), PS1 ALM (t test, t = −2.843, df = 58, P = .006), and PS2 ALM (t test, t = −2.231, df = 58, P = .030). ALI indicates allostatic load index; ALM, multimethod approach, allostatic load estimated using primarily 1-tailed quartiles but also some 2-tailed split quartiles (top and bottom 12.5%), depending on the biomarker (eg, cortisol); ALQ, allostatic load estimated using traditional 1-tailed quartiles for each biomarker; PS1, pooled sample 1, allostatic load estimated at each zoo independently prior to pooling the sample; PS2, pooled sample 2, allostatic load estimated after pooling biomarkers from all locations.
Table 5.
Risk of mortality in western lowland gorillas (n = 60) was analyzed using binomial generalized linear models (GLMs) with logit links for a baseline model with age and sex only, a model with each allostatic load index only, and a full model with allostatic load and significant variables from the baseline model.
| ALI | Allostatic load |
Age |
Sex |
AICc | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | ||
| Baseline | .007 | 1.07 | 1.02-1.12 | .955 | 0.97 | 0.30-3.18 | 74.2 | |||
| PS1 ALQ | .017 | 1.49 | 1.09-2.12 | 74.3 | ||||||
| .098 | 1.34 | 0.95-1.94 | .030 | 1.06 | 1.01-1.11 | 71.4 | ||||
| PS2 ALQ | .021 | 1.48 | 1.08-2.12 | 74.8 | ||||||
| .112 | 1.34 | 0.94-1.94 | .027 | 1.06 | 1.01-1.11 | 71.7 | ||||
| PS1 ALM | .012 | 1.67 | 1.15-2.58 | 73.1 | ||||||
| .093 | 1.45 | 0.95-2.29 | .049 | 1.05 | 1.00-1.11 | 71.3 | ||||
| PS2 ALM | .035 | 1.41 | 1.04-1.98 | 75.8 | ||||||
| .242 | 1.23 | 0.87-1.77 | .029 | 1.06 | 1.01-1.11 | 72.8 | ||||
Abbreviations: AICc, Akaike’s information criterion, adjusted for small sample size; ALI, allostatic load index; ALM, multimethod approach, allostatic load estimated using primarily 1-tailed quartiles but also some 2-tailed split quartiles (top and bottom 12.5%), depending on the biomarker (eg, cortisol); ALQ, allostatic load estimated using traditional 1-tailed quartiles for each biomarker; CI, confidence intervals; OR, odds ratio; PS1, pooled sample 1, allostatic load estimated at each zoo independently prior to pooling the sample; PS2, pooled sample 2, allostatic load estimated after pooling biomarkers from all locations.
Reference groups: all-cause mortality, 1 = deceased; sex, male. Models with better goodness of fit than the baseline model, as determined by the lower AICc values, are in bold.
Discussion
Allostatic load was consistently higher in gorillas with at least 1 chronic condition, although these differences were not statistically significant, but there was little to no difference in allostatic load between those affected and unaffected by cardiac disease. When only allostatic load was included as a predictor, it had or neared a significant association with increased risk of all-cause morbidity in PS1 ALQ and PS2 ALM ALIs, predicting 34% to 38% higher odds of disease. Allostatic load was not a significant predictor in any models for cardiac disease. For both all-cause morbidity and cardiac disease, the model with the lowest AICc value contained only age and sex. According to these baseline models, each 5-year increase in age predicts 75% and 25% higher odds of all-cause morbidity and cardiac disease, respectively. In addition, these baseline models indicate males are 9× more likely to have a chronic condition and 10× more likely to have cardiac disease than females. In contrast to disease, allostatic load was a strong predictor of mortality. Best fit models for mortality contained allostatic load and age, with P ⩽ .10 for allostatic load for both PS1 ALIs. With age included, a 1-unit increase in allostatic load was associated with 23% to 45% higher odds of mortality. Each 5-year increase in age predicted 30% to 35% greater likelihood of mortality.
Although the effect of allostatic load on all-cause morbidity in gorillas is weaker when age and sex are included, results from PS1 ALQ and PS2 ALM models are consistent with research in humans, which has established allostatic load as a predictor of morbidity across multiple populations.20-33 The strong effect of allostatic load on mortality in gorillas also is consistent with human research demonstrating higher allostatic load is associated with increased mortality risk.20,34-45 However, allostatic load does not predict cardiac disease in gorillas despite doing so in humans.20-26 This is consistent with our previous research indicating an inverse association between allostatic load and odds of developing cardiac disease,65 although the reason for this unexpected relationship is unclear. Also consistent with research in humans, older age predicts increases in both disease and mortality. Sex was an important predictor of all-cause morbidity and cardiac disease, with males being more likely to develop chronic conditions, but not mortality. In humans, men usually have shorter lifespans but suffer fewer chronic conditions than women.97-101 However, the increased odds of cardiac disease observed here is consistent with research indicating it is the cause of death for 70% of silverbacks aged 30 years or older,59,60,102,103 with males estimated as being 8× more likely to develop the condition than females.63
When compared with previous ALIs constructed for this sample,65 results for associations between allostatic load and all-cause morbidity suggest including cholesterol and triglycerides in ALIs may improve disease predictions in zoo-housed gorillas. Although the contributions of age and sex remain relatively constant between our previous ALIs and those presented here (eg, age OR = 1.14-1.16 for both), including cholesterol and triglycerides increased effect sizes observed for PS2 ALIs (without lipid markers: OR = 0.84-1.11; with lipid markers: OR = 0.92-1.38), although the original PS1 ALIs have higher ORs than those with lipids. However, AICc values were consistently lower for all ALIs with lipids, indicating better goodness of fit compared with the ALIs without lipids. This suggests cholesterol and triglycerides may play an important role in chronic conditions other than cardiac disease. For example, total cholesterol may predict risk of rheumatoid arthritis in women.104 Although our sample size is too small to subdivide health concerns aside from cardiac disease, arthritis is the second most common chronic condition in this sample.
Predictions of cardiac disease also may be improved by adding total cholesterol and triglycerides to our original ALIs,65 particularly for PS1 ALQ and PS2 ALM (without lipid markers: OR = 0.64-1.12; with lipid markers: OR = 1.06-1.24). In addition, as with all-cause morbidity, ALIs with lipids have lower AICc values than those without lipids for all models tested. As lipid markers are frequently included in human ALIs,17,19 these results are not unexpected, but their inclusion in human ALIs is mostly predicated on their importance as predictors of atherosclerosis and coronary artery disease (CAD), the primarily diagnosed type of cardiac disease in humans. Conversely, severe atherosclerosis is rare in gorillas and other great apes.57,59,60 More commonly, great apes develop fibrosing cardiomyopathy (FCM), wherein increases in connective tissue in the heart reduces contractility and conductivity.59,60,63,64 Although data on lipid markers are few,55,56,105-108 especially in relation to health outcomes, lipid markers by themselves do not appear to be strong predictors of cardiac disease (Edes et al, submitted), which may partially explain why adding cholesterol and triglycerides to ALIs for gorillas improved predictions of all-cause morbidity more than cardiac disease specifically. Interestingly, lower total cholesterol, as opposed to higher, was recently observed to be significantly correlated with indicators of left ventricular hypertrophy (LVH) in male gorillas,109 a common symptom of FCM. This is consistent with the nearly significant inverse association observed between allostatic load in the PS2 ALM and total cholesterol, and supports our use of a 2-tailed quartile for total cholesterol even though results were similar when a 1-tailed quartile was used for comparison with most of the studies in humans (see Supplemental Material).
As we observed previously,65 allostatic load was a stronger predictor of mortality than disease. Mortality was similarly predicted by ALIs which included cholesterol and triglycerides as it was compared with ALIs without lipids,65 as evidenced by similar effect sizes for allostatic load (without lipid markers: OR = 1.17-1.71; with lipid markers: OR = 1.23-1.67). However, as observed for the 2 previous health outcomes, all ALIs with lipids have lower AICc values than ALIs without lipids. Although statistical significance is not equivalent to clinical importance, this conclusion is further supported by P ⩽ .10 for allostatic load in the full PS1 models with lipids despite small sample size. ALIs were designed to be preclinical predictors of poor health outcomes.20 Studying health and determining the timing of disease onset is complicated for many animals, as hiding sick behavior is often adaptive and individuals may not show symptoms until conditions are dire.110 Given the difficulty of diagnosing health conditions in captive wildlife, our sample of deceased gorillas likely includes both those with diagnosed conditions and those without. As such, the increased sensitivity of ALIs for predicting higher odds of mortality may be partially due to the inclusion of gorillas who had not been diagnosed with chronic conditions despite being affected.
Based on the mixed patterns observed for both all-cause morbidity and cardiac disease, we recommend testing both pooling strategies for combining individuals from multiple institutions when using allostatic load as a predictor of disease. There also were inconsistent results between indices estimated using only 1-tailed quartiles and those that had a 2-tailed split quartile for cortisol and total cholesterol. We suggest testing both 1-tailed and 2-tailed quartiles to see which works best for the biomarker in question. Such minor variations in methodologies may be useful for refining ALIs for certain health outcomes without changing their underlying composition.
One limitation of this research is the lack of biomarkers that have been explicitly linked to cardiac disease in gorillas, such as blood pressure,59,64,107,111 brain natriuretic peptide (BNP),59,64,112 and leptin and adiposity;109 including some or all of these biomarkers may be key to developing an ALI that strongly predicts risk for and potentially monitors progression of cardiac disease in great apes. The timing of sample collection presents another limitation. Samples from some individuals were collected when they were at younger ages and prior to any disease onset, whereas others had already developed chronic conditions. Larger sample sizes may permit controlling for this in the future, but as previously discussed, timing disease onset is difficult in captive wildlife. However, as great apes are routinely monitored for signs of cardiac disease, assessing timing of onset may be more feasible for this particular health outcome. Given the strong association between social status and health and longevity in humans and nonhuman primates,113-120 including rank as a mediator in the future may improve these analyses, although dominance hierarchies among female gorillas are not strong and can shift regularly.121-124 Unfortunately, as many of these data are historical, it is often not possible to know social rank at the time of sample collection because these types of social data are not routinely recorded in zoo records. Measuring changes in allostatic load over time also may be beneficial. Longitudinal studies are infrequent even in human research, but studies have shown increased mortality risk in association with both greater changes in allostatic load over time37 and higher allostatic load at baseline.45 Finally, although binomial GLMs are frequently used to assess odds of disease and mortality, we have a smaller sample size than is recommended for performing these types of analyses, although it is still possible to obtain large estimated effects despite higher P values and wider confidence intervals than may be observed with larger samples.
Conclusions
Measures of allostatic load predict mortality risk and, to a lesser extent, odds of developing chronic conditions, but currently do not predict cardiac disease in gorillas. Including total cholesterol and triglycerides in ALIs improves predictions of all-cause morbidity, cardiac disease, and mortality in zoo-housed western lowland gorillas, as evidenced by larger effect sizes for some ALIs and better goodness of fit for all models. We recommend testing different strategies for pooling data from multiple institutions and whether 1-tailed or 2-tailed split quartiles are more appropriate for the various biomarkers selected for inclusion. The ALI discussed herein is not intended to serve as the definitive model for western lowland gorillas, especially as we have estimated allostatic load using only a fraction of potential biomarkers and there may be better methods for determining which ones to include. We continue to encourage others to do as much biomarker exploration and discovery as possible and to test different methods when studying allostatic load in animals. Such efforts are key to advancing ALIs as powerful tools for predicting disease and mortality across taxa.
Supplemental Material
Supplemental material, Final_Edes_et_al_ALIs_with_lipids_supplement_02122020_xyz343751ef94532 for Allostatic Load Indices With Cholesterol and Triglycerides Predict Disease and Mortality Risk in Zoo-Housed Western Lowland Gorillas (Gorilla gorilla gorilla) by Ashley N Edes, Katie L Edwards, Barbara A Wolfe, Janine L Brown and Douglas E Crews in Biomarker Insights
Acknowledgments
The authors thank research assistants Rebecca Makii, Michelle Forman, Balbine Jourdan, and Jessica Schuster. This research would not have been possible without the assistance of veterinarians, keepers, and staff at the Columbus Zoo and Aquarium, Louisville Zoo, and Omaha’s Henry Doorly Zoo. The authors thank 4 anonymous reviewers whose feedback improved this manuscript.
Footnotes
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a Conservation and Research Grant from the Columbus Zoo and Aquarium, the Department of Anthropology at The Ohio State University, and the Endocrinology Research Laboratory at the Smithsonian Conservation Biology Institute.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: ANE conceived and designed the study, analyzed the data, and drafted and revised the manuscript; ANE and KLE performed the laboratory assays of the lipid markers and interpreted the results; KLE, BAW, JLB, and DEC revised and approved the manuscript. All authors read and approved this final manuscript.
Data Availability Statement: Data supporting the findings of this study are available on request from the corresponding author (ANE). Individually identifiable data are not publicly available due to confidential agreements made between the corresponding author (ANE) and each cooperating zoological institution.
ORCID iD: Ashley N Edes  https://orcid.org/0000-0003-2127-1179
https://orcid.org/0000-0003-2127-1179
Supplemental Material: Supplemental material for this article is available online.
References
- 1. McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med. 1993;153:2093-2101. [PubMed] [Google Scholar]
- 2. Schulkin J. Allostasis, Homeostasis, and the Costs of Physiological Adaptation. New York: Cambridge University Press; 2004. [Google Scholar]
- 3. Charmandari E, Tsigos C, Chrousos G. Endocrinology of the stress response. Annu Rev Physiol. 2005;67:259-284. [DOI] [PubMed] [Google Scholar]
- 4. Kalantaridou SN, Zoumakis E, Makrigiannakis A, Lavasidis LG, Vrekoussis T, Chrousos G. Corticotropin-releasing hormone, stress and human reproduction: an update. J Reprod Immunol. 2010;85:33-39. [DOI] [PubMed] [Google Scholar]
- 5. Möstl E, Palme R. Hormones as indicators of stress. Domest Anim Endocrinol. 2002;23:67-74. [DOI] [PubMed] [Google Scholar]
- 6. Breuner CW, Patterson SH, Hahn TP. In search of relationships between the acute adrenocortical response and fitness. Gen Comp Endocrinol. 2008;157: 288-295. [DOI] [PubMed] [Google Scholar]
- 7. Bonier F, Moore IT, Martin PR, Robertson RJ. The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen Comp Endocrinol. 2009;163:208-213. [DOI] [PubMed] [Google Scholar]
- 8. Busch DS, Hayward LS. Stress in a conservation context: a discussion of glucocorticoid actions and how levels change with conservation-relevant variables. Biol Conserv. 2009;142:2844-2853. [Google Scholar]
- 9. Cockrem JF. Individual variation in glucocorticoid stress responses in animals. Gen Comp Endocrinol. 2013;181:45-58. [DOI] [PubMed] [Google Scholar]
- 10. Crespi EJ, Williams TD, Jessop TS, Delehanty B. Life history and the ecology of stress: how do glucocorticoid hormones influence life-history variation in animals? Funct Ecol. 2013;27:93-106. [Google Scholar]
- 11. Dickens MJ, Romero LM. A consensus endocrine profile for chronically stressed wild animals does not exist. Gen Comp Endocrinol. 2013;191:177-189. [DOI] [PubMed] [Google Scholar]
- 12. Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ. Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv Physiol. 2014;2:cou023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Madliger CL, Love OP. The need for a predictive, context-dependent approach to the application of stress hormones in conservation. Conserv Biol. 2014;28: 283-287. [DOI] [PubMed] [Google Scholar]
- 14. MacDougall-Shackleton SA, Bonier F, Romero LM, Moore IT. Glucocorticoids and “stress” are not synonymous [published online ahead of print March 13, 2020]. Integr Org Biol. doi: 10.1093/iob/obz017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McEwen BS. What is the confusion with cortisol? Chronic Stress. 2019;3:1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Edwards KL, Edes AN, Brown JL. Stress, well-being and reproductive success. In: Comizzoli P, Brown JL, Holt WV, eds. Reproductive Sciences in Animal Conservation. 2nd ed. Cham: Springer; 2019:91-162. [Google Scholar]
- 17. Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35:2-16. [DOI] [PubMed] [Google Scholar]
- 18. Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs. 2012;14:311-346. [DOI] [PubMed] [Google Scholar]
- 19. Edes AN, Crews DE. Allostatic load and biological anthropology. Am J Phys Anthropol. 2017;162:44-70. [DOI] [PubMed] [Google Scholar]
- 20. Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation—allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997;157:2259-2268. [PubMed] [Google Scholar]
- 21. Mattei J, Demissie S, Falcon LM, Ordovas JM, Tucker K. Allostatic load is associated with chronic conditions in the Boston Puerto Rican Health Study. Soc Sci Med. 2010;70:1988-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Seeman TE, Singer BH, Ryff CD, Dienberg Love G, Levy-Storms L. Social relationships, gender, and allostatic load across two age cohorts. Psychosom Med. 2002;64:395-406. [DOI] [PubMed] [Google Scholar]
- 23. Karlamangla AS, Singer BH, McEwen BS, Rowe JW, Seeman TE. Allostatic load as a predictor of functional decline. MacArthur studies of successful aging. J Clin Epidemiol. 2002;55:696-710. [DOI] [PubMed] [Google Scholar]
- 24. Nelson KM, Reiber G, Kohler T, Boyko EJ. Peripheral arterial disease in a multiethnic national sample: the role of conventional risk factors and allostatic load. Ethn Dis. 2007;17:669-675. [PubMed] [Google Scholar]
- 25. Logan JG, Barksdale DJ. Allostasis and allostatic load: expanding the discourse on stress and cardiovascular disease. J Clin Nurs. 2008;17:201-208. [DOI] [PubMed] [Google Scholar]
- 26. Sabbah W, Watt RG, Sheiham A, Tsakos G. Effects of allostatic load on the social gradient in ischaemic heart disease and periodontal disease: evidence from the Third National Health and Nutrition Examination Survey. J Epidemiol Community Health. 2008;62:415-420. [DOI] [PubMed] [Google Scholar]
- 27. Slade GD, Sanders AE, By K. Role of allostatic load in socio-demographic patterns of pain prevalence in the U.S. population. J Pain. 2012;13:666-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gruenewald TL, Seeman TE, Karlamangla AS, Sarkisian CA. Allostatic load and frailty in older adults. J Am Geriatr Soc. 2009;57:1525-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Szanton SL, Allen JK, Seplaki CL, Bandeen-Roche K, Fried LP. Allostatic load and frailty in the women’s health and aging studies. Biol Res Nurs. 2009;10:248-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zota AR, Shenassa ED, Morello-Frosch R. Allostatic load amplifies the effect of blood lead levels on elevated blood pressure among middle-aged U.S. adults: a cross-sectional study. Environ Health. 2013;12:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ottino-González J, Jurado MA, Garcia-Garcia I, et al. Allostatic load is linked to cortical thickness changes depending on body-weight status. Front Hum Neurosci. 2017;11:639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borrell LN, Crawford ND. Social disparities in periodontitis among U.S. adults: the effect of allostatic load. J Epidemiol Community Health. 2011;65:144-149. [DOI] [PubMed] [Google Scholar]
- 33. Chen X, Redline S, Shields AE, Williams DR, Williams MA. Associations of allostatic load with sleep apnea, insomnia, short sleep duration, and other sleep disturbances: findings from the National Health and Nutrition Examination Survey 2005 to 2008. Ann Epidemiol. 2014;24:612-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A. 2001;98:4770-4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Seeman TE, Glei DA, Goldman N, Weinstein M, Singer BH, Lin Y-H. Social relationships and allostatic load in Taiwanese elderly and near elderly. Soc Sci Med. 2004;59:2245-2257. [DOI] [PubMed] [Google Scholar]
- 36. Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci U S A. 2006;103:14158-14163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk: MacArthur studies of successful aging. Psychosom Med. 2006;68:500-507. [DOI] [PubMed] [Google Scholar]
- 38. Borrell LN, Dallo FJ, Nguyen N. Racial/ethnic disparities in all-cause mortality in U.S. adults: the effect of allostatic load. Public Health Rep. 2010;125:810-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duru OK, Harawa NT, Kermah D, Norris KC. Allostatic load burden and racial disparities in mortality. J Natl Med Assoc. 2012;104:89-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Glei DA, Goldman N, Rodríguez G, Weinstein M. Beyond self-reports: changes in biomarkers as predictors of mortality. Popul Dev Rev. 2014;40:331-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hwang A-C, Peng L-N, Wen Y-W, et al. Predicting all-cause and cause-specific mortality by static and dynamic measurements of allostatic load: a 10-year population-based cohort study in Taiwan. J Am Med Dir Assoc. 2014;15: 490-496. [DOI] [PubMed] [Google Scholar]
- 42. Levine ME, Crimmins EM. A comparison of methods for assessing mortality risk. Am J Hum Biol. 2014;26:768-776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Howard JT, Sparks PJ. The effects of allostatic load on racial/ethnic mortality differences in the United States. Popul Res Policy Rev. 2016;35:421-443. [Google Scholar]
- 44. Robertson T, Beveridge G, Bromley C. Allostatic load as a predictor of all-cause and cause-specific mortality in the general population: evidence from the Scottish Health Survey. PLoS ONE. 2017;12:e0183297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Castagné R, Garès V, Karimi M, et al. Allostatic load and subsequent all-cause mortality: which biological markers drive the relationship? findings from a UK birth cohort. Eur J Epidemiol. 2018;33:441-458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Howard JT, Sparks PJ. Does allostatic load calculation method matter? evaluation of different methods and individual biomarkers functioning by race/ethnicity and educational level. Am J Hum Biol. 2016;28:627-635. [DOI] [PubMed] [Google Scholar]
- 47. Gustafsson PE, Janlert U, Theorell T, Westerlund H, Hammarström A. Socioeconomic status over the life course and allostatic load in adulthood: results from the Northern Swedish Cohort. J Epidemiol Community Health. 2011;65: 986-992. [DOI] [PubMed] [Google Scholar]
- 48. Read S, Grundy E. Allostatic Load—A Challenge to Measure Multisystem Physiological Dysregulation. London, England: National Centre for Research Methods; 2012:1-9. [Google Scholar]
- 49. Gallo LC, Fortmann AL, Mattei J. Allostatic load and the assessment of cumulative biological risk in biobehavioral medicine: challenges and opportunities. Psychosom Med. 2014;76:478-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mauss D, Li J, Schmidt B, Angerer P, Jarczok MN. Measuring allostatic load in the workforce: a systematic review. Ind Health. 2015;53:5-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Delpierre C, Barbosa-Solis C, Torrisani J, et al. Origins of health inequalities: the case for allostatic load. Longit Life Course Stud. 2016;7:79-103. [Google Scholar]
- 52. Duong MT, Bingham BA, Aldana PC, Chung ST, Sumner AE. Variation in the calculation of allostatic load score: 21 examples from NHANES. J Racial Ethn Health Disparities. 2017;4:455-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wallace ME, Harville EW. Allostatic load and birth outcomes among white and black women in New Orleans. Matern Child Health J. 2013;17:1025-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prior L, Manley D, Jones K. Stressed out? an investigation of whether allostatic load mediates associations between neighbourhood deprivation and health. Health Place. 2018;52:25-33. [DOI] [PubMed] [Google Scholar]
- 55. Baitchman EJ, Calle PP, Clippinger TL, et al. Preliminary evaluation of blood lipid profiles in captive western lowland gorillas. J Zoo Wildl Med. 2006;37: 126-129. [DOI] [PubMed] [Google Scholar]
- 56. Schmidt DA, Ellersieck MR, Cranfield MR, Karesh WB. Cholesterol values in free-ranging gorillas (Gorilla gorilla gorilla and Gorilla beringei) and Bornean orangutans (Pongo pygmaeus). J Zoo Wildl Med. 2006;37:292-300. [DOI] [PubMed] [Google Scholar]
- 57. Varki N, Anderson D, Herndon JG, et al. Heart disease is common in humans and chimpanzees, but is caused by different pathological processes. Evol Appl. 2009;2:101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Videan EN, Heward CB, Chowdhury K, Plummer J, Su Y, Cutler RG. Comparison of biomarkers of oxidative stress and cardiovascular disease in humans and chimpanzees (Pan troglodytes). Comp Med. 2009;59:287-296. [PMC free article] [PubMed] [Google Scholar]
- 59. McManamon R, Lowenstine L. Cardiovascular disease in great apes. In: Miller RE, Fowler ME, eds. Fowler’s Zoo and Wild Animal Medicine: Current Therapy. Vol 7 St. Louis, MO: Elsevier; 2012:408-415. [Google Scholar]
- 60. Lowenstine LJ, McManamon R, Terio KA. Comparative pathology of aging great apes: bonobos, chimpanzees, gorillas, and orangutans. Vet Pathol. 2016; 53:250-276. [DOI] [PubMed] [Google Scholar]
- 61. Strong VJ, Grindlay D, Redrobe S, Cobb M, White K. A systematic review of the literature relating to captive great ape morbidity and mortality. J Zoo Wildl Med. 2016;47:697-710. [DOI] [PubMed] [Google Scholar]
- 62. Strong VJ, Baiker K, Brennan ML, et al. A retrospective review of western lowland gorilla (Gorilla gorilla gorilla) mortality in European zoologic collections between 2004 and 2014. J Zoo Wildl Med. 2017;48:277-286. [DOI] [PubMed] [Google Scholar]
- 63. Strong VJ, Martin M, Redrobe S, White K, Baiker K. A retrospective review of great ape cardiovascular disease epidemiology and pathology. Int Zoo Yearbook. 2018;52:113-125. [Google Scholar]
- 64. Murphy HW, Danforth MD, Clyde VL. The great ape heart project. Int Zoo Yearbook. 2018;52:103-112. [Google Scholar]
- 65. Edes AN, Wolfe BA, Crews DE. Testing a method to improve predictions of disease and mortality risk in western lowland gorillas (Gorilla gorilla gorilla) using allostatic load. Stress. In press. [DOI] [PubMed] [Google Scholar]
- 66. Edes AN, Wolfe BA, Crews DE. Assessing stress in zoo-housed western lowland gorillas (Gorilla gorilla gorilla) using allostatic load. Int J Primatol. 2016; 37:241-259. [Google Scholar]
- 67. Edes AN, Wolfe BA, Crews DE. Rearing history and allostatic load in adult western lowland gorillas (Gorilla gorilla gorilla) in human care. Zoo Biol. 2016;35:167-173. [DOI] [PubMed] [Google Scholar]
- 68. Edes AN, Wolfe BA, Crews DE. The first multi-zoo application of an allostatic load index to western lowland gorillas (Gorilla gorilla gorilla). Gen Comp Endocrinol. 2018;266:135-149. [DOI] [PubMed] [Google Scholar]
- 69. Harder JD. Reproduction and hormones. In: Silvy NJ, ed. The Wildlife Techniques Manual. Vol 1 Baltimore, MD: Johns Hopkins University Press; 2012:502-525. [Google Scholar]
- 70. Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59:551-555. [DOI] [PubMed] [Google Scholar]
- 71. Latendresse G, Ruiz RJ. Bioassay research methodology: measuring CRH in pregnancy. Biol Res Nurs. 2008;10:54-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Linton EA, McLean C, Nieuwenhuyzen Kruseman AC, Tilders FJ, Van der Veen EA, Lowry PJ. Direct measurement of human plasma corticotropin-releasing hormone by “two-site” immunoradiometric assay. J Clin Endocrinol Metab. 1986;64:1047-1053. [DOI] [PubMed] [Google Scholar]
- 73. Tworoger SS, Hankinson SE. Collection, processing, and storage of biological samples in epidemiologic studies: sex hormones, carotenoids, inflammatory markers, and proteomics as examples. Cancer Epidemiol Biomarkers Prev. 2006;15:1578-1581. [DOI] [PubMed] [Google Scholar]
- 74. Shih WJ, Bachorik PS, Haga JA, Myers GL, Stein EA. Estimating the long-term effects of storage at -70 degrees C on cholesterol, triglyceride, and HDL-cholesterol measurements in stored sera. Clin Chem. 2000;46: 351-364. [PubMed] [Google Scholar]
- 75. Jansen EH, Beekhof PK, Schenk E. Long term stability of parameters of lipid metabolism in frozen human serum: triglycerides, free fatty acids, total-, HDL- and LDL-cholesterol, Apolipoprotein-A1 and B. J Mol Biomark Diagn. 2014;5:182. [Google Scholar]
- 76. Arts EEA, Popa CD, Smith JP, et al. Serum samples that have been stored long-term (>10 years) can be used as a suitable data source for developing cardiovascular risk prediction models in large observational rheumatoid arthritis cohorts. Biomed Res Int. 2014;2014:930925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ellis MJ, Livesey JH, Donald RA. Circulating plasma corticotrophin-releasing factor-like immunoreactivity. J Endocrinol. 1988;117:299-307. [DOI] [PubMed] [Google Scholar]
- 78. Linton EA, Perkins AV, Hagan P, et al. Corticotrophin-releasing hormone (CRH)-binding protein interference with CRH antibody binding: implications for direct CRH immunoassay. J Endocrinol. 1995;146:45-53. [DOI] [PubMed] [Google Scholar]
- 79. Levine S, Coe CL, Wiener SG. Psychoneuroendocrinology of stress: a psychobiological perspective. In: Brush FR, Levine S, eds. Psychoendocrinology. San Diego, CA: Academic Press; 1989:341-377. [Google Scholar]
- 80. Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554-1565. [DOI] [PubMed] [Google Scholar]
- 81. Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000;25:1-35. [DOI] [PubMed] [Google Scholar]
- 82. Badanes LS, Watamura SE, Hankin BL. Hypocortisolism as a potential marker of allostatic load in children: associations with family risk and internalizing disorders. Dev Psychopathol. 2011;23:881-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kark JD, Smith AH, Hames CG. The relationship of serum cholesterol to the incidence of cancer in Evans County, Georgia. J Chronic Dis. 1980;33: 311-332. [DOI] [PubMed] [Google Scholar]
- 84. Schatzkin A, Hoover RN, Taylor PR, et al. Serum cholesterol and cancer in the NHANES I epidemiologic followup study. National Health and Nutrition Examination Survey. Lancet. 1987;330:298-301. [DOI] [PubMed] [Google Scholar]
- 85. Forette B, Tortrat D, Wolmark Y. Cholesterol as risk factor for mortality in elderly women. Lancet. 1989;1:868-870. [DOI] [PubMed] [Google Scholar]
- 86. Isles CG, Hole DJ, Gillis CR, Hawthorne VM, Lever AF. Plasma cholesterol, coronary heart disease, and cancer in the Renfrew and paisley survey. BMJ. 1989;298:920-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Iso H, Jacobs DR, Jr, Wentworth D, Neaton JD, Cohen JD. Serum cholesterol levels and six-year mortality from stroke in 350,977 men screened for the multiple risk factor intervention trial. N Engl J Med. 1989;320:904-910. [DOI] [PubMed] [Google Scholar]
- 88. Harris T, Feldman JJ, Kleinman JC, Ettinger WH, Jr, Makuc DM, Schatzkin AG. The low cholesterol-mortality association in a national cohort. J Clin Epidemiol. 1992;45:595-601. [DOI] [PubMed] [Google Scholar]
- 89. Kronmal RA, Cain KC, Ye Z, Omenn GS. Total serum cholesterol levels and mortality risk as a function of age. A report based on the Framingham data. Arch Intern Med. 1993;153:1065-1073. [PubMed] [Google Scholar]
- 90. Muldoon MF, Rossouw JE, Manuck SB, Glueck CJ, Kaplan JR, Kaufmann PG. Low or lowered cholesterol and risk of death from suicide and trauma. Metabolism. 1993;42:45-56. [DOI] [PubMed] [Google Scholar]
- 91. Weverling-Rijnsburger AWE, Blauw GJ, Lagaay AM, Knook DL, Meinders AE, Westendorp RGJ. Total cholesterol and risk of mortality in the oldest old. Lancet. 1997;350:1119-1123. [DOI] [PubMed] [Google Scholar]
- 92. Schatz IJ, Masaki K, Yano K, Chen R, Rodriguez BL, Curb JD. Cholesterol and all-cause mortality in elderly people from the Honolulu Heart Program: a cohort study. Lancet. 2001;358:351-355. [DOI] [PubMed] [Google Scholar]
- 93. Horwich TB, Hamilton MA, Maclellan WR, Fonarow GC. Low serum total cholesterol is associated with marked increase in mortality in advanced heart failure. J Card Fail. 2002;8:216-224. [DOI] [PubMed] [Google Scholar]
- 94. McShane BB, Gal D, Gelman A, Robert C, Tackett JL. Abandon statistical significance. Am Stat. 2019;73:235-245. [Google Scholar]
- 95. R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- 96. Bates D, Maechler M, Bolker B, et al. lme4: linear mixed-effects models using “Eigen” and S4. R package version 1; 2015:1-19. https://cran.r-project.org/package=lme4. Published 2015. [Google Scholar]
- 97. Verbrugge LM. Sex differentials in health. Public Health Rep. 1982;97:417-437. [PMC free article] [PubMed] [Google Scholar]
- 98. Verbrugge LM, Wingard DL. Sex differentials in health and mortality. Health Matrix. 1987;5:3-19. [PubMed] [Google Scholar]
- 99. Crimmins EM, Hayward MD, Saito Y. Differentials in active life expectancy in the older population of the United States. J Gerontol B Psychol Sci Soc Sci. 1996;51:S111-S120. [DOI] [PubMed] [Google Scholar]
- 100. Crimmins EM, Kim JK, Hagedorn A. Life with and without disease: women experience more of both. J Women Aging. 2002;14:47-59. [DOI] [PubMed] [Google Scholar]
- 101. Crimmins EM, Kim JK, Solé-Auró A. Gender differences in health: results from SHARE, ELSA and HRS. Eur J Public Health. 2011;21:81-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Meehan TP, Lowenstine LJ. Causes of mortality in captive lowland gorillas: a survey of the SSP population. In: Baer CK, ed. Proceedings of the American Association of Zoo Veterinarians Annual Meeting. Pittsburgh, PA: American Association Zoo Veterinarians; 1994:216-218. [Google Scholar]
- 103. Dybas CL. Out of Africa: a tale of gorillas, heart disease. . . and a swamp plant. Bioscience. 2007;57:392-397. [Google Scholar]
- 104. Turesson C, Bergström U, Pikwer M, Nilsson J-Å, Jacobsson LT. High serum cholesterol predicts rheumatoid arthritis in women, but not in men: a prospective study. Arthritis Res Ther. 2015;17:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. McClure HM, Keeling ME, Guilloud NB. Hematologic and blood chemistry data for the gorilla (Gorilla gorilla). Folia Primatol (Basel). 1972;18:300-316. [DOI] [PubMed] [Google Scholar]
- 106. McGuire JT, Dierenfeld ES, Poppenga RH, Brazelton WE. Plasma alpha-tocopherol, retinol, cholesterol, and mineral concentrations in captive gorillas. J Med Primatol. 1989;18:155-161. [PubMed] [Google Scholar]
- 107. Junge RE, Mezei LE, Muhlbauer M, Weber M. Cardiovascular evaluation of lowland gorillas. J Am Vet Med Assoc. 1998;212:413-415. [PubMed] [Google Scholar]
- 108. Crissey SD, Barr JE, Slifka KA, et al. Serum concentrations of lipids, vitamins A and E, vitamin D metabolites, and carotenoids in nine primate species at four zoos. Zoo Biology. 1999;18:551-564. [Google Scholar]
- 109. Dennis PM, Raghanti MA, Meindl RS, et al. Cardiac disease is linked to adiposity in male gorillas (Gorilla gorilla gorilla). PLoS ONE. 2019;14:e0218763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Markowitz H. Behavioral Enrichment in the Zoo. New York, NY: Van Nostrand Reinhold; 1982. [Google Scholar]
- 111. Murphy HW, Dennis P, Devlin W, Meehan T, Kutinsky I. Echocardiographic parameters of captive western lowland gorillas (Gorilla gorilla gorilla). J Zoo Wildl Med. 2011;42:572-579. [DOI] [PubMed] [Google Scholar]
- 112. Murray S, Kishbaugh JC, Hayek L-AC, et al. Diagnosing cardiovascular disease in western lowland gorillas (Gorilla gorilla gorilla) with brain natriuretic peptide. PLoS ONE. 2019;14:e0214101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310-357. [PubMed] [Google Scholar]
- 114. House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540-545. [DOI] [PubMed] [Google Scholar]
- 115. Schwarzer R, Leppin A. Social support and health: a meta-analysis. Psychol Health. 1989;3:1-15. [Google Scholar]
- 116. Uchino BN. Social support and health: a review of physiological processes potentially underlying links to disease outcomes. J Behav Med. 2006;29:377-387. [DOI] [PubMed] [Google Scholar]
- 117. Smith KP, Christakis NA. Social networks and health. Annu Rev Sociol. 2008;34:405-429. [Google Scholar]
- 118. Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Silk JB, Beehner JC, Bergman TJ, et al. Strong and consistent social bonds enhance the longevity of female baboons. Curr Biol. 2010;20:1359-1361. [DOI] [PubMed] [Google Scholar]
- 120. Archie EA, Tung J, Clark M, Altmann J, Alberts SC. Social affiliation matters: both same-sex and opposite-sex relationships predict survival in wild female baboons. Proc Biol Sci. 2014;281:20141261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Harcourt AH. Social relationships among adult female mountain gorillas. Anim Behav. 1979;27:251-264. [Google Scholar]
- 122. Mace G. Birth sex ratio and infant mortality rates in captive western lowland gorillas. Folia Primatol. 1990;55:156-165. [Google Scholar]
- 123. Watts DP. Social relationships of immigrant and resident female mountain gorillas. I. Male-female relationships. Am J Primatol. 1992;28:159-181. [DOI] [PubMed] [Google Scholar]
- 124. Watts DP. Social relationships of immigrant and resident female mountain gorillas, II: relatedness, residence, and relationships between females. Am J Primatol. 1994;32:13-30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Final_Edes_et_al_ALIs_with_lipids_supplement_02122020_xyz343751ef94532 for Allostatic Load Indices With Cholesterol and Triglycerides Predict Disease and Mortality Risk in Zoo-Housed Western Lowland Gorillas (Gorilla gorilla gorilla) by Ashley N Edes, Katie L Edwards, Barbara A Wolfe, Janine L Brown and Douglas E Crews in Biomarker Insights