Abstract
Background:
The prevalence of frailty is disproportionately increased in patients with chronic kidney disease (CKD) in comparison with non-CKD counterparts and is the highest in patients on hemodialysis (HD). While the cross-sectional measurement of frailty on HD has been associated with adverse clinical events, there is a paucity of data on longitudinal assessment of frailty and its relationship to outcomes.
Objective:
The objectives were to (1) evaluate changes in frailty status, level of independence, mood, cognition, and quality of life (QoL) over a 12-month period and (2) explore the relationship between frailty status and level of independence, mood, cognition, and QoL at 2 different time points (at baseline and at 1 year).
Design:
This is a prospective cohort study involving 100 prevalent HD patients.
Setting:
Regina General Hospital and Wascana Dialysis Unit in Regina, Saskatchewan, Canada, between January 2015 and January 2017.
Patients:
One hundred prevalent HD patients underwent frailty assessments using the Fried criteria at baseline and 1 year later.
Measurements:
Frailty was assessed using the Fried criteria, which included assessments of unintentional weight loss, weakness (handgrip strength), slowness (walking speed), and questionnaires for physical activity and self-perceived exhaustion. Cognition, mood, and QoL were measured using questionnaires (Montreal Cognitive Assessment [MoCA], Geriatric Depression Scale [GDS], and EuroQol [EQ-5D] utility scores and visual analog scale [VAS], respectively).
Methods:
Frailty status was reported as a binary variable: frail vs. nonfrail (prefrail and robust). Differences across baseline and 1-year groups were assessed using McNemar’s test or Wilcoxon signed-rank test, as appropriate. We assessed the differences between frail and nonfrail groups using the Mann–Whitney U test or chi-square test/Fisher’s exact test where appropriate.
Results:
Ninety-seven of the 100 patients had complete initial assessments. The median (interquartile range [IQR]) duration of dialysis at baseline was 35.5 (13.75-71.75 months). One year later, 22 had died, 10 refused assessments, and 3 had relocated. In comparison with baseline vs 1 year, the number of frail patients was 68.1% vs. 67.7%; prefrail 26.8% vs. 26.1%; robust 5.1% vs. 6.2%; MoCA ≥24, 69% vs. 64.5%; GDS score ≥ 2, 52.8% vs. 47.7%; median EQ-5D utility score 0.81 vs. 0.77; and median EQ-VAS 60 vs. 50. Similarly, in comparison with baseline vs. 1 year, the number of independent patients was 82% vs. 63%, independent with support 17% vs. 31%, and long-term care home 0% vs. 3.1%. Eighteen of the 22 patients (82%) who died were frail. At 1 year, the median (IQR) MoCA was 24 (19-25) vs. 25 (21-26; P = .039) and median (IQR) GDS was 2 (1-3) vs. 1(0-2; P = .034). Likewise, median (IQR) EQ-5D utility score was 0.78 (0.6-0.82) vs. 0.81 (0.78-0.85; P = .023). There were significant changes in self-care (27% vs. 0%), P = 0.006, and daily activities (68.2% vs. 38.1%), P = 0.021.
Limitations:
This is a single-center study, so direct inferences must be interpreted in the context of the demographics of the study population. Patients were undergoing dialysis for a median of 36 months before undergoing initial assessment.
Conclusions:
Frailty and prefrailty in our dialysis patients is near-ubiquitous and will need to be proactively addressed to improve subsequent health care outcomes.
Keywords: frailty, Fried frailty criteria, hemodialysis, level of independence, mood, depression, cognition, quality of life (QoL)
Abrégé
Contexte:
La prévalence de la fragilité augmente de façon disproportionnée chez les patients atteints d’insuffisance rénale chronique (IRC) comparativement aux patients non-IRC, et est encore plus élevée chez les patients hémodialysés. Bien que la mesure transversale de la fragilité en hémodialyse soit associée à des événements cliniques indésirables, très peu de données existent sur cette mesure et sur son lien avec les résultats.
Objectifs:
Les objectifs étaient: 1) évaluer les changements dans l’état de fragilité, le niveau d’indépendance, l’humeur, la cognition et la qualité de vie (QdV) sur une période de 12 mois et; 2) explorer la relation entre l’état de fragilité et ces mêmes facteurs à deux moments précis, soit à l’inclusion et après un an.
Type d’étude:
Étude de cohorte prospective portant sur 100 patients hémodialysés.
Cadre:
L’hôpital général et l’unité de dialyse Wascana Dialysis de Régina, en Saskatchewan (Canada) entre janvier 2015 et janvier 2017.
Sujets:
Les critères de Fried ont servi à évaluer la fragilité de 100 patients hémodialysés à l’inclusion et après douze mois.
Mesures:
La fragilité a été évaluée selon les critères de Fried, soit un questionnaire mesurant l’activité physique et le niveau d’épuisement perçu, ainsi que des évaluations pour une perte de poids involontaire, la faiblesse (force de préhension) et la lenteur (vitesse de marche). Des questionnaires ont servi à évaluer la cognition (Montreal Cognitive Assessment [MoCA]), l’humeur (échelle de dépression gériatrique [EDG]) et la QdV (scores d’utilité de l’EuroQol [EQ-5D] et échelle analogue visuelle [VAS]).
Méthodologie:
La fragilité a été rapportée comme une variable binaire: fragile ou non fragile (préfragile et robuste). Les différences de fragilité entre l’inclusion et un an ont été déterminées par le test McNemar ou par le test de rang de Wilcoxon, selon le cas. Les différences entre les groupes fragiles et non fragiles ont été déterminées par le test U de Mann–Whitney U test ou le test de Chi-Deux/test exact de probabilité de Fisher, le cas échéant.
Résultats:
Sur les 100 sujets retenus, 97 avaient complété les évaluations initiales. À l’inclusion, les patients étaient en hémodialyse depuis une période médiane (EIQ) de 35,5 mois (13,75 à 71,75 mois). Un an plus tard, 22 patients étaient décédés, dix ont refusé d’être évalués et trois étaient relocalisés. La proportion de patients jugés fragiles s’établissait à 68,1 % à l’inclusion et à 67,7 % après un an. Ces proportions étaient de 26,8 % contre 26,1 % pour les patients jugés préfragiles et de 5,1 % contre 6,2 % pour les patients robustes. Les patients avaient obtenu un score égal ou supérieur à 24 pour le MoCA dans une proportion de 69 % à l’inclusion contre 64,5 % un an plus tard. Ces mêmes proportions s’établissaient à 52,8 % contre 47,7 % pour un score égal ou supérieur à 2 pour l’EDG. La médiane du score d’utilité EQ-5D était de 0,81 à l’inclusion et de 0,77 un an plus tard, alors que le score médian à la VAS était de 60 contre 50. Parallèlement, la proportion de patients indépendants est passée de 82 % à l’inclusion à 63 % un an plus tard, les patients indépendants avec support sont passés de 17 à 31% et les patients en centre de soins de longue durée de 0 à 3,1 %. La grande majorité des patients décédés (18/22; 82%) étaient jugés fragiles. Après un an, le score médian (IQR) au MoCA était de 24 (19-25) pour les patients fragiles contre 25 (21-26), p=0.039 pour les non fragiles. Respectivement, le score médian (IQR) à l’EDG était de 2 (1-3) contre 1 (0-2), p=0,034, et la médiane (IQR) au score d’utilité EQ-5D était de 0,78 (0,6-0,82) contre 0,81 (0,78-0,85), p=0,023. Une différence significative a été observée dans l’autonomie des patients (27% contre 0%; p=0,006) et dans la capacité de vaquer aux activités quotidiennes (68,2 % contre 38,1 %; p=0,021).
Limites:
Il s’agit d’une étude monocentrique et ainsi, les interférences directes doivent être interprétées dans le contexte démographique de la population étudiée. Les patients étaient traités en hémodialyse depuis 36 mois (médiane) avant leur première évaluation.
Conclusion:
La fragilité et la préfragilité est omniprésente chez les patients hémodialysés et devra être adressée de façon proactive pour améliorer les résultats en santé.
What was known before
Patients on hemodialysis (HD) are disproportionately frail in comparison with age-matched non-HD counterparts. Cross-sectional measurements of frailty on HD are associated with adverse outcomes that are well documented.
What this adds
This is the first Canadian study looking at longitudinal frailty assessments in patients on hemodialysis (HD). The study explores the relationship between the frailty status and level of independence, mood, cognition, and QoL at 2 different time points (at baseline and at 1 year).
Introduction
Frailty is an all-embracing syndrome of diminished physiological reserve to stressors resulting in reduced physical ability and increased vulnerability to hospitalization and mortality.1 In the non-chronic kidney disease (CKD) population, frailty has been linked to a variety of outcomes, such as falls,2 fractures,3 dementia,4 and hospitalization.5 In addition, there are published data on cross-sectional frailty assessments and their subsequent relationship to mobility,1 quality of life (QoL),6 depression,7 cognitive decline,8 nursing home admissions,9 and disability.10 The prevalence of frailty is disproportionately increased in patients with CKD in comparison with non-CKD counterparts.11,12 It is the highest in patients with end-stage kidney disease (ESKD).13 The most widely used definition in literature is known as the Fried frailty phenotype.14 It is based on 5 physical domains that can be assessed by self-report (weight loss, low physical activity, and exhaustion) and objective measures (weakness and slow gait speed). Based on the number of deficits, individuals are characterized as robust (0), prefrail (1-2/5), and frail (≥3/5).1
In a meta-analysis by Kojima and published in 2017, the pooled prevalence of measured frailty among patients on dialysis was 36.8% and upon self-report was 67.0%.15 Most studies on patients receiving hemodialysis (HD) have used 1-time measures of frailty (either cross-sectionally or at dialysis initiation) in determining subsequent health outcomes.16,17 There is a paucity of longitudinal studies addressing changes in frailty status to level of dependence, decline in cognition, mood, and QoL. The associations between changes in frailty to the level of independence and the subsequent need for institutionalization have not yet been explored in detail. In this prospective study, we attempted to (1) evaluate changes in frailty status, level of independence, mood, cognition, and QoL over a 12-month period and (2) explore the relationship between frailty status and level of independence, mood, cognition, and QoL at 2 different time points (at baseline and at 1 year).
Methods
Study Design
We analyzed a cohort of 100 prevalent HD patients at the Regina General Hospital and Wascana Dialysis Unit in Regina, Saskatchewan, Canada, between January 2015 and January 2017. The patients underwent baseline and 1-year follow-up frailty assessments, as routine clinical care. The patients were recruited if they met the following inclusion criteria: (1) age >18 years, (2) on scheduled dialysis, (3) on dialysis for >6 months, (4) fluency in English, and (5) sufficient visual and hearing acuity. The exclusion criteria included the following: (1) patients on dialysis with acute kidney injury with a likelihood of recovery, (2) transient in center dialysis (3) concurrent treatment on peritoneal dialysis or home HD, (4) active malignancy, (5) imminent geographic relocation, (6) pregnancy, (7) significant mental illness, and (8) previous or approved transplants. The Research Ethics Board of the former Regina Qu’Appelle Health Region (REB–19–17) approved the study.
Patient Characteristics
Demographic variables (age, gender, and ethnicity) were ascertained from patient charts. Age was categorized as a binary variable (≥ or <65 years). The level of education was enquired during assessment. Body mass index (BMI) was calculated in kilogram/square meter from self-reported height and pre-HD weight at the time of assessment. Self-reported outcomes (level of independence) were recorded at each assessment. Comorbidities (diabetes [type I or II], hypertension, dyslipidemia, and peripheral vascular disease), predialysis blood pressure, and length of time since dialysis initiation were obtained from electronic medical records—Medical Information Quality System (MIQS, Denver, Colorado, USA).
Frailty was measured using the Fried frailty phenotype, a valid measure of frailty that is used as a screening tool in CKD literature. Fried frailty phenotype scores were assessed at cohort entry and 1-year follow-up (in patients who survived). All physical assessments were completed before dialysis, and questionnaires were completed during the first hour of HD treatment. The criteria include 5 physical components: slowness, weakness, weight loss, low physical activity, and exhaustion. Each component received a score of 0 or 1 based on the following criteria (Supplemental Appendix 1). Patients with a score of 3 or more were classified as frail, a score of 1 to 2 were defined as prefrail, and a score of 0 were considered robust. In addition to frailty assessments, patients underwent questionnaires for cognitive assessment, depression, and QoL. They were enquired about their levels of dependence with activities of daily living. Patients were approached by phone on nondialysis days, and a mutually agreed time and date were identified for assessment upon obtaining verbal consent for frailty assessment.
Slowness was assessed based on a 4-m walk (walking speed >5 seconds). The time taken for the patient to walk was measured. The average time of the 2 walks was used for scoring. If the patient was unable to walk, then speed was not recorded, received a score of 1, and were categorized as slow. If they walked with assistance, then the average time of 2 walks was used.
Weakness was assessed on handgrip strength measured using Jamar Hydraulic Hand Dynamometer (Model 5030J1, Sammons Preston Rolyan, Bolingbrook, IL, USA). Patients performed 2 attempts on the nonfistula arm. The highest value in kilograms (kg) for muscle strength was used to calculate the score. A score below an established cutoff, based on gender (male ≤30 kg, female ≤20kg), was considered as weakness.
Weight loss was based on unintentional ≥4.5-kg (predialysis) reduction in weight over the preceding 12 months.
Low physical activity was determined by the Paffenbarger Physical Activity Index Questionnaire (Supplemental Appendix 2). The patients were asked about the frequency and duration of activities over 1 week. A score below an established cutoff, based on gender (male <383 kcal/wk, female <270 kcal/wk), was considered as low activity.
Exhaustion was based on 2 questions from the Center for Epidemiologic Studies Depression (CES-D) scale (Supplemental Appendix 3). A score above an established cutoff (≥2) was defined as exhaustion.
Cognitive function was measured at each assessment visit using the Montreal Cognitive Assessment (MoCA, English version 7.1 available at www.mocatest.org; Supplemental Appendix 4). The test was conducted before HD or during the first hour of the session. The MoCA is highly sensitive and evaluates multiple cognitive domains, including visuospatial ability and executive function (/5), naming (/3), memory (/5), attention (/6), language (/3), abstraction (/2), and orientation (/6). The total possible score is 30 points. However, for participants with ≤12 years of formal education, 1 extra point is added. The cutoff score ≤ 24 was considered cognitive impairment.18,19
Depression
Five-item Geriatric Depression Scale (GDS) screen questionnaire was administered to identify symptoms of depression in patients at baseline and follow-up assessment. The test was developed by Hoyl et al in 199920 and is a validated tool for depression screening.21 The 5-item GDS consists of 5 questions; a score ≥2 is suggestive of depression (Supplemental Appendix 5).
Level of independence
Information on the level of independence and institutionalization status was ascertained from patients or caregivers at the time of assessment(s). Four levels of independence based on feeding, dressing, ambulation, grooming, using a toilet, and bathing were defined: (1) independent (did not require assistance with activities of daily living from family and friends), (2) independent with support (required assistance with activities of daily living from a family member/friend), (3) home care recipient (lived at home but received assistance from personal care services), and (4) long-term care home resident and assisted living.
Quality of life
Health-related QoL was measured using a self-reported questionnaire, 3-level version of EuroQol-5 dimensions (EQ-5D-3L) at the time of assessment(s) (available at www.euroqol.org; Supplemental Appendix 6). EQ-5D-3L includes 2 sections: EQ-5D descriptive system and EQ-5D visual analog scale (VAS). EQ-5D-3L measures 5 dimensions (mobility, self-care, daily activities, pain/discomfort, anxiety/depression) with 3 levels each (no/moderate/severe problem, labeled 1, 2, 3, respectively). The patient selected the most appropriate answer in each of the 5 dimensions. EQ-5D-3L health states were converted into a single index value “utility score.” Since the data for Canada are not available, we used the scores based on the USA general population perspectives. The utility score ranges between −1.109 and 1, where a score below 0 reflects a health state worse than death, 0 means death, and 1 is the best health state. We converted health state into a utility score using formulas available at https://www.economicsnetwork.ac.uk/health/EQ_5D_index_calculator.xls. For example “health state” 12321 was converted to “utility score” 0.546 in our study population. The EQ-5D VAS records the patient’s self-rated health (0-100) on a vertical scale, where 100 is the “best imaginable health state” and 0 is the “worst imaginable health state” (Supplemental Appendix 7).
Statistical Analysis
Variables were reported as count (%), mean ± standard deviation (SD), or medians with interquartile range (IQR) as appropriate. Frailty status was reported as a binary variable: frail vs. nonfrail (prefrail and robust). We also dichotomized the EQ-5D levels into “no problems” (level 1) and “problems” (levels 2 and 3) and reported the frequencies of problems. McNemar’s test was used to compare categorical variables (frailty, frailty components, cognitive impairment, depression, and EQ-5D dimensions) between baseline and 1-year follow-up. Wilcoxon signed-rank test was used to compare variables such as the MoCA score, GDS score, EQ-5D utility score, and EQ-VAS between baseline and 1-year follow-up. We tested whether frailty was associated with cognitive function, mood, QoL, and level of dependence using the chi-square test or Fisher’s exact test where appropriate. Differences in variables such as MoCA score, GDS score, EQ-5D utility score, and EQ-VAS between frail and nonfrail groups were assessed using the Mann–Whitney Test. The significance level was set as α = .05. Statistical analyses were performed with SPSS Statistics for Windows, Version 22.0 (SPSS Inc., Chicago, Illinois).
Results
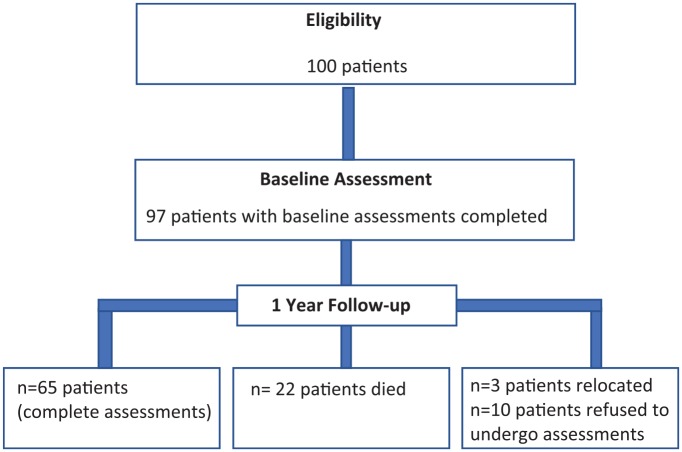
Ninety-seven of the 100 enrolled patients had complete baseline assessments. At 1-year follow-up, 22 patients died, 10 refused to follow-up assessments, and 3 were lost to follow-up, leaving 65 patients for 1-year follow-up assessment (Figure 1). At baseline, the mean age ± SD in years was 63 ± 15. A total of 58% were men, 73% were Caucasian, 51% were diabetic, and the mean BMI ± SD was 29.33 ± 7.26 (Table 1). The median time on dialysis (IQR) in months before the initial assessment was 35.5 (13.75-71.75). The prevalence of frailty, prefrailty, and robust was 68%, 27%, and 5%, respectively. Half of the frail patients were men; 54.5% were ≥65 years old (Table 2).
Figure 1.
Study flow chart.
Table 1.
Patients’ Demographics and Comorbidities at Baseline and 1-Year Follow-Up.
| Characteristics | Baseline | 1-year follow-up | N |
|---|---|---|---|
| Mean ± SD; median (interquartile range); n (%) | |||
| Age (years) | 62.86 ± 15.44 | 64.35 ± 14.88 | Baseline: n = 100; Follow-up: n = 65 |
| <65 | 51 (51%) | 34 (52.3%) | |
| ≥65 | 49 (49%) | 31 (47.7%) | |
| Gender (female) | 42 (42%) | 25 (38.5%) | Baseline: n = 100; Follow-up: n = 65 |
| Ethnicity | |||
| Caucasian | 73 (73%) | 49 (75.4%) | Baseline: n = 100; Follow-up: n = 65 |
| Aboriginal | 21 (21%) | 10 (15.4%) | |
| Asian | 6 (6%) | 6 (9.2%) | |
| Education | |||
| ≤12 years of education | 63 (63%) | 41 (63%) | Baseline: n = 100; Follow-up: n = 65 |
| >12 years of education | 37 (37%) | 24 (37%) | |
| Weight (kg) | 83.99 ± 23.41 | 84.88 ± 24.51 | Baseline: n = 100; Follow-up: n = 65 |
| Height (cm) | 168.89 ± 9.44 | 169.18 ± 8.90 | Baseline: n = 100; Follow-up: n = 65 |
| Body mass index (kg/m2) | 29.33 ± 7.26 | 29.49 ± 7.45 | Baseline: n = 100; Follow-up: n = 65 |
| Systolic blood pressure (mm Hg) | 135.28 ± 25.10 | 136.14 ± 29.42 | Baseline: n = 100; Follow-up: n = 65 |
| Diastolic blood pressure (mm Hg) | 72.97 ± 13.59 | 72.29 ± 17.02 | Baseline: n = 100; Follow-up: n = 65 |
| Comorbidities | |||
| Diabetes I or II | 44 (51.2%) | 30 (52.6%) | Baseline: n = 86; Follow-up: n = 57 |
| Peripheral vascular diseases | 7 (8.1%) | 10 (17.5%) | |
| Hypertension | 68 (79.6%) | 43 (75.4%) | |
| Dyslipidemia | 15 (17.4%) | 19 (33.3%) | |
| Vintage of dialysis (months) | 35.5 (13.75-71.75) | 47 (29-88) | Baseline: n = 100; Follow-up: n = 65 |
Table 2.
Results at Initial Assessment and 1-Year Follow-Up.
| Characteristics | Baseline | 1-year follow-up | N |
|---|---|---|---|
| Mean ± SD; median (interquartile range); n (%) | |||
| Frail (≥3) | 66 (68.1%) | 44 (67.7%) | Baseline: n = 97; Follow-up: n = 65 |
| Gender (female) | 33 (50%) | 20 (45.4%) | |
| ≥65 years | 36 (54.5%) | 21 (47.7%) | |
| Age (years) | 65.0 ± 13.86 | 64.0 ± 15.24 | |
| Prefrail (1-2) | 26 (26.8%) | 17 (26.1%) | |
| Robust (0) | 5 (5.1%) | 4 (6.2%) | |
| MoCA score (≤24) | 69 (69%) | 40 (64.5%) | Baseline: n = 100; Follow-up: n = 62 |
| Gender (female) | 26 (37.7%) | 12 (30%) | |
| ≥65 years | 42 (60.9%) | 18 (45%) | |
| Age (years) | 67.11 ± 14.04 | 64.25 ± 13.54 | |
| GDS score (≥2) | 50 (52.8%) | 31 (47.7%) | Baseline: n = 96; Follow-up: n = 65 |
| Gender (female) | 18 (36%) | 8 (25.8%) | |
| ≥65 years | 23 (46%) | 9 (29.1%) | |
| Age (years) | 61.04 ± 15.98 | 59.48 ± 13.76 | |
| EQ-5D utility score | 0.81 (0.70-0.85) | 0.77 (0.69-0.84) | Baseline: n = 100; Follow-up: n = 65 |
| EQ-VAS | 60 (44-80) | 50 (45-80) | Baseline: n = 100; Follow-up: n = 63 |
| Level of dependence | |||
| Independent | 82 (82%) | 41 (63.1%) | Baseline: n = 100; Follow-up: n = 65 |
| Independent with support | 17 (17%) | 20 (30.8%) | |
| Home care | 1 (1%) | 2 (3.1%) | |
| Long-term care home | 0 (0%) | 2 (3.1%) | |
Note. Frailty was measured using 5-item Fried frailty criteria, frail: ≥3 criteria present, prefrail: 1 or 2 criteria present, robust: 0 criteria present. Cognitive function was measured using the MoCA, cognitive impaired: MoCA score ≤ 24. Depressive symptoms were identified using 5-item GDS questionnaire, depressed: GDS score≥ 2. Quality of life was measured using EQ-5D; EQ-5D utility score: 0 means death and 1 is the best health state; EQ-VAS: 0 is the worst imaginable health state and 100 is the best imaginable health state. EQ-5D-3L = EuroQol-5 dimensions-3 levels; MoCA = Montreal Cognitive Assessment; GDS = Geriatric Depression Scale; EQ-VAS = EuroQol visual analog scale.
In comparison with baseline vs. 1 year, the number of frail patients was 68.1% vs. 67.7%, prefrail 26.8% vs. 26.1%, and robust 5.1% vs. 6.2%. Similarly, in comparison with baseline vs. 1 year, the number of independent patients were 82% vs. 63%; independent with support 17% vs. 31%; long-term care home 0% vs. 3.1%; MoCA ≤24, 69% vs. 64.5%; GDS score ≥2, 52.8% vs. 47.7%; median EQ-5D utility score 0.81 vs. 0.77; and median EQ-VAS 60 vs 50; Table 2.
Our results showed that frailty is bidirectional: at 1 year, 5 of the 65 frail patients converted to nonfrail and 7 of the 65 nonfrail patients worsened their frailty status. Among those 5 patients who improved their status, the frailty components that were most commonly affected were walking speed (3/5), energy (exhaustion; CES-D; 2/5), physical activity and weight (1/5), and strength (handgrip test; 0/5). Among those 7 patients who worsened, the most common affected components were energy (exhaustion; CES-D; 5/7), walking speed and physical strength (both 3/7), and physical activity and weight (both 2/7). Analyses showed that the vintage of dialysis was associated with a change in frailty status (P = .01). This shows that those who were not frail at baseline and were found to be frail at follow-up (worsened) had a higher vintage of dialysis (68 [37.5-135.5] months) than those who were frail (9.0 [4-20]) at baseline and became nonfrail at follow-up (improved; Supplemental Table 1).
Frailty and Outcomes
The results of characteristics of frail vs. nonfrail at baseline are demonstrated in Table 3, Figure 2, and Supplemental Table 2. Proportion of patients with ≤12 years of education in the frail group was lower than nonfrail (27.3% vs. 54.8%, P = .01). There was no statistical difference between frail and nonfrail in terms of age, gender, ethnicity, BMI, comorbidity, and vintage of dialysis (Supplemental Table 2).
Table 3.
Comparisons of Baseline Characteristics by Frailty Group.
| Characteristics | Frail | Nonfrail | P value |
|---|---|---|---|
| Median (interquartile range); n (%) | |||
| Cognitive impaired | n = 66 50 (75.8%) |
n = 31 18 (58.1%) |
P = .076, n = 97 |
| MoCA score (≤24) | n = 66 21 (15.75-24.25) |
n = 31 24 (21-26) |
P = .006, n = 97 |
| Depressive symptoms | n = 63 35 (55.6%) |
n = 31 13 (42%) |
P = .214, n = 94 |
| GDS score (≥2) | n = 63 2 (1-4) |
n = 31 1 (0-3) |
P = .068, n = 94 |
| EQ-5D-3L dimensions | n = 66 | n = 31 | |
| Mobility Problems |
52 (78.8%) | 11 (35.5%) | P < .001 |
| Self-care Problems |
9 (13.7%) | 30 (96.8%) | P = .161 |
| Daily activities Problems |
36 (54.6%) | 16 (51.6%) | P = .571 |
| Pain/discomfort Problems |
50 (75.8%) | 18 (58%) | P = .001 |
| Anxiety/depression Problems |
21 (31.8%) | 7 (22.6%) | P = .349 |
| EQ-5D utility score | n = 66 0.79 (0.71-0.83) |
n = 31 0.85 (0.79-1) |
P < .001, n = 97 |
| EQ-VAS | n = 66 50 (40-75) |
n = 31 60 (50-80) |
P = .111, n = 97 |
Note. Frailty was measured using 5-item Fried frailty criteria, frail: ≥3 criteria present, nonfrail: <3, nonfrail was considered the combination of robust and prefrail. Cognitive function was measured using the MoCA: the highest possible score is 30, cognitive impaired: MoCA score ≤24. Depressive symptoms were identified using 5-item GDS questionnaire: the worst possible score is 5, depressed: GDS score ≥ 2. Quality of life was measured using EQ-5D; problems in each dimension were considered the combination of moderate and extreme problems. EQ-5D utility score: 0 means death and 1 is the best health state; EQ-VAS: 0 is the worst imaginable health state and 100 is the best imaginable health state. EQ-5D-3L = EuroQol-5 dimensions-3 levels; MoCA = Montreal Cognitive Assessment; GDS = Geriatric Depression Scale; EQ-VAS = EuroQol visual analog scale.
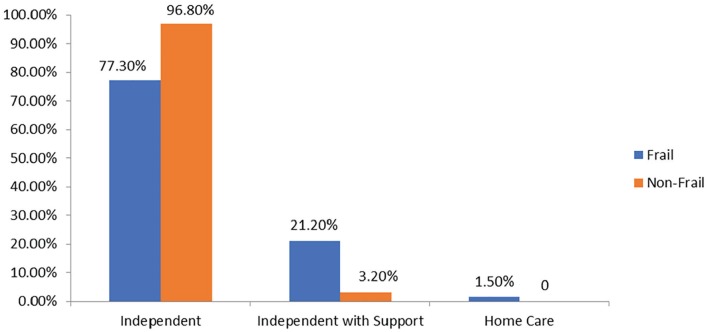
Figure 2.
Frailty and level of dependence at baseline.
Note. Frailty was measured using 5-item Fried frailty criteria: Frail: ≥3 criteria present, nonfrail: <3, nonfrail was considered the combination of robust and prefrail. Each bar shows the proportion of patients who are independent, independent with support, and home care recipient in frail and nonfrail groups (baseline). Of the 97 patients, 66 were frail. In the frail group, 51 (77.3%) were independent, 14 (21.2%) were independent with support, and 1 (1.5%) was home care recipient. In the nonfrail group, 30 (96.8%) were independent and 1 (3.2%) was independent with support.
In patients who were available for the second assessments (n = 65), we compared frail vs. nonfrail (composite of robust and prefrail) outcomes at 1 year. The median (IQR) MoCA was 24 (19-25) vs. 25 (21-26), P = .039, and median (IQR) GDS was 2 (1-3) vs. 1 (0-2), P = .034. Likewise, median (IQR) EQ-5D utility score was 0.78 (0.6-0.82) vs. 0.81 (0.78-0.85), P = .023. There were significant changes in self-care (27% vs 0%), P = .006, and daily activities (68.2% vs. 38.1%), P = .021 (Table 4 and Figure 3). At 1-year, 22 patients died, 18 of the 22 (82.0%) were frail and 3 of the 22 (18.0%) were nonfrail. Eighteen of the 66 (27.3%) frail patients and 4 of the 31 (9.7%) nonfrail patients died at 1 year, respectively. The comparison of characteristics between baseline and 1 year are shown in Supplemental Table 3.
Table 4.
Comparisons of 1-Year Follow-Up Characteristics by the Frailty Group.
| Characteristics | Frail | Nonfrail | P value |
|---|---|---|---|
| Median (interquartile range); n (%) | |||
| Cognitive impaired | n = 41 30 (73.2%) |
n = 21 10 (47.7%) |
P = .047, n = 62 |
| MoCA score (≤24) | n = 41 24 (19-25) |
n = 21 25 (21-26) |
P = .039, n = 62 |
| Depressive symptoms | n = 44 24 (54.6%) |
n = 21 7 (33.3%) |
n = 65, P = .109 |
| GDS score (≥2) | n = 44 2 (1-3) |
n = 21 1 (0-2) |
P = .034, n = 65 |
| EQ-5D-3L dimensions | n = 44 | n = 21 | |
| Mobility Problems |
40 (90.9%) | 15 (71.4%) | P = .065 |
| Self-care Problems |
12 (27.3%) | 0 (0%) | P = .006 |
| Daily activities Problems |
30 (68.2%) | 8 (38.1%) | P = .021 |
| Pain/discomfort Problems |
30 (68.2%) | 11 (52.4%) | P = .217 |
| Anxiety/depression Problems |
17 (38.6%) | 7 (33.3%) | P = .679 |
| EQ-5D utility score | n = 44 0.78 (0.6-0.82) |
n = 21 0.81 (0.78-0.85) |
P = .023, n = 65 |
| EQ-VAS | n = 43 50 (40-75) |
n = 20 65 (46.25-80) |
P = .286, n = 63 |
Note. Frailty was measured using 5-item Fried frailty criteria, frail: ≥3 criteria present, nonfrail: <3, nonfrail was considered the combination of robust and prefrail. Cognitive function was measured using the MoCA: the highest possible score is 30, cognitive impaired: MoCA score ≤24. Depressive symptoms were identified using 5-item GDS questionnaire: the worst possible score is 5, depressed: GDS score ≥ 2. Quality of life was measured using EQ-5D; problems in each dimension were considered the combination of moderate and extreme problems. EQ-5D utility score: 0 means death and 1 is the best health state; EQ-VAS: 0 is the worst imaginable health state and 100 is the best imaginable health state. EQ-5D-3L = EuroQol-5 dimensions-3 levels; MoCA = Montreal Cognitive Assessment; GDS = Geriatric Depression Scale; EQ-VAS = EuroQol visual analog scale.
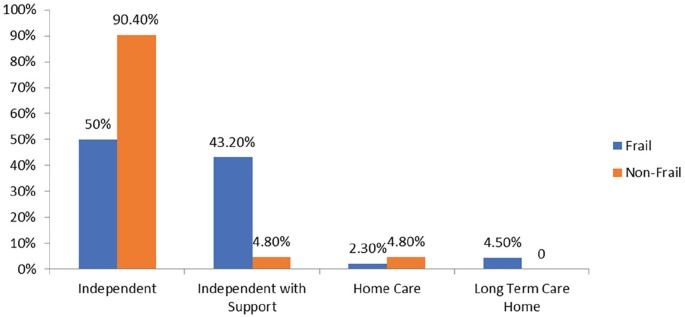
Figure 3.
Frailty and level of dependence at 1-year follow-up.
Note. Frailty was measured using 5-item Fried frailty criteria: Frail: ≥3 criteria present, nonfrail: <3, nonfrail was considered the combination of robust and prefrail. Each bar shows the proportion of patients who are independent, independent with support, home care recipient, and long-term care home residents in the frail and nonfrail groups (1 year). Of the 65 patients, 44 were frail. In the frail group, 22 (50%) were independent, 19 (43.2%) were independent with support, 1 (2.3%) was home care recipient, and 2 (4.5%) were long-term care home resident. In the nonfrail group, 19 (90.4%) were independent, 1 (4.8%) was independent with support, and 1 (4.8%) was independent with support.
Discussion
To our knowledge, this is the first Canadian study that evaluates relationship between frailty and level of independence, mood, cognition, and QoL at 2 different time points. We describe high prevalence (95%) of frail and prefrail status at baseline which remained unchanged 1 year later. Frailty was observed in 65% of patients which is similar to Bao et al17 but higher than others.22,23 We noticed that frail patients on dialysis were older and had challenges with mobility and self-care in comparison with their nonfrail counterparts. Quality of life is defined by the World Health Organization (WHO) as an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards, and concerns.24 Several studies involving patients with CKD have demonstrated an association between poor QOL measures and greater hospitalization and mortality.25,26 We found a decline at 1 year in QoL dimensions (mobility and self-care), and frail patients required greater assistance from their family members, additional home care support and were twice as likely to need support from home care and transition to nursing home care.
We observed that a significant proportion (69%) of our patients had cognitive impairment at baseline, and there was further decline after 12 months. Cognitive impairment has been shown to impair patients’ ability to adhere to HD schedules, fluid, and dietary restrictions.27 We used the MoCA test as it has been identified to assess executive functioning in patients with CKD/HD. The prevalence of cognitive impairment in our study was similar to other published studies estimated to be between 50% and 80%.28-30 The prevalence of depression in our study was 53% which is similar to Jaber et al31 but higher than other studies.22 There is a growing body of evidence that depression is associated with poor outcomes such as post dialysis fatigue, higher mortality, and hospitalizations. There was a substantial overlap among frailty, cognition, and depression. At baseline, 29% of our patients were collectively frail, cognitively impaired, and had depressive symptoms. The trajectory of depression and cognitive impairment did not change over 12-month follow-up.
Studies from the geriatric population have shown that frail older people are exceedingly vulnerable to adverse health outcomes, such as falls, prolonged hospitalization, institutionalization, and death.32-34 In non-ESKD patients, routine assessments of frailty have been shown to prognosticate risk, determine who may or may not benefit from aggressive interventions, and also to postulate when interventions are likely to be futile.35 Even though death was not a prespecified endpoint, we found that a fifth of our patients (22/100) had died by the time of the next assessment in 12 months. Eighteen of the 22 (82%) had been identified as frail, suggesting majority of the deaths had as anticipated occurred in frail patients. We did not link the data for hospitalizations, but the patients with ESKD irrespective of their frailty status are at a greater risk of hospitalizations compared with non-CKD cohorts.36 This risk is increased further in frail individuals.36 Frail patients are more susceptible to large declines in health status from minor illnesses. Hospitalizations are likely to be longer and lead to further functional decline, and subsequent requirement of additional support systems, and transition to nursing homes.37
The trajectory from robustness to frailty occurs over the journey of CKD and is associated with significant sarcopenia. On a more positive note, it has been recognized that frailty is a dynamic process and patients transition between frail to robust at different time points.32,38,39 We found that at 1 year, 5 of the 65 frail patients converted to nonfrail and 7 of the 65 nonfrail patients worsened their frailty status. Among those 5 patients who improved at 1 year, walking speed improved in 3 patients, and 2 patients no longer met the criteria for exhaustion. Among those 7 patients who worsened at 1 year, 5 patients were more exhausted, 3 walked slower and had weaker grip, and 2 had lower levels of physical activity and had unintentional weight loss). Despite data from our frailty study, we do not yet perform routine frailty assessments in our dialysis units as a means to improve clinical care and cost-effectiveness. Unfortunately, we did not specifically review the reasons for the improvement or the decline when we were conducting the assessments.
It is recognized that early intervention in geriatric frail patients improves QoL and reduces the cost of care. An international consensus group has suggested exercise (resistance and aerobic), caloric and protein support, vitamin D, and reduction in polypharmacy as targeted interventions to assist with frailty.33 Studies involving resistance training and aerobic exercise have shown to decrease hospitalizations, nursing home placements, decrease frailty progression, and disability in orthopedic and community-based programs.40-42
Further, there is literature to support that multicomponent exercise training (endurance, flexibility, balance, and resistance training) improves physical function, and sarcopenia which are important components of frailty.43 Our paper supports the need for similar interventions to be initiated in patients with HD as they are high users of emergency rooms, community resources, and hospitalizations.44 Patients with frailty are likely start to dialysis at higher estimated glomerular filtration rates (eGFRs), more likely to avoid fistulas and persist with catheters. Frail older HD and family members are increasingly seeking symptom management and reduction of polypharmacy while on HD over prolongation of life.45 Furthermore, a reduced level of independence leads to caregiver burden and alters family dynamics. As such incorporating frailty assessment and corresponding interventions into routine clinical care has the potential to improve QoL and health outcomes among dialysis patients.
Limitations
Our patients were receiving dialysis for a median of 36 months prior to undergoing their initial frailty assessments. It would have been ideal if they had undergone assessments at initiation and were followed at yearly intervals. Due to a lack of resources, we were unable to follow patients for greater than a year. Longer longitudinal data would have undoubtedly helped in exploring the associations better. We did not capture data for hospitalizations, but it is well recognized that frail patients are likely to have prolonged hospitalization and the cycle of inactivity while recovering from illness and muscle decompensation adds further to the cycle. It would have also been helpful to have explored associations between mediators of inflammation and oxidative stress in relation to frailty. We did not account for medications that could influence mood and cognition. In addition, we had a small sample size and only 65 patients had a second assessment. This is also a single-center study, so direct inferences must be interpreted in the context of the demographics of the study population.
Conclusion
Nephrologists manage primarily a geriatric population on dialysis and the majority of whom are exceedingly frail. It is also obvious that frailty and prefrailty is near-ubiquitous in our dialysis population and will have to be proactively addressed. Care teams including pharmacists to reduce polypharmacy, psychiatry to assist with mood and cognitive impairment, exercise therapists, dietitians, and social workers will have to work in cohesion to address the multiple aspects of frailty. We hope that larger studies looking at longitudinal frailty assessments will lead to the development of targeted care pathways.
Supplemental Material
Supplemental material, Feb_24Clean__Frailty__Supplementarymaterials_1 for The Burden of Frailty on Mood, Cognition, Quality of Life, and Level of Independence in Patients on Hemodialysis: Regina Hemodialysis Frailty Study by Maryam Jafari, Kaval Kour, Shelley Giebel, Idunnu Omisore and Bhanu Prasad in Canadian Journal of Kidney Health and Disease
Acknowledgments
The authors wish to acknowledge Regina Qu’Appelle Health Region, Research and Performance Support for assisting with this study.
Footnotes
Ethics Approval and Consent to Participate: The study was approved by the Research Ethics Board of the former Regina Qu’Appelle Health Region (REB 19-17).
Consent for Publication: Not applicable as there is no patient identifying information in this manuscript.
Availability of Data and Materials: The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions: B.P conceived and designed the study. He also edited the final manuscript. M.J wrote the initial draft and assisted with data analysis. K.K assisted with the drafts. S.G did all the assessments and assisted with the draft. I.O performed the statistical analysis. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Maryam Jafari  https://orcid.org/0000-0002-7261-2191
https://orcid.org/0000-0002-7261-2191
Bhanu Prasad  https://orcid.org/0000-0002-1139-4821
https://orcid.org/0000-0002-1139-4821
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M156. [DOI] [PubMed] [Google Scholar]
- 2. Kojima G. Frailty as a predictor of future falls among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2015;16(12):1027-1033. [DOI] [PubMed] [Google Scholar]
- 3. Ensrud KE, Lipschutz RC, Cauley JA, et al. Body size and hip fracture risk in older women: a prospective study. Am J Med. 1997;103(4):274-280. [DOI] [PubMed] [Google Scholar]
- 4. Kojima G, Taniguchi Y, Iliffe S, Walters K. Frailty as a predictor of Alzheimer disease, vascular dementia, and all dementia among community-dwelling older people: a systematic review and meta-analysis. J Am Med Dir Assoc. 2016;17(10):881-888. [DOI] [PubMed] [Google Scholar]
- 5. Kojima G. Frailty as a predictor of hospitalisation among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70(7):722-729. [DOI] [PubMed] [Google Scholar]
- 6. Kojima G, Iliffe S, Jivraj S, Walters K. Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Community Health. 2016;70(7):716-721. [DOI] [PubMed] [Google Scholar]
- 7. Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation. J Am Geriatr Soc. 2010;58(4):681-687. [DOI] [PubMed] [Google Scholar]
- 8. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12(4):840-851. [DOI] [PubMed] [Google Scholar]
- 9. Kojima G. Frailty as a predictor of nursing home placement among community-dwelling older adults: a systematic review and meta-analysis. J Geriatr Phys Ther. 2018;41(1):42-48. [DOI] [PubMed] [Google Scholar]
- 10. Kojima G. Frailty as a predictor of disabilities among community-dwelling older people: a systematic review and meta-analysis. Disabil Rehabil. 2017;39(19):1897-1908. [DOI] [PubMed] [Google Scholar]
- 11. Brown SA, Tyrer FC, Clarke AL, et al. Symptom burden in patients with chronic kidney disease not requiring renal replacement therapy. Clin Kidney J. 2017;10:788-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Roshanravan B, Khatri M, Robinson-Cohen C, et al. A prospective study of frailty in nephrology-referred patients with CKD. Am J Kidney Dis. 2012;60(6):912-921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Khan SS, Kazmi WH, Abichandani R, Tighiouart H, Pereira BJ, Kausz AT. Health care utilization among patients with chronic kidney disease. Kidney Int. 2002;62:229-236. [DOI] [PubMed] [Google Scholar]
- 14. Johansen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18:2960-2967. [DOI] [PubMed] [Google Scholar]
- 15. Kojima G. Prevalence of frailty in end-stage renal disease: a systematic review and meta-analysis. Int Urol Nephrol. 2017;49(11):1989-1997. [DOI] [PubMed] [Google Scholar]
- 16. Johansen KL, Dalrymple LS, Glidden D, et al. Association of performance-based and self-reported function-based definitions of frailty with mortality among patients receiving hemodialysis. Clin J Am Soc Nephrol. 2016;11(4):626-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bao Y, Dalrymple L, Chertow GM, Kaysen GA, Johansen KL. Frailty, dialysis initiation, and mortality in end-stage renal disease. Arch Intern Med. 2012;172(14):1071-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tiffin-Richards FE, Costa AS, Holschbach B, et al. The Montreal Cognitive Assessment (MoCA)—a sensitive screening instrument for detecting cognitive impairment in chronic hemodialysis patients. PLoS One. 2014;9(10):e106700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foster R, Walker S, Brar R, et al. Cognitive impairment in advanced chronic kidney disease: the Canadian frailty observation and interventions trial. Am J Nephrol. 2016;44(6):473-480. [DOI] [PubMed] [Google Scholar]
- 20. Hoyl MT, Alessi CA, Harker JO, et al. Development and testing of a five-item version of the Geriatric Depression Scale. J Am Geriatr Soc. 1999;47(7):873-878. [DOI] [PubMed] [Google Scholar]
- 21. Rinaldi P, Mecocci P, Benedetti C, et al. Validation of the five-item geriatric depression scale in elderly subjects in three different settings. J Am Geriatr Soc. 2003;51(5):694-698. [DOI] [PubMed] [Google Scholar]
- 22. Sy J, McCulloch CE, Johansen KL. Depressive symptoms, frailty, and mortality among dialysis patients. Hemodial Int. 2019;23(2):239-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Szeto CC, Chan GC, Ng JK, et al. Depression and physical frailty have additive effect on the nutritional status and clinical outcome of Chinese peritoneal dialysis. Kidney Blood Press Res. 2018;43(3):914-923. [DOI] [PubMed] [Google Scholar]
- 24. The World Health Organization Quality of Life assessment (WHOQOL): position paper from the World Health Organization. Soc Sci Med. 1995;41:1403-1409. [DOI] [PubMed] [Google Scholar]
- 25. Mujais SK, Story K, Brouillette J, et al. Health-related quality of life in CKD Patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009;4(8):1293-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lowrie EG, Curtin RB, LePain N, Schatell D. Medical outcomes study short form-36: a consistent and powerful predictor of morbidity and mortality in dialysis patients. Am J Kidney Dis. 2003;41(6):1286-1292. [DOI] [PubMed] [Google Scholar]
- 27. Sehgal AR, Grey SF, DeOreo PB, Whitehouse PJ. Prevalence, recognition, and implications of mental impairment among hemodialysis patients. Am J Kidney Dis. 1997;30(1):41-49. [DOI] [PubMed] [Google Scholar]
- 28. Erken E, Altunoren O, Senel ME, et al. Impaired cognition in hemodialysis patients: the Montreal Cognitive Assessment (MoCA) and important clues for testing. Clin Nephrol. 2019;91(5):275-283. [DOI] [PubMed] [Google Scholar]
- 29. Lu R, Xu C, Li Y, et al. The incidence prognosis and risk factors of cognitive impairment in maintenance haemodialysis patients. Blood Purif. 2019;47(1-3):101-108. [DOI] [PubMed] [Google Scholar]
- 30. Dasgupta I, Patel M, Mohammed N, et al. Cognitive function declines significantly during haemodialysis in a majority of patients: a call for further research. Blood Purif. 2018;45(4):347-355. [DOI] [PubMed] [Google Scholar]
- 31. Jaber BL, Lee Y, Collins AJ, et al. Effect of daily hemodialysis on depressive symptoms and postdialysis recovery time: interim report from the FREEDOM (Following Rehabilitation, Economics and Everyday-Dialysis Outcome Measurements) Study. Am J Kidney Dis. 2010;56(3):531-539. [DOI] [PubMed] [Google Scholar]
- 32. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381:752-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013;14:392-397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cerreta F, Eichler HG, Rasi G. Drug policy for an aging population—the European Medicines Agency’s geriatric medicines strategy. N Engl J Med. 2012;367(21):1972-1974. [DOI] [PubMed] [Google Scholar]
- 35. Lindman BR, Alexander KP, O’Gara PT, Afilalo J. Futility, benefit, and transcatheter aortic valve replacement. JACC Cardiovasc Interv. 2014;7(7):707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garcia-Canton C, Rodenas A, Lopez-Aperador C, et al. Frailty in hemodialysis and prediction of poor short-term outcome: mortality, hospitalization and visits to hospital emergency services. Ren Fail. 2019;41(1):567-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hall RK, McAdams-DeMarco MA. Breaking the cycle of functional decline in older dialysis patients. Semin Dial. 2018;31(5):462-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: a review. Eur J Intern Med. 2016;31:3-10. [DOI] [PubMed] [Google Scholar]
- 39. Cheung JTK, Yu R, Wu Z, Wong SYS, Woo J. Geriatric syndromes, multimorbidity, and disability overlap and increase healthcare use among older Chinese. BMC Geriatr. 2018;18(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Singh NA, Quine S, Clemson LM, et al. Effects of high-intensity progressive resistance training and targeted multidisciplinary treatment of frailty on mortality and nursing home admissions after hip fracture: a randomized controlled trial. J Am Med Dir Assoc. 2012;13(1):24-30. [DOI] [PubMed] [Google Scholar]
- 41. Yamada M, Arai H, Sonoda T, Aoyama T. Community-based exercise program is cost-effective by preventing care and disability in Japanese frail older adults. J Am Med Dir Assoc. 2012;13(6):507-511. [DOI] [PubMed] [Google Scholar]
- 42. Theou O, Stathokostas L, Roland KP, et al. The effectiveness of exercise interventions for the management of frailty: a systematic review. J Aging Res. 2011;2011:569194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hogan DB, MacKnight C, Bergman H. Models, definitions, and criteria of frailty. Aging Clin Exp Res. 2003;15(3 suppl.):1-29. [PubMed] [Google Scholar]
- 44. Nixon AC, Bampouras T, Pendleton N, Mitra S, Brady M, Dhaygude A. Frailty is independently associated with worse health-related quality of life in chronic kidney disease: a secondary analysis of the “frailty assessment in chronic kidney disease” study. Cli Kidney J. 2020;13:85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Unruh ML, Hess R. Assessment of health-related quality of life among patients with chronic kidney disease. Adv Chronic Kidney Dis. 2007;14:345-352. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Feb_24Clean__Frailty__Supplementarymaterials_1 for The Burden of Frailty on Mood, Cognition, Quality of Life, and Level of Independence in Patients on Hemodialysis: Regina Hemodialysis Frailty Study by Maryam Jafari, Kaval Kour, Shelley Giebel, Idunnu Omisore and Bhanu Prasad in Canadian Journal of Kidney Health and Disease