Abstract
Background
Gulf War Illness (GWI) is a poorly understood condition characterized by a constellation of mood, cognitive, and physical symptoms. A growing body of evidence demonstrates autonomic nervous system (ANS) dysfunction. Few published treatment studies exist for GWI.
Method
We recently completed a randomized controlled trial comparing a 10-week group yoga intervention to 10-week group cognitive behavioral therapy (CBT) for veterans with GWI. Here, we present exploratory data on ANS biomarkers of treatment response from a small pilot exploratory neurophysiological add-on study (n = 13) within that larger study.
Results
Findings suggest that veterans with GWI receiving either yoga or CBT for pain improved following treatment and that changes in biological ANS—especially for the yoga group—moved in the direction of healthy profiles: lower heart rate, higher square root of the mean squared differences between successive R-R intervals (RMSSD), greater parasympathetic activation/dominance (increased high-frequency heart rate variability [HF-HRV], decreased low-frequency/high-frequency [LF/HF] ratio), reduced right amygdala volume, and stronger amygdala-default mode/amygdala-salience network connectivity, both immediately posttreatment and at 6-month follow-up. Biological mechanisms of CBT appeared to underlie improvements in more psychologically loaded symptoms such as self-reported fatigue and energy. Higher tonic arousal and/or more sympathetic dominance (higher skin conductance, lower RMSSD, lower HF-HRV, higher LF/HF ratio) pretreatment predicted greater treatment-related improvements in self-reported ANS for both the yoga and CBT group.
Conclusion
These exploratory pilot data provide preliminary support for the suggestion that treatment (yoga, CBT) is associated with improvements in both biological and self-reported ANS dysfunctions in GWI. The major limitation for these findings is the small sample size. Larger and more controlled studies are needed to replicate these findings and directly compare biomarkers of yoga versus CBT.
Keywords: Gulf War Illness, heart rate variability, amygdala, default mode network, salience network, yoga, cognitive behavioral therapy
Introduction
Gulf War Illness (GWI) is a poorly understood condition experienced by many veterans who served in the Gulf War in 1990 to 1991.1 GWI is characterized by a constellation of symptoms, including mood disruption, cognitive complaints, chronic pain, chronic fatigue/fibromyalgia, and gastrointestinal problems such as irritable bowel syndrome (IBS).2,3 A growing body of evidence demonstrates autonomic nervous system (ANS) dysfunction in individuals with GWI on both subjective and objective measures. First, veterans with GWI self-report clinically significantly elevated levels of ANS dysfunction.4 Second, diurnal disruptions in cardiac function—specifically high-frequency heart rate variability (HF-HRV)—are consistently observed in individuals with GWI.5,6 Third, structural brain abnormalities are observed in individuals with GWI in regions implicated in ANS regulation such as the amygdala, brain stem, insula, and cingulate cortices.7,8 To date, no published studies have examined resting-state functional connectivity in GWI, though both the default mode network (DMN) and salience network (SN) are implicated in ANS regulation.9
Few published treatment studies exist for GWI, which begs the question whether ANS dysfunction improves with treatment and/or impedes the progress of treatment. In clinical settings, the most common intervention is cognitive behavioral therapy (CBT), the standard (first-line) evidence-based psychological treatment for many of the independent symptoms of GWI, such as mood disturbance,10 chronic pain,11,12 chronic fatigue/fibromyalgia,13 and IBS.14 Outside the GWI field, there is a small but growing body of literature suggesting that CBT may be associated with positive effects on the ANS, including heart rate (HR),15 HRV,16 and amygdala volume,17 though findings are mixed for amygdala-DMN/amygdala-SN connectivity18–20 and further investigation is warranted for HR/HRV.21
Recently, researchers have begun to explore complementary and integrative interventions for GWI, such as yoga. Yoga is an ancient practice that combines mindfulness meditation with controlled breathing and physical postures.22 Increasingly, research supports the efficacy of yoga for improved psychological well-being, including symptoms associated with GWI such as depression,23 anxiety,24 chronic pain,25,26 cognitive disturbance,27 chronic fatigue/fibromyalgia,28 and IBS.29 Yoga is also associated with improvements in ANS regulation manifesting in a more balanced and less reactive ANS (reduced blood pressure and HR, increased HRV)30,31 as well as increased inhibitory regulation of the amygdala by cortical brain regions (prefrontal cortex, anterior cingulate cortex, and insula).32
We recently completed a randomized controlled trial (RCT) comparing a 10-week group yoga intervention to 10-week group CBT for 74 veterans with GWI33 (ClinicalTrials.gov NCT02378025). Briefly, yoga but not CBT was associated with significant improvement in symptoms of chronic pain and fatigue, both at posttreatment and 6-month follow-up. We received pilot funds to conduct an exploratory add-on ANS biomarker neurophysiological study in a small subsample (n = 13; pretreatment and posttreatment) of veterans who participated in the larger RCT. Due to the putative associations between GWI and ANS function, we examined exploratory biomarkers of treatment-related ANS function. Specifically, we investigated cardiac function (HR, HRV) as an index of parasympathetic activation5,6 and sympathetic/parasympathetic balance34 and skin conductance (SCL) as a measure of tonic (resting) arousal.35 We used a region of interest (ROI) approach to focus on structural amygdala volume and resting-state amygdala-DMN/amygdala-SN connectivity due to the robustness of their associations with ANS function and regulation, particularly in GWI.7–9 Due to the limited sample size, we did not directly compare CBT to yoga but rather examined treatment effects separately by group. In view of the extant literature, we hypothesized (i) treatment-related changes in self-reported ANS symptoms (pretreatment vs posttreatment; pretreatment vs 6-month follow-up) would be associated with treatment-related changes in biological ANS function (pre vs post) and (ii) pretreatment biological ANS function would be associated with treatment-related changes in self-reported ANS symptoms (pretreatment vs posttreatment; pre-treatment vs 6-month follow-up).
Methods
Participants
Participants were veterans of the Gulf War recruited from the San Francisco Bay Area via flyers and advertisements. All participants met Fukuda et al.2 criteria for GWI and took part in the larger RCT “Treating Chronic Pain in Gulf War Illness” (see Bayley et al.33 for more details; ClinicalTrials.gov NCT02378025; N = 74 randomized). Thirteen adults (10 M:3F; 50.31 years [5.07]; yoga = 6) completed the battery of ANS measures pretreatment, with 11 (yoga = 6) returning for the posttreatment assessment battery (1 participant withdrew from the larger RCT prior to completion, 1 participant declined to return for this add-on pilot study) and 8 (yoga = 5) returning for the 6-month follow-up (self-report assessment battery only; 2 participants were lost to follow-up). The groups did not differ pretreatment in age, sex, race-ethnicity, years of education, marital status (Table 1), or self-reported autonomic dysfunction (Table 2). Pretreatment data were not available for 1 participant on the cardiac (equipment failure: device slipped off participant, possibly due to movement), 1 participant on the SCL (equipment failure: device slipped off participant, possibly due to movement), 3 participants on the structural magnetic resonance imaging (MRI) (participant movement), and 7 participants on the functional MRI (fMRI) (participant movement1) measures. Posttreatment data were not available for 1 participant on the Composite Autonomic Symptom Score (COMPASS) (did not complete), 6 participants on the SCL (equipment failure/human error2), and 4 participants on the fMRI (participant movement) measures.
Table 1.
Group Demographics.
| Characteristics |
Yoga |
CBT |
All Participants |
|||
|---|---|---|---|---|---|---|
| n/M | %/SD | n/M | %/SD | N/M | %/SD | |
| Age | 52.17 | 6.43 | 48.71 | 3.25 | 50.31 | 5.07 |
| Male | 6 | 100.00 | 4 | 57.14 | 10 | 76.92 |
| White | 3 | 50.00 | 4 | 57.14 | 7 | 53.85 |
| Non-Hispanic | 6 | 100.00 | 6 | 85.71 | 12 | 92.31 |
| Education (years) | 14.67 | 3.50 | 16.86 | 1.46 | 15.85 | 2.73 |
| Married/Defacto | 5 | 83.33 | 3 | 42.86 | 8 | 61.54 |
Abbreviations: CBT, cognitive behavioral therapy; M, mean; n, number; SD, standard deviation.
Table 2.
Self-Reported Symptoms of Autonomic Nervous System Dysfunction.
| Measure |
Yoga |
CBT |
All Participants |
|||
|---|---|---|---|---|---|---|
| n/M | %/SD | n/M | %/SD | n/M | %/SD | |
| Pretreatment | 6 | 46.15 | 7 | 53.85 | 13 | 100.00 |
| COMPASS | ||||||
| Total | 18.90 | 11.12 | 21.45 | 15.52 | 20.27 | 13.18 |
| Orthostatic intolerance | 5.33 | 8.64 | 6.29 | 7.95 | 5.85 | 7.94 |
| Vasomotor | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Secretomotor | 3.93 | 4.16 | 6.43 | 4.29 | 5.27 | 4.25 |
| Gastrointestinal | 5.95 | 4.33 | 5.36 | 4.64 | 5.63 | 4.32 |
| Bladder | 1.30 | 2.67 | 1.43 | 1.24 | 1.37 | 1.93 |
| Pupillomotor | 2.39 | .83 | 1.95 | 1.27 | 2.15 | 1.07 |
| POMS | ||||||
| Tension | 7.17 | 7.57 | 9.57 | 7.21 | 8.46 | 7.17 |
| Vigora | 6.83 | 4.62 | 5.29 | 4.39 | 6.00 | 4.37 |
| Fatigue | 6.83 | 6.37 | 10.14 | 6.36 | 8.62 | 6.33 |
| Posttreatment | 6 | 60.00 | 5 | 40.00 | 11 | 100.00 |
| COMPASS | ||||||
| Total | 24.21 | 18.58 | 36.37 | 13.51 | 29.08 | 17.09 |
| Orthostatic intolerance | 12.00 | 10.73 | 16.00 | 5.66 | 13.60 | 8.88 |
| Vasomotor | 0.28 | 0.68 | 0.42 | 0.83 | 0.33 | 0.70 |
| Secretomotor | 2.50 | 2.11 | 5.36 | 5.67 | 3.64 | 3.92 |
| Gastrointestinal | 6.40 | 5.88 | 9.15 | 6.24 | 7.50 | 5.85 |
| Bladder | 0.93 | 0.84 | 2.78 | 2.13 | 1.67 | 1.68 |
| Pupillomotor | 2.11 | 0.83 | 2.67 | 0.54 | 2.33 | .75 |
| POMS | ||||||
| Tension | 4.33 | 6.50 | 10.20 | 6.42 | 7.00 | 6.86 |
| Vigora | 5.00 | 3.69 | 7.00 | 6.33 | 5.91 | 4.89 |
| Fatigue | 8.67 | 5.54 | 9.20 | 6.98 | 8.91 | 5.91 |
| 6-Month follow-up | ||||||
| COMPASS | 5 | 62.50 | 3 | 37.50 | 8 | 100.00 |
| Total | 21.78 | 21.87 | 23.85 | 16.96 | 22.56 | 18.88 |
| Orthostatic intolerance | 8.80 | 12.13 | 5.33 | 9.24 | 7.50 | 10.57 |
| Vasomotor | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Secretomotor | 3.00 | 3.59 | 5.00 | 4.46 | 3.75 | 3.76 |
| Gastrointestinal | 6.25 | 5.89 | 8.93 | 5.58 | 7.25 | 5.53 |
| Bladder | 1.33 | 1.45 | 2.59 | 1.70 | 1.81 | 1.56 |
| Pupillomotor | 2.40 | .60 | 2.00 | .88 | 2.25 | .68 |
| POMS | ||||||
| Tension | 9.80 | 8.04 | 8.33 | 7.57 | 9.25 | 7.34 |
| Vigora | 5.60 | 1.82 | 2.33 | 4.04 | 4.38 | 3.07 |
| Fatigue | 8.40 | 7.27 | 10.00 | 6.56 | 9.00 | 6.57 |
Abbreviations: CBT, cognitive behavioral therapy; COMPASS, Composite Autonomic Symptom Score (COMPASS 31); M: mean; n: number; POMS, Short Form of the Profile of Mood States (POMS-SF); SD: standard deviation.
aReverse-scored (higher scores indicate worse functioning).
Procedure
The protocol was approved by the Stanford University Institutional Review Board (IRB-21337). The full procedure for the RCT is described elsewhere.33 Briefly, veterans with GWI (including at least moderate to severe chronic pain and 1 or more symptoms from the fatigue and cognition-mood GWI symptom clusters) were randomized into a 10-week 60-minute manualized group treatment intervention for pain (yoga or CBT). All participants were administered multiple clinician-delivered and self-report measures (including the COMPASS and Profile of Mood States [POMS] described below) at pretreatment, posttreatment, and 6-month follow-up.
Following commencement of the larger RCT, one of the study staff (DCM) designed an exploratory add-on ANS biomarker neurophysiological study and obtained pilot funds for a small subsample (n = 13; pretreatment and posttreatment) of veterans who participated in the larger RCT. This smaller study is reported here. Eligible participants with no contraindications for MRI were invited to participate in this biomarker add-on study, which involved a 1-hour MRI scanning session at Stanford University both pretreatment and posttreatment, in addition to the primary visits made to the study site as part of the larger RCT. Participants received an additional $100 for participation in this add-on study.
During the scanning session, participants were oriented to the MRI scanner and prepped for the scan. The MRI battery consisted of structural (T1 and T2), resting-state functional (8 min, during which SCL and HR were measured concurrently), and diffusion tensor imaging protocols. Only the structural and functional MRI results are reported here.
Self-Report Measures
Composite Autonomic Symptom Score
The COMPASS 3136 is a brief 31-item self-report measure designed to provide a global autonomic symptom severity score (total; maximum 100) as well as 6 subdomains of autonomic dysfunction: orthostatic intolerance (dizziness and feeling faint), vasomotor (color changes in the skin, such as red, white, and purple), secretomotor (excess sweat or dryness), gastrointestinal (bloating, abdominal cramps/pain, diarrhea, and constipation), bladder (loss of control and difficulty urinating), and pupillomotor (sensitivity to bright light and difficulty focusing eyes). It is based on the longer, well-established 169-item Autonomic Symptom Profile37 and demonstrates high internal consistency (Cronbach’s α = .62–.92).36 The internal consistency was also high for this study (Cronbach’s α = .72–.93).
Short Form of the POMS
The Short Form of the POMS (POMS-SF)38 is a brief, 37-item self-report measure of psychological distress based on the longer, well-established 65-item Profile of Mood States.39 Here, we assessed only the POMS tension (tense, on edge, uneasy, restless, nervous, and anxious), POMS vigor (lively, active, energetic, cheerful, full of pep, and vigorous; reverse-scored), and POMS fatigue (worn out, fatigued, exhausted, weary, and bushed) subscales, due to the high loading of autonomic function-related symptoms. The POMS-SF demonstrates high internal consistency (Cronbach’s α = .76–.95).38 The internal consistency was also high for this study (Cronbach’s α = .81–.96).
Biological ANS Data Acquisition
Peripheral measures
Resting-state HR and SCL were recorded concurrently during the 8-minute resting state fMRI acquisition. Pulse was recorded via a photopulse sensor (eliciting photoplethysmogram [PPG]) attached to the proximal hallux of the right foot and automatically synched to MRI scanner start and stop times. SCLs were recorded in microSiemens (µS) from BIOPAC EDA100C-MRI Smart Amplifier via 2 Ag-AgCl electrodes (with isotonic NaCl electrode paste) placed on the distal phalanges of digits II and IV of the left hand. Recording was triggered by Mac Terminal synchronized with the MRI scanner trigger, and data were digitized with a LabJack UE9 DAQ device.
Magnetic resonance imaging
MRI data were collected on a 3T GE Discovery™ MR750 scanner (GE Healthcare, Chicago, IL) with a 32-channel head coil at the Stanford Center for Cognitive and Neurobiological Imaging. T1-weighted high-resolution structural images (repetition time = 7.24 ms; echo time = 2.784 ms; flip angle 12°, field of view = 23 cm; matrix size = 256 × 256; voxel dimensions = .9 × .9 × .9 mm; 176 slices) and T2*-weighted echoplanar images (EPI) using simultaneous multislice (SMS) EPI, gradient-echo spiral pulse sequence, axial sections (anterior commissure-posterior commissure aligned; repetition time = 7.1 ms; echo time = 3 ms; flip angle = 54°; field of view = 22 cm; matrix size 92 × 92; voxel dimensions = 2.4 × 2.4 × 2.4 mm; SMS factor 6; 10 muxed slices [60 unmuxed slices, 14.4 cm]) were acquired. During the 8-minute resting-state fMRI, participants were instructed to close their eyes and rest quietly while remaining awake.
Peripheral Data Processing
All data were extracted from an 8-minute epoch beginning 30 seconds after the resting-state fMRI scan trigger.
Cardiac measures
The raw PPG waveforms were processed using Kubios HRV Premium 3.1.0 (©Kubios, 2018) and the R-R intervals were visually inspected for artifacts, per standard recommendations.40 We extracted mean HR (bpm), time domain HRV (square root of the mean squared differences between successive R-R intervals [RMSSD] [ms]), and frequency-domain HRV (absolute [ms2] HF-HRV power, relative [n.u.] HF-HRV power, and low-frequency/high-frequency [LF/HF] ratio). Mean HR and RMSSD reflect general autonomic function (typically lower and higher values, respectively, indicate healthier function41). HF-HRV values were used as indices of parasympathetic activation5,6 and LF/HF ratio was used as an index of sympathetic/parasympathetic balance.34
Skin conductance
Data were recorded with the amplifier gain set to 5 µS/V, so raw data extracted in Excel were multiplied by 5 to obtain SCL in microSiemens. Data outside the range of 0 to 40 µS were removed following standard practice in the field and as recommended by the equipment manufacturer.42 Mean SCL (µS) was extracted as a measure of tonic (resting) arousal.35 As mean SCL may be artificially elevated by spontaneous responses, we also extracted minimum SCL as a confirmatory measure of tonic (resting) arousal.42
MRI Data Processing
Structural MRI (amygdala)
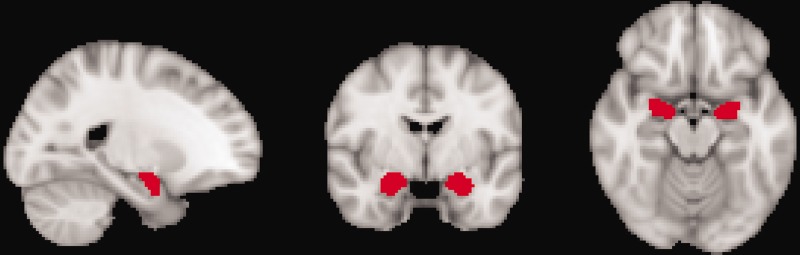
ROI analyses were conducted using voxel-based morphometry in FMRIB’s Software Library (FSL) v.4.1.8 (http://www.fmrib.ox.ac.uk/uk/fsl/). Preprocessing included removal of nonbrain tissues using Brain Extraction Tool (BET), segmentation into gray matter, white matter, and cerebrospinal fluid using FMRIB’s Automated Segmentation Tool (FAST4), and normalization to an average template using a linear registration tool (Montreal Neurologic Institute [MNI] 152 standard 2 mm template [voxel size = 2 × 2 × 2 mm]). Jacobian modulation and spatial smoothing with Gaussian kernels (full width at half maximum [FWHM] = 2 × 2.3 = 4.6 mm) were applied. A mask for amygdala was constructed using the Harvard-Oxford subcortical structure atlas (Figure 1) and the mean volume for each patient extracted. Pretreatment versus posttreatment change scores were computed.
Figure 1.
ROI Mask for Amygdala (Harvard-Oxford Subcortical Structure Atlas).
fMRI (resting-state connectivity)
Analyses were conducted using FSL v.4.1.8 (http://www.fmrib.ox.ac.uk/uk/fsl/). Preprocessing included conversion from Digital Imaging and Communications in Medicine (DICOM) and reorientation to standard space, motion scrubbing using a voxel-specific mean frame wise displacement threshold of 0.5,43 and removal of nonbrain tissues using BET. Functional data were collected with reversed phase-encode blips. The susceptibility-induced off-resonance field was estimated in FSL44,45 from the resultant pairs,46 producing in a single corrected image. Further preprocessing using FSL’s MELODIC included motion correction (MCFLIRT) with 6 movement parameters (3 translations and 3 rotations), high-pass filter of 100, nonlinear registration between fMRI, T1-weighted anatomical and standard MNI 152 space (2 mm3) images, and spatial smoothing (Gaussian kernel of 5 mm FWHM). No slice-time correction or intensity normalization was performed.
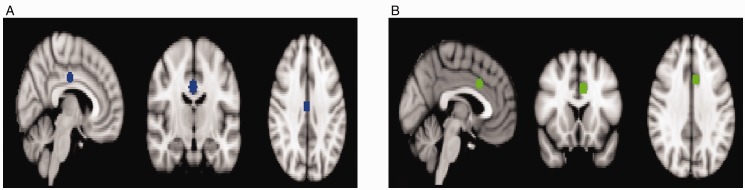
Sphere masks for DMN (xyz = 2, −14, 34; Figure 2(A)) and SN (xyz = −6, 14, 32; Figure 2(B)) were created in MNI standard space based on peak MNI coordinates from a previous study.47 Amygdala masks were created as per the structural analysis (Figure 1). Functional data were linearly transformed from native into standard space, and intensity normalization was run with a whole brain mode value of 10 000. Functional data at rest were obtained by extracting the mean time series from the amygdala, DMN, and SN ROIs. Spearman’s rho correlations were computed between ROIs (amygdala-DMN and amygdala-SN) for each participant’s pretreatment and posttreatment scan. Fisher z transformations were applied to each pretreatment/posttreatment correlation to obtain an estimate of the strength of functional connectivity.
Figure 2.
ROI Sphere Masks for (A) DMN (xyz = 2, −14, 34) and (B) SN (xyz = −6, 14, 32).47
Analyses
All analyses were conducted in IBM SPSS Statistics 21 with significance threshold set at P < .05. Due to the exploratory nature of the study, we did not control for multiple comparisons and we report trend-level associations where .05 < P < .10 and r or β > .50 (ie, ≥ moderate effect size), to inform future studies with larger sample size. While sample size precluded direct comparison of differences between the treatment interventions (ie, yoga vs CBT), all analyses were conducted separately by treatment group to highlight differential patterns for further investigation in larger studies.
For hypothesis 1 (treatment-related changes in self-reported ANS will be associated with treatment-related changes in biological ANS function), separate exploratory bivariate correlations were conducted between change scores (pretreatment minus posttreatment, pretreatment minus 6-month follow-up) of self-reported ANS (COMPASS and POMS) and change scores (pretreatment minus posttreatment) of biological ANS (mean HR, RMSSD, HF-HRV, LF/HF ratio, mean SCL, minimum SCL, amygdala volume, resting-state amygdala-DMN connectivity, and resting-state amygdala-SN connectivity). All self-reported ANS measures were scored such that higher scores indicate worse functioning; thus, the higher the change score value, the greater the treatment-related improvement in functioning.
For hypothesis 2 (pretreatment biological ANS function will be associated with treatment-related changes in self-reported ANS), separate exploratory regression analyses were conducted with pretreatment biological ANS (mean HR, RMSSD, HF-HRV, LF/HF ratio, mean SCL, minimum SCL, amygdala volume, resting-state amygdala-DMN connectivity, and resting-state amygdala-SN connectivity) as the independent (predictor) variables and pre/posttreatment change scores (pretreatment minus posttreatment) and pre/follow-up change scores (pretreatment minus 6-month follow-up) on self-reported ANS (COMPASS, POMS) as the dependent variables.
Results
Hypothesis 1 (Biological and Self-Reported ANS Treatment-Related Associations): Treatment-Related Changes in Self-Reported ANS Symptoms Will Be Associated With Treatment-Related Changes in Biological ANS Function
Yoga group
Change in general autonomic function from pretreatment to posttreatment was associated with treatment-related change on total COMPASS (global autonomic dysfunction severity; pre- to 6-month follow-up [RMSSD]: r = −.86, P = .030), COMPASS secretomotor subscale (excess sweat or dryness; pretreatment to posttreatment [mean HR; trend]: r = −.76, P = .080), COMPASS bladder (loss of control, difficulty urinating; pretreatment to posttreatment [mean HR; trend]: r = .76, P = .080; pre- to 6-month follow-up [mean HR]: r = .93, P = .024; [RMSSD]: r = −.99, P = .001), POMS tension (tense, on edge, uneasy, restless, nervous, and anxious; pre- to 6-month follow-up [mean HR]: r = .98, P = .002; [RMSSD; trend]: r = −.86, P = .062), POMS vigor (lively, active, energetic, cheerful, full of pep, and vigorous; pre- to 6-month follow-up [RMSSD]: r = .92, P = .025), and POMS fatigue (worn out, fatigued, exhausted, weary, and bushed; pretreatment to posttreatment [mean HR]: r = −.85, P = .033; [RMSSD; trend]: r = .73, P = .098; Table 3).
Table 3.
Summary of Associations Between Biological and Self-Reported ANS by Treatment Group (Hypothesis 1).
|
Yoga (Pre to Post) |
CBT (Pre to Post) |
Yoga (Pre to 6 Months) |
CBT (Pre to 6 months) |
|||||
|---|---|---|---|---|---|---|---|---|
| Biological ANS (n Pre/n Post) | ΔCOMPASS (r) | ΔPOMS (r) | ΔCOMPASS (r) | ΔPOMS (r) | ΔCOMPASS (r) | ΔPOMS (r) | ΔCOMPASS (r) | ΔPOMS (r) |
| ΔMeanHR (12/10) | −.76^ to .76^ | −.85* | N/A | .93* | .98** | N/A | N/A | |
| ΔRMSSD (12/10) | .73^ | N/A | −.86* to −.99*** | −.86^ to .92* | N/A | N/A | ||
| ΔHF-HRV (12/10) | −.73^ to −.91* | N/A | −1.00** to 1.00* | −.88* to −.99** | −.86^ to .88^ | N/A | N/A | |
| ΔLF/HF (12/10) | .77^ | −.77^ | N/A | .88* | N/A | N/A | ||
| ΔSCL (12/4) | .99^ | N/A | N/A | N/A | N/A | |||
| ΔAmygdala (10/10) | .82* | −1.00^ | .87* to .97** | −.87^ | N/A | N/A | ||
| ΔAmyg-DMN (6/6) | −.82^ to −.90* | N/A | N/A | −81^ to −.99*** | .91* to .93* | N/A | N/A | |
| ΔAmyg-SN (6/6) | −.81^ to .90* | N/A | N/A | −.82^ to −.86^ | N/A | N/A | ||
Abbreviations: Amyg, amygdala volume; Amyg-DMN, amygdala-default mode network connectivity; Amyg-SN, amygdala-salience network connectivity; ANS, autonomic nervous system; CBT, cognitive behavioral therapy; COMPASS, Composite Autonomic Symptom Score (COMPASS 31); Δ, change (pre minus post; pre minus 6 months); HF-HRV, high-frequency-domain heart rate variability; HR, heart rate; hypothesis 1 = biological and self-reported ANS treatment-related associations: treatment-related changes in self-reported ANS symptoms (pre to post [left], pre to 6 months [right]) will be associated with treatment-related changes (pre to post) in biological ANS function; LF/HF, ratio low-frequency to high-frequency-domain heart rate variability; N/A, analyses were not possible due to missing data; n pre/n post, number at pretreatment/posttreatment; POMS, Short Form of the Profile of Mood States (POMS-SF); RMSSD, square root of the mean squared differences between successive R-R intervals, SCL, skin conductance level.
All self-reported ANS measures are scored such that higher scores indicate worse functioning; thus, the higher the change score value, the greater the treatment-related improvement in functioning.
^P < .10. *P < .05. **P < .01. ***P < .001.
Change in parasympathetic activation was associated with change on total COMPASS (pretreatment to posttreatment [absolute HF-HRV, ms2; trend]: r = −.75, P = .084; pre- to 6-month follow-up [absolute HF-HRV, ms2]: r = −.92, P = .009), COMPASS orthostatic intolerance (pretreatment to posttreatment [absolute HF-HRV, ms2; trend]: r = −.79, P = .063; pre- to 6-month follow-up [absolute HF-HRV, ms2]: r = −.99, P = .002), COMPASS vasomotor (pretreatment to posttreatment [relative HF-HRV (n.u.)]: r = −.91, P = .011), COMPASS secretomotor (pretreatment to posttreatment [absolute HF-HRV, ms2; trend]: r = .73, P = .099), COMPASS bladder (pre- to 6-month follow-up [relative HF-HRV (n.u.)]: r = −.88, P = .049), POMS tension (pre- to 6-month follow-up [relative HF-HRV (n.u.); trend]: r = −.86, P = .064), and POMS vigor (pre- to 6-month follow-up [absolute HF-HRV, ms2; trend]: r = .88, P = .051).
Change in sympathetic/parasympathetic balance (LF/HF ratio) was associated with change on COMPASS bladder (pretreatment to posttreatment [trend]: r = .77, P = .076; pre- to 6-month follow-up: r = .88, P = .047) and POMS fatigue (pretreatment to posttreatment [trend]: r = −.77, P = .073).
Change in tonic arousal (mean SCL) was associated with change on POMS fatigue (pretreatment to posttreatment [trend]: r = .99, P = .083).
Change in amygdala volume was associated with change on total COMPASS (pretreatment to posttreatment [right]: r = .82, P = .048; pre- to 6-month follow-up [right]: r = .87, P = .023), COMPASS orthostatic intolerance (pre- to 6-month follow-up [right]: r = .97, P = .006), COMPASS pupillomotor (sensitivity to bright light, difficulty focusing eyes; pre- to 6-month follow-up [left]: r = .92, P = .028), and POMS vigor (pre- to 6-month follow-up [right; trend]: r = −.87, P = .052).
Change in amygdala-DMN connectivity was associated with change on total COMPASS (pretreatment to posttreatment [right; trend]: r = −.82, P = .092; pre- to 6-month follow-up [right]: r = −.97, P = .005; [left]: r = −.89, P = .045), COMPASS orthostatic intolerance (pre- to 6-month follow-up [right]: r = −.99, P < .001), COMPASS gastrointestinal (bloating, abdominal cramps/pain, diarrhea, constipation; pre- to posttreatment [left] r = −.90, P = .038; pre- to 6-month follow-up [left]: r = −.94, P = .017), COMPASS bladder (pre- to 6-month follow-up [left; trend]: r = −.82, P = .086), COMPASS pupillomotor (pre- to 6-month follow-up [left; trend]: r = −.81, P = .093), and POMS vigor (pre- to 6-month follow-up [right]: r = .91, P = .030; [left]: r = .93, P = .023).
Change in amygdala-SN connectivity was associated with change on COMPASS orthostatic intolerance (pretreatment to posttreatment [right; trend]: r = −.81, P = .096; pre- to 6-month follow-up [trend]: [right]: r = −.82, P = .090; [left]: r = −.86, P = .063) and COMPASS secretomotor (pretreatment to posttreatment [left]: r = .90, P = .037). There were no other treatment-related change associations between biological and self-reported ANS measures for the yoga group.
CBT group
Due to missing data, correlations were not possible for the CBT group between COMPASS pretreatment to posttreatment change scores and the cardiac, SCL, or fMRI measures, nor between any self-reported ANS symptom (COMPASS/POMS) pre- to 6-month follow-up change scores and any biological ANS function measures.
Change in parasympathetic activation (absolute HF-HRV [ms2]) was associated with change pretreatment to posttreatment on POMS vigor (r = 1.00, P = .029) and POMS fatigue (r = −1.00, P = .004; Table 3). Change in right amygdala volume was associated with change pretreatment to posttreatment on COMPASS secretomotor (trend: r = −1.00, P = .061). There were no other treatment-related change associations between biological and self-reported ANS measures for the CBT group.
Hypothesis 2 (Biological ANS Treatment Markers): Pretreatment Biological ANS Function Will Be Associated With Treatment-Related Changes in Self-Reported ANS Symptoms
Yoga group
Pretreatment general autonomic function predicted treatment-related change on total COMPASS (pre- to 6-month follow-up: [RMSSD]: R2 = .76, β = −.87, P = .024), COMPASS secretomotor (pretreatment to posttreatment [mean HR]: R2 = .99, β = −1.00, P < .001), COMPASS gastrointestinal (pretreatment to posttreatment [RMSSD]: R2 = .75, β = −.87, P = .025; pre- to 6-month follow-up [RMSSD]: R2 = .89, β = −.94, P = .017), COMPASS bladder (pre- to 6-month follow-up [mean HR]: R2 = .78, β = .88, P = .047), COMPASS pupillometer (pre- to 6-month follow-up [RMSSD; trend]: R2 = .67, β = −.82, P = .092), and POMS vigor (pre- to 6-month follow-up [RMSSD]: R2 = .85, β = .92, P = .027; Table 4).
Table 4.
Summary of Biological ANS Treatment Markers for Self-Reported ANS by Treatment Group (Hypothesis 2).
|
Yoga (Pre to Post) |
CBT (Pre to Post) |
Yoga (Pre to 6 months) |
CBT (Pre to 6 months) |
|||||
|---|---|---|---|---|---|---|---|---|
| Biological ANS (n Pre/n Post) | ΔCOMPASS (β) | ΔPOMS (β) | ΔCOMPASS (β) | ΔPOMS (β) | ΔCOMPASS (β) | ΔPOMS (β) | ΔCOMPASS (β) | ΔPOMS (β) |
| Mean HR (12/10) | −.996*** | .88* | N/A | N/A | ||||
| RMSSD (12/10) | −.87* | −.93^ | −.82^ to −.94* | .92* | N/A | N/A | ||
| HF-HRV (12/10) | −.80^ to −.84* | −.88* to −.94* | N/A | N/A | ||||
| LF/HF (12/10) | −.99^ | .91* | N/A | N/A | ||||
| SCL (12/4) | −.95* to .98* | .82^ | .83* to .88* | .99^ to 1.00* | ||||
| Amygdala (10/10) | .81* to .95** | .86* to .95* | N/A | N/A | ||||
| Amyg-DMN (6/6) | .93* to .94* | −.82^ to −.98** | N/A | N/A | .97** | N/A | N/A | |
| Amyg-SN (6/6) | N/A | N/A | .97** to .99** | −.93* | N/A | N/A | ||
Abbreviations: Amyg, amygdala volume; Amyg-DMN, amygdala-default mode network connectivity; Amyg-SN, amygdala-salience network connectivity; ANS, autonomic nervous system; CBT, cognitive behavioral therapy; COMPASS, Composite Autonomic Symptom Score (COMPASS 31); Δ, change (pre minus post; pre minus 6 months); HF-HRV, high-frequency-domain heart rate variability; HR, heart rate; hypothesis 2 = biological ANS treatment markers: pretreatment biological ANS function will be associated with treatment-related changes in self-reported ANS symptoms (pre to post [left], pre to 6 months [right]); LF/HF, ratio low-frequency to high-frequency-domain heart rate variability; N/A, analyses were not possible due to missing data; n pre/n post, number at pretreatment/posttreatment; POMS, Short Form of the Profile of Mood States (POMS-SF); RMSSD, square root of the mean squared differences between successive R-R intervals, SCL, skin conductance level.
All self-reported ANS measures are scored such that higher scores indicate worse functioning; thus, the higher the change score value, the greater the treatment-related improvement in functioning.
^P < .10. *P < .05. **P < .01. ***P < .001.
Pretreatment parasympathetic activation predicted treatment-related change on COMPASS vasomotor (pretreatment to posttreatment [relative HF-HRV (n.u.)]: R2 = .71, β = −.84, P = .036), COMPASS gastrointestinal (pretreatment to posttreatment [absolute HF-HRV, ms2; trend]: R2 = .64, β = −.80, P = .057; pre- to 6-month follow-up [absolute HF-HRV, ms2]: R2 = .81, β = −.90, P = .039), and COMPASS pupillometer (pre- to 6-month follow-up [absolute HF-HRV, ms2]: R2 = .78, β = −.88, P = .049; [relative HF-HRV (n.u.)]: R2 = .88, β = −.94, P = .019).
Pretreatment sympathetic/parasympathetic balance (LF/HF ratio) predicted change pre- to 6-month follow-up on COMPASS pupillometer (R2 = .82, β = .91, P = .034).
Pretreatment tonic arousal (minimum SCL) predicted change pre- to 6-month follow-up on COMPASS orthostatic intolerance (trend: R2 = .67, β = .82, P = .089).
Pretreatment amygdala volume predicted treatment-related change on total COMPASS (pretreatment to posttreatment [right]: R2 = .66, β = .81, P = .049; pre- to 6-month follow-up [right]: R2 = .73, β = .86, P = .030), COMPASS orthostatic intolerance (pre- to 6-month follow-up [right]: R2 = .91, β = .95, P = .013), and COMPASS vasomotor (pretreatment to posttreatment [left]: R2 = .90, β = .95, P = .004).
Pretreatment amygdala-DMN connectivity predicted treatment-related change on total COMPASS (pretreatment to posttreatment [left]: R2 = .88, β = .94, P = .018), COMPASS orthostatic intolerance (pretreatment to posttreatment [left]: R2 = .95, β = .93, P = .025), POMS tension (pretreatment to posttreatment [left]: R2 = .95, β = −.98, P = .005), POMS vigor (pretreatment to posttreatment [right; trend]: R2 = .68, β = −.82, P = .087), and POMS fatigue (pre- to 6-month follow-up [left]: R2 = .95, β = .97, P = .005).
Pretreatment left amygdala-SN connectivity predicted change pre- to 6-month follow-up on total COMPASS (R2 = .94, β = .97, P = .006), COMPASS orthostatic intolerance (R2 = .97, β = .99, P = .002), and POMS vigor (R2 = .86, β = −.93, P = .023). There were no other biological ANS predictors of self-reported ANS treatment-related change for the yoga group.
CBT group
Due to missing data, regression analyses were not possible for the CBT group for the cardiac or structural MRI predictors of long-term treatment-related changes (pre- to 6-month follow-up) in self-reported ANS symptoms (COMPASS/POMS), nor any of the fMRI predictors of short- (pretreatment to posttreatment) or long-term (pre- to 6-month follow-up) treatment-related change.
Pretreatment general autonomic function predicted change pretreatment to posttreatment on POMS vigor (RMSSD; trend: R2 = .86, β = −.93, P = .075; Table 4). Pretreatment sympathetic/parasympathetic balance (LF/HF ratio) predicted change pretreatment to posttreatment on COMPASS vasomotor (trend: R2 = .99, β = −.99, P = .078). Pretreatment tonic arousal predicted treatment-related change on the total COMPASS score (pretreatment to posttreatment [minimum SCL; trend]: R2 = .65, β = .81, P = .053; pre- to 6-month follow-up [minimum SCL]: R2 = .70, β = .83, P = .039; [mean SCL]: R2 = .77, β = .88, P = .021), COMPASS orthostatic intolerance (pretreatment to posttreatment [minimum SCL]: R2 = .96, β = .98, P = .021; [mean SCL; trend]: R2 = .89, β = .94, P = .057), COMPASS vasomotor (pretreatment to posttreatment [minimum SCL]: R2 = .91, β = −.95, P = .046; [mean SCL]: R2 = .97, β = −.99, P = .013), and POMS tension (pre- to 6-month follow-up [minimum SCL]: R2 = 1.00, β = 1.00, P = .019; [mean SCL; trend]: R2 = .98, β = .99, P = .070).
Discussion
Findings from this small exploratory pilot add-on study suggest that veterans with GWI receiving either yoga or CBT for pain gained improvements in biological ANS (changes moved in the direction of healthy profiles) and these improvements were associated with self-reported symptom improvements in both the short term (immediately posttreatment) and long term (6-month follow-up). Associations were generally stronger, more statistically significant, and more consistent for the yoga group, possibly due to the higher proportion of missing data in the CBT group (lost to follow-up, equipment failure, and movement artifact).
Specifically, for the yoga group, treatment-related improvements in self-reported ANS dysfunction were associated with treatment-related improvements in general biological ANS function (lower average HR and higher HRV [RMSSD]), increased parasympathetic activation (HF-HRV), greater parasympathetic dominance (lower LF/HF ratio), reduced amygdala volume, and stronger amygdala-DMN and amygdala-SN connectivity, both short term and long term. Pretreatment biological ANS predictors of short- and long-term treatment-related improvements in self-reported ANS for the yoga group were poorer general biological ANS function (higher average HR, lower HRV [RMSSD]), lower parasympathetic activation (HF-HRV) and greater sympathetic dominance (higher LF/HF ratio), larger amygdala volume, and stronger left amygdala-DMN/left amygdala-SN connectivity. There were, however, some discrepancies for the yoga group in that improvements in self-reported energy (POMS vigor and fatigue) were more strongly associated with treatment-related increases in amygdala volume and arousal (higher average HR, lower RMSSD, lower HR-HRV, and higher LF/HF ratio) and decreases in amygdala-DMN connectivity. Weaker left amygdala-DMN connectivity at pretreatment predicted short-term improvements in self-reported restlessness and anxiety and higher HRV and weaker left amygdala-SN connectivity at pretreatment predicted long-term improvements in self-reported energy.
For the CBT group, treatment-related improvements in self-reported fatigue were associated with treatment-related increases in parasympathetic activation (HF-HRV), while improvements in self-reported energy were associated with decreased parasympathetic activation (ie, higher arousal). Higher pretreatment tonic arousal (mean/minimum SCL) predicted greater treatment-related improvements in self-reported ANS function both short term and long term. There was also a trend for poorer pretreatment general biological ANS function (lower HRV [RMSSD]) to predict short-term improvements in self-reported energy.
Higher resting HR is associated with poorer physical48 and mental49 health. Similarly, lower RMSSD, lower HF-HRV, and higher LF/HF ratio are associated with poorer physical34,50 and mental51,52 health, independent of aging.53 The present findings of treatment-related improvements—reductions in average resting HR, increases in HRV and parasympathetic activation/dominance—for the yoga group are consistent with previous yoga30,31 and exercise intervention54,55 studies. Increases in parasympathetic activation were also observed in the CBT group, which supports a small but growing body of literature suggesting CBT may also be associated with these positive effects on the ANS.15,16
Extant literature are mixed regarding relative amygdala volume size and associations with psychopathology, though one recent study demonstrated reduced amygdala volume in veterans with GWI.7 The present findings of associations between improved self-reported ANS and treatment-related reductions in amygdala volume in the yoga group are therefore intriguing and difficult to interpret. Treatment-related reductions in amygdala volume have been observed following yoga and meditation,56 CBT for social anxiety,17 and mindfulness-based stress reduction,57 suggesting the current findings are consistent with existing treatment-intervention literature outside the GWI field.
Increased amygdala-DMN/amygdala-SN connectivity in the yoga group was associated with—and predicted—short- and long-term improvements in self-reported ANS function, consistent with past observations during mindfulness practice.58,59 Although missing data precluded analyses for the CBT group, previous findings for CBT are mixed, with studies demonstrating either decreased18 or increased19,20 amygdala-DMN/amygdala-SN connectivity, supporting further investigation. The present findings highlight the importance of concurrently exploring both structural and functional brain responses to treatment.
Interestingly, where inconsistencies occurred in the pattern of findings for the yoga group, these were typically for self-reported energy and fatigue on the POMS (vs self-reported symptoms of autonomic dysfunction on the COMPASS). At the same time, where treatment-related associations between biological and self-reported ANS occurred for the CBT group, these were also typically for the POMS rather than COMPASS. While we chose only those subscales with the highest loading of autonomic (dys)function (tension, vigor, and fatigue), the POMS is more accurately a measure of psychological distress. Thus, for CBT, biological mechanisms of treatment action may underlie more psychologically loaded symptom improvements, whereas for yoga, these biomarkers appear to demonstrate different patterns of association for symptoms with—versus without—psychological loading. Due to the small sample and high proportion of missing data, further investigation is needed to confirm this theory.
Data for both the yoga and CBT group suggest that veterans with GWI who have higher tonic arousal and/or more sympathetic dominance—higher SCL, lower RMSSD, lower HF-HRV, and higher LF/HF ratio—may obtain the greatest benefits from treatment. Given that higher SCL is associated with anxiety60,61 and lower RMSSD, lower HF-HRV, and higher LF/HF ratio are associated with poorer physical and mental health,34,50–52 one interpretation of these findings is that poorer pretreatment biological ANS function predicts greater relative treatment response. There is an overall paucity of studies examining pretreatment predictors of response to yoga and extant findings for CBT are mixed: lower symptom severity and more adaptive ANS function pretreatment may be predictive of either better62–64 or worse65,66 treatment outcome, highlighting the need for further exploration.
These exploratory pilot data provide preliminary support for the suggestion that treatment (yoga, CBT) is associated with improvements in both biological and self-reported ANS dysfunctions in GWI. The major limitations for these findings are the small sample size and large amount of missing data, which also prevented group analyses directly comparing treatment-related changes in ANS between yoga and CBT. It should also be noted that since the MRI environment is potentially anxiety-inducing, it is possible that some of the treatment-related improvements in biological ANS measures were driven by habituation to the MRI environment rather than treatment per se.67,68 Although outside the scope of this study, other studies using a nontreatment (wait-list) or healthy control comparison group have observed stronger amygdala effects for the active treatment condition (CBT) in individuals with emotional disorders,69 suggesting that treatment effects occur over and above potential test–retest habituation. Larger and more controlled studies are needed to replicate these findings in individuals with GWI and directly compare biomarkers of yoga versus CBT.
Acknowledgments
The author(s) would like to thank the participants for their time. The authors also thank Louise A Mahoney, Rachael H Cho, Maheen M Adamson, James H Bishop, R Jay Schulz-Heik, Linda M Collery, Timothy J Avery, Melinda S Wong, Julia S Tang, Michael Stanton, Marcelle A Friedman, and Jennifer Hanft for assistance with data collection and treatment intervention.
Notes
The rate of fMRI data loss from participant movement is high given our small sample size. Individuals with chronic pain are known to be more susceptible to movement artifact in fMRI than healthy controls. There is a paucity of fMRI studies on veterans with GWI so it remains to be seen whether their pain is qualitatively or quantitatively different from individuals with non-GWI chronic pain; this small pilot study suggests they may be at least be more susceptible to fMRI movement artifact.
We share the MRI scanner with multiple research teams at Stanford University. One user made unauthorized changes to the Mac Terminal settings that control SCL recordings via synchronization with the MRI scanner trigger resulting in no SCL recordings (ie, data loss) across all saved paradigms. This human error affected multiple projects and teams (including our study) and was not discovered by Stanford Cognitive and Neurobiological Imaging staff until months later.
Authors’ Contributions
DCM was responsible for writing the manuscript with significant contributions from all other authors. PJB is principal investigator and executive manager of this randomized controlled trial (RCT). PJB conceptualized and designed the RCT, and DCM conceptualized and designed this brain and physiological autonomic nervous system measures add-on study. DCM was one of the cognitive behavioral therapy providers and collected the data. DCM, CME, and DDD analyzed the data. DS provided consultation on data analysis and content. All authors read and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a Department of Defense Congressionally Directed Medical Research Program grant (11488016; PJB) and a Stanford Center for Cognitive and Neurobiological Imaging Innovation Seed Grant (DCM). DCM was supported by a Veterans Affairs Advanced Fellowship in the War Related Illness and Injury Study Center, a National Veteran Affairs Post-Deployment Health Resource. Funding bodies have not and will not participate in the study design, the collection, management, analysis, or interpretation of data, nor the writing of findings for publication. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
ORCID iD
Danielle C Mathersul https://orcid.org/0000-0003-2372-8654
References
- 1.Eisen SA, Kang MK, Murphy FM, et al. Gulf War veterans’ health: medical evaluation of a U.S. cohort. Ann Int Med. 2005; 142:881–890. [DOI] [PubMed] [Google Scholar]
- 2.Fukuda K, Nisenbaum R, Stewart G, et al. Chronic multisymptom illness affecting Air Force veterans of the Gulf War. J Am Med Assoc. 1998. ;280:981–988. [DOI] [PubMed] [Google Scholar]
- 3.Steele L. Prevalence and patterns of Gulf War Illness in Kansas veterans: association of symptoms with characteristics of person, place, and time of military service. Am J Epidemiol. 2000; 152:992–1002. [DOI] [PubMed] [Google Scholar]
- 4.Fox A, Helmer D, Tseng CL, Patrick-DeLuca L, Osinubi O. Report of autonomic symptoms in a clinical sample of veterans with Gulf War Illness. Mil Med. 2018; 183:e179–e185. [DOI] [PubMed] [Google Scholar]
- 5.Haley RW, Charuvastra E, Shell WE, et al. Cholinergic autonomic dysfunction in veterans with Gulf War Illness: confirmation in a population-based sample. JAMA Neurol. 2013; 70:191–200. [DOI] [PubMed] [Google Scholar]
- 6.Haley RW, Vongpatanasin W, Wolfe GI, et al. Blunted circadian variation in autonomic regulation of sinus node function in veterans with Gulf War syndrome. Am J Med. 2004; 117:469–478. [DOI] [PubMed] [Google Scholar]
- 7.Christova P, James LM, Engdahl BE, Lewis SM, Carpenter AF, Georgopoulos AP. Subcortical brain atrophy in Gulf War Illness. Exp Brain Res. 2017; 235:2777–2786. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Avery TJ, Vakhtin AA, et al. Brainstem atrophy in Gulf War Illness. NeuroToxicology. 2020; 78:71–79. [DOI] [PubMed] [Google Scholar]
- 9.Beissner F, Meissner K, Bär KJ, Napadow V. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci. 2013; 33:10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hofmann SG, Asnaani A, Vonk IJJ, Sawyer AT, Fang A. The efficacy of cognitive behavioral therapy: a review of meta-analyses. Cognit Ther Res. 2012; 36:427–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman BM, Papas RK, Chatkoff DK, Kerns RD. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol. 2007; 26:1–9. [DOI] [PubMed] [Google Scholar]
- 12.Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev. 2012; 11:CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrot S, Russell IJ. More ubiquitous effects from non-pharmacologic than from pharmacologic treatments for fibromyalgia syndrome: a meta-analysis examining six core symptoms. Eur J Pain. 2014; 18:1067–1080. [DOI] [PubMed] [Google Scholar]
- 14.Laird KT, Tanner-Smith EE, Russell AC, Hollon SD, Walker LS. Comparative efficacy of psychological therapies for improving mental health and daily functioning in irritable bowel syndrome: a systematic review and meta-analysis. Clin Psychol Rev 2017; 51:142–152. [DOI] [PubMed] [Google Scholar]
- 15.Carney RM, Freedland KE, Stein PK, Skala JA, Hoffman P, Jaffe AS. Change in heart rate and heart rate variability during treatment for depression in patients with coronary heart disease. Psychosom Med. 2000; 62:639–647. [DOI] [PubMed] [Google Scholar]
- 16.Garakani A, Martinez JM, Aaronson CJ, Voustianiouk A, Kaufmann H, Gorman JM. Effect of medication and psychotherapy on heart rate variability in panic disorder. Depress Anxiety. 2009; 26:251–258. [DOI] [PubMed] [Google Scholar]
- 17.Månsson KNT, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2016; 6:e727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuan M, Zhu H, Qiu C, et al. Group cognitive behavioral therapy modulates the resting-state functional connectivity of amygdala-related network in patients with generalized social anxiety disorder. BMC Psychiatry. 2016; 16:198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shou H, Yang Z, Satterthwaite TD, et al. Cognitive behavioral therapy increases amygdala connectivity with the cognitive control network in both MDD and PTSD. NeuroImage Clin. 2017; 14:464–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Straub J, Metzger CD, Plener PL, Koelch MG, Groen G, Abler B. Successful group psychotherapy of depression in adolescents alters fronto-limbic resting-state connectivity. J Affect Disord. 2017; 209:135–139. [DOI] [PubMed] [Google Scholar]
- 21.Rive MM, Van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013; 37:2529–2553. [DOI] [PubMed] [Google Scholar]
- 22.Khalsa SB, Cohen L, McCall T, Telles S. Principles and Practice of Yoga in Health Care. Edinburgh, Scotland: Handspring; 2016. [Google Scholar]
- 23.Cramer H, Lauche R, Langhorst J, Dobos G. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety. 2013; 30:1068–1083. [DOI] [PubMed] [Google Scholar]
- 24.Hofmann SG, Andreoli G, Carpenter JK, Curtiss J. Effect of hatha yoga on anxiety: meta-analysis. J Evid Based Med. 2016; 9:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Büssing A, Ostermann T, Lüdtke R, Michalsen A. Effects of yoga interventions on pain and pain-associated disability: a meta-analysis. J Pain. 2012; 13:1–9. [DOI] [PubMed] [Google Scholar]
- 26.Posadzki P, Ernst E, Terry R, Lee MS. Is yoga effective for pain? A systematic review of randomized clinical trials. Complement Ther Med. 2011; 19:281–287. [DOI] [PubMed] [Google Scholar]
- 27.Wu C, Yi Q, Zheng X, et al. Effects of mind-body exercises on cognitive function in older adults: a meta-analysis. J Am Geriatr Soc. 2019; 67:749–758. [DOI] [PubMed] [Google Scholar]
- 28.Langhorst J, Klose P, Dobos GJ, Bernardy K, Häuser W. Efficacy and safety of meditative movement therapies in fibromyalgia syndrome: a systematic review and meta-analysis of randomized controlled trials. Rheumatol Int. 2013; 33:193–207. [DOI] [PubMed] [Google Scholar]
- 29.Schumann D, Anheyer D, Lauche R, Dobos G, Langhorst J, Cramer H. Effect of yoga in the therapy of irritable bowel syndrome: a systematic review. Clin Gastroenterol Hepatol. 2016; 14:1720–1731. [DOI] [PubMed] [Google Scholar]
- 30.Saoji AA, Raghavendra BR, Manjunath NK. Effects of yogic breath regulation: a narrative review of scientific evidence. J Ayurveda Integr Med. 2019; 10:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stark R, Schienle A, Walter B, Vaitl D. Effects of paced respiration on heart period and heart period variability. Psychophysiology. 2000; 37:302–309. [PubMed] [Google Scholar]
- 32.Chiesa A, Serretti A, Jakobsen JC. Mindfulness: top-down or bottom-up emotion regulation strategy? Clin Psychol Rev. 2013; 33:82–96. PMC][ 10.1016/j.cpr.2012.10.006] [23142788] [DOI] [PubMed] [Google Scholar]
- 33.Bayley PJ, Cho RH, Schulz-Heik RJ, et al. Yoga is effective in treating symptoms of Gulf War Illness: a randomized clinical trial. Manuscript submitted for publication. [DOI] [PubMed]
- 34.Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol. 2010; 141:122–131. [DOI] [PubMed] [Google Scholar]
- 35.Barry RJ, Sokolov EN. Habituation of phasic and tonic components of the orienting reflex. Int J Psychophysiol. 1993; 15:39–42. [DOI] [PubMed] [Google Scholar]
- 36.Sletten DM, Suarez GA, Low PA, Mandrekar J, Singer W. COMPASS 31: a refined and abbreviated composite autonomic symptom score. Mayo Clin Proc. 2012; 87:1196–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suarez GA, Opfer-Gehrking TL, Offord KP, Atkinson EJ, O’Brien PC, Low PA. The autonomic symptom profile a new instrument to assess autonomic symptoms. Neurology. 1999; 52:523–528. [DOI] [PubMed] [Google Scholar]
- 38.Curran SL, Andrykowski MA, Studts JL. Short Form of the Profile of Mood States (POMS-SF): psychometric information. Psychol Assess. 1995; 7:80–83. [Google Scholar]
- 39.McNair DM, Lorr M, Droppleman LF. Manual: Profile of Mood States. 2nd ed. San Diego, CA: Educational and Industrial Testing Service; 1992. [Google Scholar]
- 40.Malik M, Camm AJ, Bigger JT, Jr, et al. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996; 17:354–381. [PubMed] [Google Scholar]
- 41.Shaffer F, Ginsberg JP. An overview of heart rate variability metrics and norms. Front Public Health. 2017; 5:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Braithwaite JJ, Watson DG, Jones R, Rowe M. A Guide for Analysing Electrodermal Activity (EDA) & Skin Conductance Responses (SCRs) for Psychological Experiments. University of Birmingham, England: Selective Attention & Awareness Laboratory (SAAL) Behavioural Brain Sciences Centre; 2013. [Google Scholar]
- 43.Muschelli J, Nebel MB, Caffo BS, Barber AD, Pekar JJ, Mostofsky SH. Reduction of motion-related artifacts in resting state fMRI using aCompCor. NeuroImage. 2014; 96:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002; 17:143–155. [12391568] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004; 23:S208–S219. [DOI] [PubMed] [Google Scholar]
- 46.Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003; 20:870–888. [DOI] [PubMed] [Google Scholar]
- 47.Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex. 2012; 22:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aune D, Sen A, ó’Hartaigh B, et al. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality—a systematic review and dose–response meta-analysis of prospective studies. Nutr Metab Cardiovasc Dis. 2017; 27:504–517. [DOI] [PubMed] [Google Scholar]
- 49.Latvala A, Kuja-Halkola R, Rück C, et al. Association of resting heart rate and blood pressure in late adolescence with subsequent mental disorders: a longitudinal population study of more than 1 million men in Sweden. JAMA Psychiatry. 2016; 73:1268–1275. [DOI] [PubMed] [Google Scholar]
- 50.Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD. A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev. 2012; 36:747–756. [DOI] [PubMed] [Google Scholar]
- 51.Chalmers JA, Quintana DS, Abbott MJA, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: a meta-analysis. Front Psychiatry. 2014; 5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010; 67:1067–1074. [DOI] [PubMed] [Google Scholar]
- 53.Tan JPH, Beilharz JE, Vollmer-Conna U, Cvejic E. Heart rate variability as a marker of healthy ageing. Int J Cardiol. 2019; 275:101–103. [DOI] [PubMed] [Google Scholar]
- 54.Besnier F, Labrunée M, Pathak A, et al. Exercise training-induced modification in autonomic nervous system: an update for cardiac patients. Ann Phys Rehabil Med. 2017; 60:27–35. [DOI] [PubMed] [Google Scholar]
- 55.Raffin J, Barthélémy JC, Dupré C, et al. Exercise frequency determines heart rate variability gains in older people: a meta-analysis and meta-regression. Sports Med. 2019; 49:719–729. [DOI] [PubMed] [Google Scholar]
- 56.Gotink RA, Vernooij MW, Ikram MA, et al. Meditation and yoga practice are associated with smaller right amygdala volume: the Rotterdam study. Brain Imaging Behav. 2018; 12:1631–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hölzel BK, Carmody J, Evans KC, et al. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2009; 5:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Doll A, Hölzel BK, Mulej Bratec S, et al. Mindful attention to breath regulates emotions via increased amygdala-prefrontal cortex connectivity. NeuroImage. 2016; 134:305–313. [DOI] [PubMed] [Google Scholar]
- 59.Kral TRA, Schuyler BS, Mumford JA, Rosenkranz MA, Lutz A, Davidson RJ. Impact of short- and long-term mindfulness meditation training on amygdala reactivity to emotional stimuli. NeuroImage. 2018; 181:301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Croft RJ, Gonsalvez CJ, Gander J, Lechem L, Barry RJ. Differential relations between heart rate and skin conductance, and public speaking anxiety. J Behav Ther Exp Psychiatry. 2004; 35:259–271. [DOI] [PubMed] [Google Scholar]
- 61.Rosebrock LE, Hoxha D, Norris C, Cacioppo JT, Gollan JK. Skin conductance and subjective arousal in anxiety, depression, and comorbidity: implications for affective reactivity. J Psychophysiol. 2017; 31:145–157. [Google Scholar]
- 62.Soder HE, Wardle MC, Schmitz JM, Lane SD, Green C, Vujanovic AA. Baseline resting heart rate variability predicts post-traumatic stress disorder treatment outcomes in adults with co-occurring substance use disorders and post-traumatic stress. Psychophysiology. 2019; 56:e13377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor CT, Knapp SE, Bomyea JA, Ramsawh HJ, Paulus MP, Stein MB. What good are positive emotions for treatment? Trait positive emotionality predicts response to cognitive behavioral therapy for anxiety. Behav Res Ther. 2017; 93:6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolitzky-Taylor KB, Arch JJ, Rosenfield D, Craske MG. Moderators and non-specific predictors of treatment outcome for anxiety disorders: a comparison of cognitive behavioral therapy to acceptance and commitment therapy. J Consult Clin Psychol. 2012; 80(5):786–799. [DOI] [PubMed] [Google Scholar]
- 65.Davies CD, Niles AN, Pittig A, Arch JJ, Craske MG. Physiological and behavioral indices of emotion dysregulation as predictors of outcome from cognitive behavioral therapy and acceptance and commitment therapy for anxiety. J Behav Ther Exp Psychiatry. 2015; 46:35–43. [DOI] [PubMed] [Google Scholar]
- 66.Rizvi SL, Vogt DS, Resick PA. Cognitive and affective predictors of treatment outcome in cognitive processing therapy and prolonged exposure for posttraumatic stress disorder. Behav Res Ther. 2009; 47:737–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chapman HA, Bernier D, Rusak B. MRI-related anxiety levels change within and between repeated scanning sessions. Psychiatry Res. 2010; 182:160–164. [DOI] [PubMed] [Google Scholar]
- 68.Coen SJ, Gregory LJ, Yágüez L, et al. Reproducibility of human brain activity evoked by esophageal stimulation using functional magnetic resonance imaging. Am J Physiol Gastrointest Liver Physiol. 2007; 293:G188–G197. [DOI] [PubMed] [Google Scholar]
- 69.Barsaglini A, Sartori G, Benetti S, Pettersson-Yeo W, Mechelli A. The effects of psychotherapy on brain function: a systematic and critical review. Prog Neurobiol. 2014; 114:1–4. [DOI] [PubMed] [Google Scholar]