Abstract
Background:
Patients treated with maintenance dialysis are at high risk of polypharmacy given their many comorbidities as well as complications from their disease state and treatment. The prescribing patterns and burden of polypharmacy in patients treated with maintenance dialysis, and specifically the difference between hemodialysis (HD) and peritoneal dialysis (PD) prescribing, are not well characterized.
Objectives:
The objectives of this study were to review the prescribing patterns for patients treated with maintenance dialysis, to compare prescribing pattern between HD and PD, and to identify opportunities for deprescription.
Design:
This is a retrospective cohort study.
Setting:
This study was conducted in all dialysis centers in British Columbia, Canada.
Patients:
Patients who were receiving chronic dialysis (>120 days on the same dialysis modality) between June 3 and October 1, 2015, and registered in the British Columbia (BC) Renal Patient Records and Outcomes Management Information System.
Measurements:
Patient demographics as well as both prescription and non-prescription medications were collected. Comparison of discrete and continuous variables was made by chi-square analysis and independent t test, respectively. All statistical tests were 2-sided, and a P value of <.05 was considered statistically significant.
Methods:
Medications were classified by indication: (1) management of renal complications, (2) cardiovascular (CV) medications, (3) diabetes medications, or (4) management of symptoms, and then classified as to whether they were a “potentially inappropriate medication” (PIM) or not. Ethics approval was granted from the University of British Columbia Research and Ethics Board.
Results:
In total, 3017 patients met inclusion criteria (2243 HD, 774 PD). The mean age was 66.2 ± 14.8 years. The HD group had more patients over 80 years old (22.1% vs 12.5%) and more patients with diabetes and CV disease. The mean number (standard deviation [SD]) of discrete prescribed medications was 17.71 (5.72) overall with more medications in the HD group versus the PD group. The mean number of medications increased with dialysis vintage in both groups. HD patients were on more medications for renal complications and management of symptoms than PD patients. Of the total number of medications prescribed, 5.02 (2.78) were classified as a PIM, with the number of PIMs higher in HD vs PD patients: 5.37 (2.83) versus 4.02 (2.37).
Limitations:
In BC, some of the medications are prescribed through standardized protocols and may not be comparable with other Canadian provinces. We report here prescribing patterns, not utilization patterns, as we are not able to ascertain actual consumption of prescribed medication.
Conclusion:
This study reviews and characterizes both the prescription and non-prescription medication prescribed to HD patients and PD patients in BC. Pill burden in both groups is high, as is the prescription of PIMs. Patients receiving maintenance HD receive more overall medications and more PIMs. These results highlight areas of opportunities for future systematic and patient-informed deprescription initiatives in both patient groups.
Keywords: polypharmacy, potentially inappropriate medication, hemodialysis, peritoneal dialysis, deprescription, medication safety, end-stage kidney disease
Abrégé
Contexte:
Les patients sous dialyse à long terme, en raison de leurs nombreuses comorbidités et des complications inhérentes à leur état de santé et à leur traitement, s’exposent à un plus grand risque de polypharmacie. On en sait toutefois peu sur le fardeau qu’elle représente pour ces patients et sur leurs profils de prescription, particulièrement sur les possibles différences entre les patients traités par hémodialyse ou par dialyse péritonéale.
Objectifs:
Comparer les profils de prescription des patients traités par hémodialyse (HD) et par dialyse péritonéale (DP), et cerner les possibilités de déprescription.
Type d’étude:
Étude de cohorte rétrospective.
Cadre:
Tous les centres de dialyse de la Colombie-Britannique (Canada).
Sujets:
Les patients sous dialyse chronique (plus de 120 jours avec la même modalité) entre le 3 juin et le 1er octobre 2015, et inscrits dans la base de données Renal Patient Records and Outcomes Management Information System de Colombie-Britannique.
Mesures:
Les caractéristiques démographiques des patients et la liste des médicaments, prescrits ou non. Une analyse du chi-carré (variables discontinues) et un test t indépendant (variables continues) ont été employés pour comparer les différentes variables. Tous les tests statistiques étaient bilatéraux. Une valeur de P inférieure à 0,05 a été jugée significative.
Méthodologie:
Les médicaments ont été classés par indication : (1) traitement des complications rénales, (2) contre les maladies cardiovasculaires (3) contre le diabète et (4) traitement des symptômes. Ils ont ensuite été classés comme étant ou non un « médicament potentiellement inapproprié » (MPI). L’approbation déontologique a été octroyée par le comité d’éthique de la recherche de l’Université de la Colombie-Britannique.
Résultats:
Un total de 3 017 patients, dont l’âge moyen était de 66,2 ± 14,8 ans, satisfaisaient les critères d’inclusion (2243 HD, 774 DP). Le groupe HD comportait davantage de patients âgés de plus de 80 ans (22,1 % contre 12,5 %) et de patients souffrant de diabète et de maladies cardiovasculaires. Le nombre moyen de prescriptions (écart-type) s’élevait à 17,71 (5,72) avec des nombres globaux plus élevés dans le groupe HD. Le nombre moyen de médicaments augmentait avec le temps passé en dialyse dans les deux groupes. Les patients HD prenaient davantage de médicaments pour traiter les symptômes et les complications rénales que les patients DP. Dans l’ensemble, une moyenne de 5,02 (2,78) médicaments ont été classés MPI, et leur nombre était plus élevé dans le groupe HD que dans le groupe DP (5,37 [2,83] contre 4,02 [2,37]).
Limites:
En C.-B., certains médicaments sont prescrits selon des protocoles standardisés, et ceci pourrait ne pas être comparable aux autres provinces canadiennes. L’article présente des profils de prescription et non des schémas de prise de médicaments, car nous ne pouvions vérifier la consommation réelle des médicaments prescrits.
Conclusion:
Cette étude examine et caractérise les médicaments sous ordonnance et en vente libre qui sont prescrits aux patients britanno-colombiens traités par HD et DP. La charge médicamenteuse est élevée dans les deux groupes, de même que le nombre d’ordonnances de MPI. Les patients traités par HD se voient prescrire davantage de médicaments et de MPI. Ces résultats montrent que des initiatives de déprescription systématiques et informées sont possibles pour ces deux groupes de patients.
What was known before
Patients treated with maintenance dialysis experience polypharmacy, which is associated with a decreased quality of life, decreased adherence to treatment, and an increased mortality risk.
What this adds
Previous contemporary comparisons between peritoneal dialysis (PD) and hemodialysis (HD) patients have not been published. We describe that patients receiving maintenance HD were prescribed more potentially inappropriate medications (PIMs) than patients on PD (5.37 ± 2.83 vs 4.02 ± 2.37). One third of the prescribed medications were classified as being used for symptom management, which represents an important target area for intervention. As symptoms may wane over time, deprescription may be appropriate with minimal sequelae.
Introduction
Patients with end-stage kidney disease (ESKD) receiving dialysis treatment are complex and are prone to polypharmacy. Polypharmacy in this population reflects the high number of comorbidities, as well as the multiple complications related to kidney failure and dialysis treatment. Previous studies have shown that these patients are prescribed at least 8 to 12 different medications that they take on a daily basis.1 A recent study looking at Ontario prescription pattern for in-center hemodialysis (HD) patients showed that an average of 11 medications per patient was dispensed.2 Dialysis patients are also at increased risk of adverse effects related to medication because drug pharmacokinetic parameters are changed in this population; drug interactions are common; and these patients are rarely included in clinical trials, which bring uncertainty regarding medication efficacy and safety. Furthermore, studies have reported that a high number of prescribed medications in HD patients is associated with a decrease in their quality of life, a decrease in their adherence to treatment, and an increase in their mortality risk.3,4
Deprescribing is defined as “the process of tapering, stopping, discontinuing or withdrawing drugs, with the goals of managing polypharmacy and improving outcomes.” Deprescribing strategies often target “potentially inappropriate medications” (PIMs), medications with either no clear evidence-based indication, a higher risk of adverse effects, or which may not be cost-effective.5 This methodology has been trialed in the elderly population and certain specific patient groups such as palliative care and heart failure.6-8
In the elderly population, deprescribing has been associated with decreased risk of death, decreased referral to nursing home, and lower drug costs. Importantly, these studies have also demonstrated improvements in patients’ perception of their health. One study used deprescribing tools to decrease medications taken by HD patients. They were successful in deprescribing 1 medication in 71% of patients, and at 6 months, only 16% of medication stopped were represcribed.6
The objectives of this study were to review the prescribing patterns for dialysis patients, to analyze any differences between HD and PD (peritoneal dialysis) prescribing patterns, and to identify opportunities for deprescription in this population.
Participants and Methods
All patients who receive dialysis in British Columbia (BC) are all registered in the Patient Records and Outcomes Management Information System (PROMIS).9 This database contains demographic data, comorbidities, laboratory values, as well as a complete and real-time medication profile. This medication profile includes prescription and over-the-counter medications.
We retrospectively extracted demographic data and medication profile information from PROMIS for patients who were on chronic dialysis (>120 days on the same dialysis modality) during the study period (defined as June 3-October 1, 2015). Medication profiles for these patients were considered accurate because medication reconciliation is performed every 6 months and PROMIS medication profile is updated regularly with orders written in the unit or with patient communication. Medication list for each patient was extracted at 1 time point only.
Dialysis modalities included were HD (facility-dependent HD, home conventional HD, home short HD, home nocturnal HD, facility independent HD) and PD. Dialysis vintage was defined as the number of years between first dialysis ever done as a chronic patient under the BC dialysis program and the study period. Pediatric patients (<18 years of age at study date) were excluded from this study.
Comorbidities were obtained from PROMIS and were defined as follows:
Diabetes based on renal diagnosis, comorbidity condition, or labs (A1C > 6.5%, fasting blood glucose >7 mmol/L, or non-fasting blood glucose >11.1 mmol/L).
Cardiovascular (CV) disease includes cardiac arrest, congestive heart failure, myocardial infarction, angina, cardiac devices, coronary revascularization, coronary angiography, cerebrovascular accident, transient ischemic attacks, coronary artery disease, other CV diseases, pulmonary hypertension, dysrhythmias, valvular heart disease, peripheral vascular disease, myocarditis, endocarditis, cardiomyopathy, or left ventricular hypertrophy.
Medications were classified as their main indication being for (1) management of renal complications, (2) CV disease, (3) diabetes, (4) management of symptoms, or (5) other. Table 1 shows the list of medication classes by category. Table 2 outlines a classification system for PIMs for renal patients. This classification was previously developed by a group of expert Canadian nephrology health professionals and patients (Can-SOLVE CKD working group) working on deprescription initiatives.10 This classification was inspired from the American Society of Geriatrics Beers criteria for PIM use in adult older than 65 years,5 which would also include the majority of the dialysis population. The goal of this initiative is to improve the care of patients by reducing their exposure to “potentially” inappropriate medications that have an unfavorable balance of benefits and harms compared with alternative treatment options, including non-pharmacotherapeutic ones.5 However, it is recognized that prescribing decisions are not always clear-cut and need to take into consideration individualized patient circumstances to allow individualized patient-center care.
Table 1.
List of Medication Classes by Main Indication Categories.
| Categories | List of medication, medication class |
|---|---|
| Management of renal complications | Activated vitamin D, phosphorus binders, iron supplement, erythropoietin-stimulating agents (ESAs), potassium binders, cinacalcet, renal vitamins, sodium bicarbonate, tolvaptan |
| Cardiovascular medications | Amiodarone, diuretics, beta-blockers, antiplatelets, anticoagulants, angiotensin-converting-enzyme (ACE) inhibitors, angiotensin II receptor blockers (ARBs), calcium channel blockers, lipid-lowering therapies, vasodilators, digoxin, anti-arrhythmic, nitrates, alpha-blockers, clonidine, minoxidil, aldosterone antagonists |
| Diabetes medications | Acarbose, metformin, insulins, sodium-glucose transport protein 2 (SGLT-2) inhibitors, sulfonylurea, glucagon-like peptide-1 (GLP-1) agonists, dipeptidyl peptidase 4 (DPP-4) inhibitors, thiazolidinediones, meglitinides |
| Medications for symptom management | Antacid, dopamine agonists, fiber supplements, opioids, acetaminophen, non steroidal anti-inflammatory drugs (NSAIDs), alpha-1 blocker, benzodiazepines, antidepressants, antipsychotics, muscle relaxants, antidiarrheal drugs, levodopa/carbidopa, betahistine, laxatives, analgesic cream, carbamazepine, gabapentin/pregabalin, antihistamines, hypnotics, proton pump inhibitors (PPIs), promotility agents, 5-HT3 receptor antagonists, quinine, megace, hypnotics, tramadol, cannabinoids |
| Other medications | All other medications not captured in previous categories (eg, bisphosphonates, immunosuppressants, eye drops) |
Table 2.
List of PIMs Categorized by Indication.
| Categories | PIMs | Rationale |
|---|---|---|
| Cardiovascular medications | Antiplatelets | Increase risk of bleeding risk may outweigh benefits |
| Anticoagulants | Increase risk of bleeding risk may outweigh benefits | |
| Digoxin | Increase risk of toxicity due to decrease renal clearance | |
| Diuretics | Lack of efficacy in ESKD patients with limited urine output | |
| Statins, ezetimibe, niacin, and fibrates | Lack of efficacy data and potential risk of increase risk of adverse events (especially with niacin and fibrates) | |
| Diabetes medications | Metformin | Increase of toxicity in ESKD patients |
| Medications for symptom management | Allopurinol | Need to be reassessed because dialysis reduces serum uric acid level |
| Alpha-1 blocker | Increase risk of postural hypotension; if indication was to improve urinary flow, indication needs to be reassessed | |
| Antacid | Increase risk of toxicity due to decrease renal clearance | |
| Antidepressants (non-Tricyclic antidepressants (TCAs)) | Increase risk of adverse events in ESKD, indication needs to be reassessed regularly | |
| Antihistamines | Highly anti-cholinergic, high risk of adverse events like confusion, dry mouth, constipation | |
| Antipsychotics | Lack of safety data in ESKD patients may increase risk of stroke and risk of decrease cognition | |
| Benzodiazepines | Increase risk of adverse events like falls, fracture, decrease cognition | |
| Cannabinoids | Lack of safety data in ESKD patients may increase risk of falls and risk of decrease cognition | |
| Dimenhydrinate | Highly anti-cholinergic, high risk of adverse events like confusion, dry mouth, constipation | |
| Dopamine agonists | Indication needs to be reassessed regularly, increase risk of adverse events like dizziness, sudden sleep attack, hallucinations | |
| Gabapentin/pregabalin | Indication needs to be reassessed regularly, increase risk of adverse events like dizziness, drowsiness, decrease cognition, edema | |
| Hypnotics | Increase risk of adverse events like falls, fracture, decrease cognition | |
| Laxatives | Indication needs to be reassessed regularly, increase pill burden | |
| Levodopa/carbidopa | Indication needs to be reassessed regularly, increase risk of adverse events like dizziness, nausea, trouble sleeping | |
| Megestrol | Lack of efficacy data, risk of adverse events like hypertension, insomnia, deep vein thrombosis/pulmonary embolism | |
| Muscarinic blocker | Highly anti-cholinergic, high risk of adverse events like confusion, dry mouth, constipation; indication needs to be reassessed if limited urine output | |
| NSAIDs | Increase risk of adverse events like bleeding, hypertension, edema | |
| Narcotics | Increase risk of adverse events like increase risk of fall, risk of fracture, risk of decrease cognition, risk of constipation | |
| proton pump inhibitors (PPIs) | Increase risk of Clostridium difficile infection and increase bone loss; indication needs to be reassessed | |
| GI motility stimulants | Increase risk of adverse events like arrhythmia, extra-pyramidal symptoms | |
| Quinine | Increase risk of adverse events like thrombocytopenia, aplastic anemia, arrhythmia | |
| TCAs | Highly anti-cholinergic, high risk of adverse events like confusion, dry mouth, constipation; risk of cardiac toxicity | |
| Others | Bisphosphonates | Potential increase risk of toxicity and lack of efficacy data |
Note. PIMs = potentially inappropriate medications; ESKD = end-stage kidney disease ; TCAs = Tricyclic antidepressants; NSAIDs = non steroidal anti-inflammatory drugs.
Annual cost of PIMs was estimated based on the British Columbia PharmaCare Formulary, including an average monthly dispensing fee of Can$12.00.11
This study was supported by a grant from BC Renal (www.bcrenalagency.ca).
Statistical Analysis
Comparison of discrete and continuous variables was made by chi-square analysis and independent t test, respectively. All statistical tests were 2-sided, and a P value of <.05 was considered statistically significant. All analyses were performed using SPSS. Ethics approval was granted from the University of British Columbia Research and Ethics Board.
Results
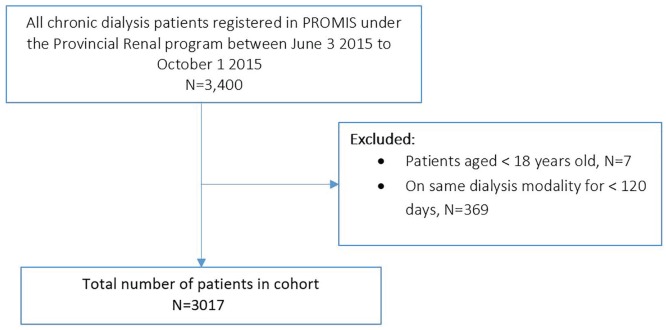
Figure 1 describes the cohort selection for this study. Table 3 describes the demographics of the cohort of interest. In total, 3017 patients met our inclusion criteria (2243 HD, 774 PD). The mean age was 66.2 ± 14.8 years. Patients on HD were older and with longer dialysis vintage than PD patients. Table 4 shows that dialysis patients are prescribed a mean (standard deviation [SD]) of 17.7 (5.7) discrete medications. The mean (SD) number of prescribed medications in the HD group, 18.1 (5.9), was greater than the PD group, 16.7 (5.0) (P < .0001). As expected, in both HD and PD populations, the mean number of prescribed medications increased with longer dialysis vintage. The mean (SD) number of “as-needed” medications in the HD and PD groups was 5.7 (3.2) and 4.2 (2.2), respectively. On average, HD and PD patients were prescribed 6.0 and 5.2 discrete medications for symptom management. Hemodialysis patients were on more medications for management of renal complications and for management of symptoms than PD patients.
Figure 1.
Cohort selection.
Note. PROMIS = Patient Records and Outcomes Management Information System.
Table 3.
Demographics of Study Patients.
| Overall | Hemodialysis | Peritoneal dialysis | P value | |
|---|---|---|---|---|
| Final cohort (patients): n | 3017 | 2243 | 774 | |
| Age: mean (SD) | 66.2 (14.8) | 67.7 (14.7) | 64.2 (14.4) | <.0001 |
| Age group: n (%) | <.0001 | |||
| 18 to <40 years | 183 (6.1%) | 132 (5.9%) | 51 (6.6%) | |
| 40 to <65 years | 1041 (34.5%) | 740 (33.0%) | 301 (38.9%) | |
| 65 to <80 years | 1201 (39.8%) | 876 (39.1%) | 325 (42.0%) | |
| ≥80 years | 592 (19.6%) | 495 (22.1%) | 97 (12.5%) | |
| Male sex: n (%) | 1824 (60.5%) | 1336 (59.6%) | 488 (63.0%) | .0883 |
| Race: n (%) | .0190 | |||
| Caucasian | 1730 (57.3%) | 1285 (57.3%) | 445 (57.5%) | |
| Asian Oriental | 610 (20.2%) | 426 (19.0%) | 184 (23.8%) | |
| Asian Indian | 396 (13.1%) | 315 (14.0%) | 81 (10.5%) | |
| First Nations | 125 (4.1%) | 96 (4.3%) | 29 (3.7%) | |
| Black | 33 (1.1%) | 25 (1.1%) | 8 (1.0%) | |
| Other/multiracial | 82 (2.7%) | 67 (3.0%) | 15 (1.9%) | |
| Unknown | 41 (1.4%) | 29 (1.3%) | 12 (1.6%) | |
| Comorbidities: n (%) | ||||
| Cardiovascular disease | 1741 (57.7%) | 1335 (59.5%) | 406 (52.5%) | .889 |
| Diabetes | 2098 (69.5%) | 1588 (70.8%) | 510 (65.9%) | .011 |
| Dialysis vintage: years | ||||
| Median | 3.3 | 3.8 | 2.4 | |
| Interquartile range | [1.7-6.1] | [1.8-7.1] | [1.3-3.9] | |
| Dialysis vintage group: n (%) | <.00001 | |||
| ≤1 year | 419 (13.9%) | 283 (12.6%) | 136 (17.6%) | |
| 1-3 years | 982 (32.5%) | 631 (28.1%) | 351 (45.3%) | |
| >3 years | 1616 (53.6%) | 1329 (59.3%) | 287 (37.1%) | |
Table 4.
Medication Data.
| Overall | Hemodialysis | Peritoneal dialysis | P value | |
|---|---|---|---|---|
| Final cohort (patients): n | 3017 | 2243 | 774 | |
| Patients on any medication: n (%) | 3016 (99.97%) | 2242 (99.96%) | 774 (100%) | |
| Patients on as needed (PRN) medications: n (%) | 2929 (97.1%) | 2169 (96.7%) | 760 (98.2%) | .0336 |
| Number of medications per patient | ||||
| Mean (SD) | 17.7 (5.7) | 18.1 (5.9) | 16.7 (5) | .0001 |
| Number of non-regularly scheduled medications per patient | ||||
| Mean (SD) | 12.4 (4.2) | 12.3 (4.2) | 12.5 (4.2) | .2725 |
| Number of as needed (PRN) medications per patient | ||||
| Mean (SD) | 5.3 (3.1) | 5.7 (3.2) | 4.2 (2.2) | .0001 |
| Patients on medications by category: n (%) | ||||
| Cardiovascular | 2862 (94.9%) | 2127 (94.8%) | 735 (95.0%) | .8852 |
| Diabetes | 1147 (38.0%) | 843 (37.6%) | 304 (39.3%) | .4028 |
| Renal | 3013 (99.9%) | 2240 (99.9%) | 773 (99.9%) | .9760 |
| Symptoms | 2958 (98.0%) | 2194 (97.8%) | 764 (98.7%) | .1220 |
| Other | 2763 (91.6%) | 1996 (89.0%) | 767 (99.1%) | <.00001 |
| Number of medications by patient: mean (SD) | ||||
| Cardiovascular | 3.5 (2.0) | 3.5 (2.02) | 3.6 (2.0) | .1927 |
| Diabetes | 0.6 (0.8) | 0.6 (0.83) | 0.7 (0.9) | .0107 |
| Renal | 4.7 (1.4) | 5.0 (1.33) | 3.9 (1.2) | <.0001 |
| Symptoms | 5.8 (3.0) | 6.0 (3.21) | 5.2 (2.2) | <.0001 |
| Other | 3.1 (2.3) | 3.0 (2.33) | 3.5 (2.1) | <.0001 |
| Patients with diabetes | ||||
| Number of medications: mean (SD) | 18.5 (5.7) | 18.7 (5.9) | 17.8 (4.8) | .0002 |
| Number of diabetes medications: mean (SD) | 0.9 (0.9) | 0.8 (0.9) | 1.0 (0.9) | <.0001 |
| Patients without diabetes | ||||
| Number of medications: mean (SD) | 15.9 (5.5) | 16.5 (5.7) | 14.6 (4.6) | <.0001 |
| Number of medications by age group: mean (SD) | ||||
| 18 to < 40 years | 15.4 (6.1) | 16.1 (6.4) | 13.4 (4.8) | <.0001 |
| 40 to < 65 years | 17.7 (5.8) | 18.1 (6.1) | 16.5 (4.8) | <.0001 |
| 65 to < 80 years | 18.4 (5.6) | 18.7 (5.8) | 17.3 (5.0) | <.0001 |
| ≥80 years | 17.3 (5.4) | 17.2 (5.4) | 17.3 (5.1) | .8570 |
| Number of medications by dialysis vintage group: mean (SD) | ||||
| ≤1 year | 16.9 (5.7) | 17.3 (5.9) | 16.1 (5.0) | <.0001 |
| 1 to 3 years | 17.6 (5.6) | 18.2 (5.8) | 16.5 (4.9) | <.0001 |
| >3 years | 18.0 (5.8) | 18.2 (6.0) | 17.3 (5.0) | .0006 |
Table 5 shows that 2936 (97.3%) patients in our cohort were prescribed a PIM with a mean (SD) of 5.0 (2.8) per patient. The number of PIMs increased with age, with 3.6 (2.9) PIMs per patient in those 18 to 39 years of age compared with 5.3 (2.6) PIMs per patients in those 65 to 79 years of age. Hemodialysis patients were on more PIMs than PD patients, with a mean (SD) of 5.4 (2.8) PIMs and 4.0 (2.4) PIMs, respectively.
Table 5.
PIMs data.
| Overall | Hemodialysis | Peritoneal dialysis | P value | |
|---|---|---|---|---|
| Final cohort (patients): n | 3017 | 2243 | 774 | |
| Patients on PIMs: n (%) | 2936 (97.3%) | 2200 (98.1%) | 736 (95.1%) | <.00001 |
| Patients on PIMs by group: n (%) | ||||
| Cardiovascular | 2308 (76.5%) | 1666 (74.3%) | 642 (83.0%) | <.00001 |
| Diabetes | 0 | 0 | 0 | NA |
| Symptom management | 2759 (91.5%) | 2125 (94.7%) | 634 (81.9%) | <.00001 |
| Other | 551 (18.3%) | 481 (21.4%) | 70 (9.0%) | <.00001 |
| Number of PIMs by patient: mean (SD) | ||||
| Any PIM | 5.0 (2.8) | 5.4 (2.8) | 4.0 (2.4) | <.0001 |
| Cardiovascular | 1.5 (1.2) | 1.4 (1.1) | 1.8 (1.3) | <.0001 |
| Diabetes | 0 | 0 | 0 | NA |
| Symptom management | 3.3 (2.3) | 3.7 (2.3) | 2.1 (1.8) | <.0001 |
| Other | 0.2 (0.4) | 0.2 (0.4) | 0.1 (0.3) | <.0001 |
| Number of PIMs by age group: mean (SD) | ||||
| 18 to <40 years | 3.6 (2.9) | 4.2 (3.0) | 2.1 (2.0) | <.0001 |
| 40 to <65 years | 4.9 (2.9) | 5.4 (3.0) | 3.9 (2.3) | <.0001 |
| 65 to <80 years | 5.3 (2.6) | 5.7 (2.7) | 4.4 (2.2) | <.0001 |
| ≥80 years | 5.1 (2.7) | 5.2 (2.7) | 4.3 (2.7) | <.0001 |
| Number of PIMs by dialysis vintage group: mean (SD) | ||||
| ≤1 year | 4.5 (2.8) | 5.0 (2.8) | 3.5 (2.4) | <.0001 |
| 1-3 years | 4.9 (2.7) | 5.5 (2.8) | 4.0 (2.3) | <.0001 |
| > 3 years | 5.2 (2.8) | 5.4 (2.8) | 4.3 (2.4) | <.0001 |
Note. PIMs = potentially inappropriate medications; NA = not applicable.
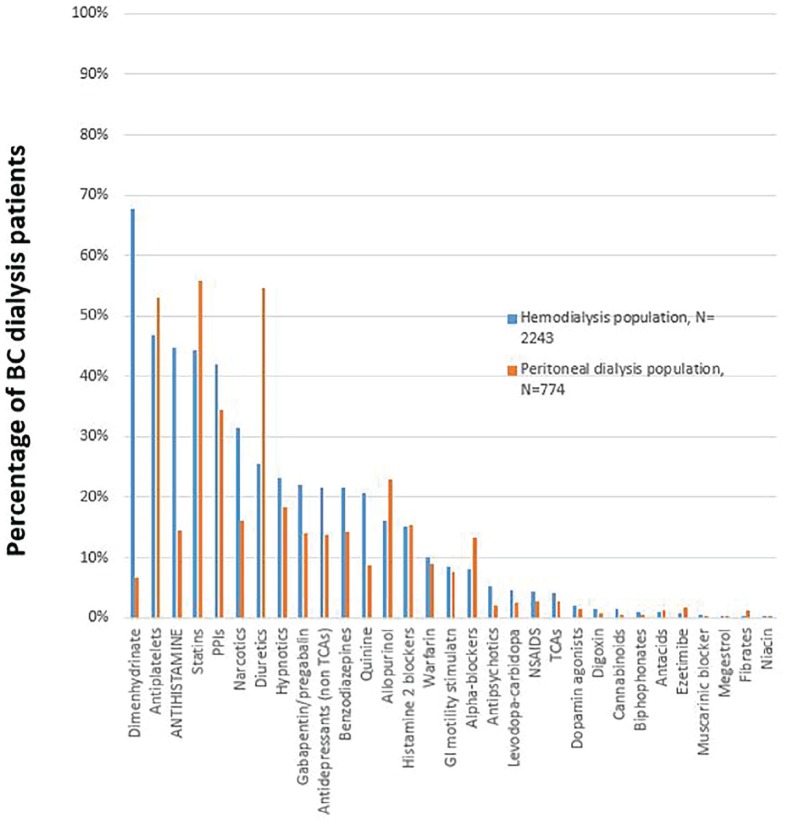
Figure 2 illustrates the most common classes of medications that were prescribed potentially inappropriately. The most commonly prescribed PIMs were antiplatelets (50.2%), statins (47.3%), PPIs (40.1%), antihistamines (37.0%), loop diuretics (29.7%), narcotics (27.6%), hypnotics (22.0%), benzodiazepines (19.7%), and antidepressants (19.7%). Interestingly, some PIMs like dimenhydrinate are widely prescribed for HD patients (67.7%) but not for PD patients. (6.72%). Patient age and dialysis vintage were associated with an increased number of PIMs prescribed. Table 6 shows the potential cost saving associated with deprescribing the 5 most frequent PIMs in our population, with variable success rate of deprescription.
Figure 2.
Percentage of potentially inappropriate medication in the British Columbia (BC) dialysis population.
Note. NSAIDS = non steroidal anti-inflammatory drugs; PPIs = proton pump inhibitors; TCAs = Tricyclic antidepressants.
Table 6.
Annual Potential Cost Saving With PIMs Deprescription.
| PIMs | No. of patients | Annual potential cost saving based on deprescription success rate | ||
|---|---|---|---|---|
| 100% | 75% | 50% | ||
| Antiplatelets | 1871 | Can$282 877 | Can$212 158 | Can$141 439 |
| Dimenhydrinate | 1614 | Can$261 047 | Can$195 785 | Can$130 524 |
| Antihistamine | 1088 | Can$254 403 | Can$190 802 | Can$127 202 |
| Statin | 1467 | Can$305 435 | Can$229 076 | Can$152 718 |
| PPIs | 1230 | Can$255 013 | Can$191 260 | Can$127 507 |
| Total | Can$1 358 775 | Can$1 019 081 | Can$679 388 | |
Note. PIMs = potentially inappropriate medications; PPIs = proton pump inhibitors.
Discussion
This study describes the current state of polypharmacy for patients on dialysis, using a robust and complete data set from a single province in Canada. This contemporary cohort of patients on both HD and PD reflect current practice and highlight that when non-prescription and as-needed medications are accounted for, patients are prescribed more medications than previously reported in published studies.1,3 A US study using the USRDS database demonstrated that the mean number of prescribed medications in HD patients was 12.1 Our results show that the overall median number of prescribed medications was 17. The discrepancy between the reported literature and the current cohort is due to inclusion of over-the-counter medications in our analysis, and thus may be more reflective of true practice.
Quantification of the number of medications that dialysis patients receive, and systematic evaluation of those that are essential and those that are “potentially inappropriate,” is an important component of delivering safe and patient-centered care. We identify here a large number of PIMs in both HD and PD patients that could potentially reduce the pill burden by at least 25%. Given that patients on dialysis are at greater risk than the general population of experiencing adverse drug events due to impaired drug clearance, polypharmacy, and the increased number of comorbidities,12 increased attention to prescribing patterns is an imperative. The evidence of benefit of most PIMs in dialysis patients is lacking. A number of initiatives are underway to assess the best method by which to assess benefit and risk, and to systematically address the need for medications to address the many symptoms related to their kidney disease and treatment. Regular reassessment of efficacy may not currently be part of routine care plans, nor is review of cessation of medications a regular metric followed by clinicians. This analysis of a contemporary large group of HD and PD patients in a single province describes a large burden of PIMs, and offers opportunity for implementation of deprescribing strategies and evaluation of those on patient outcomes and satisfaction.
Our study is the first, to our knowledge, that compares prescribing patterns for HD and PD patients. We demonstrate that the mean number of prescribed medications in the HD cohort was greater than the PD cohort. We acknowledge that the PD and HD populations are quite different; however, the number of medications increased with dialysis vintage in both the HD and PD cohorts. This is likely due to both an increased symptom burden with duration of dialysis and potentially due to the fact that once a medication is started, it is not routinely reassessed to determine efficacy or continued need. As over one third of the prescribed medications were classified as symptom management medications, this represents an important area to focus on to ensure all medications prescribed to improve symptoms are providing benefit to the patient.
One strategy to decrease polypharmacy and adverse effects related to medication in the dialysis population is to identify PIMs and try to minimize them when appropriate. A study by Kondo et al assessed prescribing patterns of PIMs and determined that 57% of their elderly Japanese HD population was prescribed a PIM.12 They also reported that some of the most frequent PIMs in their population were antiplatelet (19%), alpha-blockers (13%), and benzodiazepines (11%). In comparison, in our study, almost all patients were prescribed a PIM, with antiplatelets prescribed in 50.1%, alpha-blockers in 9.4%, and benzodiazepines in 19.7% of our dialysis cohort.
We found that the use of hypnotics (22.0%), benzodiazepines (19.7%), and opioids (27.6%) were high in our population. These results are comparable with usage reported by the Wyne et al systematic review, which summarized the use of opioids and benzodiazepines in ESKD patients. They found that opioid use ranged from 5% to 36%, and benzodiazepines use ranged from 8% to 26% in the 12 included studies.13 They also reported that the use of these medications increased with dialysis vintage. This group also described an association between benzodiazepine use and an increased risk of mortality, as well as an increased risk of falls and fractures with use of opioid. We also found that hypnotics, benzodiazepines, and opioids were more likely to be prescribed to HD patients than PD patients. In addition to the above risks, some HD patients drive home post treatment, reminding the prescriber of the need for clear guidelines for the use of these agents during HD treatments.
Deprescription protocols can be useful tools to reassess medications that may lack benefit in a specific population or have questionable safety. The use of antiplatelets and statins in the ESKD patients are 2 good examples as there is very little data to support the use of an antiplatelet or statin in this population. Furthermore, the use of an antiplatelet has been associated with an increased risk of bleeding in these patients.14 We report that about half of our population were prescribed a statin and/or an antiplatelet. Furthermore, 5.7% of patients were on dual antiplatelet therapy. Although there may be valid indications for these agents in select patients, this also provides an opportunity for prescribers to determine on an individual basis whether these prescribed medications are indeed being used in an appropriate, evidence, and patient-informed manner.
Deprescribing medications that do not have a clear indication or demonstration of benefit can also be cost saving. As shown in Table 6, PIMs have a considerable impact on our drug budget. We estimated a potential annual cost saving of Can$1 million if 75% of these medications are successfully deprescribed. By reducing the prescribing of PIMs in general, it allows funding of medications that are effective, safe, and evidence-based in our dialysis population. Not to be overlooked, the cost of the medication is low in comparison with the cost of potential adverse events, falls, and hospitalizations related to the use of some of these most prescribed PIMs. Further research is needed to quantify the potential cost savings of deprescription initiatives.
Finally, quinine is an example of how health care team can successfully deprescribe a potentially harmful medication. Quinine has been previously widely used for leg cramps despite limited evidence of efficacy. In September 2010, Health Canada published a black box warning on quinine due to an increase in serious adverse drug reaction reports related to this drug. Health Canada specifically reminded prescribers that quinine is only indicated for malaria, and use for other indication should be discouraged.15 Despite this recommendation, 21.5% of dialysis patients in our study were prescribed this medication, which represents an approximate cost of Can$93 000 annually.11 BC Renal removed quinine from the provincial formulary in 2015, citing these concerns. Since that time, virtually all quinine in the province has been deprescribed in dialysis patients, highlighting that deprescribing initiatives can be successfully implemented on a broad scale without adverse effects.
The strengths of this study include the complete data collection on all HD and PD patients in British Columbia. In addition, the capture of prescription, non-prescription, and as-needed medications have not been readily available in other registries. Routine medication reconciliation performed and documented by renal pharmacists helps to ensure that medication lists are an accurate reflection of what the patient is actually taking, versus solely what is prescribed.
Limitations of the study include the fact that this represents prescribing only in British Columbia and may not reflect prescription pattern in other jurisdictions. The medication profiles, which were pulled from PROMIS database, are dependent on accurate data entry. Finally, we were not able to determine how many of the as-needed medications were actually being taken, as HD and PD patients have some medications that are automatically prescribed by protocol when initiating dialysis. This may overestimate the number of medications that are actually being taken.
Conclusion
In conclusion, based on the prescribing patterns observed in this contemporary analysis, we confirm that HD and PD patients are prescribed an extraordinary number of medications, of which 25% are PIMs. Older patients and patient with a longer dialysis vintage have increased evidence of polypharmacy in both HD and PD cohorts. Hemodialysis patients take more medications than PD patients, and have more PIMs prescribed. Description of the baseline burden of medications allows a comparison for future studies. Relationship of PIMs to adverse outcomes requires further study. The cost of PIMs can be substantial and represent an area of potentially significant cost savings.
A systematic method which includes a deprescribing tool or process may be of great benefit to our outpatient HD and PD patient populations. Last, evaluating the patients’ perspective on these types of initiatives as well as their impact on their quality of life should be assessed in future studies. Current research and initiatives such as Can-SOLVE CKD are addressing this need.10
Footnotes
Ethics Approval and Consent to Participate: Ethics approval for this reasearch was received from the UBC - Research Ethics Board (16--00956).
Consent for Publication: All authors consent to the publication of this manuscript.
Availability of Data and Materials: Data queries can be addressed to Dr.Judith Marin via email at jmarin@providencehealth.bc.ca.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Judith G. Marin  https://orcid.org/0000-0001-9317-8809
https://orcid.org/0000-0001-9317-8809
References
- 1. Manley HJ, Garvin CG, Drayer DK, et al. Medication prescribing patterns in ambulatory haemodialysis patients: comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant. 2004;19(7):1842-1848. [DOI] [PubMed] [Google Scholar]
- 2. Battistella M, Jandoc R, Ng JY, McArthur E, Garg AX. A Province-wide, Cross-Sectional Study of Demographics and Medication Use of Patients in Hemodialysis Units Across Ontario [published online ahead of print March 13, 2018]. Can J Kidney Health Dis. doi: 10.1177/2054358118760832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tozawa M. Analysis of drug prescription in chronic haemodialysis patients. Nephrol Dial Transplant. 2002;17(10):1819-1824. [DOI] [PubMed] [Google Scholar]
- 4. Chiu Y-W, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009;4(6):1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. By the American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63(11):2227-2246. [DOI] [PubMed] [Google Scholar]
- 6. McIntyre C, McQuillan R, Bell C, Battistella M. Targeted deprescribing in an outpatient hemodialysis unit: a quality improvement study to decrease polypharmacy. Am J Kidney Dis. 2017;70(5):611-618. [DOI] [PubMed] [Google Scholar]
- 7. Bermingham M, Ryder M, Travers B, et al. The St Vincent’s potentially inappropriate medicines study: development of a disease-specific consensus list and its evaluation in ambulatory heart failure care. Eur J Heart Fail. 2014;16(8):915-922. [DOI] [PubMed] [Google Scholar]
- 8. Lindsay J, Dooley M, Martin J, Fay M, Kearney A, Barras M. Reducing potentially inappropriate medications in palliative cancer patients: evidence to support deprescribing approaches. Support Care Cancer. 2014;22(4):1113-1119. [DOI] [PubMed] [Google Scholar]
- 9. BC Renal 2019. PROMIS [Internet]. Provincial Health Services Authority. http://www.bcrenalagency.ca/health-professionals/professional-resources/promis.
- 10. Can-SOLVE CKD Network. Targeted de-prescribing in patients with chronic kidney disease to decrease polypharmacy [Internet]. Innovative Care; 2019. https://www.cansolveckd.ca/research/theme-3/deprescribing. Accessed March 2, 2020.
- 11. British Columbia Government. BC PharmaCare Formulary Search [Internet]. https://pharmacareformularysearch.gov.bc.ca. Accessed March 2, 2020.
- 12. Kondo N, Nakamura F, Yamazaki S, et al. Prescription of potentially inappropriate medications to elderly hemodialysis patients: prevalence and predictors. Nephrol Dial Transplant. 2015;30(3):498-505. [DOI] [PubMed] [Google Scholar]
- 13. Wyne A, Rai R, Cuerden M, Clark WF, Suri RS. Opioid and benzodiazepine use in end-stage renal disease: a systematic review. Clin J Am Soc Nephrol. 2011;6(2):326-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jain N, Hedayati SS, Sarode R, Banerjee S, Reilly RF. Antiplatelet therapy in the management of cardiovascular disease in patients with CKD: what is the evidence? Clin J Am Soc Nephrol. 2013;8(4):665-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Health Canada. Quinine sulfate and serious adverse reactions. Can Adverse React Newsl. 2011;21(2):5. [Google Scholar]