Abstract
We investigated the clinical course of individuals with 2019 novel coronavirus disease (COVID-19) who were transferred from the Diamond Princess cruise ship to 12 local hospitals. The conditions and clinical courses of patients with pneumonia were compared with those of patients without pneumonia. Among 70 patients (median age: 67 years) analyzed, the major symptoms were fever (64.3%), cough (54.3%), and general fatigue (24.3%). Forty-three patients (61.4%) had pneumonia. Higher body temperature, heart rate, and respiratory rate as well as higher of lactate dehydrogenase (LDH), aspartate aminotransferase (AST), and C-reactive protein (CRP) levels and lower serum albumin level and lymphocyte count were associated with the presence of pneumonia. Ground-glass opacity was found in 97.7% of the patients with pneumonia. Patients were administered neuraminidase inhibitors (20%), lopinavir/ritonavir (32.9%), and ciclesonide inhalation (11.4%). Mechanical ventilation and veno-venous extracorporeal membrane oxygenation was performed on 14 (20%) and 2 (2.9%) patients, respectively; two patients died. The median duration of intubation was 12 days. The patients with COVID-19 transferred to local hospitals during the outbreak had severe conditions and needed close monitoring. The severity of COVID-19 depends on the presence of pneumonia. High serum LDH, AST and CRP levels and low serum albumin level and lymphocyte count were found to be predictors of pneumonia. It was challenging for local hospitals to admit and treat these patients during the outbreak of COVID-19. Assessment of severity was crucial to manage a large number of patients.
Keywords: Coronavirus, SARS-CoV-2, Outbreak, COVID-19, Diamond Princess cruise ship
A novel coronavirus disease (COVID-19) caused by SARS-CoV-2 in early 2020 abruptly spread within three months from the east-Asian countries to other parts of the world. In Japan, the first outbreak of COVID-19 was reported in a giant cruise ship called the Diamond Princess. This cruise ship had a gross tonnage of 115,000 tons with a maximum capacity of 2706 passengers and 1100 operating staffs. The cruise started on January 20, 2020, from Yokohama stopped at Kagoshima (Southern Japan), Hong Kong, Da Nan and Chan May (Vietnam), Ha Long bay (Vietnam), Keelung (Taiwan) and Okinawa (Southern Japan). At the cruise ship's arrival at Yokohama near the Tokyo metropolitan area early morning on February 3, 2020, 10 people were confirmed to be positive for SARS-CoV-2 [1]. Finally, 3618 individuals were screened out of 3711 individuals (2666 guests and 1045 crew) on board on February 3; in total, 696 individuals (19.2%) were confirmed positive for SARS-CoV-2 [2]. A Japanese quarantine officer, the Disaster Medical Assistance Team, and a small number of infection control specialists were dispatched to quarantine the individuals on board to assess the health status of the individuals on the cruise ship. Hundreds of asymptomatic passengers who were confirmed or suspected to be infected with SARS-CoV-2 were kept isolated on the cruise ship, and they were allowed to disembark from the cruise ship only after the quarantine observation for two weeks. Disembark of 3711 individuals on the cruise ship was completed on March 1. Meanwhile, individuals who required medical care, mainly those with severe symptoms during February 3 and March 1, were transferred to local hospitals near the Yokohama city area, which is the largest city in the Kanagawa Prefecture for medical care [3]. In this study, we describe the clinical conditions, treatment, and the clinical course of the patients positive for SARS-CoV-2 who were transferred from the Diamond Princess cruise ship for further medical care to the participating hospitals in this study.
The patients' vital signs, laboratory data, chest radiographs, or computed tomography (CT) findings at the time of admission and treatment, and the data on the clinical course and prognosis were collected using case report forms. Case report forms were collected on March 11 and March 19, 2020. The clinical findings of the patients without pneumonia were compared with those of the patients with pneumonia. The patient race was determined based on the estimation of his/her ancestor by each participating physician. Patient severity was defined according to World Health Organization criteria [4]. No patient was excluded because of bacterial or any other type of viral pneumonia by additional laboratory tests and sputum culture. The specimens for reverse transcription PCR for SARS-CoV-2 was obtained first when the patients were on the Diamond Princess cruise ship or otherwise at the time of transfer to the hospitals, and were submitted to the municipal or governmental laboratories and examined according to the instructions of the National Institute of Infectious Diseases (version 2.8 and previous versions). The use of drugs unapproved for the treatment of COVID-19 or severe lower respiratory tract infection in Japan was approved by the clinical ethical committee at each institution. This study was approved by the ethics committees at the Yokohama City University Hospital (approval number: B200200047) and the participating hospitals. Continuous data were presented as medians, and interquartile ranges were presented as (Q1–Q3). Categorical data were presented as numbers and percentages. Data were analyzed using a two-tailed Mann–Whitney U test for comparisons of continuous variables among two or three groups and by Fisher's exact test for comparisons of categorical data. Statistical analyses were performed with Prism 8 (GraphPad Software, San Diego, CA, USA). P-values of <0.05 were considered statistically significant.
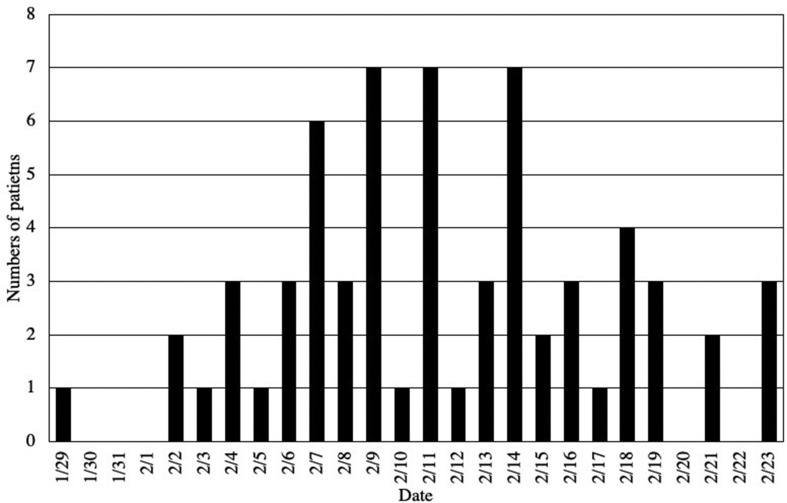
Seventy patients positive for SARS-CoV-2 were included in our study. The dates of onset of COVID-19 in these patients are summarized in Fig. 1 (date of onset among six patients were missing). The predisposing conditions, general status, vital signs at admission, and laboratory data are shown in Table 1 . The median age of the patients was 67 years; 67.1% of the patients were males, and 80% of the patients were Asians. The predisposing conditions were diabetes (24.3%) and hypertension (22.9%). Very few patients had active malignancy and renal failure. No one had HIV infections and liver dysfunction. The major symptoms of all the 70 patients were fever (64.3%), cough (54.3%), and general fatigue (24.3%). The median duration from onset of COVID-19 to admission was 4 days (2–6 days). Patients with pneumonia had significantly higher body temperatures than those without pneumonia (37.7 °C vs. 36.7 °C, P < 0.001). Heart rates (88 vs. 78/min) and respiratory rates (20 vs. 16/min) were significantly higher in the patients with pneumonia. Further, 14.3% of the patients with or without pneumonia required supplemental oxygen at the time of admission. Laboratory data taken at admission showed that the serum albumin levels were lower in patients with pneumonia than in those without pneumonia (3.9 vs. 4.2 g/dL), whereas the levels of lactate dehydrogenase (LDH) (265 vs. 206 U/L) and aspartate aminotransferase (AST) (36 vs. 26 U/L) were significantly higher in the patients with pneumonia than in those without pneumonia. The peripheral lymphocyte count was significantly lower in the patients with pneumonia than in those without pneumonia (957 vs. 1461/μL). C-reactive protein (CRP) levels were significantly higher in the patients with pneumonia than in those without pneumonia (3.7 vs. 0.4 mg/dL). Among the 43 patients with pneumonia, consolidation on chest CT was found in 27.9% and ground-glass opacity (GGO) was found in 97.7% of the patients with pneumonia. Notably, GGOs were bilateral (93.0%), multifocal (74.4%), and peripheral dominant (90.7%).
Fig. 1.
Dates of onset of COVID-19 and number of patients who developed symptoms in our study.
Table 1.
Predisposing conditions and laboratory data of the study patients.
| All patients (N = 70) | Patients with pneumonia (n = 43) | Patients without pneumonia (n = 27) | P-value | |
|---|---|---|---|---|
| Age (years) | 67 (62–71) | 69 (65–72) | 65 (54–71) | 0.547 |
| Male sex | 47 (67.1%) | 32 (74.4%) | 15 (55.6%) | 0.122 |
| Race | ||||
| Japanese | 32 (45.7%) | 23 (53.5%) | 9 (33.3%) | 0.140 |
| Asian (excluding Japanese) | 24 (34.3%) | 13 (30.2%) | 11 (40.7%) | 0.441 |
| European/American | 14 (20.0%) | 7 (16.3%) | 7 (25.9%) | 0.368 |
| Underlying conditions | ||||
| Diabetes | 17 (24.3%) | 12 (27.9%) | 5 (18.5%) | 0.409 |
| Hypertension | 16 (22.9%) | 13 (30.2%) | 3 (11.1%) | 0.083 |
| Cardiovascular disease | 1 (1.4%) | 1 (2.3%) | 0 (0%) | |
| Malignancy | 1 (1.4%) | 0 (0%) | 1 (3.7%) | |
| Cerebrovascular disease | 1 (1.4%) | 1 (2.3%) | 0 (0%) | |
| COPD | 2 (2.9%) | 1 (2.3%) | 1 (3.7%) | |
| Chronic renal disease | 1 (1.4%) | 1 (2.3%) | 0 (0%) | |
| General status and vital signs | ||||
| Body temperature (°C) | 37.3 (36.7–38.1) | 37.7 (37.1–38.5) | 36.7 (36.4–37.1) | <0.001 ∗ |
| Heart rate (/min) | 84 (77–92) | 88 (80–92) | 78 (70–85) | <0.001 ∗ |
| Systolic blood pressure (mmHg) | 134 (124–148) | 133 (124–150) | 136 (122–145) | 0.761 |
| Respiratory rate (/min) | 18 (16–20) | 20 (16–22) | 16 (14–18) | 0.005 ∗ |
| Supplemental oxygen | 10 (14.3%) | 8 (18.6%) | 2 (7.4%) | 0.297 |
| Major symptoms | ||||
| Fever | 45 (64.3%) | 30 (69.8%) | 15 (55.6%) | 0.306 |
| Cough | 38 (54.3%) | 22 (51.2%) | 16 (59.3%) | 0.624 |
| Wet cough | 4 (5.7%) | 2 (4.7%) | 2 (7.4%) | 0.637 |
| General fatigue | 17 (24.3%) | 12 (27.9%) | 5 (18.5%) | 0.409 |
| Shortness of breath | 12 (17.1%) | 9 (20.9%) | 3 (11.1%) | 0.347 |
| Diarrhea | 10 (14.3%) | 6 (14%) | 4 (14.8%) | 1.0 |
| Sputum | 9 (12.9%) | 4 (9.3%) | 5 (18.5%) | 0.292 |
| Appetite loss | 9 (12.9%) | 7 (16.3%) | 2 (7.4%) | 0.466 |
| Sore throat | 8 (11.4%) | 6 (14%) | 2 (7.4%) | 0.472 |
| Headache | 6 (8.6%) | 5 (11.6%) | 1 (3.7%) | 0.394 |
| Myalgia | 3 (4.3%) | 1 (2.3%) | 2 (7.4%) | 0.555 |
| Laboratory data | ||||
| Total protein (g/dL) | 7 (6.7–7.4) | 7.0 (6.6–7.3) | 7.3 (6.7–7.6) | 0.218 |
| Albumin (g/dL) | 3.9 (3.5–4.2) | 3.9 (3.4–4.1) | 4.2 (3.7–4.6) | 0.001 ∗ |
| LDH (U/L) | 252 (200–358) | 265 (232–392) | 206 (172–266) | 0.001 ∗ |
| AST (U/L) | 29 (25–41) | 36 (25–47) | 26 (22–31) | 0.005 ∗ |
| ALT (U/L) | 26 (20–43) | 32 (21–44) | 20 (18–36) | 0.075 |
| ALP (U/L) | 198 (178–251) | 191 (175–227) | 224 (180–286) | 0.107 |
| BUN (mg/dL) | 15.5 (12.1–20.0) | 15.5 (12.4–19.1) | 15.5 (12.0–22.5) | 0.924 |
| Serum creatinine (mg/dL) | 0.81 (0.67–0.97) | 0.86 (0.66–1.06) | 0.74 (0.67–0.82) | 0.104 |
| Total bilirubin (mg/dL) | 0.6 (0.4–0.7) | 0.6 (0.4–0.7) | 0.6 (0.5–0.7) | 0.649 |
| C-reactive protein (mg/dL) | 2.1 (0.4–5.3) | 3.7 (1.7–7.3) | 0.4 (0.32–1.13) | <0.001 ∗ |
| Bicarbonate (HCO3−, mmol/L) | 24.0 (23.0–25.8) (N = 32) |
23.9 (22.5–25.1) (N = 25) |
25.7 (23.8–28.3) (N = 7) |
|
| Lactate level (mmol/L) | 1.2 (0.9–1.2) (N = 28) |
1.2 (1.1–1.3) (N = 21) |
0.9 (0.9–1.3) (N = 7) |
|
| White blood cell count (/μL) | 5600 (4075–7600) | 5750 (4125–7535) | 5600 (3975–7775) | 0.705 |
| Absolute neutrophil count (/μL) | 3700 (2611–5605) | 4170 (2639–5605) | 2991 (1943–6348) | 0.127 |
| Lymphocyte count (/μL) | 1071 (832–1428) | 957 (796–1195) | 1461 (1042–1912) | 0.003 ∗ |
| Hemoglobin level (g/dL) | 14.5 (13.2–15.4) | 14.0 (13.2–15.4) | 14.7 (13.1–15.7) | 0.785 |
| Platelet count ( × 103/μL) | 197 (163–250) | 185 (158–232) | 228 (168–262) | 0.064 |
| Procalcitonin level (ng/mL) | 0.06 (0.04–0.14) (N = 16) |
0.10 (0.05–0.16) (N = 9) |
0.03 (0.02–0.06) (N = 7) |
|
| Positive urinary ketone | 6 (0.146) (N = 41) |
6 (19.4%) (N = 31) |
0 (0%) (N = 10) |
0.307 |
Data are expressed as n (%) or median (interquartile range Q1–Q3).
Abbreviations: COPD, chronic obstructive pulmonary disease; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; BUN, blood urea nitrogen.
∗ statistically significant.
Regarding treatments and clinical courses, the median observation period was 16 days (12–21 days) among all cases included in this study. Forty-one (58.6%) patients including 34 with pneumonia and 7 without pneumonia were administered antibiotics. The selected antibiotics are shown in Table 2 . Ceftriaxone and azithromycin were mostly chosen as the antibiotics for the initial treatment. With regard to the anti-viral drugs, the following neuraminidase inhibitors were administered in 14 patients: oseltamivir (n = 6), peramivir (n = 4), baloxavir (n = 1), and a combination of oseltamivir and baloxavir (n = 3). LPV/r was administered to 24 patients. Two patients with pneumonia were treated with a combination of neuraminidase inhibitors, LPV/r, and ribavirin. In addition, ciclesonide inhalation, which was suggested to have anti-coronavirus activity, was administered in 8 patients with pneumonia. Fourteen patients with pneumonia required mechanical ventilation; among them, 2 patients required veno-venous extracorporeal membrane oxygenation (V–V ECMO). Two patients died on day 17 and day 21 without extubation. The two patients who underwent V–V ECMO were both alive at the end of the observation period: one patient was successfully extubated on the day 13 of ventilation and the other had been intubated for 23 days using V–V ECMO. Three patients with pneumonia remained intubated for 19–25 days on the mechanical ventilator at the time of end of observation. The remaining 7 patients were successfully extubated after a median of 12 days (6–18 days) on mechanical ventilation. The median duration of hospital admission was 18 days (16–20 days) in 48 patients who were discharged from hospitals during the study period. The median duration between the first positive PCR result and the first negative PCR confirmation was 15 days (10–18 days).
Table 2.
Treatments and supportive care for the study patients.
| Total (N = 70) | Patients with pneumonia (n = 43) | Patients with mild illness (n = 27) | |
|---|---|---|---|
| Antibiotics | |||
| Ampicillin/Amoxicillin + BLI | 3 (4.3%) | 2 (7.0%) | 1 (3.7%) |
| Ceftriaxone | 6 (8.6%) | 5 (11.6%) | 1 (3.7%) |
| Ceftriaxone + Levofloxacin | 7 (10.0%) | 7 (16.3%) | 0 (0%) |
| Cefepime + Azithromycin | 10 (14.3%) | 9 (20.9%) | 1 (3.7%) |
| Meropenem + Azithromycin | 2 (2.9%) | 1 (2.3%) | 1 (3.7%) |
| Levofloxacin | 6 (8.6%) | 5 (11.6%) | 1 (3.7%) |
| Azithromycin | 7 (10.0%) | 5 (11.6%) | 2 (7.4%) |
| No antibiotic was administered | 29 (41.4%) | 9 (20.9%) | 20 (74.1%) |
| Antiviral drugs | |||
| Lopinavir/ritonavir | 24 (34.2%) | 23 (53.5%) | 1 (3.7%) |
| Neuraminidase inhibitor | 14 (20.0%) | 13 (30.2%) | 1 (3.7%) |
| Ciclesonide | 8 (11.4%) | 8 (18.6%) | 0 (0%) |
| Favipiravir | 3 (4.3%) | 3 (7.0%) | 0 (0%) |
| Rivabirin | 2 (2.9%) | 2 (4.7%) | 0 (0%) |
| Other supportive care | |||
| Mechanical ventilation | 14 (20.0%) | 14 (32.6%) | 0 (0%) |
| Steroid pulse therapy | 2 (2.9%) | 2 (4.7%) | 0 (0%) |
| Intravenous immunoglobulin | 6 (8.6%) | 6 (14.0%) | 0 (0%) |
| V–V ECMO | 2 (2.9%) | 2 (4.7%) | 0 (0%) |
Data are expressed as n (%).
Abbreviations: BLI, β-lactamase inhibitors; V–V ECMO, veno-venous extracorporeal membrane oxygenation.
Notably, the severity of this disease in the patients transferred from the cruise ship was very high compared with that previously reported in the general population in China [5,6]. The one reason was attributed to advanced age [6]. Generally, the average age of the Japanese passengers on Asian cruise ships is reported to be 57 years [7]. Most of the individuals on the Diamond Princess on February 5, 2020, were aged ≥60 years of age [2]. The crowded and closed environment on the cruise ship was possibly a suitable condition for high transmission of respiratory infections [8]. In these individuals with COVID-19, the severity of the disease varied widely from almost asymptomatic to critical cases such as those requiring ventilator support. The initial symptoms —fever, dry cough and general fatigue, were quite nonspecific. The severity of the disease depended on the presence of pneumonia. The findings of our study show that it is critical to differentiate patients with pneumonia from those without pneumonia to predict their clinical course and treatment plan. High LDH and AST levels possibly reflect the interstitial lung inflammation due to COVID-19. Low lymphocyte count and high LDH level were considered key to predict pneumonia in patients with COVID-19 because their levels serve as predictors of severe conditions [9]. The bilateral GGOs was helpful for diagnosing COVID-19. Chest CT is more sensitive than chest radiographs, and the bilateral GGOs in the lower lung lobes are reported to be specific to COVID-19 [10,11]. We suggest that chest CT should be considered if a patient is suspected to have pneumonia due to COVID-19 but PCR findings were still under examination. No treatment regimen has yet been established for the treatment of COVID-19. In the participating hospitals, unapproved drugs were used for the treatment. Treatment with LPV/r, neuraminidase inhibitors, ciclesonide inhalation, favipiravir and rivabirin were attempted. Our colleagues reported that ciclesonide inhalation is effective for the treatment of COVID-19 [12,13].
Our study has several limitations. First, data were collected from the case report forms from each attending physician in the participating hospitals. The diagnosis and treatment were depended on the clinical decision of each physician. Second, this study was descriptive and we did not properly evaluate the efficacies of the drugs or the supportive care. Further studies should be performed to assess the efficacy of these drugs for the treatment of severe cases of COVID-19. Third, we were not able to investigate all cases of COVID-19 on the Diamond Princess cruise. The environment inside the cruise ship is suitable for high transmission of respiratory infections [8]. Because individuals with severe conditions during an outbreak are possibly transferred to nearby local hospitals, managing patients during an outbreak is a big challenge to local medical systems. We believe that our findings of patients with COVID-19 during its outbreak on a cruise ship can help to manage a large number of patients infected within a short period of time. Assessment of disease severity was crucial to manage a large number of patients.
Funding
We did not receive any funding support for this study.
Ethical standards
This study was approved by the ethics committee at each participating institution.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgement
We thank our medical staffs and colleagues who were involved in caring for the patients from the cruise ship. We sincerely appreciate our families for supporting our jobs. We also appreciate the municipal officers of the Yokohama City and the Kanagawa Prefecture for cooperating with us in managing the patients. The authors would like to thank Enago (www.enago.jp) for the English language review.
Footnotes
All authors meet the ICMJE authorship criteria.
References
- 1.Diamond Princess Updates 2020. https://www.princess.com/news/notices_and_advisories/notices/diamond-princess-update.html Feb 6 [cited 2020 Mar 7]
- 2.National Institute of Infectious Diseases . 2020. Field briefing: Diamond princess COVID-19 cases.https://www.niid.go.jp/niid/en/2019-ncov-e/9417-covid-dp-fe-02.html 20 Feb Update. Feb 21. [cited 2020 Mar 7] [Google Scholar]
- 3.Takeuchi I. COVID-19 first stage in Japan - how we treat Diamond princess cruise ship with 3700 passengers? Acute Med Surg. 2020;7(1):e506. doi: 10.1002/ams2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization . 2020. Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected.https://apps.who.int/iris/handle/10665/331446 Interim guidance 13 March 2020. Mar 13 [cited Mar 19] [Google Scholar]
- 5.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan W.J., Ni Z.Y., Hu Y., Fan G., Liu Y., Liu Z., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruise Lines International Association . 2017. 2017 Asia cruise report.https://cruising.org/-/media/research-updates/research/asia-cruise-trends/asia-cruise-trends-2017.pdf Jul [cited 2020 Mar 7] [Google Scholar]
- 8.Rocklöv J., Sjödin H., Wilder-Smith A. COVID-19 outbreak on the Diamond Princess cruise ship: estimating the epidemic potential and effectiveness of public health countermeasures. J Trav Med. 2020 doi: 10.1093/jtm/taaa030. taaa030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan F., Ye T., Sun P., Gui S., Liang B., Li Lingli, et al. Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. 2020:200370. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie X., Zhong Z., Zhao W., Zheng C., Wang F., Liu J. Chest CT for typical 2019-nCoV pneumonia: relationship to negative RT-PCR testing. Radiology. 2020:200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakajima K., Ogawa F., Sakai K., Uchiyama M., Oyama Y., Kato H., et al. A case of coronavirus disease 2019 treated with ciclesonide. Mayo Clin Proc. 2020 doi: 10.1016/j.mayocp.2020.04.007. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iwabuchi K., Yoshie K., Kurakami Y., Takahashi K., Kato Y., Morishita T. Therapeutic potential of ciclesonide inahalation for COVID-19 pneumonia: report of three cases. J Infect Chemother. 2020 doi: 10.1016/j.jiac.2020.04.007. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]