Abstract
Coronavirus disease 2019 has spread to every inhabited continent in the world. So far, plain radiography and computed tomography have been the mainstay of imaging methods used. The present analytical paper on the role of point-of-care lung ultrasound in this pandemic examines its diagnostic accuracy, clinical utility, and physical practicality in the intensive care unit.
Highlights
-
•
POCUS has a high sensitivity for pulmonary manifestations of COVID-19.
-
•
POCUS minimises nosocomial spread.
-
•
POCUS can prognosticate and assess response to therapy.
-
•
Healthcare providers with skills in POCUS are encouraged to help provide this service.
Introduction
In the coronavirus disease 2019 (COVID-19) pandemic, many sick patients require intensive care support. So far, chest radiography and computed tomography (CT) have been the main imaging methods for the assessment of the cardiorespiratory system. The present study examines the role of point-of-care ultrasound (POCUS) of the lungs in the intensive care unit (ICU).
Background
POCUS is the performance and interpretation of ultrasound at the bedside. POCUS emerged in the late 1980s following the development of compact, high-quality, portable ultrasound machines. The best-known use of POCUS has been in the detection of haemoperitoneum in abdominal trauma. First described by Shackford in 1993, it is also called the Focused Assessment with Sonography For Trauma (FAST) scan.1 Several studies have shown POCUS is safe and efficacious, and it is now increasingly used in emergency departments.2
Lung ultrasound was pioneered by Daniel Lichtenstein, an intensivist in Paris, who noted that sonographic artefacts could differentiate between common lung diseases, and subsequently, developed the BLUE protocol for dyspnoeic patients being admitted to the ICU.3 The BLUE protocol diagnosed six common respiratory diseases, including pulmonary oedema and pneumonia, with 90.5% accuracy. The FALLS protocol (Fluid Administration Limited by Lung Sonography) from the same study group showed that ultrasound can assess the volume status in the critically ill, enabling rapid decisions in shocked patients.4
Today, POCUS is used in the ICU for acutely dyspnoeic patients, cardiopulmonary monitoring in circulatory failure, cardiac arrest, and undifferentiated hypotension. POCUS has been used at Mount Everest base camp,5 the International Space Station,6 and during the Iraq War.7
Why should POCUS be a priority in COVID?
POCUS has many practical advantages over other imaging methods in the COVID-19 pandemic. POCUS is a bedside test; therefore, it can be completed without moving the patient from the ICU or ward. Lack of transfer reduces the exposure of other healthcare staff, patients, and visitors to the virus. Further, the transfer of intubated, critically unwell patients is fraught with risk; POCUS avoids this risk. Staff who would ordinarily be involved in the transfer are freed to undertake other duties. Lastly, the common advantages of ultrasound still apply: the test is cheap, uses no ionising radiation, and the results are available instantly.8 , 9
The literature on POCUS in COVID-19 has grown in the pandemic, although it comprises predominantly of case reports, opinion pieces, and tutorials. The present analysis stems from seven articles, outlined in Box 1 . These constitute all of the articles present on Medline regarding POCUS in COVID-19 at the time of writing. Evidence was also included from articles on POCUS published prior to COVID-19 where this would supplement the readers' understanding of the topic.
Box 1. List of Medline articles on POCUS in the COVID-19 pandemic, in chronological order.
Peng et al. 9
A letter to the editor describing their initial experience with POCUS in China. They were the first group to describe the ultrasound imaging features of COVID-19 and suggest it as an alternative to other imaging methods
Buonsenso et al. 10
A case report of a young man with COVID-19 in Italy, describing acquisition technique and imaging findings
Soldati et al. 11
A letter asking the question “Is there a role for lung ultrasound during the COVID-19 pandemic?” The article covers the imaging features, acquisition technique, and practical advantages of using POCUS
Moro et al. 12
In this article, the authors wrote a tutorial on how to perform lung ultrasound in pregnant women with suspected COVID-19, primarily targeted at an audience of gynaecologists
Soldati et al. 13
Based on their clinical experience in Italy, Soldati et al. set out to propose an international standardised approach to POCUS in COVID-19. The authors describe a reproducible acquisition protocol and scoring system
Thomas et al. 14
The first POCUS paper from North America during the COVID-19 pandemic. Thomas et al. describe the case of a 64-year-old woman with COVID-19, confirming the imaging findings of previous authors
Vetrugno et al. 15
The authors reported their experience using lung ultrasound scoring in Italy
Kalafat et al. 16
The authors report the case of a pregnant woman in Turkey admitted to ICU due to COVID-19. POCUS contributed to early clinical decisions in the ICU
Alt-text: Box 1
POCUS findings in COVID-19
Interstitial syndrome and consolidation
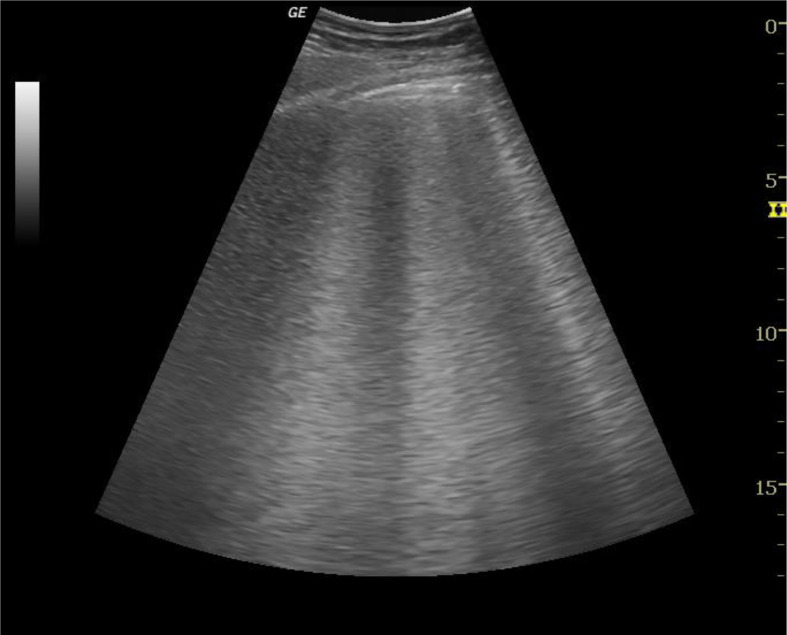
Interstitial syndrome refers to processes (water, infection, infiltration) in the pulmonary interstitium. In COVID-19, interstitial syndrome most likely results from acute respiratory distress syndrome (ARDS) or pneumonia.11 Interstitial syndrome is characterised by B lines (Fig 1 ), which are vertical hyperechoic reverberations between the ribs, in contrast to the horizontally oriented A lines, which are seen in the normal lung. Further, subpleural consolidation presents as indistinct hyperechoic areas with surrounding B lines. In severe cases, consolidation can resemble the liver, which is known as hepatisation of the lungs.17 Although non-specific, B lines are common in COVID-19, with Peng et al. 9 first to report their appearance. This was confirmed by other groups.10 , 14, 15, 16 In severe disease with significant oedema or consolidation, a “white lung” can be present .11
Figure 1.
POCUS image showing interstitial oedema. Case courtesy of Dr Maulik S Patel, Radiopaedia.org, rID: 35793.
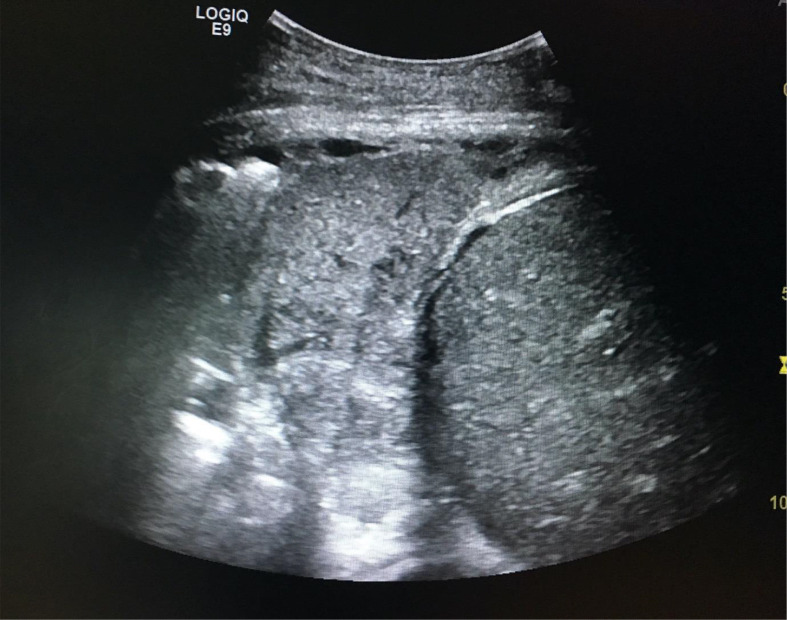
For the detection of interstitial syndrome in non-COVID patients, the accuracy of ultrasound (95%; sensitivity 98%; specificity 88%) surpasses chest radiography (72%; sensitivity 60%, specificity 100%) and auscultation (55%; sensitivity 34%; specificity 90%).18 Ultrasound can also distinguish between cardiogenic and non-cardiogenic pulmonary oedema(8) and can be deployed rapidly to exclude alternative causes of hypoxia in intensive care.19 For consolidation (Fig 2 ), ultrasound has an accuracy of 97% (sensitivity 93%; specificity 93%), compared with 75% for chest radiography (sensitivity 68%; specificity 95%) and 36% for auscultation (sensitivity 8%; specificity 100%).18
Figure 2.
POCUS image showing consolidation. Case courtesy of Dr Ian Bickle, Radiopaedia.org, rID: 59328.
Pleural inflammation
Inflammation of the pleura causes pleural thickening and disruption, which can be visualised on ultrasound. Pleural thickening has been observed in COVID-19 pneumonitis.10, 11, 12 , 14, 15, 16 Although pleural thickening appears to be sensitive for COVID-19 pneumonitis, it is non-specific, being present to some degree in all forms of pneumonia.
Treatment response and recovery
There is a paucity of literature on the ultrasonographic assessment of recovery from COVID-19. Peng et al. 9 report the re-appearance of A lines following treatment. Their re-appearance indicates a reduction in interstitial infiltration. Before the COVID-19 outbreak, ultrasound had been used in critical care to assess treatment response and for prognostication. In a clinical trial, Bouhemad et al. 20 showed that POCUS could titrate ventilator settings in positive end-expiratory pressure (PEEP)-induced lung recruitment. Haddam et al. 21 showed that POCUS enables monitoring of aeration during prone ventilation; however, it did not predict oxygenation response. Lastly, the lung ultrasound score (LUS), a quantitative measurement of non-cardiogenic pulmonary oedema, quantifies disease severity and is prognostic in ventilated ICU patients with ARDS.22 It is reasonable to suggest that these findings will also be true in COVID patients, who otherwise have POCUS findings similar to other forms of pneumonia.
Exclusion of alternative diagnoses
There are several causes of hypoxia in the ICU. Distinguishing between them is important as it enables the correct treatment to be given. The present review will demonstrate the advantage of using POCUS to distinguish between ARDS and pleural effusion will be used to illustrate this. Pleural effusion appears white on a chest radiograph and can be difficult to differentiate from consolidation. Furthermore, effusion of <500 ml is difficult to detect on chest radiography; in a ventilated ICU patient lying on their back, the fluid will be even more difficult to detect due to its dependent nature.17 These factors result in a diagnostic accuracy of 47% (sensitivity 39%; specificity 85%) for pleural effusion diagnosis on chest radiography.18 Conversely, pleural effusion is an anechoic rectangular region between the visceral and parietal pleura. The diagnostic accuracy of POCUS for pleural effusions is 93% (sensitivity 92%; specificity 93%), superior to chest radiography (above) and auscultation (accuracy 61%; sensitivity 42%; specificity 90%).18 Pleural effusions are uncommon in COVID-19.9 Therefore, its presence may indicate that another diagnosis should be considered, such as bacterial pneumonia or congestive cardiac failure.
Practical barriers to POCUS use
There are barriers to using POCUS in hospitals. Firstly, ultrasound is inherently user-dependent. Inadequate training could lead to inadequate assessment and high inter-operator variability. In a report on the Italian experience of POCUS in COVID-19, Vetrugno et al. 15 suggested that basic training and 25 supervised examinations was minimum to achieve basic proficiency. Although this training is time-consuming in the short-term, it provides greater yields in the long-term.
On the question of time, decontamination of equipment between patients may add to the workload of intensive care physicians; however, compared to a portable X-ray or CT machine, ultrasound machines are faster to decontaminate due to their small size. Italian physicians have minimised decontamination time by using portable, hand-held ultrasound probes attached to sheathed tablet devices.10 , 13
HOW can hospitals implement POCUS?
The implementation of POCUS requires ultrasound machines, which can range from handheld devices to freestanding machines on wheels. All are portable, easy to decontaminate, and are a fraction of the cost of CT machines. Hospitals can re-purpose ultrasound machines from departments running on reduced capacity due to COVID-19, such as in outpatient clinics, and also re-deploy technologists/radiologists to ICUs.
Conclusion
POCUS has a high sensitivity for the pulmonary manifestations of COVID-19, such as ARDS and consolidation. Furthermore, POCUS can be used to monitor treatment response. POCUS is an asset to hospitals as it minimises nosocomial spread of the disease. Healthcare Providers with skills in POCUS are encouraged to help provide this service.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Scalea T.M., Rodriguez A., Chiu W.C. Focused assessment with sonography for Trauma (FAST): results from an international consensus conference. J Trauma. 1999;46:466–472. doi: 10.1097/00005373-199903000-00022. [DOI] [PubMed] [Google Scholar]
- 2.Chen L., Malek T. Point-of-care ultrasonography in emergency and critical care medicine. Crit Care Nurs Q. 2018;41:94–101. doi: 10.1097/CNQ.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 3.Lichtenstein D.A., Mezière G.A. Relevance of lung ultrasound in the diagnosis of acute respiratory failure the BLUE protocol. Chest. 2008;134:117–125. doi: 10.1378/chest.07-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lichtenstein D. FALLS-protocol: lung ultrasound in hemodynamic assessment of shock. Heart Lung Vessel. 2013;5:142–147. [PMC free article] [PubMed] [Google Scholar]
- 5.Otto C., Hamilton D.R., Levine B.D. Into thin air: extreme ultrasound on Mt Everest. Wilderness Environ Med. 2009;20:283–289. doi: 10.1580/08-WEME-BR-228R2.1. [DOI] [PubMed] [Google Scholar]
- 6.Sargsyan A.E., Hamilton D.R., Jones J.A. FAST at MACH 20: clinical ultrasound aboard the International Space Station. J Trauma. 2005;58:35–39. doi: 10.1097/01.ta.0000145083.47032.78. [DOI] [PubMed] [Google Scholar]
- 7.Brooks A.J., Price V., Simms M. FAST on operational military deployment. Emerg Med J. 2005;22:263–265. doi: 10.1136/emj.2004.014308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Copetti R., Soldati G., Copetti P. Chest sonography: a useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc Ultrasound. 2008;6:16. doi: 10.1186/1476-7120-6-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peng Q.Y., Wang X.T., Zhang L.N. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med. 2020 Mar 12 doi: 10.1007/s00134-020-05996-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buonsenso D., Piano A., Raffaelli F. Point-of-care lung ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24:2776–2780. doi: 10.26355/eurrev_202003_20549. [DOI] [PubMed] [Google Scholar]
- 11.Soldati G., Smargiassi A., Inchingolo R. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med. 2020 doi: 10.1002/jum.15284. Mar 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moro F., Buonsenso D., Moruzzi M.C. How to perform lung ultrasound in pregnant women with suspected COVID-19 infection. Ultrasound Obstet Gynecol. 2020;55:593–598. doi: 10.1002/uog.22028. [DOI] [PubMed] [Google Scholar]
- 13.Soldati G., Smargiassi A., Inchingolo R. Proposal for international standardization of the use of lung ultrasound for COVID-19 patients; a simple, quantitative, reproducible method. J Ultrasound Med. 2020 Mar 30 doi: 10.1002/jum.15285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas A., Haljan G., Mitra A. Lung ultrasound findings in a 64-year-old woman with COVID-19. CMAJ. 2020 doi: 10.1503/cmaj.200414. cmaj.200414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vetrugno L., Bove T., Orso D. Our Italian experience using lung ultrasound for identification, grading and serial follow-up of severity of lung involvement for management of patients with COVID-19. Echocardiography. 2020;37:625–627. doi: 10.1111/echo.14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalafat E., Yaprak E., Cinar G. Lung ultrasound and computed tomographic findings in pregnant woman with COVID-19. Ultrasound Obstet Gynecol. 2020 Apr 6 doi: 10.1002/uog.22034. [DOI] [PubMed] [Google Scholar]
- 17.Miller A. Practical approach to lung ultrasound. BJA Educ. 2016;16:39–45. [Google Scholar]
- 18.Lichtenstein D., Goldstein I., Mourgeon E. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Sekiguchi H., Schenck L.A., Horie R. Critical care ultrasonography differentiates ARDS, pulmonary edema, and other causes in the early course of acute hypoxemic respiratory failure. Chest. 2015;148:912–918. doi: 10.1378/chest.15-0341. [DOI] [PubMed] [Google Scholar]
- 20.Bouhemad B., Brisson H., Le-Guen M. Bedside ultrasound assessment of positive end-expiratory pressure-induced lung recruitment. Am J Respir Crit Care Med. 2011;183:341–347. doi: 10.1164/rccm.201003-0369OC. [DOI] [PubMed] [Google Scholar]
- 21.Haddam M., Zieleskiewicz L., Perbet S. Lung ultrasonography for assessment of oxygenation response to prone position ventilation in ARDS. Intensive Care Med. 2016;42:1546–1556. doi: 10.1007/s00134-016-4411-7. [DOI] [PubMed] [Google Scholar]
- 22.Zhao Z., Jiang L., Xi X. Prognostic value of extravascular lung water assessed with lung ultrasound score by chest sonography in patients with acute respiratory distress syndrome. BMC Pulm Med. 2015;15:98. doi: 10.1186/s12890-015-0091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]