Abstract
Methamidophos (MET) is a pesticide that has toxic properties, including effects on fertility. This study aimed to assess the joint action of treatment time and exposure to methamidophos on the male reproductive system. MET was orally administered to adult male Swiss mice at a dose of 0.004 mg.kg−1 for 15 and 50 consecutive days. The following parameters were evaluated: weight of reproductive organs, spermatogenesis, sperm and Sertoli cell count, daily sperm production and sperm transit time. Short-term exposure to methamidophos induced a decrease in epididymal weight. The frequency of stages V–VI of spermatogenesis increased and the frequency of stage IX decreased. In the epididymis, sperm transit time (caput/corpus) was reduced and the relative sperm number (cauda) increased. Long-term exposure induced an increase in the frequencies of stages I–IV and V-VI and decreased the stages VII-VIII and IX. The number of Sertoli cells with evident nucleoli was reduced in both exposures. These results confirm the reproductive toxicity of MET.
Keywords: Pesticides, Organophosphates, Epididymis, Sertoli cells, Muscarinic receptors
1. Introduction
Organophosphates (OP) are one of the most important classes of pesticides. In addition to their use in agriculture, OP are also used in both domestic and industrial settings and in public health applications, such as the control of mosquito-borne diseases [1]. The illegal use of poisoned baits containing OP has also been reported [2]. This group of pesticides has been reported to be a risk factor in respect of biodiversity and terrestrial ecosystems [3], causing contamination and being toxic to animal and human life. Furthermore, in countries where regulations are not stringent, or where the implementation and enforcement of laws are not effective, the likelihood of pesticide poisoning may be greater [4].
Methamidophos (MET) is used on crops, including sweet pepper, grapes, strawberries and tomatoes [5,6]. It has been detected in pollen samples from honeybee colonies [7]. MET has also been shown to inhibit acetylcholinesterase (AChE) activity at cholinergic binding sites within the central and peripheral nervous systems, which can lead to signs of toxicity in animals [8,9]. In addition, it has both hydrophilic and hydrophobic domains [10] and can pass through the blood-brain and placental barriers [11] and diffuse easily through cell membranes. Thus, these chemical properties could also allow MET to pass the blood-testicular barrier.
Previous studies have shown that MET exposure may lead to developmental neurotoxicity in aquatic systems [12] and attack behavior in non-aggressive mice [13]. MET exposure has also been shown to be a factor in some reproductive disorders. Deleterious effects on male reproductive endpoints such as testosterone levels, semen quality [14,15] and ADN sperm [16,17], leading to infertility and/or structural and functional changes [9,15] have also been reported. These reproductive changes caused by contact with MET may compromise the survival of the exposed species. In this context, the present study was designed to evaluate the effects of the joint action of treatment time and exposure to methamidophos on the weight of reproductive organs (testes, epididymides and seminal vesicles), the dynamics of spermatogenesis, daily sperm production (DSP), the Sertoli cell number with evident nucleoli in the germinal epithelium and sperm transit time in the epididymis of adult Swiss mice.
2. Materials and methods
2.1. Test compound
Commercial methamidophos (O,S-dimethylphosphoroamidothioate) formulation was diluted in distilled water to obtain a 0.004 mg.kg−1 dose prior to use. Its molecular formula is C2H8NO2PS, molecular weight 141.125 g/mol, and CAS no. 10265-92-6.
2.2. Animals and experimental groups
All experimental protocols were approved by the Ethics Committee on the Use of Animals of the Universidade Federal de Goiás (protocol #047/2016-CEUA/UFG) in accordance with the National Council for the Control of Animal Experimentation (Concea) guidelines. Healthy 50-day-old adult male Swiss mice (Mus musculus), weighing 42 g, obtained from the Central Animal House of the Universidade Federal de Goiás were housed in standard polypropylene cages (40 × 30 × 16 cm) at 23 °C, with a light/dark cycle of 12 h (lights on at 6:00 am) in the Laboratory of Physiology and Toxicological Biochemistry, Universidade Estadual de Goiás, Ceres, GO, Brazil. Food (commercial rodent diet Presence®, Presence Alimentos, Paulínia, SP, Brazil) and filtered tap water were available ad libitum.
Animals were randomly allocated to two experimental groups and received 0.004 mg.kg−1 of MET dissolved in water (100 μL) daily by gavage for 15 (short-term exposure group, n = 9/group, age 65 days at the end of treatment) and 50 (long-term exposure group, n = 11/group, age 100 days at the end of treatment) consecutive days. A control group received only water. The dose was selected in accordance with the acceptable daily intake (ADI) in food for humans allowed by the World Health Organization [18] and the Agência Nacional de Vigilância Sanitária [19] in Brazil, which is 0.004 mg.kg−1.
2.3. Organ weights
On the day after the end of treatment, the mice were killed by decapitation. The testes, epididymides (separated into caput/corpus and cauda) and seminal vesicles (with fluid) were removed to determine absolute and relative weights.
2.4. Spermatogenesis and Sertoli cell count
Right testes were fixed in paraformaldehyde (10 %) fixative solution by immersion for 24 h. After embedding in paraffin (Histosec®, Merck KGaA, Darmstadt, Germany), 5-μm sections were stained with hematoxylin and eosin. Spermatogenesis and Sertoli cell count were evaluated under a light microscope (Olympus Biological Microscope Model CH30, Olympus Optical Co., LTD., Tokyo, Japan) at 400× magnification. The dynamics of spermatogenesis was determined by estimating the frequency of the stages: I–IV and V–VI (two generations of spermatids), VII–VIII (mature spermatids), IX (only one generation of spermatids), X–XI (two generations of spermatids), XII (secondary spermatocyte) in 105 transverse sections of seminiferous tubules per animal (Supplementary Data 1 and 2). Furthermore, the total number of Sertoli cells with evident nucleoli (the two meiotic divisions that occur in stage XII increase Sertoli cell activity, and present the most evident nucleoli) was calculated by averaging counts from 10 seminiferous tubules per mouse at stage XII of spermatogenesis (Supplementary Data 3) [20].
2.5. Sperm number, daily sperm production and sperm transit time
The left testes and epididymides were weighed, stored at −20 °C and subsequently used to estimate the number of mature spermatids, daily sperm production (DSP) and sperm transit time in tissue homogenates [21]. In short, the testicular parenchyma was homogenized in 3 ml of solution containing NaCl 0.9 % and Triton X-100 at 0.5 %, followed by sonication for 2 min. The epididymides were homogenized with 1 ml of the same solution for each 20 and 10 mg of tissue for the caput/corpus and cauda, respectively. Then 400 μl of these homogenates was added to 600 μl of the solution. The samples (testis and epididymis homogenates) were placed in a haemocytometer (Neubauer chamber, 0.0025 mm2, depth 0.100 mm) to count homogenization-resistant spermatids (step 16) under a light microscope at 400× magnification. Counts were performed in four replicates, and each replicate was evaluated by averaging counts from five different squares, each with 16 fields (Supplementary Data 4). DSP was calculated by dividing the total number of spermatids resistant to homogenization found per testis by 4.84 (the number of days spermatids are present in the seminiferous epithelium) and the sperm transit time was estimated from the ratio of the number of sperm in each epididymal segment (caput/corpus and cauda) and the DSP.
2.6. Statistical analysis
Data were evaluated using Factorial ANOVA, and homogeneity was assessed using the Levene’s test. The Tukey post-test was applied to detect significant effects of the parameters of MET exposure and duration of MET exposure. For testing the significance of sperm number, relative sperm number and sperm transit time data in the cauda epididymis, the Wald chi-square statistic (Wald χ2), computed by the Generalized Linear/Nonlinear (GLZ) Models, was used. Poisson and Identity were applied for the distribution and link function of predicted values. The level of significance used was P < 0.05.
3. Results
3.1. Somatic parameters
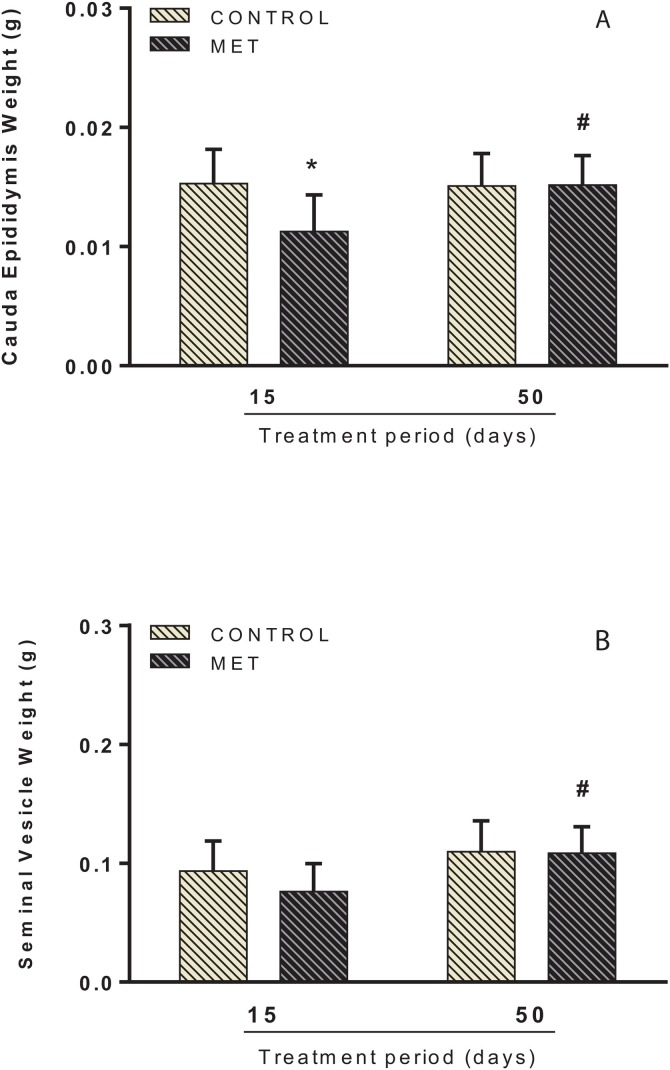
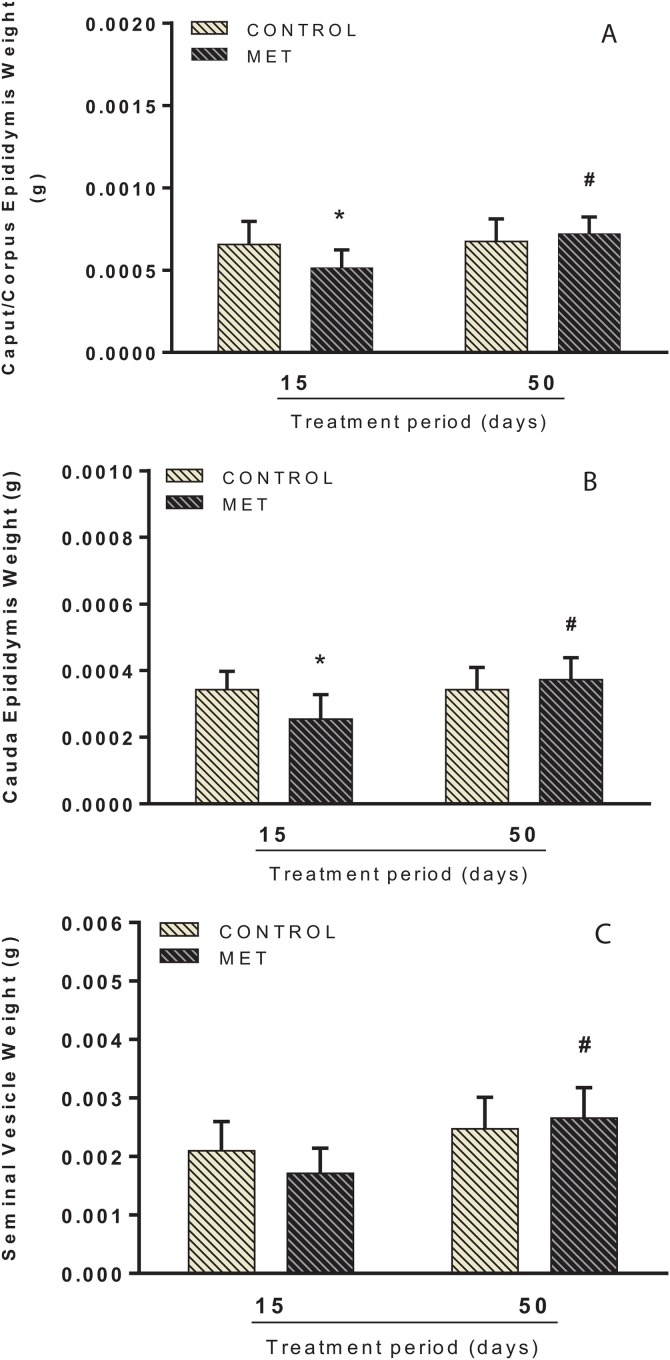
Administration of 0.004 mg.kg−1 of MET changed absolute and relative weight of the epididymides. Factorial analysis showed an interaction between time and MET exposure. Following short-term treatment, there was a decrease in the absolute weight of the cauda epididymis in the MET-exposed mice (F(1, 35) = 5.258; P < 0.05; Fig. 1 A) when compared to the control group. Relative weight reductions in the caput/corpus (F(1, 35) = 5.639; P < 0.05; Fig. 2 A) and cauda epididymis (F(1, 35) = 7.782; P < 0.05; Fig. 2B) were also observed. Following long-term exposure, no significant changes in the absolute/relative weights of reproductive organs were noted in the MET group, compared to control. However, the analysis between the MET groups in respect of exposure time showed that long-term treatment caused an increase in the absolute weight of the cauda epididymis (F(1, 35) = 4.333; P < 0.05; Fig. 1A) and seminal vesicles with fluid (F(1, 35) = 9.733; P < 0.05; Fig. 1B) when compared to short-term treatment. This increase was also observed in the relative weight of the caput/corpus (F(1, 35) = 8.116; P < 0.05; Fig. 2A) and cauda epididymis (F(1, 35) = 7.801; P < 0.05; Fig. 2B) and seminal vesicle with fluid (F(1, 35) = 16.798; P < 0.001; Fig. 2C). No difference was found in the final body and testes weight in both short and long-term exposure (Supplementary Data 5).
Fig. 1.
Absolute weight of cauda epididymis (A) and seminal vesicle with fluid (B) of adult male Swiss mice orally treated for 15 (Control: n = 9; MET: n = 9) and 50 (Control: n = 10; MET: n = 11) days with methamidophos 0.004 mg.kg−1. Values are mean ± standard deviation. Asterisk indicates a significant difference from control. Hash indicates a significant difference between 15 and 50 days of MET treatment. Factorial ANOVA and Tukey post-test (* P < 0.05, #P < 0.05).
Fig. 2.
Relative weight of caput/corpus epididymis (A), cauda epididymis (B) and seminal vesicle with fluid (C) of adult male Swiss mice orally treated for 15 (Control: n = 9; MET: n = 9) and 50 (Control: n = 10; MET: n = 11) days with methamidophos 0.004 mg.kg−1. Values are mean ± standard deviation. Asterisk indicates a significant difference from control. Hash indicates a significant difference between 15 and 50 days of MET treatment. Factorial ANOVA and Tukey post-test (* P < 0.05, #P < 0.05).
3.2. Spermatogenesis
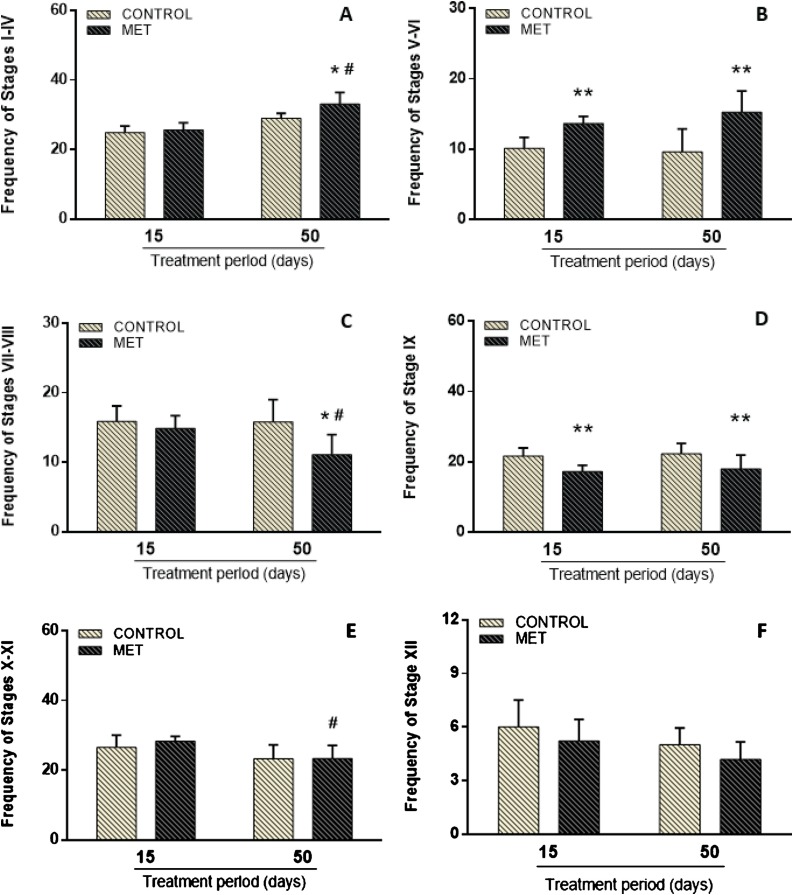
Significant differences were found among the germinal epithelium stages of the control and MET groups (Fig. 3 ). Following short-term exposure, an increase in the frequency of stages V-VI (F(1,34) = 31.970; P < 0.001; Fig. 3B) and a decrease of stage IX (F(1,34) = 20.268; P < 0.001; Fig. 3D) were observed in the MET-treated mice compared to the control. In the long-term exposure group, there was an increase of stages I-IV (F(1,34) = 4.665; P < 0.05; Fig. 3A) and V-VI (F(1,34) = 31.970; P < 0.001; Fig. 3B) and a reduction of stages VII-VIII (F(1,34) = 4.632; P < 0.05; Fig. 3C) and IX (F(1,34) = 20.268; P < 0.001; Fig. 3D). Moreover, in respect of the analysis comparing duration of MET exposure, the germinative epithelium was affected. In the long-term treatment, there was an increase in stages I-IV (F(1,34) = 57.165; P < 0.001; Fig. 3A) and a reduction in stages VII-VIII (F(1,34) = 5.012; P < 0.05; Fig. 3C) and X-XI (F(1,34) = 13.816; P < 0.001; Fig. 3E). No difference was found in stage XII (Fig. 3F) in both short and long-term exposure. Interaction between time and MET exposure was observed only in stages I-IV and VII-VIII.
Fig. 3.
Frequency of testicular transverse sections (stages I-IV (A), V-VI (B), VII-VIII (C), IX (D), X-XI (E) and XII (F) of the seminiferous epithelium) of adult Swiss mice orally treated for 15 (Control: n = 8; MET: n = 9) and 50 (Control: n = 10; MET: n = 11) days with methamidophos 0.004 mg.kg−1. Values are mean ± standard deviation. Asterisk indicates a significant difference from control. Hash indicates a significant difference between 15 and 50 days of MET treatment. Factorial ANOVA and Tukey post-test (* P < 0.05, ** P < 0.001, #P < 0.05).
3.3. Sertoli cells
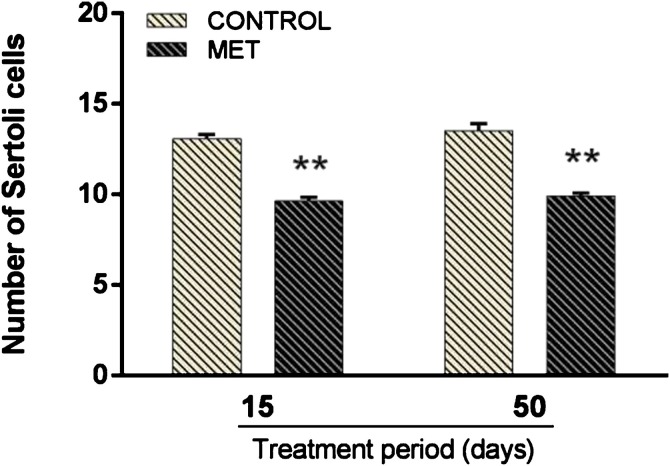
The total number of Sertoli cells with nucleoli evident per seminiferous tubule of mice is shown in Fig. 4 . There was no significant interaction between MET exposition and time of treatment. However, both short and long-term exposure to MET produced a decrease in Sertoli number (F(1,34) = 184.390; P < 0.001) by 23 % and 29 %, respectively, when compared to the control group.
Fig. 4.
Number of Sertoli cells with evident nucleoli per section of seminiferous tubules at stage XII of adult Swiss mice orally treated for 15 (Control: n = 8; MET: n = 9) and 50 (Control: n = 10; MET: n = 11) days with methamidophos 0.004 mg.kg−1. Values are mean ± standard error. Asterisk indicates a significant differences from control. Factorial ANOVA and Tukey post-test (** P < 0.001).
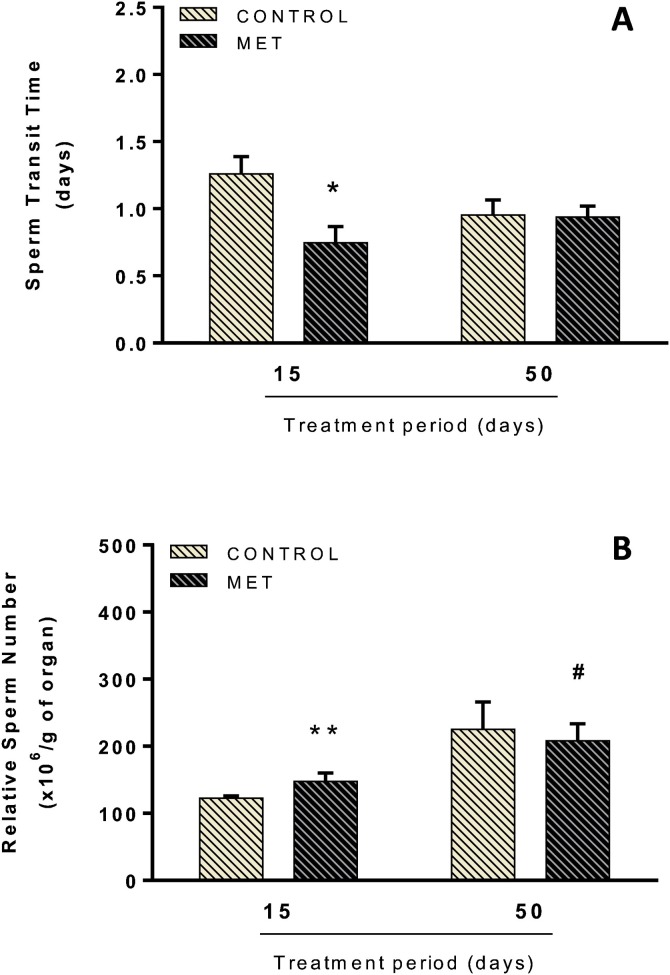
3.4. Sperm number, daily sperm production and sperm transit time
The lower dose of MET did not promote significant alterations in testicular sperm counts (number of mature spermatids) or in DSP during the short and long-term treatments (Table 1 ). On the other hand, changes in epididymal parameters were found. In the MET-treated mice, sperm transit (caput/corpus) decreased (F(1,24) = 5.143; P < 0.05; Fig. 5 A) and relative sperm count (cauda) increased (Wald X2 (1) = 19.798; P < 0.001; Fig. 5B) during short-term exposure. Interaction between time of treatment and MET exposure was observed only in the relative sperm count parameter. In respect of the analysis comparing duration of MET exposure, relative sperm count increased (Wald X2 (1) = 300.96; P < 0.001; Fig. 5B) following long-term exposure compared to short-term exposure.
Table 1.
Testicular sperm counts and daily sperm production of adult Swiss mice orally treated for 15 and 50 days with methamidophos 0.004 mg.kg−1 (methamidophos group) and control group.
| Parameters | Frequency |
|||
|---|---|---|---|---|
| Control |
Methamidophos |
|||
| 15 (n = 7) | 50 (n = 7) | 15 (n = 7) | 50 (n = 7) | |
| Sperm count in the testis | ||||
| Spermatid number (x106/organ) |
15.6 ± 2.3 | 18.4 ± 2.7 | 21.2 ± 3.2 | 16.6 ± 1.6 |
| Relative spermatid number (x106/g of organ) |
126.8 ± 17.5 | 155.6 ± 25.6 | 182.8 ± 28.2 | 141.4 ± 15.8 |
| Daily sperm production (x106/testis/day) |
3.2 ± 0.5 | 3.8 ± 0.6 | 4.4 ± 0.7 | 3.4 ± 0.3 |
| Relative daily sperm production (x106/testis/g/day) |
26.2 ± 3.6 | 32.2 ± 5.3 | 37.8 ± 5.8 | 29.2 ± 3.3 |
Values are mean ± standard error (P > 0.05). Not statistically significant according to Factorial ANOVA.
Fig. 5.
Sperm transit time in the caput/corpus epididymis (A) and relative sperm number in the cauda epididymis (B) of adult Swiss mice orally treated for 15 (Control: n = 7; MET: n = 8) and 50 (Control: n = 7; MET: n = 6) days with methamidophos 0.004 mg.kg−1. Values are mean ± standard error. Asterisk indicates a significant difference from control. Hash indicates a significant difference between 15 and 50 days of MET treatment. (A) Factorial ANOVA and Tukey post-test, (B) GLZ (Poisson, Identity), * P < 0.05, ** P < 0.001, #P < 0.001.
4. Discussion
We analyzed the effects of short- and long-term exposure to MET on the male reproductive system. Short-term exposure induced a decrease in the absolute (cauda) and relative (caput/corpus and cauda) weight of the epididymides. The frequency of stages V–VI of spermatogenesis increased and the frequency of stage IX decreased. In the epididymis, sperm transit time (caput/corpus) was reduced and the relative number of sperm (cauda) increased. Long-term exposure induced an increase in the frequencies of stages I–IV and V–VI and decreased the stages VII–VIII and IX. The number of Sertoli cells was reduced in both exposures.
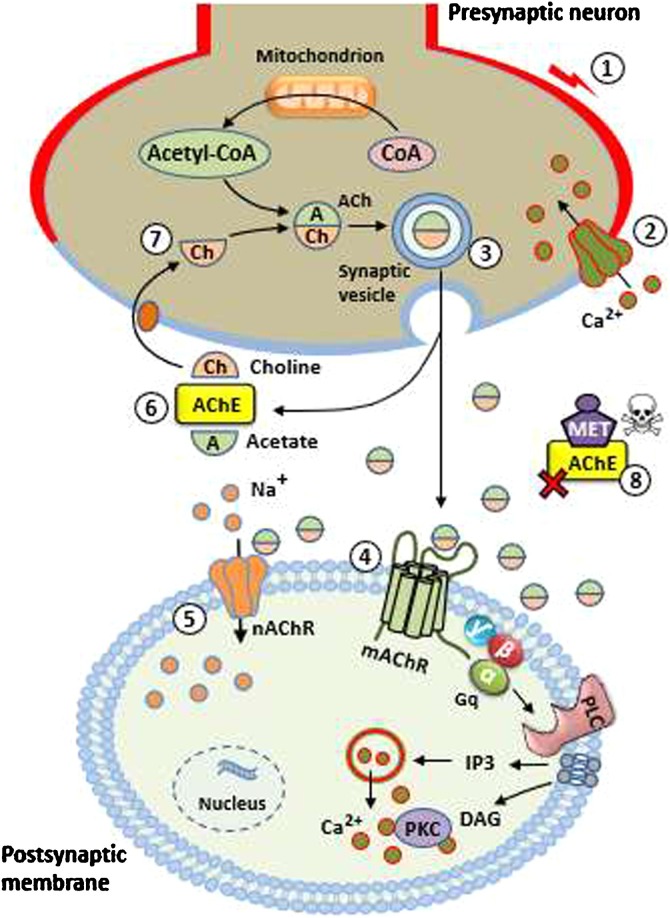
The reduction of caput/corpus weight observed in the short-term exposure group may be the result of the accelerated transit time in this epididymal unit, which promoted the release of spermatozoa. However, there was also a reduction in the cauda epididymis weight, even with the increased number of spermatozoa. MET has the potential to interfere with the cholinergic system [8] and promote acetylcholinesterase (AChE) inhibition (being considered an AChE inhibitor (AChEI)), thus increasing the supply of acetylcholine (ACh) in the muscarinic and nicotinic synapses (Fig. 6 ), which causes OP poisoning [22]. Additionally, studies suggest that both acute and chronic OP intoxication disrupt the redox processes, changing the activities of antioxidative enzymes and causing enhancement of lipid peroxidation in several organs, leading to tissue damage due to AChE inhibition [23,24].
Fig. 6.
Events occurring before and after MET action. (1) An arriving action potential depolarizes the presynaptic neuron; (2) Ca2+ ions enter the cytoplasm; (3) After a brief delay, acetylcholine (ACh) is released through the exocytosis of synaptic vesicles; (4) ACh binds to muscarinic receptors (mAChR) in the postsynaptic membrane and activates Gq protein. This event increases phospholipase C (PLC) activity, which degrades membrane phospholipids into inositol triphosphate (IP3) and diacylglycerol (DAG). IP3 leads to release of intracellular Ca2+. DAG has a role in the late (tonic) phase of the cell response; (5) In the nicotinic receptors (nAChR), ACh promotes a graded depolarization of postsynaptic membrane with Na+ input. This event results in cell response; (6) ACh action ceases when it is broken down into acetate and choline by acetylcholinesterase (AChE) enzyme; (7) Choline reuptake occurs from the synaptic cleft into the presynaptic neuron, being used to synthesize new molecules of ACh; (8) Methamidophos (MET) inhibits AChE and prolongs duration of ACh at the synaptic terminals.
On the other hand, an increase was observed in the epididymis (caput/corpus and cauda) and seminal vesicle weights following long-term exposure when compared to short-term exposure. This may be related to the downregulation process. It is known that chronic stimulation of muscarinic receptors (mAChR) induces receptor downregulation, resulting in loss of cellular response to future agonist stimulations in cultured cells [25]. Thus, a decrease in receptor density may be responsible for the weight increase observed in this study. Furthermore, downregulation may have occurred in the vas deferens musculature and hindered ejaculation, since mAChR are expressed in the vas deferens of rodents [26,27]. This event, in turn, may have promoted the accumulation of spermatozoa in the epididymides, resulting in the increased weight of this tissue observed after long-term exposure. However, little is known about the mechanisms underlying the events involving MET and mAChR in the male reproductive tract and the consequences for male fertility.
Exposure to MET caused significant changes in spermatogenic kinetics. Studies with other OP have shown that rodent spermatogenesis stages are sensitive to the action of these substances [28,29]. In the present study, the mitotic stages V–VI and IX were more susceptible to the action of MET following short-term exposure to 0.004 mg.kg−1, whereas long-term exposure accentuated the mitotic alterations, with an increase in stages I–IV and V–VI, and a reduction in stage IX. Long-term treatment also reduced the spermiation stages (VII–VIII), which are essential in the dynamics of spermatogenesis. Spermiation is the final stage of spermatogenesis and involves the release of the spermatozoa from the Sertoli cell into the lumen of the seminiferous epithelium. This process is also related to the transfer of spermatozoa to the epididymis [30]. The reduction of these stages results in there being less spermatozoa available for fertilization. Farag et al. [15] reported a reduction in spermatozoa in mice treated with high doses of MET (2.0 and 3.0 mg.kg−1 for 4 weeks).
The effects of MET on spermatogenic dynamics may be the result of a direct action of this pesticide on the germinal epithelium. Previous studies have shown that sperm cells at different stages of spermatogenesis are a target of OP exposure, particularly their nuclei. In rats, the fungicide carbendazim appears to target pachytene spermatocytes and spermatozoa at step 14 of spermatid differentiation at the time of first exposure [31]. Damage to the sperm DNA of mice treated intraperitoneally with MET at doses of 3.75–7.0 mg.kg−1 for 4 days has been observed [16,17].
Other OP, such as the insecticides diazinon [32] and methyl-parathion [33,34] have shown the ability to cause changes in chromatin and DNA integrity and the potential of mitochondrial spermatozoa. One of the most well-known mechanisms of OP toxicity is protein phosphorylation [35]. Diazinon phosphorylated nuclear sperm proteins (protamines) in mice, thus altering the structure of sperm chromatin [32]. The ability of MET to phosphorylate proteins in spermatozoa was also evaluated. Urióstegui-Acosta et al. [17] reported sperm phosphorylation (in serine and tyrosine residues) in adult male mice treated with MET. Therefore, these changes in proteins may explain some of the effects observed in the spermatogenesis stages of mice exposed to MET.
It is important to note that, as the treatment period was increased, in addition to the changes in stages V–VI and IX, promoted by short-term exposure, stages I–IV and VII–VIII also changed with treatment. However, we did not find structural modifications of the germ epithelium, for example, so the absence of this kind of alteration indicates that the findings with long-term exposure to MET could be reversible.
Our results also showed that short- and long-term exposure to MET reduced the number of Sertoli cells with evident nucleoli. It is known that pesticides can directly affect Sertoli cells and promote morphological and functional changes, resulting in the death of these cells [36,37]. In fact, MET has the ability to promote apoptosis. He et al. [12] reported that MET treatment promoted a significant increase in apoptotic cells in the brain of zebrafish larvae. MET possesses both hydrophilic and hydrophobic domains [10], so it is reasonable to expect that this pesticide can pass through the blood-testis barrier by diffusion and cause death of the Sertoli cells.
Somatic cells are crucial in the coordination of the multiple events of spermatogenesis. Studies have shown that in the seminiferous epithelium, each Sertoli cell transports micronutrients to support germ cell development [38]. They send signals between germ cells to accurately regulate cell cycle progression through the process of mitotic renewal of spermatogonia, meiosis, spermiogenesis, germ cell movement through the epithelium, and degenerate germ cell apoptosis [39,40]. Therefore, it is possible that the disturbances in spermatogenic dynamics observed in this study may have been caused by the direct action of MET on Sertoli cells.
In the evaluation of testicular sperm counts and DSP, no differences between MET-treated mice and the control group were observed. These results are consistent with the normal testes weight. However, it is interesting to note that both the relative number of mature spermatids and relative DSP were higher (44 %) in the MET group compared to the control group during short-term exposure. Our data showed that the mitotic stages (responsible for sperm cell proliferation) are more susceptible to short-term MET exposure, evidenced by the elevated number of stages V–VI (stages preceding spermiation). This result may explain the increase in the testicular sperm counts and DSP in the short-term exposure group.
An increase of about 23 % in the number of relative testicular spermatids and relative DSP in the long-term control group was also observed, when compared to the short-term control group. On the other hand, the same analysis with MET time exposure showed an opposite effect, a 23 % decrease. Although these results were not statistically significant, our data showed that long-term MET exposure promoted a reduction in stages VII–VIII, responsible for the release of spermatids, which may explain this finding.
Our analyses showed that MET administration caused significant epididymal changes. In fact, Urióstegui-Acosta et al. [16] reported that the epididymis and epididymal sperm cells are targets of MET toxicity. It is possible, therefore, that the sperm transit reduction in the caput/corpus epididymis in MET-treated mice in the short-term exposure group is a result of excess ACh in mAChR. These receptors are expressed in different cell types in the epididymides of rodents and are involved in the modulation of smooth muscle contraction and epithelial cell activity [26,27]. Thus, hyperstimulation of the mAChR and cholinergic overload may have increased contractility and promoted the release of spermatozoa from the epididymis. However, although not statistically significant, the sperm number in the caput/corpus was 23 % lower in the MET group (2.8 ± 0.3) when compared to the control group (3.7 ± 0.7) following short-course treatment. This reduction may also have influenced the acceleration of sperm transit time.
Sperm transit time through the epididymides plays an important role in the maturation of spermatozoa. They acquire the capacity for progressive motility, and develop the ability to undergo the acrosome reaction, becoming able to recognize and penetrate the female gamete [41]. The acceleration of sperm transit time can harm normal sperm maturation, thus reducing sperm quality and leading to fertility impairment [42]. In turn, the speed of the spermatozoa release from the caput/corpus epididymis can explain the increase in the relative sperm count of the cauda epididymis following short-term exposure to MET, which may also have prevented an additional reduction in the weight of this tissue, as discussed earlier.
Finally, changes in the epididymal transit may be indirectly responsible for the spermatogenic effects produced by short-term exposure to MET. An increase in the speed of sperm transit through the caput/corpus epididymides promotes a paracrine signal to the testes, thereby affecting the spermatogenic dynamic, as was observed.
The present study has a number of limitations. The first being the absence of measurements of FSH and LH levels, as well as information on metabolic proteins/mRNA levels (e.g., Insl3, Star, Hsd3b1, Hsd17b3, Cyp11a1). Second, the use of immunostaining to count Sertoli cells could have been a useful addition to the study. Unfortunately, due to the current exceptional public health situation relating to Covid 19, as well as the lack of technical availability, we were not able to perform these analyses in the current study, but will certainly undertake this in future studies.
5. Conclusion
In summary, our results indicate that short- and long-term exposure to methamidophos, even at an acceptable daily intake dose, induced changes in the somatic and spermatogenic parameters of adult mice. The epididymal transit time was also modified after short-term treatment. Although these changes did not significantly affect daily sperm production, the sperm quality may have been impaired due to the alterations found, which may result in male infertility. This shows that the decision to prohibit methamidophos use and marketing in some countries was prudent. However, where the use of this organophosphate is still permitted, there is a need for awareness and adequate technical advice about its use, and effective policies to regulate its supervision, control, and monitoring to avoid the possible toxic effects in the general population.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors gratefully acknowledge the technical contributions of the students from the Laboratory of Physiology and Toxicological Biochemistry of the Universidade Estadual de Goiás, Campus Ceres and the Associação Fundo de Incentivo à Pesquisa (AFIP) in the conduct of this study. This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. R.K.C., T.C.R. and G.M.P.M were supported by CAPES; M.L.A. was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/PQ1D) and R.M.C. was supported by the Programa de Educação Tutorial/Secretaria de Educação Superior - Ministério da Educação (PET/SESu-MEC).
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.repbio.2020.05.003.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Karunamoorthi K., Sabesan S. Insecticide resistance in insect vectors of disease with special reference to mosquitoes: a potential threat to global public health. J Health Scope. 2013;2:4–18. doi: 10.17795/jhealthscope-9840. [DOI] [Google Scholar]
- 2.Chiari M., Cortinovis C., Vitale N., Zanoni M., Faggionato E., Biancardi A., et al. Pesticide incidence in poisoned baits: a 10-year report. Sci Total Environ. 2017;601–602:285–292. doi: 10.1016/j.scitotenv.2017.05.158. [DOI] [PubMed] [Google Scholar]
- 3.Mahmood I., Imadi S.R., Shazadi K., Gul A., Hakeem K.R. In: Plant, soil and microbes: vol.1: implications in crop science. Hakeem K.R., Akhtar M.S., Abdullah S.N.A., editors. Springer International Publishing; Switzerland: 2016. Effects of pesticides on environment; pp. 253–269. [DOI] [Google Scholar]
- 4.Silva J.M., Novato-Silva E., Faria H.P., Pinheiro T.M.M. Agrotóxico e trabalho: uma combinação perigosa para a saúde do trabalhador rural. Cien Saude Colet. 2005;10:891–903. doi: 10.1590/S1413-81232005000400013. [DOI] [Google Scholar]
- 5.Andrade G.C., Monteiro S.H., Francisco J.G., Figueiredo L.A., Botelho R.G., Tornisielo V.L. Liquid chromatography-electrospray ionization tandem mass spectrometry and dynamic multiple reaction monitoring method for determining multiple pesticide residues in tomato. Food Chem. 2015;175:57–65. doi: 10.1016/j.foodchem.2014.11.105. [DOI] [PubMed] [Google Scholar]
- 6.Jardim A.N.O., Brito A.P., van Donkersgoed G., Boon P.E., Caldas E.D. Dietary cumulative acute risk assessment of organophosphorus, carbamates and pyrethroids insecticides for the Brazilian population. Food Chem Toxicol. 2018;112:108–117. doi: 10.1016/j.fct.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Overmyer J., Feken M., Ruddle N., Bocksch S., Hill M., Thompson H. Thiamethoxam honey bee colony feeding study: linking effects at the level of the individual to those at the colony level. Environ Toxicol Chem. 2018;37:816–828. doi: 10.1002/etc.4018. [DOI] [PubMed] [Google Scholar]
- 8.Hussain M.A., Mohamad R.B., Oloffs P.C. Studies on the toxicity, metabolism, and anticholinesterase properties of acephate and methamidophos. J Environ Sci Health B. 1985;20:129–147. doi: 10.1080/03601238509372472. [DOI] [PubMed] [Google Scholar]
- 9.Castro V.L.S., Chiorato S.H. Effects of separate and combined exposure to the pesticides methamidophos and chlorothalonil on the development of suckling rats. Int J Hyg Environ Health. 2007;210:169–176. doi: 10.1016/j.ijheh.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Singh A.K., White T., Spassova D., Jiang Y. Physicochemical, molecular-orbital and electronic properties of acephate and methamidophos. Comp Biochem Physiol C, Pharmacol Toxicol Endocrinol. 1998;119:107–117. doi: 10.1016/S0742-8413(98)00002-4. [DOI] [PubMed] [Google Scholar]
- 11.Bradman A., Barr D.B. B.G. Claus Henn, T. Drumheller, C. Curry, B. Eskenazi, Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: a validation study. Environ Health Perspect. 2003;111:1779–1782. doi: 10.1289/ehp.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He X., Gao J., Dong T., Chen M., Zhou K., Chang C., et al. Developmental neurotoxicity of methamidophos in the embryo-larval stages of zebrafish. Int J Environ Res Public Health. 2017;14:1–12. doi: 10.3390/ijerph14010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nascimento C.P., Maretto G.X., Marques G.L.M., Passamani L.M., Abdala A.P., Schenberg L.C., et al. Methamidophos, an organophosphorus insecticide, induces pro-aggressive behaviour in mice. Neurotox Res. 2017;32:398–408. doi: 10.1007/s12640-017-9750-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maia L.O., Júnior W.D., Carvalho L.S., Jesus L.R., Paiva G.D., Araujo P., et al. Association of methamidophos and sleep loss on reproductive toxicity of male mice. Environ Toxicol Pharmacol. 2011;32:155–161. doi: 10.1016/j.etap.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 15.Farag A.T., Radwan A.H., Eweidah M.H., ElMazoudy R.H., El-Sebae A.E. Evaluation of male-mediated reproductive toxic effects of methamidophos in the mouse. Andrologia. 2012;44:116–124. doi: 10.1111/j.1439-0272.2010.01113.x. [DOI] [PubMed] [Google Scholar]
- 16.Urióstegui-Acosta M., Hernández-Ochoa I., Solís-Heredia M.J., Martínez-Aguilar G., Quintanilla-Vega B. Comparative effect of technical and commercial formulations of methamidophos on sperm quality and DNA integrity in mice. Environ Toxicol. 2012;29:942–949. doi: 10.1002/tox.21822. [DOI] [PubMed] [Google Scholar]
- 17.Urióstegui-Acosta M., Hernández-Ochoa I., Sánchez-Gutiérrez M., Piña-Guzmán B., Rafael-Vázquez L., Solís-Heredia M.J., et al. Methamidophos alters sperm function and DNA at different stages of spermatogenesis in mice. Toxicol Appl Pharmacol. 2014;279:391–400. doi: 10.1016/j.taap.2014.06.017. [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization . 2005. Toxicological monographs and monograph addenda, Acephate 3-16, JMPR.http://www.inchem.org/documents/jmpr/jmpmono/v2005pr02.pdf (Accessed 28 November 2016) [Google Scholar]
- 19.Agência Nacional de Vigilância Sanitária . 2017. Índice monográfico do metamidofós.http://portal.anvisa.gov.br/documents/10181/2989308/CONSULTA+PUBLICA+N+348+GGTOX.pdf/0257754e-25e0-40a6-a03b-5d6e540ffd6d (Accessed 1 January 2018) [Google Scholar]
- 20.Hess R.A., Franca L.R. Spermatogenesis and cycle of the seminiferous epithelium. Adv Exp Med Biol. 2008;636:1–15. doi: 10.1007/978-0-387-09597-4_1. [DOI] [PubMed] [Google Scholar]
- 21.Souza M.R., Carvalho R.K., Carvalho L.S., Sá S., Andersen M.L., Araújo E.G., et al. Effects of subchronic exposure to Caryocar brasiliense peel ethanolic extract on male reproductive functions in Swiss mice. Reprod Toxicol. 2019;87:118–124. doi: 10.1016/j.reprotox.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 22.Čolović M.B., Krstić D.Z., Lazarević-Pašti T.D., Bondžić A.M., Vasić V.M. Acetylcholinesterase inhibitors: pharmacology and toxicology. Curr Neuropharmacol. 2013;11:315–335. doi: 10.2174/1570159X11311030006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Buyukokuroglu M.E., Cemek M., Yurumez Y., Yavuz Y., Aslan A. Antioxidative role of melatonin in organophosphate toxicity in rats. Cell Biol Toxicol. 2008;24:151–158. doi: 10.1007/s10565-007-9024-z. [DOI] [PubMed] [Google Scholar]
- 24.Fortunato J.J., Agostinho F.R., Reéus G.Z., Petronilho F.C., Dal-Pizzol F., Quevedo J. Lipid peroxidative damage on malathion exposure in rats. Neurotox Res. 2006;9:23–28. doi: 10.1007/BF03033304. [DOI] [PubMed] [Google Scholar]
- 25.Bernard V., Décossas M., Liste I., Bloch B. Intraneuronal trafficking of G-protein-coupled receptors in vivo. Trends Neurosci. 2006;29:140–147. doi: 10.1016/j.tins.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 26.Miranda H.F., Duran E., Fernandez E., Pinardi G. Muscarinic receptor subtypes in the bisected vas deferens of the rat. Gen Pharmacol. 1995;26:387–391. doi: 10.1016/0306-3623(94)00185-P. [DOI] [PubMed] [Google Scholar]
- 27.Avellar M.C., Siu E.R., Yasuhara F., Maróstica E., Porto C.S. Muscarinic acetylcholine receptor subtypes in the male reproductive tract: expression and function in rat efferent ductules and epididymis. J Mol Neurosci. 2010;40:127–134. doi: 10.1007/s12031-009-9268-6. [DOI] [PubMed] [Google Scholar]
- 28.Bustos-Obregón E., Yucra S., Gonzales G.F. Lepidium meyenii (Maca) reduces spermatogenic damage induced by a single dose of malathion in mice. Asian J Androl. 2005;7:71–76. doi: 10.1111/j.1745-7262.2005.00006.x. [DOI] [PubMed] [Google Scholar]
- 29.Garagna S., Vasco C., Merico V., Esposito A., Zuccotti M., Redi C.A. Effects of a low dose of bentazon on spermatogenesis of mice exposed during foetal, postnatal and adult life. Toxicology. 2005;212:165–174. doi: 10.1016/j.tox.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 30.Saito K., O’Donnell L., McLachlan R.I., Robertson D.M. Spermiation failure is a major contributor to early spermatogenic suppression caused by hormone with drawal in adult rats. Endocrinology. 2000;141:2779–2785. doi: 10.1210/endo.141.8.7628. [DOI] [PubMed] [Google Scholar]
- 31.Kadalmani B., Girija R., Faridha A., Akbarsha M.A. Male reproductive toxic effects of carbendazim: hitherto unreported targets in testis. Indian J Exp Biol. 2002;40:40–44. [PubMed] [Google Scholar]
- 32.Piña-Guzmán B., Solís-Heredia M.J., Quintanilla-Vega B. Diazinon alters sperm chromatin structure in mice by phosphorylating nuclear protamines. Toxicol Appl Pharmacol. 2005;202:189–198. doi: 10.1016/j.taap.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Piña-Guzmán B., Solís-Heredia M.J., Rojas-García A.E., Urióstegui-Acosta M., Quintanilla-Vega B. Genetic damage caused by methyl-parathion in mouse spermatozoa is related to oxidative stress. Toxicol Appl Pharmacol. 2006;216:216–224. doi: 10.1016/j.taap.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 34.Piña-Guzmán B., Sánchez-Gutiérrez M., Marchetti F., Hernández-Ochoa I., Solís-Heredia M.J., Quintanilla-Vega B. Methyl-parathion decreases sperm function and fertilization capacity after targeting spermatocytes and maturing spermatozoa. Toxicol Appl Pharmacol. 2009;238:141–149. doi: 10.1016/j.taap.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 35.Tacal O., Lockridge O. Methamidophos, dichlorvos, O-methoate and diazinon pesticides used in Turkey make a covalent bond with butyrylcholinesterase detected by mass spectrometry. J Appl Toxicol. 2010;30:469–475. doi: 10.1002/jat.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Monsees T.K., Franz M., Gebhardt S., Winterstein U., Schill W.B., Hayatpour J. Sertoli cells as a target for reproductive hazards. Andrologia. 2000;32:239–246. doi: 10.1046/j.1439-0272.2000.00391.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhao W.H., Zhai H., Wang L., Shu L., Zhou L.H. The protective effects of tea polysaccharides on injury and apoptosis of mouse Sertoly cells induced by glyphosate. Curr Top Nutraceutical Res. 2016;14:81–90. [Google Scholar]
- 38.Sylvester S.R., Griswold M.D. The testicular iron shuttle: a “nurse” function of the Sertoli cells. J Androl. 1994;15:381–385. doi: 10.1002/j.1939-4640.1994.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 39.Cheng C.Y., Wong E.W., Yan H.H., Mruk D.D. Regulation of spermatogenesis in the microenvironment of the seminiferous epithelium: new insights and advances. Mol Cell Endocrinol. 2010;315:49–56. doi: 10.1016/j.mce.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.França L.R., Hess R.A., Dufour J.M., Hofmann M.C., Griswold M.D. The Sertoli cell: one hundred fifty years of beauty and plasticity. Andrology. 2016;4:189–212. doi: 10.1111/andr.12165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson G.E., Nagdas S.K., Winfrey V.P. In: The epididymis: from molecules to clinical practice. Robaire B., Hinton B.T., editors. Kluwer Academic Plenum Publisher; New York: 2002. Structural differentiation of spermatozoa during post-testicular maturation; pp. 371–387. [Google Scholar]
- 42.Fernandez C.D., Porto E.M., Arena A.C., Kempinas W.G. Effects of altered epididymal sperm transit time on sperm quality. Int J Androl. 2008;31:427–437. doi: 10.1111/j.1365-2605.2007.00788.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.