Highlights
-
•
The reuse of disposable masks is limited in terms of the number of uses and unwanted effects.
-
•
The use of H2O2 is considered the most effective method for industrial disinfection of Face Masks.
-
•
The use of hot air is considered the most effective method for home disinfection of Face Masks.
-
•
Surgical masks are slightly less effective than PPE masks.
-
•
Homemade or non-certified disposable masks have a very low effectiveness compared to certified ones.
Keywords: PPE, SARS-COV-2, Coronavirus, Filtering facepiece respirators, Decontamination, Reuse
Abstract
The COVID-19 pandemic is posing a huge global health threat. To deal with this problem, in addition to research and work in the medical field, the main health measures being taken in the workplace and at home involve the establishment of safety protocols, which include distance measures, hygiene and the use of personal protective equipment, such as masks, etc. The WHO still does not recommend the use of masks for the general population. However, their successful use in China, South Korea and the Czech Republic has encouraged their widespread use, and the shortage that already existed. This has caused that companies and individuals are looking at the best way to reuse them, and to manufacture, homemade or not, of non-certified masks. This paper is based on two objectives: to consult the scientific literature to identify the main strategies for disinfecting them, and to determine the effectiveness of non-certified disposable masks. A rapid review has been conducted in which the main publications and other information available online have been analyzed. Results showed that the most promising methods are those that use hydrogen peroxide vapor, ultraviolet radiation, moist heat, dry heat and ozone gas. Soapy water, alcohol, bleach immersion, ethylene oxide, ionizing radiation, microwave, high temperature, autoclave or steam are not fully recommended. Regarding the effectiveness of surgical masks compared to PPE, the former have been seen to be slightly less effective than PPE. As for other types of masks the effectiveness of homemade or non-certified masks is very low.
1. Introduction
The COVID-19 pandemic caused by the virus SARS-COV-2, which first emerged in Wuhan, China, in the province of Huebei in December 2019, is posing a huge global health threat. The total number of global deaths on the date of submitting this paper (24 April 2020) has risen to 191.263, with 2.717.004 infected (European Centre for Disease Prevention and Control, 2020c). The economic impact will be undoubtedly colossal and we are still unaware of the real consequences this will have on each country's economy and on the labor market. It is not the first pandemic that we have suffered, but the dimensions of this one are especially shocking and only comparable to the flu pandemic of 1918, a little over a century ago. It is certainly a public health threat that goes beyond occupational health and safety, and it transgresses the borders of business organizations, being of primary concern to virologists and epidemiologists, but also to technicians, specialists and academics in the field of safety who can make a significant contribution to its prevention. In addition to the past and present research carried out to create detection tests, vaccines, antivirals and other treatments, the main measures used in the health, work and domestic spheres have focused on social distancing and lockdown, as well as on the monitoring of safety protocols, the adoption of hygiene measures, and the use of personal protection equipment such as masks, gloves, etc. This has meant that terms that were previously used by occupational health and safety professionals alone, such as FFP2, FFP3, N95, KN95, etc., have become part of the common language and have had a positive effect in the field of health and safety by popularizing and increasing the culture of prevention of society as a whole.
However, we cannot forget the importance that the World Health Organization (WHO) has played in this context. It initially made different recommendations for the groups including healthcare personnel, people in direct contact with the infected and people with symptoms (Holland et al., 2020, Jansson et al., 2020) on the one hand, and for the rest of the people on the other. While recommending the use of masks for the first three groups of people, it did not do the same for individuals, where it focused on measures of social distancing, minimum interpersonal distance, and personal hygiene, more specifically the adequate washing of hands, since airborne propagation was ruled out under normal conditions (WHO, 2020a). To date, although the WHO acknowledges that “wearing a medical mask is one of the prevention measures that can limit the spread of certain respiratory viral diseases, including COVID-19” (WHO, 2020c) it also says that “however, the use of a mask alone is insufficient to provide an adequate level of protection, and other measures should also be adopted”. Therefore, on 20 April 2020, it still recommends that “If you are healthy, you only need to wear a mask if you are taking care of a person with COVID-19” (WHO, 2020b). Nevertheles, it advises that each country apply a risk-based approach, that is, considering the benefits (possibility of reducing the potential risk of exposure during the presymptomatic period) as well as the potential risks (self-contamination, false sense of security, impact on mask shortages), when deciding whether to recommend the use of masks by the general population (WHO, 2020c).
In this sense, the success of policies followed by countries such as South Korea, China and the Czech Republic regarding the use of masks from the very beginning, in addition to other measures, have demonstrated their benefits (World Economic Forum, 2020). News of this has spread through social networks and the media, and has meant that the use of masks has become widespread in countries such as Italy, Spain and others hit hard by the pandemic, despite the fact that to date the WHO still does not explicitly recommend it.
This has led to the European Center for Disease Prevention and Control (European Centre for Disease Prevention and Control, 2020a) to recognize that the use of masks by the population could reduce the spread of the infection, but it remembers that this should be a complementary measure to preventive hygiene measures. Likewise, the Centers for Disease Control and Prevention (2020b) recommends the use of cloth face coverings to help slow the spread of COVID-19 and the Government of Spain recommends the use of hygienic masks by the population (Ministry of Health of Spain, 2020b).
These circumstances together with the mass use of masks by health workers, essential service companies, cleaners, supermarkets and other people in food supply, security, transport, etc., have caused a shortage, leading the authorities in different countries to confiscate PPE and medical masks, among other means, that are used to fight the disease. Furthermore, this situation is expected to persist for some time, since the WHO itself estimates that approximately 89 million medical masks are needed each month to respond to COVID-19 (WHO, 2020d).
This is why governments, hospitals, companies and even individuals, have also begun to look for solutions of all kinds, including the reuse, cleaning and disinfection of certified disposable masks, either Personal Protection Equipment or medical, and the manufacture of homemade or non-certified ones (European Centre for Disease Prevention and Control, 2020a). In addition, at the time of submission of this paper, a lockdown is in place in many countries around the world, but once it is lifted, a greater number of masks will be required to meet increasing needs.
Consequently, prevention professionals, companies and individuals, from all over the world need to know how to disinfect and sterilize masks that, in principle, were designed, manufactured and certified for short-term use and subsequent disposal, and also to be aware of the effectiveness of homemade or non-certified ones. Thus, in this publication we conducted a rapid review of scientific publications, preprints, protocols, guides and other information available online with two objectives. On the one hand, to identify in the scientific literature the effectiveness of disposable or non-certified masks. On the other hand, to identify the main strategies for their disinfection and/or sterilization, as well as their advantages and disadvantages.
2. Types of masks
Table 1 presents a classification of the different types of disposable masks available according to the use for which they are intended, such as Personal Protection Equipment (PPE) or medical use. Likewise, other types of masks have been included in the classification in order to obtain a complete overview of those that are currently being used due to the situation caused by COVID-19 such as cloth, hygienic, homemade or non-medical masks. This classification has been developed based on the definitions proposed by the European Center for Disease Prevention and Control (European Centre for Disease Prevention and Control, 2020a), although it has been adapted to include new types of masks such as hygienic masks according to Specification UNE 0064:2020 and UNE 0065:2020 recently developed by the Spanish Association for Standardization (UNE, 2020a, UNE, 2020b, UNE, 2020d). These masks are intended for people or children without symptoms who are not susceptible to using surgical, medical or PPE/filter masks to protect them against particles, in accordance with the restrictions established by the Government of Spain, which currently recommends these latest types of masks for healthcare workers and people infected or with symptoms of COVID-19. Therefore, Table 1 focus on disposable mask such as PPE, medical mask and others that can be single use (non-reusable hygienic mask) or some uses (barrier mask, reusable hygienic mask, cloth mask…). Reusable face mask respirators, such as reusable half mask or full face mask, that allow long-term use by changing their filters are not included in this classification (see Fig. 3).
Table 1.
Classification of disposable face masks for particle filtration. (Adapted from: European Centre for Disease Prevention and Control, 2020a).
| Types | Description |
|---|---|
| Filtering facepiece (FFP) respirators (also known as respirator) | It is classified as personal protective equipment (PPE) designed to protect the wearer from exposure to airborne contaminants. Filtering facepiece respirators comply in Europe with requirements defined in Regulation (EU) 2016/425 through European Standard EN 149:2001 + A1:2009. In other countries with similar standards such as NIOSH-42CFR84 in the United States or GB2626-2006 in China. |
| Medical face mask (also known as surgical mask or procedure mask) | It is classified as a medical device that covers the mouth, nose and chin ensuring a barrier that limits the transition of an infective agent between the hospital staff and the patient. Medical masks comply with requirements defined in Directive 93/42 CE or Regulations UE/2017/745 through European Standard EN 14683:2019 + AC:2019 or with similar standards in other countries such as ASTM F2100- 11 in the United States or YY 0469 in China. |
| Other face masks (also commonly known as non-medical, home made, cloth, fabric, ‘community’, hygienic or barrier masks) | This type of mask includes various forms of self-made or commercial masks or face covers made of cloth, other textiles or other materials such as paper. Within this group most are not standardized except those that are manufactured according to AFNOR SPEC S76-001 or Specifications UNE 0064-1:2020, UNE 0064-2:2020 and UNE 0065:2020. In any case, these masks are not intended for use in healthcare settings, or by healthcare professionals nor for workers. The European Centre for Disease Prevention and Control (2020a) calls these masks non-medical face masks or “community” masks and the Centers for Disease Control and Prevention (2020b) call them cloth face coverings. Masks manufactured according to the AFNOR Specification (2020) are called barrier masks and those manufactured according to the UNE Specifications (UNE, 2020c, UNE, 2020e) are called hygienic masks. |
Fig. 3.
Non-Disposable Face Mask FFP3. Source: Marcapl (2020).
Disposable filtering facepiece particulate respirators, including reusable and disposable ones, are Personal Protective Equipment (hereinafter PPE) in European working environments, and are regulated by Regulation (EU) 2016/425 of the European Parliament and of the Council of March 9, 2016, on personal protective equipment and repealing Council Directive 89/686/EEC (European Parliament and the Council of the European Union, 2016) which obliges the manufacturer to apply the CE marking and to follow the procedure for evaluating and complying with the requirements for that marking specifically established in that directive (see Fig. 1, Fig. 2, Fig. 3 ). For this purpose, the manufacturer will have a set of standards, the main one being EN 149:2001 + A1:2009, entitled “Respiratory Protective Devices. Filtering half mask to protect against particles. Requirements, testing, marking” (AENOR, 2010). Additionally, the manufacturer should abide by a series of standards, among which the following should be highlighted: EN 132: 1999. “Respiratory protective devices - Definitions of terms and pictograms” (AENOR, 1999); EN 134: 1998. “Respiratory protective devices - Nomenclature of components” (AENOR, 1998), EN143:2001. “Respiratory protective devices. Particle filters. Requirements, testing, marking.” (AENOR, 2001), and EN 13274-7: 2008. “Respiratory protective devices – Test methods - Part 7: Determination” (AENOR, 2008). This summary of standards will facilitate the presumed compliance of the manufacturer, and will usually lead to the certification of the mask according to the standard. There are also other countries outside Europe with their own similar certification or homologation systems, such as the United States (42 CFR 84), China (GB2626-2006), South Korea (KMOEL-2017-64), Australia/New Zealand (AS/NZA 1716:2012), Japan (JMHLW-2000), etc. (see Table 3). For example, Fig. 4 shows a disposable N95 face mask according to 42 CFR 84 used in the United States.
Fig. 1.
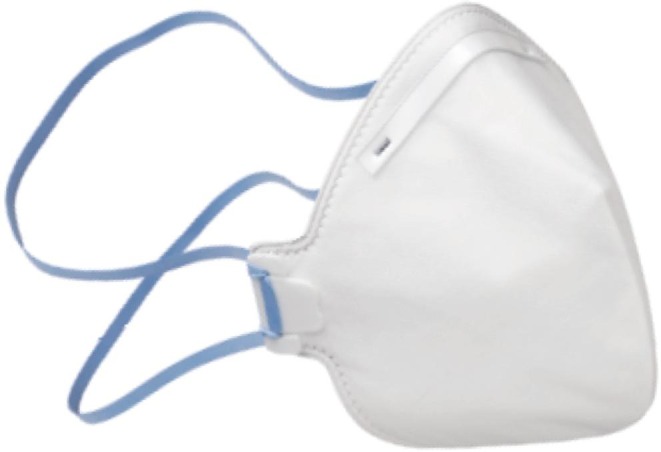
Disposable Face Mask FFP2. Source: Bimedica, 2020a.
Fig. 2.
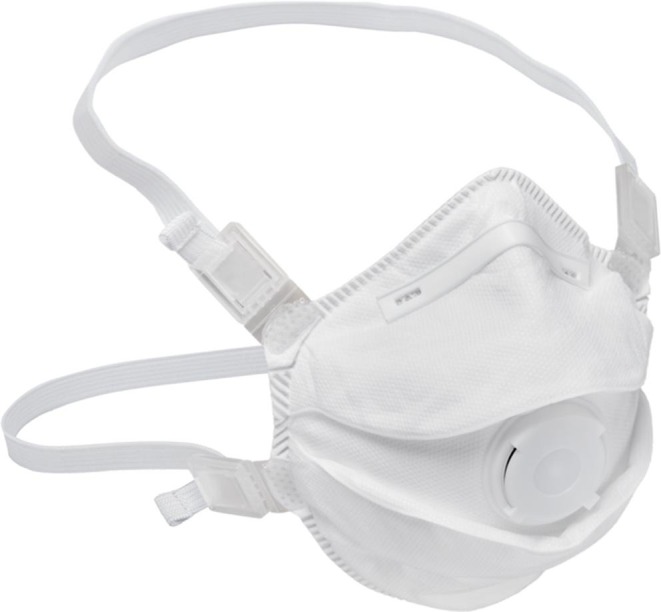
Disposable Face Mask FFP3. Source: Bimedica, 2020b.
Table 3.
Performance requirements for surgical masks according to EN 14683:2019 + AC:2019.
| Test | Type I a | Type II | Type IIR |
|---|---|---|---|
| Bacterial Filtration Efficiency (BFE), (%) | ≥ 95 | ≥ 98 | ≥ 98 |
| Differential pressure (Pa/cm2) | < 40 | < 40 | < 60 |
| Splash resistance pressure (kPa) Not required Not required 3 16.0 | Not required | Not required | 16 |
| Microbial cleaning (ufc/g) | ≤ 30 | ≤ 30 | ≤ 30 |
Fig. 4.
Disposable Face Mask N95.
Source: Battelle (2016).
Additionally, European healthcare systems use disposable medical or surgical masks, which are not PPE but “surgical/medical apparatus and devices”, which are standardized by EN 14683:2019 + AC:2019 “Medical face masks - Requirements and test methods” (UNE, 2019) (see Fig. 5 ). As in the case of PPE, there are also different certifications in other countries such as ASTM F2100-11 in the United States or YY 0469 in China.
Fig. 5.
Medical or surgical mask. Source: own elaboration.
Due to the public health threat caused by SARS-COV-2, there has been a lack of disposable masks certified to meet European technical standards. In this situation, the European Commission published the Commission Recommendation (EU) 2020/403 of March 13, 2020 on conformity assessment and market surveillance procedures within the context of the COVID-19 threat to allow, as long as the shortage lasts, the commercialization of PPE or medical devices that comply with non-European standards even if they do not have the CE marking. WHO recommendations may be followed for their selection, but an adequate level of protection must be guaranteed and the corresponding notifying authority immediately informed (European Commission, 2020). Similarly and subsequently, on March 28, 2020 and April 3, 2020 (Food and Drug Administration, 2020a, Food and Drug Administration, 2020b), the United States government published authorizations to import Non-NIOSH-Approved filtering facepiece respirators from other countries (see Table 3).
This situation of widespread shortages has led the civil society in different countries to dedicate itself to making all kinds of improvised facemasks without any guarantee of certification or homologation (see Fig. 6 ). Initially, individuals began to do this and the idea spread across social networks, which resulted in misunderstandings and sometimes including intentional fakes or hoaxes by trolls. Thus, individuals and even organizations began to prepare masks from different types of materials that allow users to cover their mouth and nose. Subsequently, the authorities or institutions themselves have released their own documents to maximize the effectiveness of masks and minimize the negative impact of not being manufactured in accordance with the quality standards established by international standardization and certification bodies.
Fig. 6.
Homemade or non-certified disposable Face mask. Source: El País (2020).
Faced with this situation, some standardization organizations began to develop reference documents. In this regard, it should be noted that, at the end of March, the French Association for Standardization published AFNOR SPEC S76-001 Barrier masks. Guide to minimum requirements, test methods, manufacture and use for mass manufactured and homemade masks (AFNOR, 2020). However, this document contains recommendations for design and use but does not allow conformity assessment by notified bodies or laboratories. On the other hand, the Spanish Association for Standardization (UNE) published some key specifications to facilitate the manufacture of hygienic masks that, if made under this specification, would offer people protection against the COVID-19 pandemic (UNE, 2020c, UNE, 2020e). In that way, on April 2020, the Specification UNE 0064 for non-reusable hygienic masks and the Specification UNE 0065 for reusable hygienic masks were published. The first is made up of two parts: Specification UNE 0064-1. Non-reusable hygienic masks. Materials, design, manufacturing, marking and usage requirements. Part 1: For adult use and Specification UNE 0064-2. Non-reusable hygienic masks. Materials, design, manufacturing, marking and usage requirements. Part 2: For children use and establishes specifications for manufacture and use. The second is made up of a single part: Specification UNE 0065. Reusable hygienic masks for adults and children. Materials, design, manufacturing, marking and use requirements (UNE, 2020d). The interesting fact is that these Specifications allow conformity assessment to be certified by a laboratory based on technical specifications UNE-EN 14683:2019 + AC: 2019, or another equivalent, which offers greater guarantees to wearers.
The advantage of hygienic masks compared to barrier masks is that the former have been tested following some of the procedures of EN 14683:2019 + AC:2019, achieving good results in the acceptance criteria for the effectiveness of bacterial filtration and breathability, as explained in the next section.
The development of this Specification is positive due to the effect it may have on the availability of masks for the general population, or rather people that are healthy or asymptomatic, since many countries have begun to announce that they will require the use of masks despite the current shortage. However, it still takes time for hygienic masks to reach the population. As it mentioned above, on April 8, 2020, the European Centre for Disease Prevention and Control recommended the use of masks by the population as a complementary preventive measure (European Centre for Disease Prevention and Control, 2020a) and there are countries, such as Spain, that recommend their use beginning April 13, 2020, without sufficient stock to cover the entire population. This is why “improvised” or “community” masks have become crucial and must be used with caution, since their effectiveness has not been proven.
3. Effectiveness of the masks
The effectiveness of disposable masks is different depending on the type and certification standard. In particular, the EN 149:2001-A1:2010 standard (AENOR, 2010), establishes 3 levels of protection depending on the leakage of all particles into the interior, either through the adjustment of the mask to the face, by the exhalation valve if any, or penetration through the filter, always measured according to the arithmetic measurement of the laboratory tests carried out by carriers. These are:
-
•
22% for FFP1.
-
•
8% for FFP2.
-
•
2% for FFP3.
In the case of the North American standard 42 CFR Part 84 developed by NIOSH (NIOSH, 1995), nine types of filters are established, composed of three levels of minimum filtration efficiency and three categories of resistance to the degrading effects of the oil at the workstation. These resistance categories are: “N” Non-oil resistant, “R” Oil resistant and “P” Oil-proof. The levels of efficiency of filtration against aerosols have been determined considering a 0.3 µm aerodynamic mass median diameter and these are:
-
•
95% for N95, R95, P95.
-
•
99% for N99, R99, P99.
-
•
99.97% for N100, R100 and P100.
Logically, other regulations also establish their particular level of effectiveness. Table 2 below summarizes the FFP respirators that are recommended as PPE against COVID-19, according to the standards of different countries (Food and Drug Administration, 2020a, Food and Drug Administration, 2020b). It should therefore be noted that masks with an exhalation valve, regardless of their level of effectiveness, are not recommended as PPE against SARS-COV-2, since exhaled air is released directly into the environment without any type of filtration and would favor the spreading of the coronavirus (European Centre for Disease Prevention and Control, 2020a, Ministry of Health of Spain, 2020a).
Table 2.
Filtering Face Piece (FFP) respirators that could be used as PPE against COVID-19. ().
| Country | Performance Standard | Acceptable Product Classification | Standards/Guidance Documents | Protection Factor ≥ 10 |
|---|---|---|---|---|
| Australia | AS/NZS 1716:2012 | P3, P2 | AS / NZS 1715: 2009 | YES |
| Brazil | ABNT/NBR 13698:2011 | PFF3, PFF2 | Fundacentro CDU 614.894 | YES |
| China | GB 2626-2006, GB 2626-2019 |
KN100 KP100 KN95 KP95 | GB / T 18664-2002 | YES |
| Europe | EN 149-2001 | FFP3 FFP2 | EN 529: 2005 | YES |
| Japan | JMHLW-2000 | DS/DL3 DS/DL2 | JIS T8150: 2006 | YES |
| Korea | KMOEL-2017-64 | Especial Primero | GUÍA KOSHA H-82-2015 | YES |
| Mexico | NOM-116-2009 | N100, P100, R100 N99, P99, R99 N95, P95, R95 | NOM-116 | YES |
| USA | NIOSH 42 CFR 84 | N100, P100, R100 N99, P99, R99 N95, P95, R95 |
OSHA 29CFR1910.134 | YES |
Regarding the effectiveness of surgical or medical masks, the EN 14683:2019 + AC:2019 (UNE, 2019) standard establishes two types depending on their bacterial filtration efficiency and breathability. In addition, within Type II there are two categories depending on whether they are splash resistant or not, as shown in Table 3 . Type I masks are only recommended for patients and other people including healthcare professionals to reduce the risk of spreading infection.
The effectiveness of homemade or cloth masks is unknown a priori, as they are not manufactured according to a standard. However, in the case of hygienic masks abiding by the Specifications UNE 0064 and UNE 0065 (UNE, 2020a, UNE, 2020b, UNE, 2020d), tests have been carried out and some of the operating requirements established in EN 14683:2019 + AC:2019 (UNE, 2019) have been analyzed. The results are presented in Table 4 .
Table 4.
Acceptance criteria for hygienic masks according to the Specifications UNE 0064:2020 and UNE 0065:2020.
| Test | Acceptance criteria for non-reusable hygienic masks | Acceptance criteria for reusable hygienic masks |
|---|---|---|
| Bacterial Filtration Efficiency (BFE), (%) | ≥ 95 | ≥ 90 |
| Differential pressure (Pa/cm2) | < 60 | < 60 |
Although the effectiveness of the masks depends substantially on their correct use, and they can often be overestimated (Garrigou et al, 2020), it is especially interesting to know the comparative effectiveness of different types of masks, assuming their correct use, to determine the level of protection in each case. In particular, the bibliography regarding the comparative effectiveness of PPE-certified, surgical and other masks has been reviewed below.
Thus, in a HSE study, Gawn et al. (2008) compared in a laboratory the protection for airborne particles of surgical masks against FFP respirators and found the lowest level of respiratory protection in surgical masks against FFP respirators. Specifically, they calculated a reduction factor for exposure to live aerosolized influenza virus as the ratio of the particle concentration inside and outside for each mask. Their results indicated that a properly adjusted FFP respirator can provide a mean reduction factor in exposure of 100 while a surgical mask would provide a mean reduction factor of 6. On the other hand, Lee et al (2008) have undertaken the task of comparing the N95 respirators and surgical masks against particles representing bacterial and viral size ranges. As a result, their study found that around 29% of N95 respirators and 100% of surgical masks had a protection factor < 10 (protection factor set by OSHA for that type of mask). So, they concluded that the N95s may not offer the expected protection level against bacteria and viruses. However, in 2016 we found the work of Smith et al. (2016) in which they compared the effectiveness of N95 respirators versus surgical masks in protecting healthcare workers from acute respiratory infection. Their study did not find sufficient evidence to uniquely determine that N95 respirators are superior to surgical masks as protection for healthcare workers against acute respiratory infections in clinical settings. However, they pointed out that in the laboratory setting, the N95 respirators seemed to offer greater protection than the surgical masks. A similar study was carried out by Radonovich et al (2019) to compare the effect of N95 respirators vs. surgical masks to protect healthcare workers against influenza and other viral respiratory infections. They found no significant difference between the two masks in the incidence of influenza in the laboratory setting.
Regarding the comparison with non-certified or homemade masks, Rengasamy et al (2010) carried out an evaluation of the filtration efficiency of this type of mask against particles. To do this, they tested these masks for 20–1000 nm size particles, specifically, polydisperse and monodisperse aerosols, at two speeds. Since there are a wide variety of homemade masks with various characteristics, they used the five main types made of sweatshirts, T-shirts, towels, scarves, and cloth in the test. Furthermore, they compared the results with the penetration levels presented by N95 respirators. Their results indicated that these masks had 40–90% instantaneous penetration levels at a low speed and between 9 and 98% at a higher speed. Subsequently, MacIntyre et al. (2015) compared the effectiveness of cloth mask vs. medical mask in a hospital. In this study, 1607 healthcare workers participated, who wore the masks for 4 weeks during their shifts in the hospital and performed procedures where aerosols are usually generated, such as suctioning of airways, sputum induction, endotracheal intubation and bronchoscopy. Their results indicate that the penetration of the medical mask by the particles was 44% while the penetration for the cloth mask was almost 97%.
In addition to the above, some organizations indicate that these improvised masks should be the last solution and for low-risk cases, but that they can even increase the risk of infection due to humidity, liquid diffusion and virus retention (European Centre for Disease Prevention and Control, 2020b).
4. Reuse, disinfection and sterilization of disposable masks
To analyze reuse, disinfection and sterilization, we must differentiate between the different types of marks presented in Table 1.
We must remember that either PPE (-FFP respirators) or medical face masks manufactured according to technical standards, require that they be discarded after use, since they are heat sensitive and are not designed to undergo a process as severe as sterilization (Rowan and Laffey, 2020). This is why manufacturers like 3M initially advised against the sterilization process (3M, 2020a). However, due to the shortage of these masks caused by the COVID-19 crisis, manufacturers, including 3M, governments and related agencies and institutions began to analyze the reuse, disinfection or sterilization of PPE (– FFP respirators).
As far as reuse of PPE is concerned, the first thing to know is how long the SARS-COV-2 remains on surfaces. Kampf et al. (2020) analyzed 22 studies focused on this question and studied different human coronaviruses, such as Severe Acute Respiratory Syndrome (SARS) coronavirus, Middle East Respiratory Syndrome (MERS) coronavirus or endemic human coronaviruses (HCoV). They concluded that human coronaviruses remain on inanimate surfaces such as metal, wood, paper, glass or plastic for up to 9 days, but they can be efficiently inactivated through disinfection with 62–71% ethanol, 0.5% hydrogen peroxide or 0.1% sodium hypochlorite within 1 min of exposure. However, it is important to note that none of the studies analyzed by Kampf et al. (2020) focused specifically on SARS-COV-2 and that only one of them showed 9-day persistence of the coronavirus, all the others indicated 5 days at most. In another recent study, tests were conducted with the SARS-CoV-2 and SARS-CoV-1 in aerosols and on various surfaces. Their results indicate that SARS-CoV-2 remains on plastic, stainless steel, copper and cardboard surfaces for up to 72 h (Van Doremalen et al., 2020).
Based on this study and given the need to reuse FFP respirators, the US government has recommended that each healthcare worker receives five FFP respirators and uses one per day in a specific order. At the end of the workday, the FFP respirator must be kept in a breathable paper bag and stored by order of use. If the worker stores and uses their FFP respirators in order each day, a minimum of five days between the use of each FFP respirator elapses (Centers for Disease Control and Prevention, 2020a). However, these recommendations should be treated with caution, since FFP respirators are designed for single-use and could be damaged, lose some of their properties and become ineffective as a barrier against contagion. Furthermore, due to the aforementioned shortage, five FFP respirators may not be available per healthcare worker. Therefore, it is necessary to study disinfection or sterilization methods.
In this regard, it should be noted that any method used for the disinfection of FFP respirators must guarantee effectiveness against COVID-19, that the mask is not damaged in terms of the adjustment to the face or its filtration capacity, and that it is not harmful to the person wearing the respirator (3M, 2020b). Therefore, governments, manufacturers, scientists or experts in the field are working towards finding the most effective method for disinfecting FFP respirators against COVID-19.
Under these premises, multiple potential methods for disinfection and sterilization have begun to be studied. Some based on chemical methods, such as the use of H2O2 hydrogen peroxide, chlorine dioxide, bleach, alcohol, soap solutions, ethylene oxide, ozone decontamination, etc., and physical methods, such as the use of heat with steam or with dry air, UV rays, gamma irradiation, microwave, etc. (Mohapatra, 2017), although strict follow-up of procedures is undoubtedly of vital importance, and this is not always the case (Bessesen et al., 2015).
Like all respiratory equipment used for protection against particles, filtering FFP2 respirators are thermally tested for their marking and certification, which entails subjecting the equipment to 70 °C for 24 h, followed by another 24 h at 30 °C (Ministry of Labor and Social Economy, 2020). It therefore appears that this method is of singular importance. Therefore, Song et al. (2020) carried out a study on FFP respirators, but with the flu virus, and used an oven for 30 min at 56 °C as well as hot air from a hair dryer for 30 min. These researchers obtained total inactivation in the case of the dryer and partial inactivation of the virus in the case of the oven, without affecting filtering capacity. Based on this, both the Spanish Ministry of Labor and Social Economy (2020) and the International Medical Center of Beijing (2020) indicate that FFP respirators maintain their filtration efficiency after being disinfected at 70 °C for 30 min, although no effects on the fit or deformation are mentioned. According to N95DECON (2020d), it could be pointed out as an advantage that under these conditions, heat inactivates related coronavirus, although the disadvantage is that there are no data confirming that SARS-COV-2 is inactivated with dry heat and several decontamination cycles could lead to the degradation of the effectiveness of the filtration or the fit. In this way, Spanish Society of Preventive Medicine, Public Health and Hygiene (2020) recommends, as a method of decontamination for FFP respirators, dry heat <70 °C for 30 min in a convection oven to guarantee constant and uniform temperature maintenance. Likewise, Price and Chu (2020) recommend disinfection under those same conditions and they add that it could be done for 20 cycles.
Regarding decontamination of FFP respirators by ozone, Zhang et al. (2004) studied the inactivation of the SARS-CoV-1 by applying different concentrations of ozone solution disinfectant. They found that this virus could be inactivated using a high concentration of 27.73 mg/L for 4 min. Dennis et al. (2020) conducted another study where they analyzed the scientific literature available in this regard and concluded that the existing findings in other studies seem to support that ozone inactivates viruses by attacking capsid proteins. They proposed a decontamination method using ozone to inactivate SARS-CoV-2. Thus, they performed different experiments in two ozone decontamination boxes. Based on this, they proposed practical recommendations to implement a simple disinfection box system using inexpensive and readily available components that could be used for FFP respirators. The ozone concentrations required are 10–20 ppm with an exposure for at least 10 min. They noted that it is an improvised solution for situations of need such as the COVID-19 pandemic, but not an optimal long-term solution. Among the advantages of ozone gas, they indicated that its virucidal action is faster than the degradation effect of the FFP respirator, which is effective for disinfecting fibrous materials, as it is a dry virucidal, and that it reaches shadows and crevices in the process disinfection, unlike ultraviolet radiation as will be seen later.
Other methods that seem promising are Vaporized Hydrogen Peroxide, Low Temperature Moist Heat and ultraviolet C radiation (3M, 2020b, Centers for Disease Control and Prevention, 2020a, N95DECON, 2020a). Firstly, regarding the use of ultraviolet (UV) radiation, Jinadatha et al. (2015) studied disinfecting PPE prior to doffing by applying pulsed xenon ultraviolet (PX-UV) on glass carriers and PPE material contaminated with an Ebola surrogate virus. As a result, the viral load on both glass carriers and PPE materials decreased after exposure to PX-UV. Numerous studies are therefore currently being carried out to determine its effectiveness for decontamination against the coronavirus. O'Hearn et al. (2020), have developed a systematic review on the efficacy and safety of FFP respirators(N95) after decontamination with ultraviolet germicidal irradiation (UVGI). They have analyzed 13 papers and have concluded that the filtering effectiveness of FFP respirator is maintained after a UVGI cycle and they propose the use of a cumulative UV-C dose of 40,000 J/m2 in future research to validate this promising method. Likewise, they have indicated that it will be necessary to carry out fit tests. Moreover, Card et al (2020) analyzed the possibility of using biosafety cabinets for the decontamination of FFP respirators (N95) by ultraviolet germicidal irradiation (UVGI). For this purpose, they tested the UV-C radiation in two randomly chosen idle biosafety cabinets in which they measured the minimum intensity and obtained a factor of 1:71 and 1:98 less than the maximums of each biosafety cabinet, respectively. Based on the maximum observed ratio (1:98), they estimated the sterilization time for FFP respirators (N95) in a biosafety cabinet. Finally, they developed a protocol to disinfect an FFP respirator after irradiating it for 15–20 min per side with a fluence of 100 µWcm−2 (per manufacturer’s records).
Recently, another report also focusing on the use of ultraviolet germicidal irradiation (UVGI) on FFP respirators (N95) has been published online by Nebrasca Medicine (Lowe et al., 2020). They demonstrated that UVGI is effective in inactivating a large number of human pathogens, including coronaviruses, and that if UVGI is applied on FFP respirators (N95), these pathogens are inactivated. Likewise, the necessary levels of UVGI do not affect the fit and filtering effectiveness of the FFP respirators and can be safely administered after providing adequate safeguards. According to them, Hamzavi et al. (2020) recommend its use for the inactivation of SARS-CoV-2 due to the shortage of FFP respirators, highlighting that UVGI does not degrade the polymers. Thus, there are already other organizations that recommend this protocol proposed by Nebrasca Medicine as a decontamination method (N95DECON, 2020e). Similarly, Spanish Society of Preventive Medicine, Public Health and Hygiene (2020) includes among the recommended decontamination methods for FFP respirators the UVGI with double lamp (up and down) 36 W and exposure time of 148 s. They indicate an effectiveness of bacterial disinfection with 7log reduction and harmlessness on respirators.
However, it must be considered that the effectiveness of UV depends on the dose or fluence and shading, since it only inactivates by irradiating surfaces. The FFP respirators must thus be irradiated from both sides (Rowan and Laffey, 2020). The International Medical Center of Beijing (2020) indicates that UV disinfection does not affect the filtration levels of the FFP respirators, although it does not recommend its use because the inactivation effect it produces on FFP respirator fibers is unknown.
This has given rise to uncertainty about the actual decontamination capacity in the inner layers of the FFP respirators. Thus, the (Centers for Disease Control and Prevention, 2020a) warns that “UVGI is unlikely to kill all the viruses and bacteria on an filtering facepiece respirator due to shadow effects produced by the multiple layers of the filtering facepiece respiratoŕs construction”. Therefore, it would be necessary to develop methods or procedures that can eliminate this uncertainty. In this regard, Fisher and Shaffer (2011) established a method to evaluate decontamination of inner layers of FFP respirators (N95) using ultraviolet-C. Therefore, based on this study, a standard procedure could be established to evaluate the effectiveness of UVGI in the inner layers and confirm or rule out its possible use for the inactivation of the coronavirus in FFP respirators.
The use of ultraviolet radiation is currently being evaluated by 3M with a 254 mm UV Lamp for its possible use as an FFP respirator decontamination method during the COVID-19 pandemic. However, the evaluations have not yet been completed nor has its use been authorized by the FDA (3M, 2020b). According to N95DECON (2020c), the advantages of this could be that ≥1 J/cm of UV-C inactivates viruses similar to SARS-CoV-2 on N95s and the N95 maintains fit and filter performance after 10–20 cycles of 1–1.2 J/cm UV-C; and the disadvantages are that UV-C may not reach the inner layer of the FFP respirator, shadows may appear giving rise to parts of the FFP respirator that have not been decontaminated, straps may not be completely decontaminated, the strap and facepiece may be damaged after UV-C and at high UV-C doses (≥120 J/cm) the N95 can be damaged.
On the other hand, the use of Hydrogen Peroxide or H2O2 has also been recently evaluated, although there were already previous studies such as that carried out by Battelle, an FDA Contractor, which analyzed the decontamination and reuse of FFP respirators (N95) using Hydrogen Peroxide Vapor (HPV) and the Bioquell Clarus C system. In this study, only the efficacy of disinfection for the inactivation of G. stearothermophilus spores was verified. They specifically demonstrated that they had achieved a 6-log reduction in organism and that inactivation continued to occur in up to 50 decontamination cycles. Regarding mechanical integrity and performance in up to 50 cycles, the filtration capacity was maintained, but the adjustability was affected because the strap became degraded after 10–20 cycles and broke after 30 cycles. The recommended wash cycle was 480 min. In this way, they demonstrated that this process was feasible to decontaminate large amounts of FFP respirators simultaneously (up to 50 cycles) with Hydrogen Peroxide Vapor, since the filtration requirements of FFP respirators were maintained, although they recommended studying alternative materials for the straps or studying other models of FFP respirators (Battelle, 2016).
Actually, based on this study, Schwartz et al. (2020) have performed validation tests on Hydrogen Peroxide Vapor (HPV) to decontaminate FFP respirators (N95) using the Bioquell 61 Clarus™ C system with a 35% hydrogen peroxide solution for the time recommended by Battelle (2016). They have validated the method and point out that FFP respirators retain their filtering effectiveness after 50 cycles in the laboratory. In addition, they performed fit tests and the results indicate that no loss of fit occurred. Therefore, they say that they are going to start using this decontamination process, which has been internally validated and approved by the Duke Institutional Biosafety Review Committee (IBRC).
It is important to note that, on March 29, 2020, the FDA issued an Emergency Use Authorization (USA) for the emergency use of the Battelle CCDS Critical Care Decontamination System™ at the Battelle Memorial Institute for decontaminating N95 FFP respirators or the equivalent for reuse by health personnel during the COVID-19 pandemic for a maximum of 20 FFP respirators decontamination cycles (Food and Drug Administration, 2020d). Subsequently, on April 2020, the FDA also authorized the use of STERIS V-PRO 1 Plus, maX, and maX2 Low Temperature Sterilization Systems and STERRAD 100S, NX, and 100NX Sterilization Systems for the same purpose and for a maximum of 10 and 2 decontamination cycles per FFP respirator, respectively (Food and Drug Administration, 2020e, Food and Drug Administration, 2020f). All these systems use Vaporized Hydrogen Peroxide (VHP), which is the only FDA-authorized decontamination method to date. Likewise, Spanish Society of Preventive Medicine, Public Health and Hygiene (2020) recommends the use of low-pressure vaporized hydrogen peroxide for the decontamination of FFP respirators, except those containing cellulose, and may only be reprocessed a maximum of 2 times.
Similar conclusions regarding Hydrogen Peroxide Vapor have been established by the Dutch National Institute of Public Health and the Environment (RIVM) through a pilot study carried out with MATACHANA Hydrogen Peroxide sterilizers. This study has confirmed that hydrogen peroxide sterilization is a valid reprocessing method for FFP respirators (FFP2) in order to inactive coronavirus. To reach this conclusion, unused FFP respirators were reprocessed by applying different types of processes such as cleaning and drying with and without detergent or chemical disinfection, vaporized hydrogen peroxide low pressure gas sterilization applied at different times and steam sterilization. Subsequently, FFP respirators were subjected to a fit test to verify that they fit properly and that the filter material continued to be a good barrier against particles. The fit test results for an untreated FFP respirator showed an average value of 162. Therefore, a reprocessed FFP respirator must have a minimum average value of 100 to pass the fit test. The results are presented in Table 5 . Based on these, it was preliminarily concluded that “double sterilization using a short process with hydrogen peroxide gives an acceptable result” (Dutch National Institute for Public Health and the Environment, 2020).
Table 5.
Comparison of different sterilization systems and their effect on mask deformation and the fit test outcome. (Dutch National Institute for Public Health and the Environment, 2020).
| Process | Face mask deformation yes/no |
Fit test outcome +/− |
|---|---|---|
| Control | N/A | + (1 6 2) |
| 1. 60°Celsius cleaning without detergent and disinfectants | No | − (60) |
| 2. 90 °C cleaning without detergent | Yes | N/A* |
| 3. 90 °C cleaning with detergent | Yes | N/A* |
| 4. Hydrogen peroxide sterilization 1x | No | + (151) |
| Hydrogen peroxide sterilization 2x | No | + (103) |
| Hydrogen peroxide sterilization 3x | No | − (28) |
| Hydrogen peroxide sterilization 4x | Yes | N/A* |
| 5. Steam sterilization 134 °C | Yes | N/A* |
Fit test was not performed because FFP respirators were deformed and they were no longer usable.
The use of ethylene oxide, which is widespread in hospitals, is less safe than hydrogen peroxide vaporization and less environmentally friendly. Research seems to confirm that the coronavirus is highly affected by vaporization of hydrogen peroxide, which is lethal with a concentration of 0.5% in less than one minute (Rowan and Laffey, 2020). According to N95DECON (2020b) the advantages of Hydrogen Peroxide Vapor (VHP) are that it inactivates the coronavirus and that after 20 cycles it does not degrade the effectiveness of the filter, fit or straps.
Other study was also found that analyzed the use of hydrogen peroxide as plasma gas (HPGP) for disinfection. The results showed that out of the six models of FFP respirators analyzed, four of them demonstrated mean penetration levels of less than 5%. However, this has not been tested for the specific case of SARS-COV-2 (Bergman et al. 2010). According to N95DECON (2020b) a low dose HPGP for 2 cycles does not degrade fit for 3M 8822 N95s and a high dose reduces FFP respirator filtration.
Also, moist heat has been proposed as a decontamination method. This is a process based on applying heat and humidity to the FFP respirators. There are different studies that address this method for the disinfection of FFP respirators (3M, 2020b, Bergman et al., 2010, Bergman et al., 2011, Heimbuch et al., 2011, Lore et al., 2012, Viscusi et al., 2011). Among them, the study carried out by 3M is the only one that specifically focuses on the inactivation of SARS-COV-2. They used an environment chamber and introduced each FFP respirator in a high temperature self-seal pouch. A temperature of 65 ± 5 °C and 50–80% relative humidity were used for 30 min. They tested up to 10 reprocessing cycles and both the filtration efficiency and the fit were maintained. Although 3M indicates that they are still working to obtain from the U.S. Food and Drug Administration issue an Emergency Use Authorization for decontamination of FFP respirators of SARS-CoV-2 (3M, 2020b).
In relation to other disinfection or decontamination methods that could be used for SARS-VOC-2, the use of powdered alcohol, another traditional disinfectant, does not seem to be recommended, since it eliminates the electrostatic retention of the mask fibers, reducing filtration capacity by 95% (International Medical Center of Beijing, 2020, Ministry of Labor and Social Economy of Spain, 2020). Additionally, washing with soapy water can also affect the electrostatic properties of the fibers or even deform the mask (Ministry of Labor and Social Economy, 2020).
Regarding the use of gamma radiation and based on previous studies, the European Centre for Disease Prevention and Control, 2020a, European Centre for Disease Prevention and Control, 2020b indicated that coronaviruses are inactivated with a gamma radiation dose of 20 kGy (2MRad). However, possible deformations appear with a dose of 24 kGy on the FFP respirator and therefore, the inner filtering layer and the fit are compromised (Feldmann et al., 2019).
As for steam sterilization, like the Dutch National Institute for Public Health and the Environment (2020) of the Dutch Ministry of Health, Welfare and Sport, the International Medical Center of Beijing (2020) indicates on its website that there is a reduction in the efficiency of FFP respirators to below 95% and serious deformation when subjected to the steamer damp heat method with high pressure and high temperature. Nevertheless, a study by Stafondford Medicine (Price and Chu, 2020) indicates that hot water steam could be used for 3 treatment cycles or less while maintaining a filtration efficiency of >95%, but for a greater number of cycles they found that the filtration efficiency was affected. Based on this, Spanish Society of Preventive Medicine, Public Health and Hygiene (2020) indicates that water vapor could be used to cycle at 65 °C for 30 min for decontamination of FFP respirator.
There are even those who need an alternative disinfection method and propose a two-step process to maximize the effectiveness. Thus, Derraik et al. (2020) propose to store the FFP respirators for 4 or more days and then subject them to a disinfection process using ultraviolet light (UVC), dry heat treatment or chemical disinfection, although the efficiency of these has not been proven.
Summarizing, there seems to be a more or less generalized consensus on some methods that are not recommended for disinfection or sterilization such as cleaning with soapy water, alcohol, bleach immersion, ethylene oxide, ionizing radiation, microwave, high temperature, autoclave or steam because they can significantly degrade the filter, either because they alter the electrostatic properties of the filter fibers, affect particle penetration levels, or deform the FFP respirator leading to FFP respirator degradation (3M, 2020b, Centers for Disease Control and Prevention, 2020a, N95DECON, 2020a, Viscusi et al., 2007, Viscusi et al., 2009).
Masks that are not PPE, medical or surgical, such as the Spanish reutilizable hygienic mask or the French barrier mask. there are detailed instructions for machine washing. In the first case, Specification UNE 0065:2020 (UNE, 2020d) recommends washing the hygienic mask in the washing machine through a complete cycle of washing at 60 °C with the usual detergent and afterwards it must be completely dried for 2 h. after washing. Next, the hygienic mask must be visually checked (minor adjustment, deformation, wear) and if its degradation is detected it must be discarded. In the second case, AFNOR SPEC S76-001 (AFNOR, 2020) recommends that, before washing the barrier mask, the empty washing machine should be cleaned using a cold rinse with bleach or by turning it to 60 or 95 °C without spinning. The washing cycle must be equal to or longer than 30 min at 60 °C. The use of regular detergents is recommended. As for drying, it is recommended to use the dryer for two hours and then clean its filters. Once the barrier mask is completely dry, the fit should be checked.
In the case of the Spanish non-reusable hygienic mask, according to Specification UNE 0064 (UNE, 2020a, UNE, 2020b) a maximum use of 4 h is recommended, unless the mask becomes previously degraded or humid. Therefore, washing or disinfecting are not recommended.
In relation to homemade masks, cloth face coverings or non-certified masks, the Centers for Disease Control and Prevention (2020b) indicate that these can be washed in the washing machine, although no specific washing instructions are provided.
Finally, it must be considered that many of these studies and tests presented are carried out with a specific type and/or model of mask. Therefore, it is necessary to be cautious since they can have different effects on other models or types of masks and reduce their effectiveness or affect the properties of the mask or even the straps.
5. Conclusions
Although technical standards are the main reference that should be used as a guide to the manufacture and use of personal protective equipment such as disposable masks, in situations of extreme scarcity caused by epidemics, and in this particular case, the COVID 19 pandemic, other strategies should be considered. Among them, the reuse of disposable filtering facepiece respirators does not seem like a bad transitional solution until the shortage is over. Among the different methods, the available literature seems to point out that the most promising methods are those that use hydrogen peroxide vapor, ultraviolet radiation, moist heat, dry heat and ozone gas. Within them, hydrogen peroxide vapor treatment appears to be the best system and is being widely recommended. Although ultraviolet reduction has also been recommended in some countries, there are those who point to doubts about its effectiveness in the inner layers due to shadows. Dry heat also appears to be effective although it has not been widely recommended. There is even a study that points out the effectiveness of dry heat using a hair dryer for disinfection. The moist heat is currently being evaluated to verify that it does not degrade the fit or the filtration capacity. Ozone gas appears to be effective in decontaminating FFP respirators without damaging them, although it presents risks for the safety and health of workers who carry out the process if it is not handled properly
Other decontamination procedures allow reuse for a limited number of times and with certain limitations and negative side effects, including the deformation of the elastic, the metal strip to fasten it to the face, or the possibility of causing the accumulation of humidity with the consequent risk of exposure to the virus and self-infection.
In addition to effectiveness, other variables may influence the selection of one or another decontamination method by organizations, hospitals or companies that need to apply them to guarantee the supply of PPE during the COVID-19 pandemic. In fact, some methods require specific technology or resources that make the decontamination process more expensive. In this sense, dry heat seems to be cheap. Another factor to consider would be that the method chosen for disinfection must be adapted to the needs of the companies in terms of time and amounts of decontaminated FFP respirators. They must also be logistically and organizationally viable. Even it can take into account other issues such as the traceability of the process or the confidence that is generated in the workers who must reuse the FFP respirators.
Furthermore, surgical masks, compared to Personal Protection Equipment, have a similar effectiveness and are therefore a good alternative. Disinfection processes for reuse have not been described for this type of masks. The hygienic mask offers a lower level of protection than the previous ones. However, in the case of recommending the use of mask by the uninfected and asymptomatic population during the shortage of Personal Protection Equipment and surgical masks, hygienic masks seem the best option since they are certified according to a specification.
Finally, improvised homemade or non-certified masks are the worst alternative of those studied, although it seems better than using nothing at all. However, some bodies say that they may even increase the risk of infection due to humidity, the diffusion of liquids and the retention of the virus in the mask, which would facilitate self-infection. The washing machine and dryer should be used for decontamination.
Contributor Information
Juan Carlos Rubio-Romero, Email: juro@uma.es.
María del Carmen Pardo-Ferreira, Email: carmenpf@uma.es.
Juan Antonio Torrecilla-García, Email: juantorrecilla@uma.es.
Santiago Calero-Castro, Email: scc@uma.es.
References
- 3M, 2020a. Disinfection of Filtering Facepiece Respirators. M Science. Applied to life. Technical Bulletin. Available on 9th April 2020 at https://www.apsf.org/wp-content/uploads/news-updates/2020/Disinfection-of-M-Filtering-Facepiece-Respirators.pdf.
- 3M, 2020b. Decontamination Methods for M N95 Respirators. M Science. Applied to life. Technical Bulletin. Revision 4. Available on 20th of April 2020 at https://multimedia.m.com/mws/media/1824869O/decontamination-methods-for-m-n95-respirators-technical-bulletin.pdf.
- AENOR, 1998. UNE-EN 134: 1998. Respiratory protective devices - Nomenclature of components. Available on 9th April 2020 at https://www.aenor.com/normas-y-libros/buscador-de-normas/UNE?c=N0008386.
- AENOR, 1999. UNE-EN 132: 1999. Respiratory protective devices - Definitions of terms and pictograms. Available on 9th April 2020 at https://www.aenor.com/normas-y-libros/buscador-de-normas/UNE?c=N0008384.
- AENOR, 2001. UNE-EN143:2001. Respiratory protective devices. Particle filters. Requirements, testing, marking. Available on 9th April 2020 at https://www.aenor.com/normas-y-libros/buscador-de-normas/UNE?c=N0025000.
- AENOR, 2008. UNE-EN 13274-7: 2008. Respiratory protective devices - Methods of test - Part 7: Determination of particle filter penetration. Available on 9th of April 2020 at https://www.aenor.com/normas-y-libros/buscador-de-normas/UNE?c=N0042076.
- AENOR, 2010. UNE-EN 149:2001+A1:2010. Respiratory Protective Devices. Filtering half mask to protect against particles. Requirements, testing, marking. Available on 9th April 2020 at https://www.aenor.com/normas-y-libros/buscador-de-normas/UNE?c=N0044643.
- AFNOR, 2020. AFNOR SPEC S76-001 Barrier masks. Guide to minimum requirements, test methods, preparation and use for mass manufactured and homemade masks. Association française de Normalisation AFNOR. Available on 9th of April 2020 at https://iv.revistalocal.es/wp-content/uploads/AFNORSpec-S76-001-MascarillasDeProteccion.pdf.
- Battelle, 2016. Final Report for the Bioquell Hydrogen Peroxide Vapor (HPV) Decontamination for Reuse of N95 respirators. FDA, U.S. FOOD&DRUG, ADMINISTRATION. Available on 9th April 2020 at https://www.fda.gov/media/136386/download.
- Bessesen M.T., Adams J.C., Radonovich L., Anderson J. Disinfection of reusable elastomeric respirators by health care workers: A feasibility study and development of standard operating procedures. Am. J. Infect. Control. 2015;43:629–634. doi: 10.1016/j.ajic.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Bergman M.S., Viscusi D.J., Heimbuch B.K., Wander J.D., Sambol A.R., Shaffer R.E. Evaluation of multiple (3-cycle) decontamination processing for filtering facepiece respirators. J. Eng. Fiber. Fabr. 2010;5(4) 155892501000500405. [Google Scholar]
- Bergman M.S., Viscusi D.J., Palmiero A.J., Powell J.B., Shaffer R.E. Impact of three cycles of decontamination treatments on filtering facepiece respirator fit. J. Int. Soc. Respirat. Prot. 2011;28(1):48. [Google Scholar]
- Bimedica (2020a). Naturcare® Vertical FFP2. Available on 15th April 2020 at https://www.bimedica.com/catalogo-de-productos/naturcare-mascarillas-de-proteccion-vertical-ffp2.
- Bimedica (2020b). Naturcare® Cónica FFP3. Available on 15th April 2020 at https://www.bimedica.com/catalogo-de-productos/naturcare-mascarillas-de-proteccion-conica-ffp3.
- Card, K.J., Crozier, D., Dhawan, A., Dinh, M., Dolson., Farrokhian, E., Gopalakrishnan, N., Ho, V., King, E., Krishnan, N., Kuzmin, G., Maltas, J., Pelesko, J., Scarborough, J.A., Scott, J.G., Sedor, G., Weaver, D.T., 2020. UV sterilization of personal protective equipment with idle laboratory biosafety cabinets during the Covid-19 pandemic. MedRxiv. doi: 10.1101/2020.03.25.20043489. [DOI] [PMC free article] [PubMed]
- Centers for Disease Control and Prevention, 2020. Decontamination and Reuse of Filtering Facepiece Respirators. Available on 9th April 2020 at https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html.
- Centers for Disease Control and Prevention, 2020b. Use of Cloth Face Coverings to Help Slow the Spread of COVID-19. Available on 12 th April 2020 at https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/diy-cloth-face-coverings.html.
- Dennis R., Cashion A., Emanuel S., Hubbard D. Ozone gas: scientific justification and practical guidelines for improvised disinfection using consumer-grade ozone generators and plastic storage boxes. J. Sci. Med. 2020;2(1) [Google Scholar]
- Derraik J.G., Anderson W.A., Connelly E.A., Anderson Y.C. Rapid evidence summary on SARS-CoV-2 survivorship and disinfection, and a reusable PPE protocol using a double-hit process. MedRxiv. 2020 doi: 10.1101/2020.04.02.20051409. [DOI] [Google Scholar]
- Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., Lloyd-Smith J.O., de Wit E., Munster V.J. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N. Engl. J. Med. 2020 doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutch National Institute for Public Health and the Environment, 2020. Reuse of FFP2 Masks. Ministry of Health, Welfare and Sport. Available on 8th of April 2020 at https://www.rivm.nl/en/documenten/reuse-of-ffp2-masks.
- El País, 2020. Cuál es el mejor material para hacer una mascarilla en casa. Available on 20th April 2020 at https://elpais.com/elpais/2020/04/07/buenavida/1586210882_668867.html.
- European Centre for Disease Prevention and Control, 2020a. Using face masks in the community. Available on 9th April 2020 at https://www.ecdc.europa.eu/sites/default/files/documents/COVID-19-use-face-masks-community.pdf.
- European Centre for Disease Prevention and Control, 2020b. Cloth masks and mask sterilisation as options in case of shortage of surgical masks and respirators. Available on 8th April 2020 at https://www.ecdc.europa.eu/en/publications-data/cloth-masks-sterilisation-options-shortage-surgical-masks-respirators.
- European Centre for Disease Prevention and Control, 2020c. Situation update worldwide, as of 20 April 2020. Available on 20th April 2020 at https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases.
- European Commission, 2020. Commission Recommendation (EU) 2020/403 of 13 March 2020 on conformity assessment and market surveillance procedures within the context of the COVID-19 threat. https://eur-lex.europa.eu/legal-content/GA/TXT/?uri=CELEX:32020H0403.
- Feldmann F., Shupert W.L., Haddock E., Twardoski B., Feldmann H. Gamma irradiation as an effective method for inactivation of emerging viral pathogens. Am. J. Trop. Med. Hyg. 2019;100(5):1275–1277. doi: 10.4269/ajtmh.18-0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E.M., Shaffer R.E. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J. Appl. Microbiol. 2011;110(1):287–295. doi: 10.1111/j.1365-2672.2010.04881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Food and Drug Administration, 2020a. Stakeholders for Non-NIOSH-Approved Imported FFRs. Available on 10th April 2020 at https://www.fda.gov/media/136403/download.
- Food and Drug Administration, 2020b. Stakeholders for Non-NIOSH-Approved Imported FFRs Made in China. Available on 10th April 2020 at https://www.fda.gov/media/136664/download.
- Food and Drug Administration, 2020d. March 28, 2020 Emergency Use Authorization. Battelle CCDS Critical Care Decontamination System. U.S. Food & Drug Administration. Available on 11th April 2020 at https://www.fda.gov/media/136529/download.
- Food and Drug Administration, 2020e. April 9, 2020 Emergency Use Authorization. STERIS V-PRO 1 Plus, maX, and maX2 Low Temperature Sterilization Systems. U.S. Food & Drug Administration. Available on 11th April 2020 at https://www.fda.gov/media/136843/download.
- Food and Drug Administration, 2020f. April 11, 2020 Emergency Use Authorization. Advanced Sterilization Products, Inc. (ASP) STERRAD 100S, NX, and 100NX Sterilization Systems1. U.S. Food & Drug Administration. Available on 20th of April 2020 at https://www.fda.gov/media/136884/download.
- Garrigou A., Laurent C., Berthet A., Colosio C., Jas N., Daubas-Letourneux V., Jackson Filho J.M., Jouzel J.N., Samuel O., Baldi I., Lebailly P., Galey L., Goutille F., Judon N. Critical review of the role of PPE in the prevention of risks related to agricultural pesticide use. Saf. Sci. 2020;123 doi: 10.1016/j.ssci.2019.104527. [DOI] [Google Scholar]
- Gawn, J., Clayton, M., Makison, C., Crook, B. (2008). Evaluating the protection afforded by surgical masks against influenza bioaerosols: gross protection of surgical masks compared to filtering facepiece respirators. Health Safety Executive (HSE). Available on 20th April 2020 at https://www.hse.gov.uk/research/rrpdf/rr619.pdf.
- Hamzavi I.H., Lyons A.B., Kohli I., Narla S., Parks-Miller A., Gelfand J.M., Lim H.W., Ozog D. Ultraviolet germicidal irradiation: possible method for respirator disinfection to facilitate reuse during COVID-19 pandemic. J. Am. Acad. Dermat. 2020 doi: 10.1016/j.jaad.2020.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimbuch B.K., Wallace W.H., Kinney K., Lumley A.E., Wu C.Y., Woo M.H., Wander J.D. A pandemic influenza preparedness study: use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am. J. Infect. Control. 2011;39(1):e1–e9. doi: 10.1016/j.ajic.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Holland M., Zaloga D.J., Friderici C.S. COVID-19 Personal Protective Equipment (PPE) for the emergency physician. Vis. J. Emerg. Med. 2020;19 doi: 10.1016/j.visj.2020.100740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Medical Center of Beijing, 2020. Let́s talk about protective function of masks. Available on 8th April 2020 at http://www.imcclinics.com/english/index.php/news/view?id=83.
- Jansson M., Liao X., Rello J. Strengthening ICU health security for a coronavirus epidemic. Intens. Crit. Care. Nur. 2020;57 doi: 10.1016/j.iccn.2020.102812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinadatha C., Simmons S., Dale C., Ganachari-Mallappa N., Villamaria F.C., Goulding N., Tanner B., Stachowiak J., Stibich M. Disinfecting personal protective equipment with pulsed xenon ultraviolet as a risk mitigation strategy for health care workers. Am. J. Infect. Control. 2015;43:412–414. doi: 10.1016/j.ajic.2015.01.013. [DOI] [PubMed] [Google Scholar]
- Kampf G., Todt D., Pfaender S., Steinmann E. Persistence of coronaviruses on inanimate surfaces and its inactivation with biocidal agents. J. Hosp. Infect. 2020 doi: 10.1016/j.jhin.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.A., Grinshpun S.A., Reponen T. Respiratory performance offered by N95 respirators and surgical masks: human subject evaluation with NaCl aerosol representing bacterial and viral particle size range. Ann. Occup. Hyg. 2008;52:177–185. doi: 10.1093/annhyg/men005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore M.B., Heimbuch B.K., Brown T.L., Wander J.D., Hinrichs S.H. Effectiveness of three decontamination treatments against influenza virus applied to filtering facepiece respirators. Ann. Occup. Hyg. 2012;56(1):92–101. doi: 10.1093/annhyg/mer054. [DOI] [PubMed] [Google Scholar]
- Lowe, J.J., Paladino, K.D., Farke, J.D., Boulter, K., Cawcutt, K., Emodi, M., Gibbs, S., Hankins, R., Hinkle, L., Micheels, T., Schwedhelm, S., Vasa, A., Wadman, M., Watson, S., Rupp, M.E., 2020. N95 Filtering Facemask Respirator Ultraviolet Germicidal Irradiation (UVGI) Process for Decontamination and Reuse. Nebrasca Medicine. Available on 8th April 2020 at https://cleanhospital.com/wp-content/uploads/2020/03/N-95-decon-process.pdf.
- MacIntyre C.R., Seale H., Dung T.C., Hien N.T., Nga P.T., Chughtai A.A. A cluster randomised trial of cloth masks compared with medical masks in healthcare workers. BMJ Open. 2015;5(4) doi: 10.1136/bmjopen-2014-006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcapl, 2020. Equipación y vestuario de Seguridad Laboral. Catalogo 2018. Available on 12th April 2020 at https://www.marcapl.com/marca/descargas/catalogos/catalogo_marca_2018_ESP.pdf.
- Ministry of Labor and Social Economy of Spain, 2020. Occupational Risk Prevention vs. COVID-19. Non-exhaustive summary of information sources. Available on 8th April 2020 at https://www.insst.es/documents/94886/693030/Prevenci%C3%B3n+de+riesgos+laborales+vs.+COVID-19+-+Compendio+no+exhaustivo+de+fuentes+de+informaci%C3%B3n/4098124f-5324-43a6-8881-0bbd4e358de7.
- Ministry of Health of Spain, 2020a. Action procedure for occupational risk prevention services against exposure to SARS-COV-2. Available on 9th April 2020 at https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/PrevencionRRLL_COVID-19.pdf.
- Ministry of Health of Spain, 2020b. Recomendaciones sobre el uso de mascarillas en la comunidad en el contexto de COVID-19. Available on 22th of April of 2020 at https://www.mscbs.gob.es/en/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Recomendaciones_uso_mascarillas_ambito_comunitario.pdf.
- Mohapatra S. Sterilization and Disinfection in Essentials of Neuroanesthesia. Academic Press; 2017. pp. 929–944. [Google Scholar]
- N95DECON, 2020a. COVID N95 decon & reuse caution when reusing. Available on 10th April 2020 at https://static1.squarespace.com/static/5e8126f89327941b9453eeef/t/5e8e64c94955f72efb38ba27/1586390217773/200408_N95DECON_cautionsheet_v1.2_final.pdf.
- N95DECON, 2020b. COVID N95 decon & reuse. Hydrogen Peroxide Vapor & Hydrogen Peroxide Gas Plasma. Available on 10th of April of 2020 at https://static1.squarespace.com/static/5e8126f89327941b9453eeef/t/5e86da79e459ab19a0550934/1585896058356/200402_N95DECON_HPV_factsheet_v1.2_final.pdf.
- N95DECON, 2020c. COVID N95 decon & reuse. UV-C. Available on 10th April 2020 at https://static1.squarespace.com/static/5e8126f89327941b9453eeef/t/5e85902c0d94fa0516aafa2a/1585811501243/200401_N95DECON_UV_factsheet_v1.2_final.pdf.
- N95DECON, 2020d. COVID N95 decon & reuse. Heat and humidity. Available on 10th of April of 2020 at https://static1.squarespace.com/static/5e8126f89327941b9453eeef/t/5e86da14569e5d6e88818fd1/1585895957179/200402_N95DECON_Heat_factsheet_v1.2_final.pdf.
- N95DECON, 2020e. Example Processes. Available on 20th April 2020 at https://www.n95decon.org/example-processes.
- NIOSH, 1995. 42 CFR Part 84 Respiratory Protective Devices. Available at: https://www.cdc.gov/niosh/npptl/topics/respirators/pt84abs2.html. [DOI] [PubMed]
- O'Hearn, K., Gertsman, S., Sampson, M., Webster, R., Tsampalieros, A., Ng, R., et al., 2020. Decontaminating N95 masks with Ultraviolet Germicidal Irradiation (UVGI) does not impair mask efficacy and safety: A Systematic Review. [DOI] [PMC free article] [PubMed]
- Radonovich L.J., Simberkoff M.S., Bessesen M.T., Brown A.C., Cummings D.A., Gaydos C.A., Los J.G., Krosche A.E., Gibert C.L., Gorse G.J., Nyquist A., Reich N.G., Rodriguez-Barradas M.C., Price C.S., Perl T.M. N95 respirators vs medical masks for preventing influenza among health care personnel: a randomized clinical trial. JAMA. 2019;322(9):824–833. doi: 10.1001/jama.2019.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengasamy S., Eimer B., Shaffer R.E. Simple Respiratory Protection - Evaluation of the Filtration Performance of Cloth Masks and Common Fabric Materials Against 20–1000 nm Size Particles. Ann. Occup. Hyg. 2010;54:789–798. doi: 10.1093/annhyg/meq044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan N.J., Laffey J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic – Case study from the. Republic of Ireland. Sci. of the Total Environ. 2020;138532 doi: 10.1016/j.scitotenv.2020.138532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A., Stiegel M., Greeson N., Vogel A., Thomann W., Brown M., Sempowski G.D., Alderman T.S., Condreay J.P., Burch J., Wolfe C., Smith B., Lewis S. Decontamination and Reuse of N95 Respirators with Hydrogen Peroxide Vapor to Address Worldwide Personal Protective Equipment Shortages During the SARS-CoV-2 (COVID-19) Pandemic. J. ABSA Int. 2020 doi: 10.1177/1535676020919932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J.D., MacDougall C.C., Johnstone J., Copes R.A., Schwartz B., Garber G.E. Effectiveness of N95 respirators versus surgical masks in protecting health care workers from acute respiratory infection: a systematic review and meta-analysis. CMAJ. 2016;188:567–574. doi: 10.1503/cmaj.150835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanish Society of Preventive Medicine, Public Health and Hygiene (2020). Descontaminación de respiradores de partículas ante desabastecimiento debido a la pandemia COVID-19. Available on 22th April 2020 at https://www.sempsph.com/images/REPROCESADO%20FFPS%201.pdf.
- Song W., Pan B., Kan H. Evaluation of heat inactivation of virus contamination on medical mask. J. Microbes Infect. 2020;15:31–35. [Google Scholar]
- Price, A., Chu, L., 2020. Addressing COVID-19 Face Mask Shortages [v1.3]. COVID-19 Evidence Service. Stanford Medicine. Available on 20th April 2020 at https://elautoclave.files.wordpress.com/2020/03/stanford-2020.pdf.
- The European Parliament and the Council of the European Union, 2020. Regulation (EU) 2016/425 of the European Parliament and of the Council of 9 March 2016 on personal protective equipment and repealing Council Directive 89/686/EEC. Available on 9th April 2020 at http://data.europa.eu/eli/reg/2016/425/oj.
- UNE, 2019. UNE-EN 14683:2019+AC:2019. Medical face masks - Requirements and test methods. UNE Normalización Española. Available on 9th April 2020 at https://www.une.org/encuentra-tu-norma/busca-tu-norma/proyecto?c=P0048663.
- UNE, 2020a. UNE 0064-1:2020. Non-reusable hygienic masks. Materials, design, manufacturing, marking and use requirements. Part 1: For adult use. Spanish Association for Standardisation-UNE. Available on 9th April 2020 at https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0063626.
- UNE, 2020b. UNE 0064-2:2020. Non-reusable hygienic masks. Materials, design, manufacturing, marking and use requirements. Part 2: For children use. Spanish Association for Standardisation-UNE. Available on 9th April 2020 at https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0063627.
- UNE, 2020c. Press release. UNE Specification published to facilitate the manufacture of hygienic masks. Spanish Association for Standardisation-UNE. Available on 9th April 2020 at https://www.une.org/salainformaciondocumentos/NP%20UNE%200064-1%20mascarillas%20higienicas%20abr-20.pdf.
- UNE, 2020d. UNE 0065:2020. Reusable hygienic masks for adults and children. Materials, design, manufacturing, marking and use requirements. Available on 20th of April 2020 at https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma/?c=N0063661.
- UNE, 2020e. Press release. UNE Specification published to facilitate the manufacture of reusable hygienic masks. Available on 9th of April 2020 at https://www.une.org/la-asociacion/sala-de-informacion-une/noticias/especificacion-une-mascarillas.
- Viscusi D.J., King W.P., Shaffer R.E. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J. Int. Soc. Resp. Prot. 2007;24(3/4):93. [Google Scholar]
- Viscusi D.J., Bergman M.S., Eimer B.C., Shaffer R.E. Evaluation of five decontamination methods for filtering facepiece respirators. Ann. Occup. Hyg. 2009;53(8):815–827. doi: 10.1093/annhyg/mep070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viscusi D.J., Bergman M.S., Novak D.A., Faulkner K.A., Palmiero A., Powell J., Shaffer R.E. Impact of three biological decontamination methods on filtering facepiece respirator fit, odor, comfort, and donning ease. J. Occup. Environ. Hyg. 2011;8(7):426–436. doi: 10.1080/15459624.2011.585927. [DOI] [PubMed] [Google Scholar]
- WHO, 2020a. Advice on the use of masks in the community, during home care and in health care settings in the context of the novel coronavirus (2019-nCoV) outbreak. World Health Organization. Interim guidance 29 January 2020. Available on 9th April 2020 at https://apps.who.int/iris/bitstream/handle/10665/330987/WHO-nCov-IPC_Masks-2020.1-eng.pdf?sequence=1&isAllowed=y.
- WHO, 2020b. Coronavirus disease (COVID-19) advice for the public: When and how to use masks. World Health Organization. Available on 9th of April 2020 at https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public/when-and-how-to-use-masks.
- WHO, 2020c. Advice on the use of masks in the context of COVID-19. Interim guidance 6 April 2020. Available on 9th April 2020 at https://www.who.int/publications-detail/advice-on-the-use-of-masks-in-the-community-during-home-care-and-in-healthcare-settings-in-the-context-of-the-novel-coronavirus-(2019-ncov)-outbreak.
- WHO, 2020d. Shortage of personal protective equipment endangering health workers worldwide. Available on 3th of April 2020 at https://www.who.int/news-room/detail/03-03-2020-shortage-of-personal-protective-equipment-endangering-health-workers-worldwide.
- World Economic Forum, 2020. Should you wear a face mask? WHO officials weigh in at today's COVID-19 briefing. Available on 13th April 2020 at https://www.weforum.org/agenda/2020/03/who-should-wear-a-face-mask-30-march-who-briefing/.
- Zhang, Jia-min, Zheng Chong-yi, Xiao Geng-fu, Zhou Yuan-quan, Gao Rong, 2004. Examination of the Efficacy of Ozone Solution Disinfectant in Inactivating SARS Virus. Chinese J. Disinfection 2004-01.