Summary
Macrophages are tissue‐resident myeloid cells with essential roles in host defense, tissue repair, and organ homeostasis. The lung harbors a large number of macrophages that reside in alveoli. As a result of their strategic location, alveolar macrophages are critical sentinels of healthy lung function and barrier immunity. They phagocytose inhaled material and initiate protective immune responses to pathogens, while preventing excessive inflammatory responses and tissue damage. Apart from alveolar macrophages, other macrophage populations are found in the lung and recent single‐cell RNA‐sequencing studies indicate that lung macrophage heterogeneity is greater than previously appreciated. The cellular origin and development of mouse lung macrophages has been extensively studied, but little is known about the ontogeny of their human counterparts, despite the importance of macrophages for lung health. In this context, humanized mice (mice with a human immune system) can give new insights into the biology of human lung macrophages by allowing in vivo studies that are not possible in humans. In particular, we have created humanized mouse models that support the development of human lung macrophages in vivo. In this review, we will discuss the heterogeneity, development, and homeostasis of lung macrophages. Moreover, we will highlight the impact of age, the microbiota, and pathogen exposure on lung macrophage function. Altered macrophage function has been implicated in respiratory infections as well as in common allergic and inflammatory lung diseases. Therefore, understanding the functional heterogeneity and ontogeny of lung macrophages should help to develop future macrophage‐based therapies for important lung diseases in humans.
Keywords: humanized mice, lung macrophages, ontogeny, origin, single‐cell RNA‐sequencing
The lung harbors several types of macrophages that are critical for barrier immunity to airborne challenges and lung homeostasis. In this review, we discuss topical issues and concepts related to lung macrophages, covering both mouse and human studies.

Abbreviations
- BAL
bronchoalveolar lavage
- BPD
bronchopulmonary dysplasia
- GM‐CSF
granulocyte–macrophage colony‐stimulating factor
- HSPCs
hematopoietic stem and progenitor cells
- IL
interleukin
- LPS
polysaccharide
- M‐CSF
macrophage colony‐stimulating factor
- MAF
musculoaponeurotic fibrosarcoma oncogene homolog
- PAP
pulmonary alveolar proteinosis
- PPARγ
peroxisome proliferator‐activated receptor γ
- TGF‐β
transforming growth factor β
Alveolar macrophages
The lung has two main types of macrophages that reside in different anatomical compartments, namely interstitial and alveolar macrophages.1, 2, 3, 4, 5, 6 In addition, intravascular macrophages have been described in humans and other species, but they remain poorly characterized.7 Macrophage localization is related to specific tasks, thereby achieving a division of labor to best serve parenchymal organ function (Fig. 1). The tissue specialization of macrophages is shaped by instructive signals from the local environment (such as cytokines and metabolites), which induce the expression of distinct transcription factors imprinting tissue‐specific macrophage function.8, 9, 10, 11
Figure 1.

Heterogeneity of lung macrophages. Macrophages occupy distinct locations in the lung, which corresponds to a division of labor. Alveolar macrophages reside in the airways, where they promote barrier immunity and surfactant clearance. Alveolar macrophages depend on the cytokines granulocyte–macrophage colony‐stimulating factor (GM‐CSF) and transforming growth factor β (TGF‐β). Interstitial macrophages are located within the lung tissue. Studies in mice have revealed two different populations of interstitial macrophages: (i) LYVE‐1high MHC Class IIlow interstitial macrophages adjacent to blood vessels that are involved in wound healing and tissue repair. (ii) LYVE‐1low MHC Class IIhigh interstitial macrophages found near neurons that are specialized in antigen presentation. Lung macrophages can derive either from embryonic precursors or from blood monocytes. Parts of the figure have been adapted from Servier Medical Art.
Alveolar macrophages express the master transcription factor peroxisome proliferator‐activated receptor γ (PPARγ), a key regulator of lipid metabolism, which is induced by the cytokine granulocyte–macrophage colony‐stimulating factor (GM‐CSF).12 Mouse studies have shown that transforming growth factor β (TGF‐β) is another cytokine required for alveolar macrophage development and homeostasis.13 In contrast to GM‐CSF, which is secreted by alveolar type II cells, TGF‐β produced by alveolar macrophages themselves supports their homeostasis.13 As their name suggests, alveolar macrophages are located in the lumen of alveoli, where the gas exchange takes place at the alveolar–capillary membrane. Their strategic location within the distal lung allows alveolar macrophages to clear the airways of microbes, dead cells, and other airborne particles through phagocytosis, which is essential to maintain the vital oxygen uptake, e.g. during respiratory infection in mice.14, 15 The lung environment confers an anti‐inflammatory phenotype on alveolar macrophages (see review by Hussell and Bell2). Alveolar macrophages express several factors that promote immune tolerance, such as TGF‐β, as well as inhibitory receptors, restraining their pro‐inflammatory activity under steady‐state conditions.2 An important tissue‐specific function of alveolar macrophages is their ability to catabolize lung surfactant. Lack of GM‐CSF or PPARγ, and hence the lack of alveolar macrophages, results in pulmonary alveolar proteinosis (PAP),16 an inflammatory lung syndrome caused by the defective clearance of surfactant. In addition, alveolar macrophages promote regeneration of the injured mouse lung through the production of repair factors like amphiregulin.17
Alveolar macrophages are the most‐studied macrophage population in the lung because they can be relatively easily obtained by bronchoalveolar lavage (BAL). However, it is noteworthy that this technique likely samples mainly ‘motile’ alveolar macrophages, whereas their ‘sessile’ counterparts (attached to the alveolar epithelium) may be under‐represented. Interestingly, mouse studies have shown that sessile macrophages within different alveoli communicate with each other and the alveolar epithelium through connexin 43 channels.18 Another unexplored possibility is that mouse alveolar macrophages may move between adjacent alveoli through pores of Kohn,19 as not all alveoli contain macrophages at a given time. Clearly, the potential link between alveolar macrophage motility and function deserves further study. Also, there are currently no surface markers to distinguish sessile from motile alveolar macrophages. Finally, BAL macrophages may comprise airway macrophages that not only reside in alveoli, but also in larger airways, such as bronchi.
Interstitial macrophages
Interstitial lung macrophages are less studied because their isolation requires digestion of lung tissue, which is less accessible in humans than BAL fluid. They are located in the space between the lung epithelium and capillaries, where they are in contact with other immune cells, such as lymphocytes and dendritic cells. However, in most studies their exact localization is unclear, which may be in the alveolar interstitium, the submucosa, or the perivascular adventitia.4 Interstitial macrophages likely contribute to barrier immunity in the lung together with alveolar macrophages. Furthermore, they perform antigen presentation and tissue remodeling in the lung.20 A characteristic feature of both mouse and human interstitial lung macrophages seems to be their ability to produce the immunosuppressive cytokine interleukin‐10 (IL‐10).5 Interstitial lung macrophages are heterogeneous and recently two main distinct lineages have been identified in mice by the groups of Ginhoux and Jakubzick (Fig. 1).20, 21 These are LYVE‐1low MHC Class IIhigh interstitial macrophages, found adjacent to neurons, that specialize in antigen presentation; and LYVE‐1high MHC Class IIlow perivascular macrophages performing wound healing and tissue repair.20 Interestingly, the latter population regulates the permeability of blood vessels and thereby the influx of inflammatory cells in the lung, e.g. during fibrosis.20 These two interstitial macrophage subsets seem to be conserved between mice and humans, although the human LYVE‐1high MHC Class IIlow subset shares a CD206+ CD169+ MARCO+ phenotype with human alveolar macrophages (see below).20
Heterogeneity of human lung macrophages
Different monocyte–macrophage populations in the human lung have only recently been rigorously defined according to characteristic cell surface phenotypes.22, 23, 24, 25, 26, 27 Common surface markers for human lung macrophages (and monocytes) are HLA‐DR, CD11b, CD11c, and CD64. Human alveolar macrophages are large and highly autofluorescent CD14low CD16+ cells defined by the expression of the mannose receptor CD206 and the sialoadhesin CD169, as well as the scavenger receptor MARCO. Interestingly, one study suggested that two populations of CD206high CD169+ macrophages may be distinguished in lung tissue by intermediate versus high expression of CD163, the scavenger receptor for hemoglobin–haptoglobin.22 In contrast, most macrophages in BAL were CD163high.22 It remains to be explored whether CD163 expression relates to macrophage origin, localization, activation state, or motility. Human interstitial macrophages are smaller than alveolar macrophages and have the surface phenotype CD206+ CD169low CD14+ CD16+, whereas lung monocytes are CD206− CD169−. Differences between human interstitial and alveolar macrophages in terms of phagocytosis and cytokine production in response to lipopolysaccharide (LPS) have been reported.28, 29
The advent of single‐cell RNA‐sequencing has led to deeper insights into the heterogeneity of human lung macrophages. Recent studies have revealed great macrophage diversity in healthy, malignant, and fibrotic lung tissue.30, 31, 32, 33 In particular, chemokines distinguished different clusters of human lung macrophages.34 This raises the idea that different types of macrophages may act as gate keepers of immune cell recruitment to the lung (e.g. neutrophils versus T lymphocytes), thereby ensuring an optimal response to environmental challenges. Furthermore, inter‐individual variation in the relative abundance of lung macrophage clusters was observed.31 Finally, unique macrophage clusters were found to be present in human lung disease and associated with poor survival, e.g. in lung cancer.30, 31, 34 Interestingly, although the main monocyte subsets are well‐conserved between humans and mice,34, 35 the lung macrophage compartment seems to be more diverse in humans.34 Further studies are needed to clarify whether the observed macrophage clusters represent different activation states or truly independent lineages. The former possibility may be more likely because macrophage clusters were rather poorly separated. It will also be interesting to investigate whether the different populations of human macrophages correspond to macrophages occupying distinct niches in the lung.
Macrophage origin and development in the steady‐state lung
In mice, seeding of tissues with macrophage progenitors occurs in three developmental waves from yolk sac, fetal liver, and adult bone marrow.11, 36, 37, 38, 39 This also seems to apply to the mouse lung.40 Therefore, macrophages are classified according to their origin as either derived from embryonic/fetal precursors that largely self‐renew locally or from adult blood monocytes that develop from hematopoietic stem and progenitor cells (HSPCs) in the bone marrow (Fig. 1). Mouse studies have demonstrated that alveolar macrophages are predominantly of embryonic origin in steady state, as they are maintained independently of circulating monocytes.41, 42, 43, 44 Specifically, mouse alveolar macrophages originate from fetal monocytes that seed the lung and differentiate after birth into mature alveolar macrophages under the influence of GM‐CSF, TGF‐β, and PPARγ.12, 13, 43 The critical role of GM‐CSF and PPARγ in alveolar macrophage development and homeostasis is conserved between mice12, 43, 45, 46, 47, 48, 49 and humans.50, 51, 52, 53, 54, 55 Moreover, l‐plastin, a protein regulating the actin cytoskeleton, is required for the migration of mouse macrophage precursors into the alveolar space.56 It has been suggested that continued lung growth after birth might require a further contribution of bone marrow‐derived macrophages to the alveolar macrophage pool.3 This idea is supported by the finding that the contribution of HSPC‐derived monocytes to the alveolar macrophage compartment in mice steadily increases with age.57, 58 Furthermore, alveolar macrophages may be depleted with ageing, leading to a decrease in their number in old mice.59 In contrast, interstitial macrophages have a mixed origin,40 originating from blood and lung monocytes in mice60 with a minor early contribution from yolk sac macrophages. The mixed origin may relate to distinct populations of interstitial macrophages that occupy specific niches in the lung.40 In mice, both the LYVE‐1low MHC Class IIhigh and the LYVE‐1high MHC Class IIlow subset of interstitial lung macrophages are slowly replaced by circulating monocytes.20, 21 Finally, the monocytic origin of interstitial lung macrophages may explain why their energy metabolism relies on glycolysis, whereas alveolar macrophages mainly use fatty acid oxidation.12, 61
Homeostasis of lung macrophages
Macrophage development and proliferation depends on colony‐stimulating factor receptor 1 (CSFR1; CD115), the receptor for macrophage colony‐stimulating factor (M‐CSF) and IL‐34.8, 36, 62 Pioneering work by Sieweke and colleagues revealed that M‐CSF promotes the self‐renewal of mouse macrophages through a set of transcription factors (c‐MYC, KLF2, KLF4) that are shared with pluripotent stem cells.63, 64, 65 Musculoaponeurotic fibrosarcoma oncogene homolog (MAF) transcription factors inhibit M‐CSF‐induced macrophage proliferation through the suppression of c‐MYC and other self‐renewal genes.64, 65 Mouse studies demonstrated that alveolar macrophages are maintained in steady state through their stem cell‐like capacity for self‐renewal.41, 43, 44, 63, 66 Furthermore, it has been shown that human alveolar macrophages have the ability to proliferate in vitro 65, 67 and in vivo.68 Consistent with their self‐renewal ability, mouse alveolar macrophages have low expression of c‐MAF and MAFB.65 The ageing‐related reduction of alveolar macrophages in mice may be due to higher c‐MAF and lower c‐MYC expression.59 Based on mouse studies, GM‐CSF and TGF‐β are not only required for the development of alveolar macrophages, but also for their steady‐state homeostasis,13, 43 whereas M‐CSF is largely dispensable.69 Accordingly, mouse alveolar macrophages express GM‐CSF receptors (Csfr2a, Csfr2b), but little Csfr1.21 Moreover, alveolar macrophage recovery after genotoxic stress in mice is mostly dependent on GM‐CSF, whereas other tissue macrophages rely more on M‐CSF.41 Interleukin‐36γ is another cytokine that has been implicated in promoting the survival of mouse alveolar macrophages after influenza infection.70 The molecular mediators of alveolar macrophage self‐renewal are being identified in mice, such as sirtuin protein SIRT171 and BHLHE40/BHLHE41 transcription factors.72
Interstitial lung macrophages have a shorter lifespan than alveolar macrophages in rhesus macaques.73 Furthermore, mouse interstitial lung macrophages express more Csfr1, but less Csfr2a and Csfr2b, than alveolar macrophages.21 Despite their responsiveness to M‐CSF, the impaired self‐renewal capacity of interstitial lung macrophages may be explained by the observation that, in contrast to their alveolar counterparts, mouse interstitial lung macrophages express MAF transcription factors,21 likely blocking proliferation. The differential dependence on M‐CSF and GM‐CSF could allow separate regulation of the interstitial and alveolar macrophage compartments in the lung. After its depletion, the alveolar macrophage pool in mice recovers more slowly than the pool of interstitial macrophages.20 This might be due to the rapid recruitment of circulating monocytes that preferentially differentiate into interstitial lung macrophages in response to M‐CSF.
Macrophage origin in lung injury and inflammation
Mouse studies have shown that resident alveolar macrophages are depleted during severe tissue injury, e.g. after ionizing radiation, viral infection, and LPS‐induced lung injury.41, 74, 75, 76 It has been proposed that damage to the lung epithelium may lead to the loss of integrin‐dependent TGF‐β activation, thereby causing a reduction in alveolar macrophages.13, 77 Depending on the degree of injury, the alveolar macrophage pool in mice may be restored either by local proliferation of remaining resident macrophages41 or, if the injury is more severe, by the recruitment of monocytes into the alveolar niche that then differentiate into monocyte‐derived macrophages.75, 76, 78 The latter leads to a situation where alveolar macrophages of different origin (embryonic and monocytic) co‐exist in the lung. According to the ‘niche competition model’, the degree of monocytic macrophage origin is determined by accessibility of the niche and whether it is occupied.79 Furthermore, the type and timing of injury likely determine whether monocyte‐derived alveolar macrophages persist or not. Consistent with this idea, it has been reported that, in the acute LPS lung injury model, monocyte‐derived lung macrophages die after resolution of tissue damage,75 whereas they persist long‐term in mouse models of lung fibrosis or herpesvirus infection.76, 80
Resident lung macrophages can also expand after tissue damage, e.g. interstitial macrophages in the mouse lung after administration of bacterial products, such as LPS and CpG.60 This expansion was mediated by monocytes differentiating into interstitial lung macrophages, either through the CCR2‐dependent recruitment of blood monocytes (in response to LPS) or in a CCR2‐independent manner through local lung monocytes and splenic monocyte reservoirs (in response to CpG).60
The molecular pathways regulating the differentiation of monocytes into lung macrophages remain poorly defined, with the β‐catenin pathway potentially being involved, at least in mice.81 Furthermore, MAF transcription factors, highly expressed in monocytes, are progressively down‐regulated upon the differentiation of mouse monocytes into alveolar macrophages.80, 82
Impact of origin on lung macrophage function: nature versus nurture
The relative importance of cellular origin versus tissue environment9 in determining macrophage function is a topical question in the field. Mouse studies support the notion that the transcriptional identity of alveolar macrophages in steady state is largely enforced by the lung microenvironment.83, 84, 85, 86 Moreover, yolk sac macrophages, fetal monocytes, and bone marrow monocytes are all capable of reconstituting an empty alveolar niche and preventing PAP in mice.86 However, fetal monocytes repopulated the empty alveolar niche faster because of their greater proliferative ability.86 Furthermore, one mouse study found that the scavenger receptor MARCO is more highly expressed in resident embryonic‐derived than in monocyte‐derived alveolar macrophages,85 which may affect their clearance function. Therefore, it is worth testing whether fetally derived lung macrophages have greater tissue‐repair function than adult monocyte‐derived macrophages that may be more prone to support pro‐inflammatory responses in the injured lung.
The monocytic origin of lung macrophages likely matters more in the context of lung injury and inflammation than in steady‐state conditions. Specifically, epigenetic changes may result in either enhanced or reduced pro‐inflammatory activity.87 The former is called trained immunity, a form of innate memory that ensures enhanced protection against, for example, bacterial infection.87 However, it could also predispose to chronic inflammation after subsequent, repeated lung insults. This idea is supported by mouse studies of lung fibrosis. In the bleomycin‐induced lung fibrosis model, monocyte‐derived alveolar macrophages persist after the resolution of fibrosis and over time become transcriptionally and phenotypically similar to resident alveolar macrophages.80 However, during the acute phase of lung injury, monocyte‐derived alveolar macrophages have a distinct gene expression profile and they drive lung fibrosis in mice.80, 82 Similarly, single‐cell RNA‐sequencing has revealed a transitional pro‐fibrotic macrophage population in the same mouse model of lung fibrosis.88 Furthermore, a specific population of pro‐fibrotic alveolar macrophages, presumably of blood monocyte origin, is present in humans with lung fibrosis.32, 80 Finally, the gene expression profile of monocyte‐derived mouse alveolar macrophages differs from their resident counterparts during the acute inflammatory phase in the LPS‐induced lung injury model.89 On the other hand, monocyte‐derived alveolar macrophages can also acquire tolerogenic/regulatory properties that are beneficial for the host. For example, herpesvirus infection in mice causes the replacement of resident alveolar macrophages by monocyte‐derived macrophages, which protects against allergic airway inflammation.76
Ontogeny and homeostasis of human lung macrophages
In contrast, the origin and ontogeny of human lung macrophages, especially in lung diseases, is poorly understood because invasive in vivo experiments are impossible. However, useful information has been obtained from bone marrow and lung transplantation studies. In the context of allogeneic bone marrow transplantation, profound depletion of host alveolar macrophages occurs as a result of the toxic myelo‐ablative conditioning before transplantation. This causes rapid alveolar macrophage turnover, resulting in mixed host–donor chimerism, where alveolar macrophages mostly originate from donor hematopoiesis.90, 91, 92 This is consistent with the notion that blood monocytes derived from donor HSPCs replace resident alveolar macrophages. In contrast, lung transplantation is not associated with depletion of lung macrophages and therefore alveolar macrophages in the donor lung persist, although the extent of donor alveolar macrophage persistence differed markedly between studies.68, 93, 94, 95 These observations support the concept that, in the steady‐state lung, human alveolar macrophages are largely maintained by local self‐renewal, whereas during lung injury associated with macrophage depletion, alveolar macrophages are replaced by monocytes. The notion that human alveolar macrophages may be maintained independently of circulating monocytes during steady state is further supported by the finding that alveolar macrophages are present in certain leukemias, where blood monocytes are severely depleted.96 However, these studies do not determine conclusively whether human alveolar macrophages in the developing lung are of embryonic origin. Furthermore, the specific embryonic and adult progenitor of human alveolar macrophages is unknown.
Stillborn infants lack CD169+ alveolar macrophages,22 suggesting that alveolar macrophages develop postnatally in humans, which is similar to findings in mice.43 This is likely because, while GM‐CSF is already produced from gestational week ≤16, the alveolar niche is only established at birth, when the lungs are inflated, with subsequent continued maturation of the alveolar tree well into childhood. Furthermore, alveolar macrophages are present in neonates ≥48 hr after birth,97 suggesting that oxygen intake may drive their maturation.
Lung transplantation studies indicate that human interstitial macrophages (and/or lung monocytes) have a more rapid turnover than alveolar macrophages.94 Again, this does not conclusively establish the origin of human interstitial macrophages, but their origin is likely monocytic. Moreover, CD206+ CD169− macrophages are present in the lung interstitium of stillborn infants22 and therefore develop earlier than alveolar macrophages, likely because the alveolar niche is not fully established at birth. However, it is unclear whether these interstitial macrophages give rise to CD169+ alveolar macrophages or whether alveolar macrophages are derived from a distinct precursor, independently of interstitial macrophages.
A model to study human lung macrophages in vivo
The biology of lung macrophages has been difficult to investigate in humans because of the lack of in vivo experimental systems. Humanized mouse models that support the development of human lung macrophages98 offer a solution to this limitation (Fig. 2). Human immune system mice are generated by the transplantation of immunodeficient mice with human CD34+ HSPCs.99, 100 We have improved human hematopoiesis in the mouse host by providing critical human factors, such as cytokines, through gene knock‐in.101 To create an in vivo model for human lung macrophages, we engineered human gene knock‐in mice expressing GM‐CSF, which allows the development of human alveolar macrophages.102 Subsequently, by providing multiple human cytokines, we created a humanized mouse strain named, ‘MISTRG’ that expresses the human proteins M‐CSF, IL‐3/GM‐CSF, signal‐regulatory protein α, and thrombopoietin in the Rag2 −/− Il2rg −/− background.103 Human lung macrophages develop in MISTRG mice after transplantation with fetal, neonatal, and adult CD34+ cells,102, 103, 104, 105 reinforcing the concept that human GM‐CSF supports human macrophage reconstitution of an empty alveolar niche. These results show that human alveolar macrophages can derive from HSPCs of different developmental age and can therefore have a monocytic origin. Importantly, human alveolar macrophages of monocytic origin are functional because they are able to prevent PAP.102
Figure 2.

A model to study human lung macrophages in vivo. Humanized ‘MISTRG’ mice express the human proteins macrophage colony‐stimulating factor (M‐CSF), interleukin‐3 (IL‐3)/granulocyte–macrophage colony‐stimulating factor (GM‐CSF), signal‐regulatory protein α(SIRPα), and thrombopoietin (TPO) in the Rag2 −/− Il2rg −/− background. MISTRG mice support the development of human blood monocytes as well as human interstitial and alveolar macrophages after transplantation with CD34+ hematopoietic stem and progenitor cells. Parts of figure have been adapted from Servier Medical Art.
Another advantage of this model is that human lung macrophages (both interstitial and alveolar) develop in the absence of pre‐conditioning (own observation and ref. 105), which allows the study of human lung macrophage development without irradiation‐induced tissue injury and inflammation. Furthermore, all types of blood monocytes that have been described in humans106 are found in MISTRG mice after transplantation with human CD34+ cells.103 Interestingly, in the human lung CD14+ CD16+ ‘intermediate’ monocytes are the predominant subset in the airways, whereas the CD14+ CD16− ‘classical’ subset is more abundant in lung tissue.23, 25 The latter are also found in the lungs of MISTRG mice and may represent an extravascular reservoir of tissue monocytes in the lung (own observation and ref. 24), similar to what has been reported in mouse studies.60, 107 Limitations of humanized mouse models for studying human lung macrophages are the absence of human cells at birth, the interaction of human macrophages with mouse lung epithelium, and the limited lifespan of MISTRG mice after transplantation with human cells. Despite these limitations, the MISTRG model is an excellent tool to dissect the origin, development, and function of human lung macrophages in vivo.
Lung macrophage function: from cradle to grave
Macrophage function is impacted by the age of the host (Fig. 3). Alveolar macrophage adaptation to increased oxygen concentration at birth influences their maturation and function. In fact, mice lacking the oxygen‐sensing Von Hippel–Lindau protein have alveolar macrophages with an immature phenotype and impaired self‐renewal capacity.108 Furthermore, these immature alveolar macrophages are not able to prevent PAP, which is consistent with defective surfactant clearance.108 Alveolar macrophages in the newborn may also be hypo‐responsive to certain respiratory pathogens, e.g. to Pneumocystis, as has been shown in mice.109 Another study showed that exposure of neonatal mice to microbial extracts stimulates the phagocytic function of alveolar macrophages, which promotes resistance to respiratory pathogens, such as Streptococcus pneumoniae.110 Therefore, exposure to the external environment after birth likely promotes the maturation of alveolar macrophages.
Figure 3.
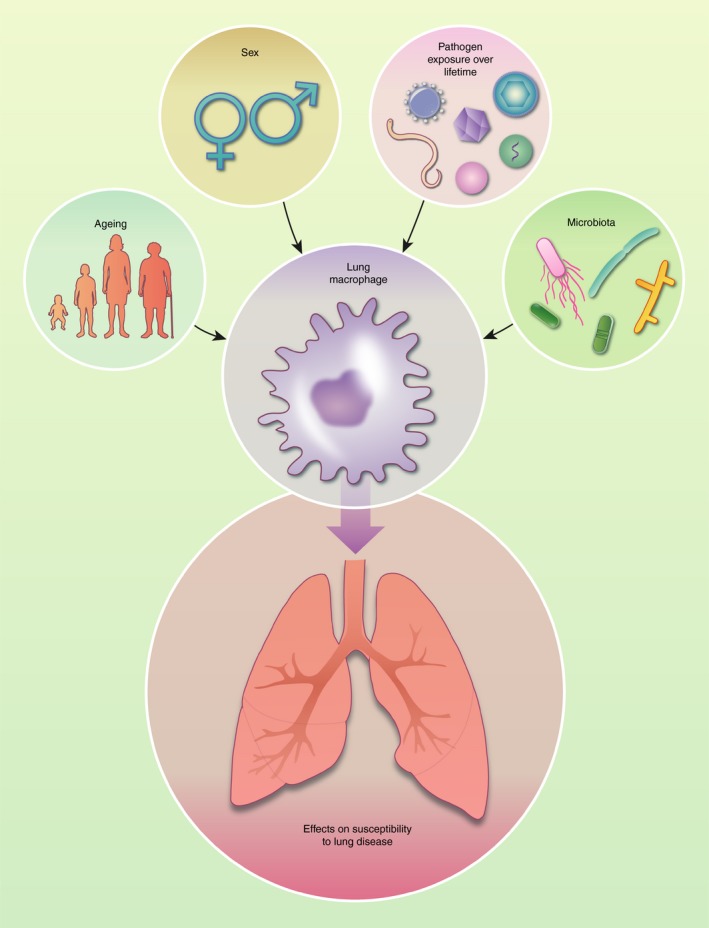
Factors regulating lung macrophage function. Environmental factors such as the gut and local lung microbiota as well as pathogen exposure over the lifetime of the host affect lung macrophage function. In addition, differences have been observed in macrophage function depending on the age and sex of the individual. By regulating the function of lung macrophages, these factors likely influence the susceptibility to lung diseases. Parts of figure have been adapted from Servier Medical Art.
On the other hand, neonatal lung inflammation, surfactant dysregulation, and altered alveolar macrophage maturity are factors involved in respiratory distress syndrome.111 Respiratory distress syndrome can develop into bronchopulmonary dysplasia (BPD), a common pulmonary complication that comes with extreme prematurity and prolonged mechanical ventilation.112 BPD is characterized by altered lung morphogenesis and increased inflammation, where the arrested development results in a reduced capacity for gas exchange that is due to a decrease in the number of alveoli and capillary formation.113 The very premature birth and the level of BPD severity in early age will have a knock‐on effect on lung function throughout adolescent life.114 Genetic impairment of alveolarization in mice rapidly increases macrophages in the neonatal lung, causing altered lung morphogenesis. Macrophage depletion rescued arrested lung development, suggesting that macrophages drive BPD pathology.115 Furthermore, macrophages in the fetal mouse lung mediate the inflammatory response to LPS by activation of the nuclear factor‐κB pathway that in turn disrupts airway morphogenesis.116 Accordingly, immature macrophage phenotype has been linked to disease severity in preterm infants with chronic lung disease/BPD in humans.117
Similarly, resident lung macrophages from old mice are phenotypically, transcriptionally, and functionally distinct from their younger counterparts with increased basal activation,118 but impaired cytokine production in response to infection.119, 120 The proliferative capacity of alveolar macrophages in old mice is also reduced as revealed in a recent single‐cell RNA‐seqencing study.121 Furthermore, the clearance of apoptotic neutrophils by mouse lung macrophages becomes impaired with age.59 Finally, alveolar macrophages from old mice are refractory to interferon‐γ, resulting in impaired killing of phagocytosed bacteria.118 Therefore, age‐related changes in lung macrophages likely favor a state of impaired host defense coupled with neutrophilic inflammation, causing excessive tissue damage in the lung. This may contribute to increased mortality after infection with important respiratory pathogens, such as S. pneumoniae 122 and influenza virus,59 as shown in mouse studies.
Environmental factors regulating lung macrophage function: the microbiota and pathogen exposure
Among environmental factors, the microbiota plays a dominant role in shaping immune activity (Fig. 3). The normal gut microbiota promotes host defense in the lung against viruses and bacteria. For example, butyrate produced by the gut microbiota enhances protection against influenza infection in mice, partly through stimulating the production of Ly6Clo monocytes that differentiate into anti‐inflammatory lung macrophages.123 Furthermore, depletion of the gut microbiota by antibiotics causes impaired resistance to respiratory infection with S. pneumoniae in mice.124 The microbiota promotes resistance against respiratory bacterial infection through enhanced production of GM‐CSF, which stimulates bacterial killing by mouse alveolar macrophages.125 These mouse studies suggest that the ‘healthy’ microbiota stimulates the phagocytic function of alveolar macrophages and their responsiveness to bacterial products, such as LPS. On the other hand, systemic dysbiosis (reduced diversity of the gut microbiota), as it occurs in human immunodeficiency virus‐infected humans, triggers lung macrophage dysfunction.126
The local microbiota also has beneficial effects on lung immunity.127, 128 The microbiota in the lower airways in humans is established within the first 2 months after birth.129 Several studies support the notion that a ‘balanced’ lung microbiota supports the anti‐inflammatory function of lung macrophages and tolerance to airborne material, which has implications for lung‐tissue remodeling130, 131 and allergic responses132 in humans, as well as for immunosurveillance of cancer in mice.133 Furthermore, bacterial components stimulate IL‐10 production by interstitial lung macrophages, which protects against allergic airway inflammation in mice.60 This finding supports the concept that the local lung microbiota contributes to the hygiene hypothesis through effects on lung macrophages.5 Conversely, lung disease states are often associated with local dysbiosis that is due to abnormal airway structure, altered mucus production, and change in pH.127, 128 This local dysbiosis could trigger a dysregulated inflammatory response, involving lung macrophages, thereby predisposing to disease exacerbations.
Studies in mice housed in specific‐pathogen‐free conditions do not reflect the situation in humans who are exposed to a multitude of airborne microbes during their lifetime. This raises the idea that continued microbial exposure in humans may lead to a lung macrophage pool that is increasingly blood‐monocyte‐derived. Consistent with this possibility, it has been shown in mice that herpesviruses, which are very common in humans, promote an alveolar macrophage compartment mainly consisting of blood monocyte‐derived macrophages.76 Accordingly, herpesvirus infection of mice can have beneficial (protection from allergic airway inflammation)76 as well as deleterious (pro‐fibrotic responses) effects134 through modulating lung macrophages. Previous pathogen exposure may also result in trained immunity to respiratory infection with viruses and parasites through effects on alveolar macrophages, at least in mice.135, 136 Conversely, lack of mouse alveolar macrophages causes increased susceptibility to secondary bacterial infection.74 Moreover, it was shown that T‐cell‐produced interferon‐γ inhibits the phagocytic function of alveolar macrophages after influenza virus infection, which impairs the resistance against secondary bacterial infection in mice.137
Concluding remarks
Macrophages are currently one of the most topical cell types in immunology and great progress has been made recently in understanding their biology in mice. As far as human lung macrophages are concerned, several interesting open questions remain that merit further investigation (Table 1). With the emergence of powerful novel technologies, such as single‐cell RNA‐sequencing, CRISPR/Cas9 genome engineering, and advanced in vivo models, we expect rapid progress in the field of lung macrophages.
Table 1.
Open questions related to human lung macrophages
|
What is the embryonic and adult progenitor of human lung macrophages? Do lung macrophages of embryonic origin have greater tissue‐repair function? |
|
What is the origin of lung macrophages in human lung disease? Are there surface markers that distinguish embryo‐derived from adult‐derived lung macrophages to track macrophage origin in human lung disease? |
|
Does continued pathogen exposure over the lifetime lead to a human lung macrophage compartment that is mainly of monocytic origin? Which molecular pathways drive the differentiation of monocytes into human lung macrophages? |
|
How can we distinguish sessile from motile human alveolar macrophages by flow cytometry? Do human macrophage clusters described in single‐cell RNA‐sequencing studies represent distinct lineages and/or occupy specific niches in the lung? |
| Do sex‐specific differences in lung macrophage function underlie gender bias in lung disease? |
Disclosures
The authors have no competing interests to declare.
Acknowledgements
Research in the Willinger laboratory is supported by a faculty‐funded career position at Karolinska Institutet, as well as by grants from the Swedish Research Council and the Center for Innovative Medicine (CIMED) financed by Region Stockholm.
References
- 1. Kopf M, Schneider C, Nobs SP. The development and function of lung‐resident macrophages and dendritic cells. Nat Immunol 2015; 16:36–44. [DOI] [PubMed] [Google Scholar]
- 2. Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue‐specific context. Nat Rev Immunol 2014; 14:81–93. [DOI] [PubMed] [Google Scholar]
- 3. Joshi N, Walter JM, Misharin AV. Alveolar macrophages. Cell Immunol 2018; 330:86–90. [DOI] [PubMed] [Google Scholar]
- 4. Garbi N, Lambrecht BN. Location, function, and ontogeny of pulmonary macrophages during the steady state. Pflugers Arch 2017; 469:561–72. [DOI] [PubMed] [Google Scholar]
- 5. Liegeois M, Legrand C, Desmet CJ, Marichal T, Bureau F. The interstitial macrophage: a long‐neglected piece in the puzzle of lung immunity. Cell Immunol 2018; 330:91–6. [DOI] [PubMed] [Google Scholar]
- 6. Schyns J, Bureau F, Marichal T. Lung interstitial macrophages: past, present, and future. J Immunol Res 2018; 2018:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dehring DJ, Wismar BL. Intravascular macrophages in pulmonary capillaries of humans. Am Rev Respir Dis 1989; 139:1027–9. [DOI] [PubMed] [Google Scholar]
- 8. Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol 2015; 15:731–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol 2016; 17:18–25. [DOI] [PubMed] [Google Scholar]
- 10. T'Jonck W, Guilliams M, Bonnardel J. Niche signals and transcription factors involved in tissue‐resident macrophage development. Cell Immunol 2018; 330:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol 2015; 33:643–75. [DOI] [PubMed] [Google Scholar]
- 12. Schneider C, Nobs SP, Kurrer M, Rehrauer H, Thiele C, Kopf M. Induction of the nuclear receptor PPAR‐γ by the cytokine GM‐CSF is critical for the differentiation of fetal monocytes into alveolar macrophages. Nat Immunol 2014; 15:1026–37. [DOI] [PubMed] [Google Scholar]
- 13. Yu X, Buttgereit A, Lelios I, Utz SG, Cansever D, Becher B et al The cytokine TGF‐β promotes the development and homeostasis of alveolar macrophages. Immunity 2017; 47:903–12 e4. [DOI] [PubMed] [Google Scholar]
- 14. Purnama C, Ng SL, Tetlak P, Setiagani YA, Kandasamy M, Baalasubramanian S et al Transient ablation of alveolar macrophages leads to massive pathology of influenza infection without affecting cellular adaptive immunity. Eur J Immunol 2014; 44:2003–12. [DOI] [PubMed] [Google Scholar]
- 15. Schneider C, Nobs SP, Heer AK, Kurrer M, Klinke G, van Rooijen N et al Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog 2014; 10:e1004053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Trapnell BC, Nakata K, Bonella F, Campo I, Griese M, Hamilton J et al Pulmonary alveolar proteinosis. Nat Rev Dis Primers 2019; 5:16. [DOI] [PubMed] [Google Scholar]
- 17. Minutti CM, Modak RV, Macdonald F, Li F, Smyth DJ, Dorward DA et al A macrophage–pericyte axis directs tissue restoration via amphiregulin‐induced transforming growth factor β activation. Immunity 2019; 50:645–54 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS et al Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 2014; 506:503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Peao MN, Aguas AP, de Sa CM, Grande NR. Morphological evidence for migration of particle‐laden macrophages through the interalveolar pores of Kohn in the murine lung. Acta Anat (Basel) 1993; 147:227–32. [DOI] [PubMed] [Google Scholar]
- 20. Chakarov S, Lim HY, Tan L, Lim SY, See P, Lum J et al Two distinct interstitial macrophage populations coexist across tissues in specific subtissular niches. Science 2019; 363:eaau0964. [DOI] [PubMed] [Google Scholar]
- 21. Gibbings SL, Thomas SM, Atif SM, McCubbrey AL, Desch AN, Danhorn T et al Three unique interstitial macrophages in the murine lung at steady state. Am J Respir Cell Mol Biol 2017; 57:66–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bharat A, Bhorade SM, Morales‐Nebreda L, McQuattie‐Pimentel AC, Soberanes S, Ridge K et al Flow cytometry reveals similarities between lung macrophages in humans and mice. Am J Respir Cell Mol Biol 2016; 54:147–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu YR, Hotten DF, Malakhau Y, Volker E, Ghio AJ, Noble PW et al Flow cytometric analysis of myeloid cells in human blood, bronchoalveolar lavage, and lung tissues. Am J Respir Cell Mol Biol 2016; 54:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Desch AN, Gibbings SL, Goyal R, Kolde R, Bednarek J, Bruno T et al Flow cytometric analysis of mononuclear phagocytes in nondiseased human lung and lung‐draining lymph nodes. Am J Respir Crit Care Med 2016; 193:614–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Baharom F, Thomas S, Rankin G, Lepzien R, Pourazar J, Behndig AF et al Dendritic cells and monocytes with distinct inflammatory responses reside in lung mucosa of healthy humans. J Immunol 2016; 196:4498–509. [DOI] [PubMed] [Google Scholar]
- 26. Patel VI, Booth JL, Duggan ES, Cate S, White VL, Hutchings D et al Transcriptional classification and functional characterization of human airway macrophage and dendritic cell subsets. J Immunol 2017; 198:1183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baharom F, Rankin G, Blomberg A, Smed‐Sorensen A. Human lung mononuclear phagocytes in health and disease. Front Immunol 2017; 8:499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fathi M, Johansson A, Lundborg M, Orre L, Skold CM, Camner P. Functional and morphological differences between human alveolar and interstitial macrophages. Exp Mol Pathol 2001; 70:77–82. [DOI] [PubMed] [Google Scholar]
- 29. Hoppstadter J, Diesel B, Zarbock R, Breinig T, Monz D, Koch M et al Differential cell reaction upon Toll‐like receptor 4 and 9 activation in human alveolar and lung interstitial macrophages. Respir Res 2010; 11:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C et al Innate immune landscape in early lung adenocarcinoma by paired single‐cell analyses. Cell 2017; 169:750–65 e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O et al Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 2018; 24:1277–89. [DOI] [PubMed] [Google Scholar]
- 32. Reyfman PA, Walter JM, Joshi N, Anekalla KR, McQuattie‐Pimentel AC, Chiu S et al Single‐cell transcriptomic analysis of human lung provides insights into the pathobiology of pulmonary fibrosis. Am J Respir Crit Care Med 2019; 199:1517–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vieira Braga FA, Kar G, Berg M, Carpaij OA, Polanski K, Simon LM et al A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med 2019; 25:1153–63. [DOI] [PubMed] [Google Scholar]
- 34. Zilionis R, Engblom C, Pfirschke C, Savova V, Zemmour D, Saatcioglu HD et al Single‐cell transcriptomics of human and mouse lung cancers reveals conserved myeloid populations across individuals and species. Immunity 2019; 50:1317–34 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J et al Single‐cell RNA‐seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science 2017; 356:eaah4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Epelman S, Lavine KJ, Randolph GJ. Origin and functions of tissue macrophages. Immunity 2014; 41:21–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ginhoux F, Jung S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat Rev Immunol 2014; 14:392–404. [DOI] [PubMed] [Google Scholar]
- 38. Ginhoux F, Guilliams M. Tissue‐resident macrophage ontogeny and homeostasis. Immunity 2016; 44:439–49. [DOI] [PubMed] [Google Scholar]
- 39. Perdiguero EG, Geissmann F. The development and maintenance of resident macrophages. Nat Immunol 2016; 17:2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tan SY, Krasnow MA. Developmental origin of lung macrophage diversity. Development 2016; 143:1318–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M et al Tissue‐resident macrophages self‐maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity 2013; 38:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M et al Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 2013; 38:79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guilliams M, De Kleer I, Henri S, Post S, Vanhoutte L, De Prijck S et al Alveolar macrophages develop from fetal monocytes that differentiate into long‐lived cells in the first week of life via GM‐CSF. J Exp Med 2013; 210:1977–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Murphy J, Summer R, Wilson AA, Kotton DN, Fine A. The prolonged life‐span of alveolar macrophages. Am J Respir Cell Mol Biol 2008; 38:380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dranoff G, Crawford AD, Sadelain M, Ream B, Rashid A, Bronson RT et al Involvement of granulocyte–macrophage colony‐stimulating factor in pulmonary homeostasis. Science 1994; 264:713–6. [DOI] [PubMed] [Google Scholar]
- 46. Shibata Y, Berclaz PY, Chroneos ZC, Yoshida M, Whitsett JA, Trapnell BC. GM‐CSF regulates alveolar macrophage differentiation and innate immunity in the lung through PU.1. Immunity 2001; 15:557–67. [DOI] [PubMed] [Google Scholar]
- 47. Baker AD, Malur A, Barna BP, Ghosh S, Kavuru MS, Malur AG et al Targeted PPARγ deficiency in alveolar macrophages disrupts surfactant catabolism. J Lipid Res 2010; 51:1325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gautier EL, Chow A, Spanbroek R, Marcelin G, Greter M, Jakubzick C et al Systemic analysis of PPARγ in mouse macrophage populations reveals marked diversity in expression with critical roles in resolution of inflammation and airway immunity. J Immunol 2012; 189:2614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Stanley E, Lieschke GJ, Grail D, Metcalf D, Hodgson G, Gall JA et al Granulocyte/macrophage colony‐stimulating factor‐deficient mice show no major perturbation of hematopoiesis but develop a characteristic pulmonary pathology. Proc Natl Acad Sci USA 1994; 91:5592–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bonfield TL, Farver CF, Barna BP, Malur A, Abraham S, Raychaudhuri B et al Peroxisome proliferator‐activated receptor‐γ is deficient in alveolar macrophages from patients with alveolar proteinosis. Am J Respir Cell Mol Biol 2003; 29:677–82. [DOI] [PubMed] [Google Scholar]
- 51. Kitamura T, Tanaka N, Watanabe J, Uchida K, Kanegasaki Shiro, Yamada Y et al Idiopathic pulmonary alveolar proteinosis as an autoimmune disease with neutralizing antibody against granulocyte/macrophage colony‐stimulating factor. J Exp Med 1999; 190:875–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tanaka N, Watanabe J, Kitamura T, Yamada Y, Kanegasaki S, Nakata K. Lungs of patients with idiopathic pulmonary alveolar proteinosis express a factor which neutralizes granulocyte‐macrophage colony stimulating factor. FEBS Lett 1999; 442:246–50. [DOI] [PubMed] [Google Scholar]
- 53. Suzuki T, Sakagami T, Rubin BK, Nogee LM, Wood RE, Zimmerman SL et al Familial pulmonary alveolar proteinosis caused by mutations in CSF2RA. J Exp Med 2008; 205:2703–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Martinez‐Moczygemba M, Doan ML, Elidemir O, Fan LL, Cheung SW, Lei JT et al Pulmonary alveolar proteinosis caused by deletion of the GM‐CSFRα gene in the X chromosome pseudoautosomal region 1. J Exp Med 2008; 205:2711–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sakagami T, Uchida K, Suzuki T, Carey BC, Wood RE, Wert SE et al Human GM‐CSF autoantibodies and reproduction of pulmonary alveolar proteinosis. N Engl J Med 2009; 361:2679–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Todd EM, Zhou JY, Szasz TP, Deady LE, D'Angelo JA, Cheung MD et al Alveolar macrophage development in mice requires l‐plastin for cellular localization in alveoli. Blood 2016; 128:2785–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L et al Tissue‐resident macrophages originate from yolk‐sac‐derived erythro‐myeloid progenitors. Nature 2015; 518:547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu Z, Gu Y, Chakarov S, Bleriot C, Kwok I, Chen X et al Fate mapping via Ms4a3‐expression history traces monocyte‐derived cells. Cell 2019; 178:1509–25 e19. [DOI] [PubMed] [Google Scholar]
- 59. Wong CK, Smith CA, Sakamoto K, Kaminski N, Koff JL, Goldstein DR. Aging impairs alveolar macrophage phagocytosis and increases influenza‐induced mortality in mice. J Immunol 2017; 199:1060–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Sabatel C, Radermecker C, Fievez L, Paulissen G, Chakarov S, Fernandes C et al Exposure to bacterial CpG DNA protects from airway allergic inflammation by expanding regulatory lung interstitial macrophages. Immunity 2017; 46:457–73. [DOI] [PubMed] [Google Scholar]
- 61. Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J Exp Med 2018; 215:1135–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature 2013; 496:445–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gentek R, Molawi K, Sieweke MH. Tissue macrophage identity and self‐renewal. Immunol Rev 2014; 262:56–73. [DOI] [PubMed] [Google Scholar]
- 64. Aziz A, Soucie E, Sarrazin S, Sieweke MH. MafB/c‐Maf deficiency enables self‐renewal of differentiated functional macrophages. Science 2009; 326:867–71. [DOI] [PubMed] [Google Scholar]
- 65. Soucie EL, Weng Z, Geirsdottir L, Molawi K, Maurizio J, Fenouil R et al Lineage‐specific enhancers activate self‐renewal genes in macrophages and embryonic stem cells. Science 2016; 351:aad5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sieweke MH, Allen JE. Beyond stem cells: self‐renewal of differentiated macrophages. Science 2013; 342:1242974. [DOI] [PubMed] [Google Scholar]
- 67. Golde DW, Byers LA, Finley TN. Proliferative capacity of human alveolar macrophage. Nature 1974; 247:373–5. [DOI] [PubMed] [Google Scholar]
- 68. Eguiluz‐Gracia I, Schultz HH, Sikkeland LI, Danilova E, Holm AM, Pronk CJ et al Long‐term persistence of human donor alveolar macrophages in lung transplant recipients. Thorax 2016; 71:1006–11. [DOI] [PubMed] [Google Scholar]
- 69. Sauter KA, Pridans C, Sehgal A, Tsai YT, Bradford BM, Raza S et al Pleiotropic effects of extended blockade of CSF1R signaling in adult mice. J Leukoc Biol 2014; 96:265–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wein AN, Dunbar PR, McMaster SR, Li ZT, Denning TL, Kohlmeier JE. IL‐36γ protects against severe influenza infection by promoting lung alveolar macrophage survival and limiting viral replication. J Immunol 2018; 201:573–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Imperatore F, Maurizio J, Vargas Aguilar S, Busch CJ, Favret J, Kowenz‐Leutz E et al SIRT1 regulates macrophage self‐renewal. EMBO J 2017; 36:2353–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rauschmeier R, Gustafsson C, Reinhardt A, A‐Gonzalez N, Tortola L, Cansever D et al Bhlhe40 and Bhlhe41 transcription factors regulate alveolar macrophage self‐renewal and identity. EMBO J 2019; 38:e101233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Cai Y, Sugimoto C, Arainga M, Alvarez X, Didier ES, Kuroda MJ. In vivo characterization of alveolar and interstitial lung macrophages in rhesus macaques: implications for understanding lung disease in humans. J Immunol 2014; 192:2821–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ghoneim HE, Thomas PG, McCullers JA. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J Immunol 2013; 191:1250–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Janssen WJ, Barthel L, Muldrow A, Oberley‐Deegan RE, Kearns MT, Jakubzick C et al Fas determines differential fates of resident and recruited macrophages during resolution of acute lung injury. Am J Respir Crit Care Med 2011; 184:547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Machiels B, Dourcy M, Xiao X, Javaux J, Mesnil C, Sabatel C et al A γ herpesvirus provides protection against allergic asthma by inducing the replacement of resident alveolar macrophages with regulatory monocytes. Nat Immunol 2017; 18:1310–20. [DOI] [PubMed] [Google Scholar]
- 77. Lambrecht BN. TGF‐β gives an air of exclusivity to alveolar macrophages. Immunity 2017; 47:807–9. [DOI] [PubMed] [Google Scholar]
- 78. Maus UA, Janzen S, Wall G, Srivastava M, Blackwell TS, Christman JW et al Resident alveolar macrophages are replaced by recruited monocytes in response to endotoxin‐induced lung inflammation. Am J Respir Cell Mol Biol 2006; 35:227–35. [DOI] [PubMed] [Google Scholar]
- 79. Guilliams M, Scott CL. Does niche competition determine the origin of tissue‐resident macrophages? Nat Rev Immunol 2017; 17:451–60. [DOI] [PubMed] [Google Scholar]
- 80. Misharin AV, Morales‐Nebreda L, Reyfman PA, Cuda CM, Walter JM, McQuattie‐Pimentel AC et al Monocyte‐derived alveolar macrophages drive lung fibrosis and persist in the lung over the life span. J Exp Med 2017; 214:2387–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sennello JA, Misharin AV, Flozak AS, Berdnikovs S, Cheresh P, Varga J et al Lrp5/β‐catenin signaling controls lung macrophage differentiation and inhibits resolution of fibrosis. Am J Respir Cell Mol Biol 2017; 56:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. McCubbrey AL, Barthel L, Mohning MP, Redente EF, Mould KJ, Thomas SM et al Deletion of c‐FLIP from CD11b(hi) macrophages prevents development of bleomycin‐induced lung fibrosis. Am J Respir Cell Mol Biol 2018; 58:66–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lavin Y, Winter D, Blecher‐Gonen R, David E, Keren‐Shaul H, Merad M et al Tissue‐resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 2014; 159:1312–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mass E, Ballesteros I, Farlik M, Halbritter F, Gunther P, Crozet L et al Specification of tissue‐resident macrophages during organogenesis. Science 2016; 353:aaf4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Gibbings SL, Goyal R, Desch AN, Leach SM, Prabagar M, Atif SM et al Transcriptome analysis highlights the conserved difference between embryonic and postnatal‐derived alveolar macrophages. Blood 2015; 126:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. van de Laar L, Saelens W, De Prijck S, Martens L, Scott CL, Van Isterdael G et al Yolk sac macrophages, fetal liver, and adult monocytes can colonize an empty niche and develop into functional tissue‐resident macrophages. Immunity 2016; 44:755–68. [DOI] [PubMed] [Google Scholar]
- 87. Saeed S, Quintin J, Kerstens HH, Rao NA, Aghajanirefah A, Matarese F et al Epigenetic programming of monocyte‐to‐macrophage differentiation and trained innate immunity. Science 2014; 345:1251086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Aran D, Looney AP, Liu L, Wu E, Fong V, Hsu A et al Reference‐based analysis of lung single‐cell sequencing reveals a transitional profibrotic macrophage. Nat Immunol 2019; 20:163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mould KJ, Barthel L, Mohning MP, Thomas SM, McCubbrey AL, Danhorn T et al Cell origin dictates programming of resident versus recruited macrophages during acute lung injury. Am J Respir Cell Mol Biol 2017; 57:294–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Thomas ED, Ramberg RE, Sale GE, Sparkes RS, Golde DW. Direct evidence for a bone marrow origin of the alveolar macrophage in man. Science 1976; 192:1016–8. [DOI] [PubMed] [Google Scholar]
- 91. Nakata K, Gotoh H, Watanabe J, Uetake T, Komuro I, Yuasa K et al Augmented proliferation of human alveolar macrophages after allogeneic bone marrow transplantation. Blood 1999; 93:667–73. [PubMed] [Google Scholar]
- 92. Wickenhauser C, Thiele J, Perez F, Varus E, Stoffel MS, Kvasnicka HM et al Mixed chimerism of the resident macrophage population after allogeneic bone marrow transplantation for chronic myeloid leukemia. Transplantation 2002; 73:104–11. [DOI] [PubMed] [Google Scholar]
- 93. Nayak DK, Zhou F, Xu M, Huang J, Tsuji M, Hachem R et al Long‐term persistence of donor alveolar macrophages in human lung transplant recipients that influences donor‐specific immune responses. Am J Transplant 2016; 16:2300–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Bittmann I, Dose T, Baretton GB, Muller C, Schwaiblmair M, Kur F et al Cellular chimerism of the lung after transplantation. An interphase cytogenetic study. Am J Clin Pathol 2001; 115:525–33. [DOI] [PubMed] [Google Scholar]
- 95. Kjellstrom C, Ichimura K, Chen XJ, Riise GC, Collins VP. The origin of alveolar macrophages in the transplanted lung: a longitudinal microsatellite‐based study of donor and recipient DNA. Transplantation 2000; 69:1984–6. [DOI] [PubMed] [Google Scholar]
- 96. Golde DW, Finley TN, Cline MJ. The pulmonary macrophage in acute leukemia. N Engl J Med 1974; 290:875–8. [DOI] [PubMed] [Google Scholar]
- 97. Alenghat E, Esterly JR. Alveolar macrophages in perinatal infants. Pediatrics 1984; 74:221–3. [PubMed] [Google Scholar]
- 98. Alisjahbana A, Mohammad I, Gao Y, Evren E, Ringqvist E, Willinger T. Human macrophages and innate lymphoid cells: tissue‐resident innate immunity in humanized mice. Biochem Pharmacol 2019; 113672 [Epub ahead of print]. 10.1016/j.bcp.2019.113672 [DOI] [PubMed] [Google Scholar]
- 99. Shultz LD, Brehm MA, Garcia‐Martinez JV, Greiner DL. Humanized mice for immune system investigation: progress, promise and challenges. Nat Rev Immunol 2012; 12:786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Rongvaux A, Takizawa H, Strowig T, Willinger T, Eynon EE, Flavell RA et al Human hemato‐lymphoid system mice: current use and future potential for medicine. Annu Rev Immunol 2013; 31:635–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Willinger T, Rongvaux A, Strowig T, Manz MG, Flavell RA. Improving human hemato‐lymphoid‐system mice by cytokine knock‐in gene replacement. Trends Immunol 2011; 32:321–7. [DOI] [PubMed] [Google Scholar]
- 102. Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ et al Human IL‐3/GM‐CSF knock‐in mice support human alveolar macrophage development and human immune responses in the lung. Proc Natl Acad Sci USA 2011; 108:2390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Rongvaux A, Willinger T, Martinek J, Strowig T, Gearty SV, Teichmann LL et al Development and function of human innate immune cells in a humanized mouse model. Nat Biotechnol 2014; 32:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Saito Y, Ellegast JM, Rafiei A, Song Y, Kull D, Heikenwalder M et al Peripheral blood CD34+ cells efficiently engraft human cytokine knock‐in mice. Blood 2016; 128:1829–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sippel TR, Radtke S, Olsen TM, Kiem HP, Rongvaux A. Human hematopoietic stem cell maintenance and myeloid cell development in next‐generation humanized mouse models. Blood Adv 2019; 3:268–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ziegler‐Heitbrock L. Blood monocytes and their subsets: established features and open questions. Front Immunol 2015; 6:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Jakubzick C, Gautier EL, Gibbings SL, Sojka DK, Schlitzer A, Johnson TE et al Minimal differentiation of classical monocytes as they survey steady‐state tissues and transport antigen to lymph nodes. Immunity 2013; 39:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Izquierdo HM, Brandi P, Gomez MJ, Conde‐Garrosa R, Priego E, Enamorado M et al Von Hippel–Lindau protein is required for optimal alveolar macrophage terminal differentiation, self‐renewal, and function. Cell Rep 2018; 24:1738–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kurkjian C, Hollifield M, Lines JL, Rogosky A, Empey KM, Qureshi M et al Alveolar macrophages in neonatal mice are inherently unresponsive to Pneumocystis murina infection. Infect Immun 2012; 80:2835–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Kasahara K, Matsumura Y, Ui K, Kasahara K, Komatsu Y, Mikasa K et al Intranasal priming of newborn mice with microbial extracts increases opsonic factors and mature CD11c+ cells in the airway. Am J Physiol Lung Cell Mol Physiol 2012; 303:L834–43. [DOI] [PubMed] [Google Scholar]
- 111. Lopez‐Rodriguez E, Gay‐Jordi G, Mucci A, Lachmann N, Serrano‐Mollar A. Lung surfactant metabolism: early in life, early in disease and target in cell therapy. Cell Tissue Res 2017; 367:721–35. [DOI] [PubMed] [Google Scholar]
- 112. Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC et al Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010; 126:443–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. May C, Prendergast M, Salman S, Rafferty GF, Greenough A. Chest radiograph thoracic areas and lung volumes in infants developing bronchopulmonary dysplasia. Pediatr Pulmonol 2009; 44:80–5. [DOI] [PubMed] [Google Scholar]
- 114. Um‐Bergstrom P, Hallberg J, Thunqvist P, Berggren‐Brostrom E, Anderson M, Adenfelt G et al Lung function development after preterm birth in relation to severity of bronchopulmonary dysplasia. BMC Pulm Med 2017; 17:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Plosa EJ, Young LR, Gulleman PM, Polosukhin VV, Zaynagetdinov R, Benjamin JT et al Epithelial β1 integrin is required for lung branching morphogenesis and alveolarization. Development 2014; 141:4751–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Blackwell TS, Hipps AN, Yamamoto Y, Han W, Barham WJ, Ostrowski MC et al NF‐κB signaling in fetal lung macrophages disrupts airway morphogenesis. J Immunol 2011; 187:2740–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Prince LR, Maxwell NC, Gill SK, Dockrell DH, Sabroe I, McGreal EP et al Macrophage phenotype is associated with disease severity in preterm infants with chronic lung disease. PLoS ONE 2014; 9:e103059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Canan CH, Gokhale NS, Carruthers B, Lafuse WP, Schlesinger LS, Torrelles JB et al Characterization of lung inflammation and its impact on macrophage function in aging. J Leukoc Biol 2014; 96:473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Albright JM, Dunn RC, Shults JA, Boe DM, Afshar M, Kovacs EJ. Advanced age alters monocyte and macrophage responses. Antioxid Redox Signal 2016; 25:805–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Jackaman C, Tomay F, Duong L, Abdol Razak NB, Pixley FJ, Metharom P et al Aging and cancer: the role of macrophages and neutrophils. Ageing Res Rev 2017; 36:105–16. [DOI] [PubMed] [Google Scholar]
- 121. Angelidis I, Simon LM, Fernandez IE, Strunz M, Mayr CH, Greiffo FR et al An atlas of the aging lung mapped by single cell transcriptomics and deep tissue proteomics. Nat Commun 2019; 10:963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Menter T, Giefing‐Kroell C, Grubeck‐Loebenstein B, Tzankov A. Characterization of the inflammatory infiltrate in Streptococcus pneumoniae pneumonia in young and elderly patients. Pathobiology 2014; 81:160–7. [DOI] [PubMed] [Google Scholar]
- 123. Trompette A, Gollwitzer ES, Pattaroni C, Lopez‐Mejia IC, Riva E, Pernot J et al Dietary fiber confers protection against Flu by shaping Ly6c− patrolling monocyte hematopoiesis and CD8+ T cell metabolism. Immunity 2018; 48:992–1005 e8. [DOI] [PubMed] [Google Scholar]
- 124. Schuijt TJ, Lankelma JM, Scicluna BP, de Sousa e Melo F, Roelofs JJTH, de Boer JD et al The gut microbiota plays a protective role in the host defence against pneumococcal pneumonia. Gut 2016; 65:575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Brown RL, Sequeira RP, Clarke TB. The microbiota protects against respiratory infection via GM‐CSF signaling. Nat Commun 2017; 8:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Shenoy MK, Fadrosh DW, Lin DL, Worodria W, Byanyima P, Musisi E et al Gut microbiota in HIV‐pneumonia patients is related to peripheral CD4 counts, lung microbiota, and in vitro macrophage dysfunction. Microbiome 2019; 7:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Marsland BJ, Gollwitzer ES. Host–microorganism interactions in lung diseases. Nat Rev Immunol 2014; 14:827–35. [DOI] [PubMed] [Google Scholar]
- 128. Wypych TP, Wickramasinghe LC, Marsland BJ. The influence of the microbiome on respiratory health. Nat Immunol 2019. [DOI] [PubMed] [Google Scholar]
- 129. Pattaroni C, Watzenboeck ML, Schneidegger S, Kieser S, Wong NC, Bernasconi E et al Early‐life formation of the microbial and immunological environment of the human airways. Cell Host Microbe 2018; 24:857–65 e4. [DOI] [PubMed] [Google Scholar]
- 130. Mouraux S, Bernasconi E, Pattaroni C, Koutsokera A, Aubert JD, Claustre J et al Airway microbiota signals anabolic and catabolic remodeling in the transplanted lung. J Allergy Clin Immunol 2018; 141:718–29 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Bernasconi E, Pattaroni C, Koutsokera A, Pison C, Kessler R, Benden C et al Airway microbiota determines innate cell inflammatory or tissue remodeling profiles in lung transplantation. Am J Respir Crit Care Med 2016; 194:1252–63. [DOI] [PubMed] [Google Scholar]
- 132. Goleva E, Jackson LP, Harris JK, Robertson CE, Sutherland ER, Hall CF et al The effects of airway microbiome on corticosteroid responsiveness in asthma. Am J Respir Crit Care Med 2013; 188:1193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Le Noci V, Guglielmetti S, Arioli S, Camisaschi C, Bianchi F, Sommariva M et al Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: a strategy to promote immunosurveillance against lung metastases. Cell Rep 2018; 24:3528–38. [DOI] [PubMed] [Google Scholar]
- 134. Mora AL, Torres‐Gonzalez E, Rojas M, Corredor C, Ritzenthaler J, Xu J et al Activation of alveolar macrophages via the alternative pathway in herpesvirus‐induced lung fibrosis. Am J Respir Cell Mol Biol 2006; 35:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Yao Y, Jeyanathan M, Haddadi S, Barra NG, Vaseghi‐Shanjani M, Damjanovic D et al Induction of autonomous memory alveolar macrophages requires T cell help and is critical to trained immunity. Cell 2018; 175:1634–50 e17. [DOI] [PubMed] [Google Scholar]
- 136. Chen F, Wu W, Millman A, Craft JF, Chen E, Patel N et al Neutrophils prime a long‐lived effector macrophage phenotype that mediates accelerated helminth expulsion. Nat Immunol 2014; 15:938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sun K, Metzger DW. Inhibition of pulmonary antibacterial defense by interferon‐γ during recovery from influenza infection. Nat Med 2008; 14:558–64. [DOI] [PubMed] [Google Scholar]


