Summary
Transforming growth factor β (TGF‐β) is a multifunctional cytokine that regulates cell growth, differentiation, adhesion, migration and death dependent on cell type, developmental stage, or tissue conditions. Various cell types secrete TGF‐β, but always as an inactive complex. Hence, for TGF‐β to function, this latent complex must somehow be activated. Work in recent years has highlighted a critical role for members of the α v integrin family, including α v β 1, α v β 3, α v β 5, α v β 6 and α v β 8 that are involved in TGF‐β activation in various contexts, particularly at barrier sites such as the gut, lung and skin. The integrins facilitating this context‐ and location‐specific regulation can be dysregulated in certain diseases, so are potential therapeutic targets in a number of disorders. In this review, we discuss the role of TGF‐β at these barrier sites with a focus on how integrin‐mediated TGF‐β activation regulates tissue and immune homeostasis, and how this is altered in disease.
Keywords: immune system, integrins, intestine, lung, skin, transforming growth factor‐β
TGF‐β is a crucial cytokine in regulation of the immune system, especially at barrier sites. Many cell types can produce TGF‐β, but always as an inactive complex that requires activating to be functional. This review highlights vital pathways that activate TGF‐β in the intestine, lung and skin, with a focus on how integrins control TGF‐β activity in a context‐specific manner.

Abbreviations
- COPD
chronic obstructive pulmonary disease
- DCs
dendritic cells
- IBD
inflammatory bowel disease
- Ig
immunoglobulin
- IPF
idiopathic pulmonary fibrosis
- IRF
interferon regulatory factor
- KLRG1+
killer-cell lectin like receptor G1
- LAP
latency‐associated peptide
- LLC
large latent complex
- LTBP
latent TGF‐β binding protein
- RALDH
retinal dehydrogenase
- SLC
small latent complex
- Smad
Similar to mothers against decapentaplegic
- TGF‐β
transforming growth factor‐β
- Th cell
T helper cell
- Treg cells
regulatory T cells
- Trm cells
tissue‐resident memory cells
Introduction
Transforming growth factor‐β (TGF‐β) is part of a large protein family comprising 33 members, including three isoforms of TGF‐β (TGF‐β 1, ‐β 2 and ‐β 3) as well as bone morphogenetic proteins, growth and differentiation factors such as Nodal, activins/inhibins, Müllerian inhibiting substance/anti‐Müllerian hormone and Lefty.1, 2 The binding of TGF‐β to TGF‐β receptors initiates a cascade of intracellular signals, which can proceed along both Smad [homologues of the Sma and mothers against decapentaplegic (Mad) proteins found in Caenorhabditis elegans and Drosophila, respectively] ‐dependent and ‐independent pathways as reviewed elsewhere.3, 4 Responses triggered by TGF‐β signalling can regulate cell growth, differentiation, adhesion, migration and death depending on target cell type, developmental stage or tissue environment.1, 2, 5 Due to this pleiotropy, the functionality of TGF‐β is tightly regulated and all three isoforms are synthesized as inactive precursors comprising an N‐terminal latency‐associated peptide (LAP) and a C‐terminal active TGF‐β moiety (Fig. 1). LAP–TGF‐β forms a homodimeric pro‐peptide complex, which is cleaved by the protease furin intracellularly.6, 7 However, following this initial cleavage event, LAP remains non‐covalently associated with active TGF‐β, which is secreted as a small latent complex (SLC). The configuration of this SLC is such that LAP masks the active TGF‐β fragment, thereby blocking its receptor binding sites and rendering it inactive (Fig. 1). Often, the SLC covalently associates with latent TGF‐β‐binding protein (LTBP) to form the large latent complex (LLC). Upon secretion, LTBP interacts with other components of the extracellular matrix and can be covalently cross‐linked to some matrix proteins.8, 9, 10, 11 However, in order for TGF‐β to bind to its receptors and trigger signalling, it must first be separated from LAP. Various mechanisms by which this process could occur have been proposed, including extremes of heat, acidic pH and reactive oxygen species, as well as the activities of serine proteases, matrix metalloproteases and thrombospondin‐1. Furthermore, recent compelling evidence suggests that key activators of TGF‐β are integrins (Fig. 1).12 These molecules are part of a large family of heterodimeric transmembrane receptors and consist of an α and a β subunit.13 Members of the αv integrin family, including α v β 1, α v β 3, α v β 5, α v β 6 can bind to the tri‐amino integrin‐binding motif RGD in LAP, while simultaneously interacting with cytoskeletal proteins to facilitate mechanical fragmentation of LAP.14, 15, 16, 17, 18, 19 Although integrin α v β 8 also binds to LAP via RGD, the activation occurs via non‐cytoskeletal‐dependent mechanisms.20 While the integrin‐binding RGD motif is present in the latent form of TGF‐β 1 and TGF‐β 3, it is not present in TGF‐β 2.12 Several of these TGF‐β‐activating integrins play fundamental roles in the preservation of normal tissue functions at barrier sites including the gut, lung and skin as discussed below.
Figure 1.
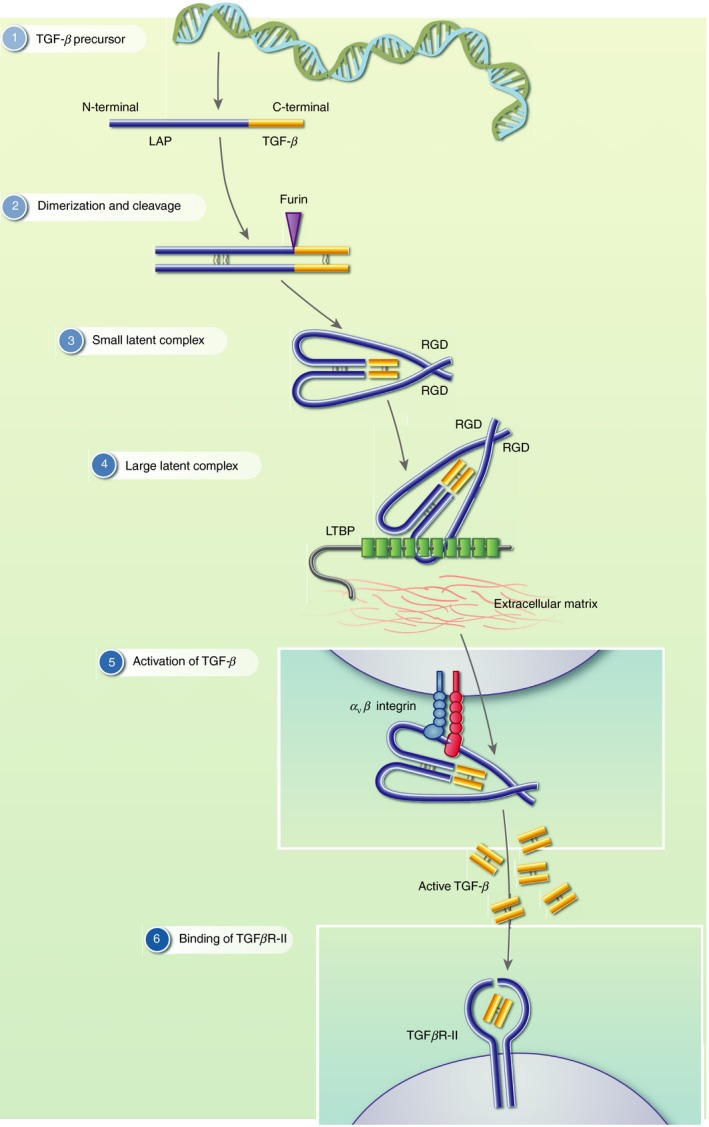
Structure of latent transforming growth factor‐β (TGF‐β) and activation by integrins. (1) TGF‐β is synthesized as a precursor that comprises an N‐terminal latency‐associated peptide (LAP) and a C‐terminal active TGF‐β moiety. (2) LAP–TGF‐β forms a homodimeric propeptide complex, which is cleaved by the protease furin intracellularly. (3) The small latent complex (SLC) comprises the cleaved LAP non‐covalently bound to active TGF‐β upon secretion. (4) Often, the SLC covalently associates with latent TGF‐β binding protein (LTBP) to form the large latent complex (LLC) together with the extracellular matrix. (5) α v integrins important activators of TGF‐β binds to LAP at an arginine‐glycine‐aspartic acid (RGD) site, leading to the dissociation of LAP and the release of active TGF‐β. (6) Active TGF‐β first binds to the TGFβRII dimer.
In this brief review, we discuss critical roles of integrin‐mediated activation of latent TGF‐β at three distinct barrier sites – the gut, lung and skin – highlighting the importance of this process in both healthy and disease states and discussing the therapeutic potential for these pathways.
Activation and function of TGF‐β in the gastrointestinal tract
Maintenance of immune equilibrium in the gastrointestinal tract is complex and multi‐faceted as harmful antigens originating from enteric pathogens must be distinguished from those that are innocuous and derived from diet or the microbiota. Central to this balance between effector and regulatory responses is TGF‐β, derived from a multitude of cells including regulatory T (Treg) cells, dendritic cells (DCs) and intestinal epithelial cells. Indeed, TGF‐β plays a central role in shaping the immunological landscape of the gut as it is an essential factor involved in the differentiation of both Treg and T helper type 17 (Th17) cells.12 Furthermore, the ability of effector T cells to respond to TGF‐β is also important in their suppression by Treg cells in models of intestinal inflammation.21 In addition to its effects on T cells, TGF‐β can also induce immunoglobulin A (IgA) production by intestinal plasma cells, which in turn helps to shape the composition of the microbiota to one which favours a tolerogenic environment.22, 23, 24, 25 Given this functional diversity, it is important to understand the mechanism(s) by which TGF‐β activation occurs in the intestine and how such processes regulate the different responses induced by TGF‐β.
TGF‐β activation by tolerogenic intestinal DCs
Dendritic cells expressing the integrin CD103 (integrin α E) appear to be particularly important in promoting intestinal tolerance owing to their co‐expression of Vitamin A‐metabolizing retinal dehydrogenase (RALDH) enzymes and TGF‐β, which enables them to promote the differentiation of Treg cells.26, 27 Furthermore, these CD103+ DCs are capable of activating latent TGF‐β via their expression of the integrin α v β 8, a feature which is essential for the maintenance of normal intestinal immune function.28, 29, 30, 31, 32 Additionally, α v β 8‐mediated TGF‐β activation by DCs can modulate different intestinal CD4+ T helper cell responses. For instance, integrin α v β 8‐mediated TGF‐β activation by DCs inhibits the differentiation of Th2 cells during infection with the intestinal parasite Trichuris muris, with mice lacking this pathway showing exacerbated Th2 cell numbers and protection from chronic infection.33 However, the ability of DC‐mediated TGF‐β activation to control responses to intestinal parasites is dependent on the pathogen, as recent data have shown that deletion of α v β 8 on DCs results in delayed expulsion of Trichinella spiralis. This delayed expulsion appears to be the result of decreased TGF‐β‐dependent Th17 cell differentiation, which resulted in impaired intestinal contractility – an important mechanism by which T. spiralis is expelled.34 Additionally, reduction in Th17 cell numbers in the gut and lymphoid tissues of mice lacking integrin α v β 8 expression on DCs has been shown to correlate with complete protection from experimental autoimmune encephalomyelitis, a Th17‐mediated pathology with a similar immune and pathological profile to multiple sclerosis.35, 36, 37 Taken together, these studies show a key role for integrin α v β 8‐mediated TGF‐β activation by DCs in controlling CD4+ T‐cell responses in the gut. Additionally, the expression of integrin α v β 8 by Peyer's patch‐derived DCs, and their resulting ability to activate latent TGF‐β, was shown by Reboldi et al. 25 to be essential for IgA class switching of activated B cells within these intestinal lymphoid structures, showing a broader role for DC expression of integrin α v β 8 in regulating adaptive immunity in the gut.
Integrin α v β 8 is also expressed by certain human intestinal DC subsets. However, whereas integrin α v β 8 is preferentially expressed by interferon regulatory factor (IRF) 8‐dependent CD103+ CD11b+ intestinal DCs in mice,32, 38 expression of α v β 8 is restricted to IRF4‐dependent CD1c+ and not IRF8‐dependent CD141+ DCs in humans and is significantly enhanced during active inflammation and in response to Toll‐like receptor ligands.39, 40
Perturbations in TGF‐β activation pathways are also observed in inflammatory bowel disease (IBD). While total concentrations of latent TGF‐β are elevated during IBD,41 decreased expression of the TGF‐β‐activating integrin α v β 8 on mature tissue macrophages and infiltrating pro‐inflammatory monocytes is a feature of intestinal inflammation in humans.42 Interestingly, this is in contrast to the pattern of expression observed on DCs isolated from intestinal biopsies from IBD patients, which exhibit enhanced levels of α v β 8 compared with those from healthy subjects.40 Although the mechanism(s) underlying the dichotomous expression of this TGF‐β‐activating integrin during intestinal inflammation remains to be elucidated, work to date suggests that alterations in the capacity of specific cell types to activate latent TGF‐β is a feature of IBD.
Suppression of intestinal inflammation by Treg cells
A reduction in the number of peripheral Treg cells, or alterations in their functionality, have also been reported in IBD.43, 44 Recent work has shown that Treg cells also activate TGF‐β through expression of integrin α v β 8.45 However, unlike DCs, conditional deletion of α v β 8 on Treg cells did not perturb immune quiescence under steady‐state conditions. Instead, ablation of integrin α v β 8 expression on Treg cells prevents these cells from resolving established, ongoing intestinal inflammation, with activated killer‐cell lectin like receptor G1 (KLRG1+) effector Treg cells expressing high levels of integrin α v β 8 rapidly expanding in inflammatory contexts.45 This pathway also appears important in humans, as administering an anti‐β 8 blocking antibody following co‐transfer of human peripheral blood mononuclear cells and autologous Treg cells to immune‐deficient mice abrogated Treg‐mediated immunosuppression and exacerbated graft‐versus‐host disease.46
TGF‐β‐dependent intestinal tissue‐resident T cells
TGF‐β has also been implicated as a critical factor involved in the establishment and maintenance of intestinal tissue‐resident memory (Trm) cells, a non‐circulating memory T‐cell population that reside in peripheral tissues.47, 48 A seminal study by Zhang and Bevan demonstrated that TGF‐β was essential for the formation and maintenance of intestinal Trm via a two‐step process by firstly inhibiting the α 4 β 7‐mediated recruitment of splenic T cells into the gut and subsequently inducing the expression of tissue residency markers, such as CD103 and CD69 on T cells situated in the intestine.49 Of note however, a bona fide population of CD103– intestinal Trm cells has also been described, whose differentiation occurs independently of TGF‐β.50 More recently, expression of the TGF‐β‐activating integrin α v β 6 by intestinal epithelial cells has also been shown to be critical for the maintenance of CD8+ Trm cells. In their study, Mohammed et al. 51 demonstrated that although the infiltration of effector cells into the epithelial layer during acute infection was comparable between wild‐type and Itgb6–/– mice, following contraction of this initial response, the numbers of antigen‐specific cells that were retained within the intestinal epithelium, but not the lamina propria, were significantly reduced in the absence of α v β 6‐mediated TGF‐β activation.
Hence, regulation of TGF‐β activation by integrins plays a crucial role in controlling a number of facets of TGF‐β function in the intestine (Fig. 2a), with several of these pathways appearing dysregulated during IBD. Hence, a better understanding of how TGF‐β activity is controlled in the gut in a context‐specific manner may identify potential targets for modulating TGF‐β function in IBD.
Figure 2.
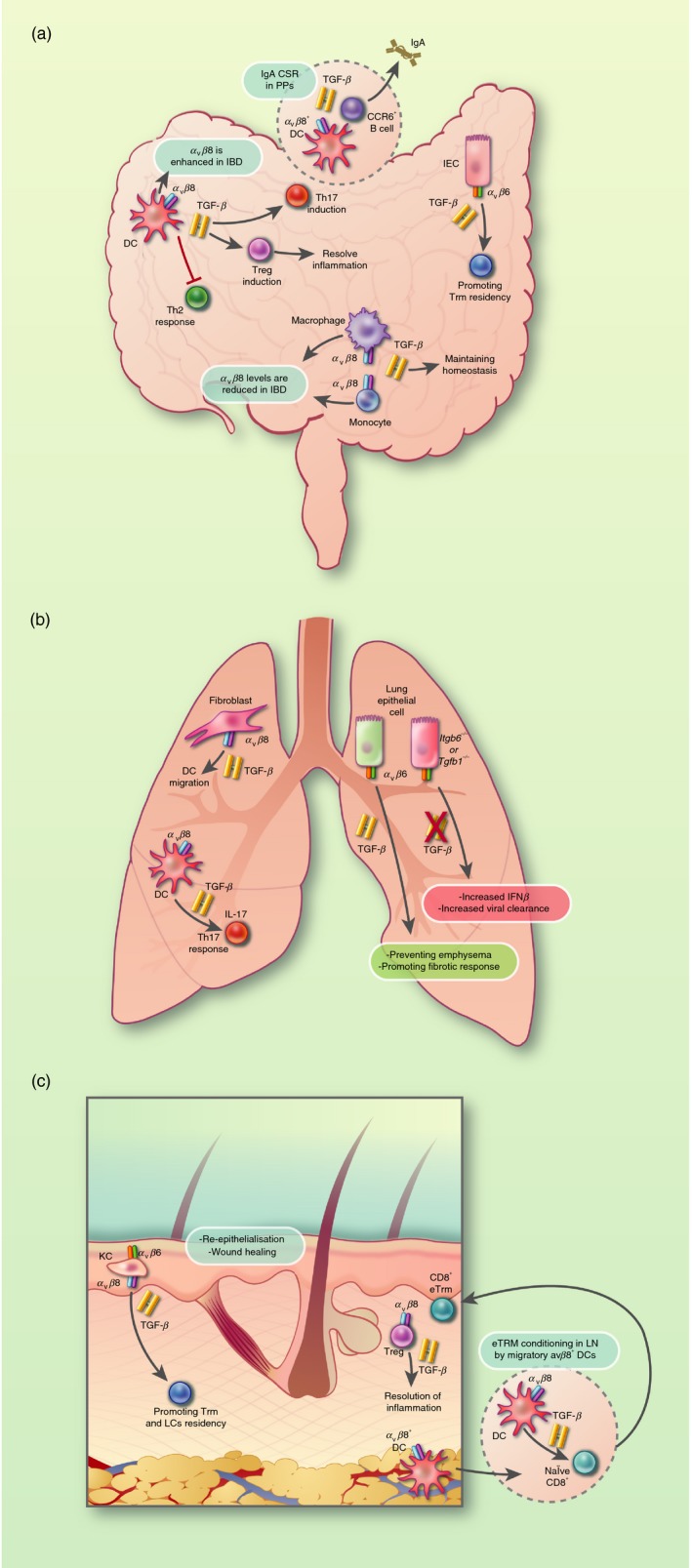
Transforming growth factor‐β (TGF‐β) activated by integrins α v β 6 and α v β 8 has important functions at barrier sites. (a) Dendritic cells (DCs) are capable of activating latent TGF‐β through their expression of the integrin α v β 8, which modulates intestinal CD4+ T helper (Th) cell responses and Foxp3+ regulatory T (Treg) cells. In inflammatory bowel disease (IBD), α v β 8 expression is enhanced on DCs, whereas it is reduced on monocytes and macrophages. TGF‐β activation by integrin α v β 6 by intestinal epithelial cells (IECs) has been shown as a critical factor involved in the maintenance of intestinal tissue‐resident memory (Trm) cells. (b) In the lung, activation of TGF‐β by α v β 8‐expressing fibroblasts has been shown to drive DC chemotaxis. Moreover, integrin α v β 8‐expressing DCs appears to be required for the differentiation of Th17 cells. Integrin α v β 6‐mediated activation of latent TGF‐β by lung epithelial cells is a critical component of pulmonary homeostasis via preventing emphysema, whereas excessive TGF‐β signalling can facilitate pulmonary fibrosis in idiopathic pulmonary fibrosis (IPF) and promote infection. (c) Integrins α v β 6 and α v β 8 play an essential role in maintaining the cutaneous barrier in the skin. α v β 6‐mediated activation of latent TGF‐β by keratinocytes (KCs) is essential for re‐epithelialization and wound healing. Moreover, activation of latent TGF‐β by integrins α v β 6 and α v β 8 by KC in the epidermis are important for the maintenance of both Trm and Langerhans cells (LCs) in the skin. α v β 8 expression on regulatory T (Treg) cells is required to prevent overt effector T‐cell responses during acute inflammation. Migration of integrin α v β 8‐expressing DCs to skin‐draining lymph nodes is also important in imprinting CD8+ T cells to become CD8+ Trm cells.
TGF‐β activation in the lung
TGFβ and its activation as a mediator of tolerance to inhalable allergens
As with other barrier sites, the pulmonary mucosa is constantly exposed to a plethora of harmless environmental antigens, which are inhaled on a daily basis and to which the immune system must remain in a state of immunological hyporesponsiveness to prevent unwarranted effector responses and maintain normal tissue function. Perturbations in the mechanisms that maintain this steady‐state tolerance to innocuous antigens, such as pollen, pet dander and dust mite excretions, can result in the development of airway hyper‐reactivity, characterized as the onset of overt inflammatory responses culminating in airway obstruction. As is the case with oral tolerance to ingested antigens in the gut, Treg cells and TGF‐β are fundamental in the prevention of exacerbated type 2 responses in the airways, which are characteristic of allergic inflammation and abrogation of TGF‐β signalling can re‐capitulate inflammatory phenotypes in a number of experimental models.52, 53, 54, 55, 56, 57 Lung‐resident macrophages, like tolerogenic intestinal DCs, have been shown to co‐express TGF‐β and RALDH constitutively and are capable of inducing the differentiation of Foxp3+ T cells from naive precursors in in vitro culture systems.58 Moreover, several studies have demonstrated that depletion of these macrophages in vivo enhances allergen‐induced responses, with Zasłona et al. reporting a concomitant decrease in TGF‐β concentrations in bronchoalveolar fluid.59, 60, 61
Interestingly, TGF‐β‐activating integrins also play a central role in maintaining the balance between effector and tolerogenic responses in the lung, as demonstrated by work showing that loss of α v β 6 resulted in enhanced metalloproteinase activity and degradation of extracellular matrix components, culminating in the development of murine emphysema.62
TGF‐β activation as a driver of disease in the lung
Despite its ordinarily tissue‐protective roles, excessive TGF‐β signalling in the lung can have detrimental consequences and can facilitate pulmonary fibrosis owing to its ability to stimulate collagen deposition and the generation of new extracellular matrix.63 In the case of idiopathic pulmonary fibrosis (IPF) this seems to occur in an integrin α v β 6‐dependent manner, as blockade or genetic ablation of α v β 6 in mice attenuated fibrotic sequelae in a variety of pre‐clinical models.14, 64, 65 This process appears to involve a positive feedback loop in which α v β 6 expression is first induced by TGF‐β, which is found at elevated concentrations in the lungs of IPF patients.66 Similarly, enhanced airway TGF‐β levels are a feature of both asthma and chronic obstructive pulmonary disease (COPD), where it is associated with airway remodelling characteristic of disease pathogenesis.67
Integrin‐mediated TGF‐β activation has also been indicated in the pathogenesis of asthma. In a pre‐clinical model of airway hyper‐responsiveness in which mice exhibit asthma‐like symptoms, Kudo et al. demonstrated that activation of latent TGF‐β by integrin α v β 8‐expressing DCs was required for the differentiation of Th17 cells following sensitization with inhaled antigens. Subsequent intra‐nasal challenge resulted in interleukin‐17A‐dependent smooth muscle contraction and airway constriction, a response that did not occur in mice lacking integrin α v β 8 on their DCs.68 Interestingly, Th2 cell numbers were not altered in mice lacking integrin α v β 8‐mediated TGF‐β activation by DCs.68 Given that TGF‐β is a known inhibitor of Th2 cell formation, these results show that TGF‐β activation by integrins allows context‐specific TGF‐β function; in this instance promoting Th17 function without affecting Th2 cells. Additionally, expression of another integrin, α v β 5, by airway smooth muscle cells has been proposed to be important in activating TGF‐β and promoting pathology in murine models of asthma.69 Hence, limiting the concentrations of bioactive TGF‐β by selectively targeting the integrins involved in its activation may represent a promising strategy by which to attenuate pulmonary fibrosis and asthma without compromising tolerance to innocuous antigens.
TGF‐β production and activation by pulmonary epithelial cells can limit effector responses during lung infection
In addition to these deleterious roles in fibrosis and asthma, recent work has suggested that TGF‐β serves to promote viral infection, and can be used to evade type I interferon‐mediated anti‐viral immune responses. Again, integrins appear to play a key role in facilitating such responses and Meliopoulos et al. 70 demonstrated enhanced protection against influenza virus, Sendai virus and Streptococcus pneumoniae in mice lacking integrin α v β 6, a phenotype that was attributed to enhanced activation status and type I IFN signalling in alveolar macrophages and could be reversed upon exogenous administration of TGF‐β. Similarly, Denney et al. 71 recently reported that conditional deletion of Tgfb1 in bronchial epithelial cells led to reduced viral burdens and infection‐associated pathology following infection with influenza virus owing to elevated interferon‐β responses. Interestingly, the same group previously demonstrated that epithelial‐derived TGF‐β actually exacerbated inflammatory sequelae by augmenting numbers of allergen‐induced pulmonary innate lymphoid cell type 2, further highlighting the dichotomous roles of TGF‐β within the lung microenvironment.72 Aside from exhibiting both pro‐inflammatory and anti‐inflammatory functions, the finding by Meliopoulos et al. 70 that the lung microenvironment of α v β 6‐deficient animals imprinted an altered developmental phenotype on alveolar macrophages, which could be reversed upon administration of exogenous TGF‐β, suggest that the activation of latent TGF‐β by integrin α v β 6 may in fact play a key role in the ontogeny of these cells.
Targeting TGF‐β activity in lung disease
As a result of its pro‐fibrotic properties, together with its potent immune suppressive functions, TGF‐β has emerged as a promising therapeutic target for the treatment of fibrosis and cancer. Pirfenidone is a small‐molecular‐weight phenyl‐substituted pyridinone with anti‐inflammatory and anti‐fibrotic effects, which include decreased fibroblast proliferation and TGF‐β signalling. It has been approved for clinical use for the treatment of IPF and has been shown to attenuate TGF‐β function and improve vital capacity in IPF patients.73 However, because of the pleiotropy of TGF‐β in maintaining normal tissue function and prevention of deleterious responses to self‐antigens or innocuous stimuli, there is a concern that systemic blockade of TGF‐β signalling might have severe complications and result in dysregulated immune responses. Therefore, an alternative approach that might attenuate, rather than completely ablate, TGF‐β responsiveness is highly desirable. Indeed several approaches targeting specific components of the TGF‐β signalling pathway are currently under investigation. One such strategy might involve targeting the activation of TGF‐β by some of the aforementioned integrins, thereby limiting the amount of active TGF‐β available to target cells. BG0001, a humanized monoclonal antibody targeting the activation of TGF‐β by integrin α v β 6 has recently demonstrated a decrease in active TGF‐β signalling in a phase 2A trial and has subsequently undergone phase 2B trials for IPF (clinical trial # NCT01371305).74
Similarly, targeting α v β 8 may be efficacious for the treatment of certain pulmonary indications. Activation of TGF‐β by α v β 8‐expressing fibroblasts has been shown to drive DC chemotaxis in response to exogenous stimuli associated with COPD.75, 76 Recently, the potential efficacy of an integrin α v β 8‐targeted therapy for pulmonary fibrosis has been demonstrated in transgenic mice expressing human α v β 8. In their study, Minagawa et al. 77 successfully attenuated fibrotic inflammation in response to a number of stimuli including allergens, tobacco smoke and pro‐inflammatory cytokines by administering a humanized monoclonal antibody specific for α v β 8. Taken together, these studies highlight the promising potential of integrin‐targeted therapies for the treatment of various lung pathologies.
Role of TGF‐β in the establishment of long‐lived lung‐resident memory T cells
Similar to the gut, another function of pulmonary TGF‐β signalling is its role in regulating the expression of tissue residency markers on T cells, thereby enabling them to differentiate into long‐lived Trm cells. The establishment of these cells in a TGF‐β‐dependent manner is one of the primary objectives of mucosal vaccination and has been shown to be essential for hetero‐subtypic protection against influenza challenge.78 In humans, the imprinting of a Trm signature, including up‐regulation of the tissue residency marker CD103, on pulmonary T cells is a specialized function attributed to CD1c+, as opposed to CD141+, DCs and is dependent on TGF‐β.79 As mentioned previously, this CD1c+ DC subset has been shown to activate TGF‐β via expression of integrin α v β 8 in the gut, a function that is essential for the prevention of intestinal immune dysfunction.40 Taken together, these findings suggest that integrin α v β 8‐mediated activation of latent TGF‐β by CD1c+ DCs may also regulate the formation of protective Trm cells at the pulmonary mucosa.
Overall, data generated to date show that TGF‐β, and the control of its activity by integrins, is vital in the regulation of responses in the lung in both health and disease (Fig. 2b). Hence, blockade of TGF‐β activation by integrins is an attractive therapeutic target in pulmonary fibrosis and COPD, as well as during pulmonary infections, with the potential to block context‐specific TGF‐β function in disease without compromising the tissue‐protective effects of the cytokine.
TGF‐β activation in the skin
TGF‐β, and several components involved in its signalling pathway, play an essential role in regulating the cutaneous barrier. Such pathways are generally up‐regulated in response to injury or inflammatory stimuli to help promote wound healing or attenuate inflammatory responses by inducing cell cycle arrest or the apoptosis of effector cells.80 Additionally, TGF‐β is critical in the formation of Langerhan's cells, and in preventing their migration out of the epidermis.81 As in gut and lung, integrin‐mediated TGF‐β activation is critical in controlling the function of TGF‐β in the skin. For example, although integrin α v β 6 and α v β 8‐mediated TGF‐β activation by keratinocytes are not required to prevent spontaneous skin inflammation,82 α v β 6‐mediated activation of latent TGF‐β by keratinocytes has been shown to be essential for re‐epithelialization and optimal wound healing both in vitro and in vivo.83 Moreover, Treg expression of α v β 8 is required to prevent overt effector T‐cell responses during acute inflammation, as mice lacking expression of integrin α v β 8 on Foxp3+ Treg cells could not restrain effector CD4+ and CD8+ cellular responses in mouse models of delayed hypersensitivity, leading to exacerbated inflammation and thickening of the cutaneous barrier.45 Additionally, integrins α v β 3 and α v β 5 are up‐regulated in dermal fibroblasts from scleroderma patients and are proposed to promote myofibroblast formation through activation of TGF‐β.15, 16
Activation of latent TGF‐β determines skin residency
As with other barrier sites, TGF‐β, in synergy with transcription factors and other cytokines such as interleukin‐15, is a key factor involved in retaining memory T cells in the skin as long‐lived Trm cells.84, 85 Co‐ordinated activation of latent TGF‐β by integrins α v β 6 and α v β 8, which are expressed in a reciprocal fashion on keratinocytes found at distinct anatomical locations in the epidermis has been implicated in the maintenance of both Trm and Langerhans cells in the skin of both mice and humans.51 Recently, the expression of αv integrins, specifically α v β 8, by migratory DCs in the skin has been shown to be essential for the formation of CD103+ skin‐resident CD8+ T cells. In their study, Mani et al. demonstrated that mice lacking expression of all α v integrins, or just α v β 8 alone, on DCs exhibited significantly reduced numbers of CD103+ epidermal Trm cells both at steady‐state and following inflammation or immunization. Interestingly, these α v integrin‐expressing DCs were shown to first migrate to the lymph node, where their ability to activate latent TGF‐β pre‐conditioned naive CD8+ T cells for subsequent tissue residency.86 In addition to this well‐established role for integrin‐activated TGF‐β in the context of tissue residency, a recent study by Dan Kaplan and colleagues has shown that local activation of latent TGF‐β in the skin also regulates the pool of recirculating memory CD8+ T cells following primary infection and is required for their persistence as peripheral or central memory T cells. In their study, complete ablation of integrins α v β 6 and α v β 8 on keratinocytes led to impaired TGF‐β activation, resulting in decreased numbers of peripheral and central memory T cells, which significantly compromised protection from a secondary challenge with Vaccinia virus.87
In summary, integrin‐mediated TGF‐β activation is key in regulating various aspects of skin biology (Fig. 2c), with further work required to determine how targeting these pathways may be useful during wound healing and infection.
Conclusion and perspectives
In this review, we have discussed pleiotropic effects of TGF‐β and the source of activation of TGF‐β that can differ in certain contexts at barrier sites. These pathways highlight the multi‐faceted manner in which barrier site homeostasis is maintained and orchestrated by several highly specialized cell types. Given the importance of TGF‐β in many different tissues and biological contexts, this cytokine has emerged as a promising therapeutic target in disorders such as fibrosis and cancer. However, given the broad importance of TGF‐β in health, global antagonism of TGF‐β signalling may have detrimental consequences, which pose a major challenge for therapeutic targeting. Therefore, TGF‐β‐activating integrins, specifically α v β 6 and α v β 8, become important potential targets to affect TGF‐β function in a more context‐specific manner. However, more work is needed to understand the relative contributions of these integrins on specific cell types, any non‐TGF‐β‐related functions they play that will also be blocked when targeted, and how their expression and function are regulated during health and disease. This will be an important step towards the translation of ‘promising’ targets into clinically effective therapeutics for disease.
Disclosures
The authors declare there are no competing interests.
Acknowledgements
Work in the Travis laboratory is supported by grants from the BBSRC (BB/R003114/1), the Kenneth Rainin Foundation (2019‐1162), the Manchester Collaborative Centre for Inflammation Research, and Defence Science Technology Laboratory. The Wellcome Centre for Cell‐Matrix Research, University of Manchester, is supported by core funding from the Wellcome Trust (grant number 203128/Z/16/Z).
References
- 1. Roberts AB, Lamb LC, Newton DL, Sporn MB, De Larco JE, Todaro GJ. Transforming growth factors: Isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci USA 1980; 77:3494–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frolik CA, Dart LL, Meyers CA, Smith DM, Sporn MB. Purification and initial characterization of a type β transforming growth factor from human placenta. Proc Natl Acad Sci USA 1983; 80:3676–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vander Ark A, Cao J, Li X. TGF‐β receptors: In and beyond TGF‐β signaling. Cell Signal 2018; 52:112–20. [DOI] [PubMed] [Google Scholar]
- 4. Kelly A, Houston SA, Sherwood E, Casulli J, Travis MA. Regulation of Innate and Adaptive Immunity by TGFβ . Adv Immunol 2017; 134:137–233. [DOI] [PubMed] [Google Scholar]
- 5. Huminiecki L, Goldovsky L, Freilich S, Moustakas A, Ouzounis C, Heldin CH. Emergence, development and diversification of the TGF‐signalling pathway within the animal kingdom. BMC Evol Biol 2009; 9:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gleizes PE, Munger JS, Nunes I, Harpel JG, Mazzieri R, Noguera I et al TGF‐β latency: biological significance and mechanisms of activation. Stem Cells 1997; 15:190–7. [DOI] [PubMed] [Google Scholar]
- 7. Munger JS, Harpel JG, Gleizes PE, Mazzieri R, Nunes I, Rifkin DB. Latent transforming growth factor‐β: Structural features and mechanisms of activation. Kidney Int 1997; 51:1376–82. [DOI] [PubMed] [Google Scholar]
- 8. Gentry LE, Lioubin MN, Purchio AF, Marquardt H. Molecular events in the processing of recombinant type 1 pre‐pro‐transforming growth factor β to the mature polypeptide. Mol Cell Biol 1988; 8:4162–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gray AM, Mason AJ. Requirement for activin A and transforming growth factor‐β 1 pro‐regions in homodimer assembly. Science 1990; 247:1328–30. [DOI] [PubMed] [Google Scholar]
- 10. Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Processing of transforming growth factor β 1 precursor by human furin convertase. J Biol Chem 1995; 270:10618–24. [DOI] [PubMed] [Google Scholar]
- 11. Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T et al Latent TGF‐β structure and activation. Nature 2011; 474:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Travis MA, Sheppard D. TGF‐β activation and function in immunity. Annu Rev Immunol 2014; 32:51–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Humphries MJ. Monoclonal antibodies as probes of integrin priming and activation. Biochem Soc Trans 2004; 32:407–11. [DOI] [PubMed] [Google Scholar]
- 14. Munger JS, Huang X, Kawakatsu H, Griffiths MJD, Dalton SL, Wu J et al The integrin α v β 6 binds and activates latent TGFβ 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999; 96:319–28. [DOI] [PubMed] [Google Scholar]
- 15. Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Increased expression of integrin α v β 3 contributes to the establishment of autocrine TGF‐β signaling in scleroderma fibroblasts. J Immunol 2005; 175:7708–18. [DOI] [PubMed] [Google Scholar]
- 16. Asano Y, Ihn H, Yamane K, Jinnin M, Mimura Y, Tamaki K. Involvement of α v β 5 integrin‐mediated activation of latent transforming growth factor β 1 in autocrine transforming growth factor β signaling in systemic sclerosis fibroblasts. Arthritis Rheum 2005; 52:2897–905. [DOI] [PubMed] [Google Scholar]
- 17. Asano Y, Ihn H, Jinnin M, Mimura Y, Tamaki K. Involvement of α v β 5 integrin in the establishment of autocrine TGF‐β signaling in dermal fibroblasts derived from localized scleroderma. J Invest Dermatol 2006; 126:1761–9. [DOI] [PubMed] [Google Scholar]
- 18. Giacomini MM, Travis MA, Kudo M, Sheppard D. Epithelial cells utilize cortical actin/myosin to activate latent TGF‐β through integrin α v β 6‐dependent physical force. Exp Cell Res 2012; 318:716–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF et al The α v β 1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med 2015; 7:288ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mu D, Cambier S, Fjellbirkeland L, Baron JL, Munger JS, Kawakatsu H et al The integrin α ν β 8 mediates epithelial homeostasis through MT1‐MMP‐dependent activation of TGF‐β 1 . J Cell Biol 2002; 157:493–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fahlén L, Read S, Gorelik L, Hurst SD, Coffman RL, Flavell RA et al T cells that cannot respond to TGF‐β escape control by CD4+ CD25+ regulatory T cells. J Exp Med 2005; 201:737–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cazac BB, Roes J. TGF‐β receptor controls B cell responsiveness and induction of IgA in vivo . Immunity 2000; 13:443–51. [DOI] [PubMed] [Google Scholar]
- 23. Kawamoto S, Maruya M, Kato L, Suda W, Atarashi K, Doi Y et al Foxp3+ T cells regulate immunoglobulin A selection and facilitate diversification of bacterial species responsible for immune homeostasis. Immunity 2014; 41:152–65. [DOI] [PubMed] [Google Scholar]
- 24. Ruane D, Chorny A, Lee H, Faith J, Pandey G, Shan M et al Microbiota regulate the ability of lung dendritic cells to induce IgA class‐switch recombination and generate protective gastrointestinal immune responses. J Exp Med 2016; 213:53–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Reboldi A, Arnon TI, Rodda LB, Atakilit A, Sheppard D, Cyster JG. Mucosal immunology: IgA production requires B cell interaction with subepithelial dendritic cells in Peyer's patches. Science 2016; 352:aaf4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coombes JL, Siddiqui KRR, Arancibia‐Cárcamo CV, Hall J, Sun C‐M, Belkaid Y et al A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF‐β– and retinoic acid–dependent mechanism. J Exp Med 2007; 204:1757–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR et al Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med 2007; 204:1775–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Travis MA, Reizis B, Melton AC, Masteller E, Tang Q, Proctor JM et al Loss of integrin α v β 8 on dendritic cells causes autoimmunity and colitis in mice. Nature 2007; 449:361–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lacy‐Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Bronson RT et al Ulcerative colitis and autoimmunity induced by loss of myeloid v integrins. Proc Natl Acad Sci 2007; 104:15823–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Worthington JJ, Czajkowska BI, Melton AC, Travis MA. Intestinal dendritic cells specialize to activate transforming growth factor‐β and induce Foxp3+ regulatory T cells via integrin α v β 8 . Gastroenterology 2011; 141:1802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Païdassi H, Acharya M, Zhang A, Mukhopadhyay S, Kwon M, Chow C et al Preferential expression of integrin α v β 8 promotes generation of regulatory T cells by mouse CD103+ dendritic cells. Gastroenterology 2011; 141:1813–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luda KM, Joeris T, Persson EK, Rivollier A, Demiri M, Sitnik KM et al IRF8 Transcription‐factor‐dependent classical dendritic cells are essential for intestinal T Cell homeostasis. Immunity 2016; 44:860–74. [DOI] [PubMed] [Google Scholar]
- 33. Worthington JJ, Klementowicz JE, Rahman S, Czajkowska BI, Smedley C, Waldmann H et al Loss of the TGFβ‐activating integrin α v β 8 on dendritic cells protects mice from chronic intestinal parasitic infection via control of type 2 immunity. PLoS Pathog 2013; 9:e1003675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Steel N, Faniyi AA, Rahman S, Swietlik S, Czajkowska BI, Chan BT et al TGFβ‐activation by dendritic cells drives Th17 induction and intestinal contractility and augments the expulsion of the parasite Trichinella spiralis in mice. PLoS Pathog 2019; 15:e1007657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Melton AC, Bailey‐Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. Expression of α v β 8 integrin on dendritic cells regulates Th17 cell development and experimental autoimmune encephalomyelitis in mice. J Clin Invest 2010; 120:4436–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Acharya M, Mukhopadhyay S, Païdassi H, Jamil T, Chow C, Kissler S et al α v Integrin expression by DCs is required for Th17 cell differentiation and development of experimental autoimmune encephalomyelitis in mice. J Clin Invest 2010; 120:4445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yoshida Y, Yoshimi R, Yoshii H, Kim D, Dey A, Xiong H et al The transcription factor IRF8 activates integrin‐mediated TGF‐β signaling and promotes neuroinflammation. Immunity 2014; 40:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Boucard‐Jourdin M, Kugler D, Endale Ahanda M‐L, This S, De Calisto J, Zhang A et al β 8 integrin expression and activation of TGF‐β by intestinal dendritic cells are determined by both tissue microenvironment and cell lineage. J Immunol 2016; 197:1968–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watchmaker PB, Lahl K, Lee M, Baumjohann D, Morton J, Kim SJ et al Comparative transcriptional and functional profiling defines conserved programs of intestinal DC differentiation in humans and mice. Nat Immunol 2014; 15:98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fenton TM, Kelly A, Shuttleworth EE, Smedley C, Atakilit A, Powrie F et al Inflammatory cues enhance TGFβ activation by distinct subsets of human intestinal dendritic cells via integrin α v β 8 . Mucosal Immunol 2017; 10:624–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Babyatsky MW, Rossiter G, Podolsky DK. Expression of transforming growth factors α and β in colonic mucosa in inflammatory bowel disease. Gastroenterology 1996; 110:975–84. [DOI] [PubMed] [Google Scholar]
- 42. Kelly A, Gunaltay S, McEntee CP, Shuttleworth EE, Smedley C, Houston SA et al Human monocytes and macrophages regulate immune tolerance via integrin α v β 8‐mediated TGFβ activation. J Exp Med 2018; 215:2725–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Eastaff‐Leung N, Mabarrack N, Barbour A, Cummins A, Barry S. Foxp3+ regulatory T cells, Th17 effector cells, and cytokine environment in inflammatory bowel disease. J Clin Immunol 2010; 30:80–89. [DOI] [PubMed] [Google Scholar]
- 44. Ueno A, Jijon H, Chan R, Ford K, Hirota C, Kaplan GG et al Increased prevalence of circulating novel IL‐17 secreting Foxp3 expressing CD4+ T cells and defective suppressive function of circulating Foxp3+ regulatory cells support plasticity between Th17 and regulatory T cells in inflammatory bowel disease patients. Inflamm Bowel Dis 2013; 19:2522–34. [DOI] [PubMed] [Google Scholar]
- 45. Worthington JJ, Kelly A, Smedley C, Bauché D, Campbell S, Marie JC et al Integrin α v β 8‐mediated TGF‐β activation by effector regulatory T cells is essential for suppression of T‐cell‐mediated inflammation. Immunity 2015; 42:903–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stockis J, Liénart S, Colau D, Collignon A, Nishimura SL, Sheppard D et al Blocking immunosuppression by human Tregs in vivo with antibodies targeting integrin α V β 8 . Proc Natl Acad Sci 2017; 114:E10161–E10168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK et al Antigen‐independent differentiation and maintenance of effector‐like resident memory T cells in tissues. J Immunol 2012; 188:4866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sheridan BS, Pham QM, Lee YT, Cauley LS, Puddington L, Lefrançois L. Oral infection drives a distinct population of intestinal resident memory CD8+ T cells with enhanced protective function. Immunity 2014; 40:747–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhang N, Bevan M. Transforming growth factor‐β signaling controls the formation and maintenance of gut‐resident memory T cells by regulating migration and retention. Immunity 2013; 39:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Bergsbaken T, Bevan MJ. Proinflammatory microenvironments within the intestine regulate the differentiation of tissue‐resident CD8+ T cells responding to infection. Nat Immunol 2015; 16:406–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mohammed J, Beura LK, Bobr A, Astry B, Chicoine B, Kashem SW et al Stromal cells control the epithelial residence of DCs and memory T cells by regulated activation of TGF‐β . Nat Immunol 2016; 17:414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ostroukhova M, Seguin‐Devaux C, Oriss TB, Dixon‐McCarthy B, Yang L, Ameredes BT et al Tolerance induced by inhaled antigen involves CD4+ T cells expressing membrane‐bound TGF‐β and FOXP3. J Clin Invest 2004; 114:28–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mucida D, Kutchukhidze N, Erazo A, Russo M, Lafaille JJ, Curotto De Lafaille MA. Oral tolerance in the absence of naturally occurring Tregs. J Clin Invest 2005; 115:1923–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell‐dependent and ‐independent control of allergic inflammation. Immunity 2008; 29:114–26. [DOI] [PubMed] [Google Scholar]
- 55. Duan W, So T, Croft M. Antagonism of airway tolerance by endotoxin/lipopolysaccharide through promoting OX40L and suppressing antigen‐specific Foxp3+ T regulatory cells. J Immunol 2008; 181:8650–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Duan W, So T, Mehta AK, Choi H, Croft M. Inducible CD4+ LAP+ Foxp3– regulatory T cells suppress allergic inflammation. J Immunol 2011; 187:6499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Josefowicz SZ, Niec RE, Kim HY, Treuting P, Chinen T, Zheng Y et al Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 2012; 482:395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Soroosh P, Doherty TA, Duan W, Mehta AK, Choi H, Adams YF et al Lung‐resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med 2013; 210:775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bang BR, Chun E, Shim EJ, Lee HS, Lee SY, Cho SH et al Alveolar macrophages modulate allergic inflammation in a murine model of asthma. Exp Mol Med 2011; 43:275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zasłona Z, Przybranowski S, Wilke C, van Rooijen N, Teitz‐Tennenbaum S, Osterholzer JJ et al Resident alveolar macrophages suppress, whereas recruited monocytes promote, allergic lung inflammation in murine models of asthma. J Immunol 2014; 193:4245–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mathie SA, Dixon KL, Walker SA, Tyrrell V, Mondhe M, O'Donnell VB et al Alveolar macrophages are sentinels of murine pulmonary homeostasis following inhaled antigen challenge. Allergy Eur J Allergy Clin Immunol 2015; 70:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G et al Loss of integrin α v β 6‐mediated TGF‐β activation causes Mmp12‐dependent emphysema. Nature 2003; 422:169–73. [DOI] [PubMed] [Google Scholar]
- 63. Kim KK, Sheppard D, Chapman HA. TGF‐β1 signaling and tissue fibrosis. Cold Spring Harb Perspect Biol 2018; 10:pii: a022293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Horan GS, Wood S, Ona V, Dan JL, Lukashev ME, Weinreb PH et al Partial inhibition of integrin α v β 6 prevents pulmonary fibrosis without exacerbating inflammation. Am J Respir Crit Care Med 2008; 177:56–65. [DOI] [PubMed] [Google Scholar]
- 65. Tatler AL, Jenkins G. TGF‐β activation and lung fibrosis. Proc Am Thorac Soc 2012; 9:130–6. [DOI] [PubMed] [Google Scholar]
- 66. Wang A, Yokosaki Y, Ferrando R, Balmes J, Sheppard D. Differential regulation of airway epithelial integrins by growth factors. Am J Respir Cell Mol Biol 1996; 15:664–72. [DOI] [PubMed] [Google Scholar]
- 67. Morty RE, Konigshoff M, Eickelberg O. Transforming growth factor‐ signaling across ages: from distorted lung development to chronic obstructive pulmonary disease. Proc Am Thorac Soc 2009; 6:607–13. [DOI] [PubMed] [Google Scholar]
- 68. Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X et al IL‐17A produced by αβ T cells drives airway hyper‐responsiveness in mice and enhances mouse and human airway smooth muscle contraction. Nat Med 2012; 18:547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tatler AL, John AE, Jolly L, Habgood A, Porte J, Brightling C et al Integrin α v β 5‐mediated TGF‐β activation by airway smooth muscle cells in asthma. J Immunol 2011; 187:6094–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Meliopoulos VA, Van de Velde LA, Van de Velde NC, Karlsson EA, Neale G, Vogel P et al An epithelial integrin regulates the amplitude of protective lung interferon responses against multiple respiratory pathogens. PLoS Pathog 2016; 12:e1005804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Denney L, Branchett W, Gregory LG, Oliver RA, Lloyd CM. Epithelial‐derived TGF‐β 1 acts as a pro‐viral factor in the lung during influenza A infection. Mucosal Immunol 2018; 11:523–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Denney L, Byrne AJ, Shea TJ, Buckley JS, Pease JE, Herledan GMF et al Pulmonary epithelial cell‐derived cytokine TGF‐β 1 is a critical cofactor for enhanced innate lymphoid cell function. Immunity 2015; 43:945–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Carter NJ. Pirfenidone: in idiopathic pulmonary fibrosis. Drugs 2011; 71:1721–32. [DOI] [PubMed] [Google Scholar]
- 74. Arefayene M, Mouded M, Stebbins C, Zhao G, Song G, Christmann R et al Phase 2B dose selection of BG00011 for the treatment of idiopathic pulmonary fibrosis (IPF). Eur Respir J 2018; 52:PA596. [Google Scholar]
- 75. Kitamura H, Cambier S, Somanath S, Barker T, Minagawa S, Markovics J et al Mouse and human lung fibroblasts regulate dendritic cell trafficking, airway inflammation, and fibrosis through integrin α v β 8 – Mediated activation of TGF‐β . J Clin Invest 2011; 121:2863–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hashimoto M, Yanagisawa H, Minagawa S, Sen D, Ma R, Murray LA et al TGF‐β‐dependent dendritic cell chemokinesis in murine models of airway disease. J Immunol 2015; 195:1182–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Minagawa S, Lou J, Seed RI, Cormier A, Wu S, Cheng Y et al Selective targeting of TGF‐β activation to treat Fibroinflammatory airway disease. Sci Transl Med 2014; 6:241ra79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wakim LM, Smith J, Caminschi I, Lahoud MH, Villadangos JA. Antibody‐targeted vaccination to lung dendritic cells generates tissue‐resident memory CD8 T cells that are highly protective against influenza virus infection. Mucosal Immunol 2015; 8:1060–71. [DOI] [PubMed] [Google Scholar]
- 79. Yu CI, Becker C, Wang Y, Marches F, Helft J, Leboeuf M et al Human CD1c+ dendritic cells drive the differentiation of CD103+ CD8+ mucosal effector T cells via the cytokine TGF‐β . Immunity 2013; 38:818–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF‐β family in wound healing, burns and scarring: a review. Int J Burns Trauma 2012; 2:18–28. [PMC free article] [PubMed] [Google Scholar]
- 81. Deckers J, Hammad H, Hoste E. Langerhans cells: sensing the environment in health and disease. Front Immunol 2018; 9:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang Y, Zenke Y, Hirai T, Kaplan DH. Keratinocyte‐derived TGFβ is not required to maintain skin immune homeostasis. J Dermatol Sci 2019; 94:290–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Duperret EK, Natale CA, Monteleon C, Dahal A, Ridky TW. The integrin α v‐TGFβ signaling axis is necessary for epidermal proliferation during cutaneous wound healing. Cell Cycle 2016; 15:2077–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. MacKay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML et al The developmental pathway for CD103+ CD8+ tissue‐resident memory T cells of skin. Nat Immunol 2013; 14:1294–301. [DOI] [PubMed] [Google Scholar]
- 85. Mackay LK, Wynne‐Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM et al T‐box transcription factors combine with the cytokines TGF‐β and IL‐15 to control tissue‐resident memory T cell fate. Immunity 2015; 43:1101–11. [DOI] [PubMed] [Google Scholar]
- 86. Mani V, Bromley SK, Äijö T, Mora‐Buch R, Carrizosa E, Warner RD et al Migratory DCs activate TGF‐β to precondition naïve CD8+ T cells for tissue‐resident memory fate. Science 2019; 366:eaav5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hirai T, Zenke Y, Yang Y, Bartholin L, Beura LK, Masopust D et al Keratinocyte‐mediated activation of the cytokine TGF‐β maintains skin recirculating memory CD8+ T cells. Immunity 2019; 50:1249–1261.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]


