Short abstract
A 30-year-old woman was admitted to a different hospital with a 2-day history of fever, cough, and expectoration. She had a history of left pulmonary tuberculosis 8 years previously. Chest computed tomography showed an infiltrate in the inferior lobe of the left lung and spot-like calcifications in the anterior lobe of the upper left lobe and lower lobe of the left lung. After antibacterial treatment, the patient’s condition deteriorated and she developed significant pleural effusion on the left side. The pleural effusion assay showed a lymphocyte-predominant exudate with a significantly increased adenosine deaminase level. The patient was transferred to our hospital with a suspected diagnosis of tuberculous pleuritis. A serum test for Mycoplasma pneumoniae-specific immunoglobulin M was positive. Because of the limitations of this test in determining the occurrence of recent infection, a thoracoscopic pleural biopsy was performed, and M. pneumoniae DNA was detected in the biopsy tissue using M. pneumoniae-specific polymerase chain reaction. Thus, the patient was diagnosed with M. pneumoniae-related parapneumonic effusion. Clinicians must be aware of the usefulness and limitations of a high adenosine deaminase level and know that lymphocyte predominance in pleural effusion does not always indicate tuberculous pleurisy, especially in areas of high tuberculosis prevalence.
Keywords: Mycoplasma pneumoniae, tuberculosis, pleurisy, Mycoplasma pneumoniae-specific immunoglobulin M, adenosine deaminase, polymerase chain reaction
Introduction
Mycoplasma pneumoniae is a common human respiratory tract pathogen that causes 6% to 30% of community-acquired pneumonia cases in adults worldwide.1 The incidence is particularly high in China, ranging from 20% to 30%.2,3 Parapneumonic effusion occurs in 7% to 20% of patients with Mycoplasma pneumoniae pneumonia.4–7 The etiology of pleural effusion varies significantly with the economic development of different countries or regions. In developed countries, congestive heart failure, malignancy, pneumonia, and pulmonary embolism are the most common causes of pleural effusion, with a proportion of >90%. In contrast, tuberculosis effusion is a common cause in developing countries.8,9 Furthermore, pleural effusion accompanying an acute Mycoplasma pneumoniae infection or tuberculous pleurisy has similar characteristics.10 Therefore, especially in countries with a high prevalence of tuberculosis, sufficient differentiation and diagnosis of Mycoplasma pneumoniae pneumonia-induced pleurisy is necessary.
The present report describes a patient with Mycoplasma pneumoniae pneumonia-induced pleurisy mimicking tuberculous pleurisy along with a review of related published studies. This study was approved by the Human Research Ethics Committees of Shandong Provincial Chest Hospital (ethics approval number: 2020XKYYEC-06). Written informed consent was obtained from the patient for publication of this case study.
Case report
On 31 October 2018, a 30-year-old woman was admitted to a different hospital with a 2-day history of fever (>39°C), cough, and expectoration without fatigue, night sweats, or weight loss. The patient had developed left pulmonary tuberculosis in 2009 and recovered after adequate anti-tuberculosis drug therapy. Chest computed tomography showed an infiltrate in the inferior lobe of the left lung and spot-like calcifications in the anterior lobe of the upper left lobe and the lower lobe of the left lung.
The patient underwent antibiotic therapy with piperacillin sodium + tazobactam sodium and moxifloxacin, but her clinical condition deteriorated and her recurrent high fever persisted. On 10 November 2018, a repeat computed tomography scan revealed massive pleural effusion in the left hemithorax with atelectasis (Figure 1). Closed thoracic drainage was performed, and the pleural effusion assay showed a lymphocyte-predominant exudate with a significantly increased adenosine deaminase (ADA) level (46 U/L). On 13 November 2018, the patient was transferred to our hospital with a suspected diagnosis of tuberculous pleuritis.
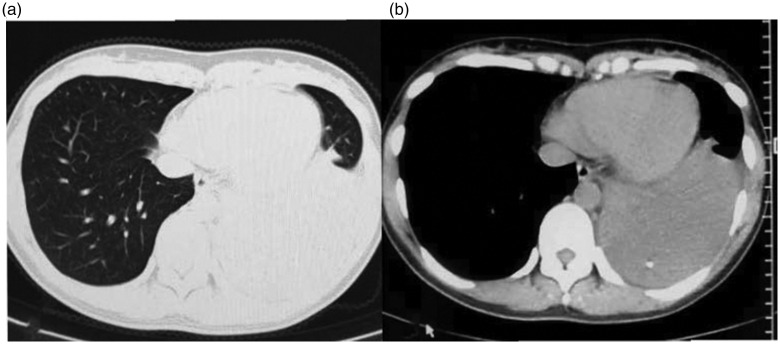
Figure 1.
Chest computed tomography scan of the patient showing massive left-sided pleural effusion with spot-like calcifications in the lower lobe of the left lung.
On admission, her vital signs were as follows: body temperature, 36.9°C; blood pressure, 115/78 mmHg; and pulse rate, 108 beats/minute. Breath sounds over the left chest were diminished. As shown in Table 1, laboratory data revealed elevations in both her C-reactive protein level and erythrocyte sedimentation rate. Her white blood cell count was normal. Normocytic anemia and a low albumin level were also noted. Anti-human immunodeficiency virus antibody was not detected. Pleural effusion and sputum specimens were tested for general and acid-fast bacteria smears and general bacterial culture, respectively, and all results were negative. The pleural fluid was gradually drained through closed thoracic drainage (Figure 2).
Table 1.
Laboratory data of the patient.
| WBC count (cells/L) | 6.21 × 109 | IgG (mg/dL) | 12.82 | Pleural effusion | |
|---|---|---|---|---|---|
| Neu (%) | 57.2 | IgM (mg/dL) | 2.79 | Appearance | Yellow |
| Mon (%) | 6.4 | IgE (IU/mL) | 52 | Predominant cell | |
| Lym (%) | 30.3 | CRP (mg/L) | 46.2 | Lymphocytes (%) | 98 |
| Eos (%) | 0.8 | HBsAg | (−) | Neutrophils (%) | 2 |
| Hb (g/dL) | 108 | HCV Ab | (−) | LDH (U/L) | 685 |
| Plt (/L) | 376 × 109 | HIV Ab | (−) | Glucose (mmol/L) | 6.33 |
| ESR (mm/h) | 34 | CEA (ng/mL) | 0.34 | Total protein (g/L) | 47.5 |
| PCT (ng/mL) | 0.13 | ADA (U/L) | 46 | ||
| TP (g/L) | 59.1 | T-SPOT.TB | (−) | ||
| Alb (g/L) | 29.6 | PPD | (−) | ||
| AST (U/L) | 42 | ANA | (−) | ||
| ALT (U/L) | 25 | ||||
| LDH (U/L) | 26 | ||||
| ALP (U/L) | 92 | ||||
| T-Bil (μmol/L) | 22.31 | ||||
| Cre (μmol/L) | 64.5 | ||||
| Na (mEq/l) | 138 | ||||
| K (mEq/l) | 4.00 | ||||
| BUN (mmol/L) | 2.62 |
WBC, white blood cell; Neu, neutrophils; Mon, monocytes; Lym, lymphocytes; Eos, eosinophils; Hb, hemoglobin; Plt, platelets; ESR, erythrocyte sedimentation rate; TP, total protein; Alb, albumin; AST, aspartate aminotransferase; ALT, alanine aminotransferase; LDH, lactate dehydrogenase; ALP, alkaline phosphatase; T-Bil, total bilirubin; Cre, creatinine; Na, sodium; K, potassium; BUN, blood urea nitrogen; Ig, immunoglobulin; CRP, C-reactive protein; HBsAg, hepatitis B surface antigen; HCV Ab, hepatitis C virus antibodies; HIV Ab, human immunodeficiency virus antibodies; CEA, carcinoembryonic antigen; PCT, procalcitonin; PPD, purified protein derivative; ANA, antinuclear antibody; ADA, adenosine deaminase.
Figure 2.
Chest computed tomography scan of the patient showing local pleural hypertrophy after the pleural fluid was drained through closed thoracic drainage.
Based on the patient’s clinical course and test findings, especially the pleural effusion assay, a diagnosis of tuberculous pleuritis was made. Daily administration of 300 mg of isoniazid, 450 mg of rifampicin, 750 mg of hydrochloride, and 1500 mg of pyrazinamide was initiated on the day of admission. Although the patient’s clinical condition had somewhat improved, she still had a recurrent mild to moderate fever that occasionally reached a high level. Both the T-SPOT.TB assay and pleural fluid TB-PCR test were negative. A tuberculin skin test (TST) was negative 72 hours after admission. Given the atypical clinical course and the negative results of the T-SPOT.TB, TB-PCR, and TST, connective tissue disease-related antibodies and Mycoplasma antibodies were further tested 4 days after admission. Serum Mycoplasma pneumoniae-specific immunoglobulin (Ig) M as measured by enzyme-linked immunosorbent assay was positive (114.70 BU/mL). Because of the limitations of IgM measurement in judging the occurrence of recent infection, further examinations using electronic endoscopy and thoracoscopy were performed. On day 7 after admission, electronic bronchoscopy revealed a few purulent secretions in the anterior basal segment of the inferior lobe of the left lung. Medical thoracoscopy showed that both the visceral and parietal pleura were smooth. Pleural biopsy was performed, and histopathological examination revealed chronic inflammation with numerous lymphocytes and mononuclear cells. Mycoplasma pneumoniae DNA was extracted in the pleural biopsy tissue using PCR (DNeasy tissue kit; CapitalBio, Beijing, China) designed to detect Mycoplasma pneumoniae.
The patient was diagnosed with Mycoplasma pneumoniae-related parapneumonic effusion. The administration of anti-tuberculous drugs was therefore discontinued. She gradually recovered thereafter with administration of intravenous moxifloxacin (400 mg once a day) for 20 days. Pleural effusion and sputum specimens were cultured several times both for common pathogens as well as Mycobacterium, but no growth occurred. The patient received no drugs after discharge, and a chest radiograph 3 months later showed no residual pleural lesions (Figure 3). She had developed no other conditions at the 6-month follow-up. Stains, cultures, and pathological examinations of the pleura were performed repeatedly before and after treatment with antibiotics, and no evidence of any other causative agents was found.
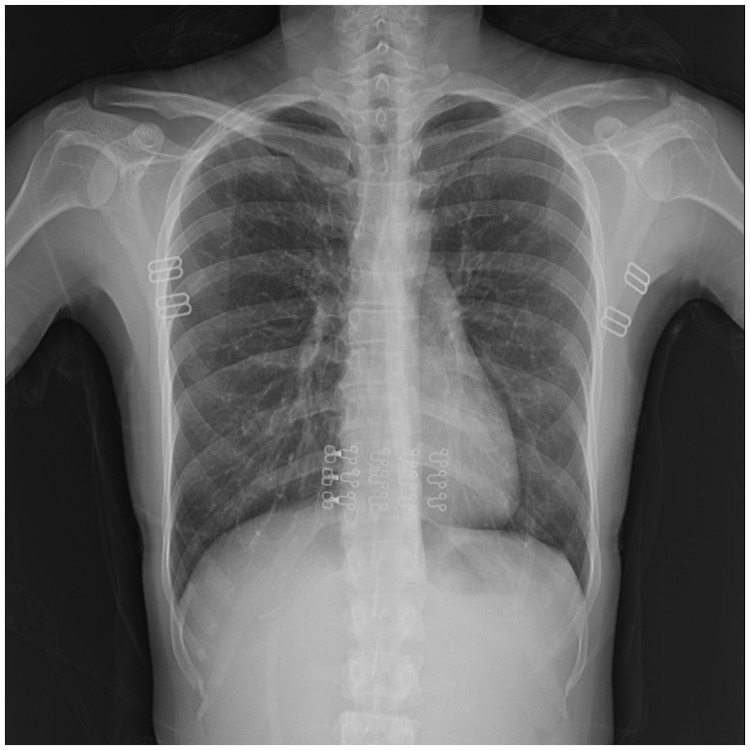
Figure 3.
Chest radiograph of the patient showing no residual pleural lesions 3 months after discharge.
Discussion
An important clinical observation in the present case is that the cause of the incorrect initial diagnosis was the elevated ADA concentration and the biochemical characteristics of the pleural fluid.
Unlike in developed countries, the most common cause of exudative effusion is tuberculosis in areas with a high prevalence of tuberculosis.8,9,11 Tuberculous pleurisy is definitively diagnosed on the basis of the following criteria: (1) a positive acid-fast bacilli smear or positive cultures of Mycobacterium tuberculosis in pleural fluid and pleural tissue, (2) chronic granulomatous inflammation in pleural tissue, and (3) a clinical response to anti-tuberculosis treatment. When a pleural biopsy is not done, patients are considered to have tuberculous pleurisy if they meet the following three conditions: (1) ADA level in pleural fluid of >45 U and/or isolation of Mycobacterium tuberculosis from sputum, (2) exclusion of any other cause known to be associated with pleural effusion, and (3) clearance of the effusion with anti-tuberculous therapy.
The diagnosis of tuberculous pleurisy can be challenging; the standard criterion is seldom met because of the paucibacillary nature of this condition. The sensitivity of a pleural fluid smear for acid-fast bacilli (0% to 1%) and Mycobacterium culture of pleural fluid and/or pleural biopsy specimens (24% to 58%), which requires a standardized laboratory, is low. This significantly limits the value of diagnostic applications.
Emerging data have shown that Mycobacterium tuberculosis is detected in only 31% of patients with tuberculous pleurisy.12 In fact, in countries with a moderate to high incidence of the disease, the diagnosis of tuberculous pleurisy has greatly relied on the use of ADA measurement13 because of the paucibacillary nature of this condition.14 An ADA level of ≥40 U/L in a lymphocyte-dominant exudate obtained via thoracentesis is a widely accepted indicator of pleurisy caused by tuberculosis.15 Notably, this is not entirely reliable and may lead to an incorrect initial diagnosis. In addition, other than tuberculous pleurisy, clinicians should be aware that other representative diseases that may cause lymphocytic pleural effusion include malignant disease, lymphoma, Mycoplasma pleurisy, and collagen disease.16
Parapneumonic effusion usually shows polymorphonuclear leukocyte predominance. In contrast, Mycoplasma pneumoniae-related parapneumonic effusion, an uncommon feature of Mycoplasma pneumoniae, occurs in 4% to 20% of patients infected by Mycoplasma pneumoniae and usually presents as a lymphocyte-dominant exudate with a high ADA level mimicking tuberculous pleurisy.10 A study by Cha et al.10 showed that all five patients with Mycoplasma pleurisy with lymphocyte-predominant pleural effusions exhibited high ADA levels in the pleural fluid (>40 IU/L). Furthermore, the clinical process of tuberculous pleurisy in young people, which tends to develop acutely,17 is similar to that of acute Mycoplasma infection.
Because of the similar clinical course and characteristics of pleural effusion between pleural effusion induced by an acute Mycoplasma pneumoniae infection and tuberculous pleurisy, misdiagnosis is likely to occur in countries with a high or low tuberculosis burden. Clinicians should be aware that a high ADA level and lymphocyte predominance in pleural effusion do not always indicate tuberculous pleurisy, especially in areas with a high tuberculosis prevalence.
Early differential diagnosis between Mycoplasma pneumoniae-related parapneumonic effusion and tuberculous pleurisy is necessary to avoid adverse effects and delayed treatment. Previous studies have revealed that most cases of Mycoplasma pneumoniae-related parapneumonic effusion are unilateral and low-volume and resolve with appropriate antimicrobial therapy.18–20 We have herein presented a case of Mycoplasma pneumoniae with an associated mass of pleural effusion that required differentiation from tuberculous pleurisy. The clinical course and pleural effusion findings supported a diagnosis of tuberculous pleurisy. However, our initial judgment was based on only a single pleural effusion sample with a high ADA level and lymphocyte predominance, which was problematic in itself. Differential diagnosis is difficult because of the similar characteristics between Mycoplasma pleurisy and tuberculous pleurisy.
Several methods, such as the IgM assay, IgG assay, Mycoplasma pneumoniae culture, and PCR, have been used as reference methods in recent studies to determine the presence of Mycoplasma infection. The IgM assay is very simple and allows the diagnosis of recent or acute Mycoplasma pneumoniae infection using a single serum specimen. After infection by Mycoplasma pneumoniae, IgM antibodies rise during the first week of the illness and reach the highest titers during the third week. However, investigators have indicated that specific IgM antibodies to Mycoplasma pneumoniae can be retained in the circulation for 1 year after a Mycoplasma pneumoniae infection and that Mycoplasma-specific IgM antibody carriers accordingly exist at a specific rate in healthy populations.21 Thus, the presence of IgM does not necessarily indicate an acute infection. The result of a single Mycoplasma pneumoniae-specific IgM antibody test is not reliable when differentiating between tuberculous pleural effusion and pleural effusion caused by Mycoplasma infection.
By identifying a four-fold increase in the IgG titer taken 2 to 4 weeks apart, clinicians can definitively diagnose a recent Mycoplasma pneumoniae infection.22 Nevertheless, obtaining convalescent serum samples and dealing with time constraints is difficult, limiting the use of this method in clinical practice. IgG antibody titer tests of paired serum samples are more often used in epidemiological investigations than as a diagnostic tool. In addition, because of the fastidious nature of this microorganism and the specialized growth media that is required, nucleic acid amplification testing is currently the most reliable technique for patient diagnosis.23 However, clinical samples obtained from relatively inaccessible sites may be paucibacillary in nature, limiting the performance of diagnostic tests.24 Few studies to date have investigated the sensitivity of PCR to detect samples from pleural tissues for diagnosis of Mycoplasma pneumoniae pneumonia-induced pleurisy. It can be speculated that PCR has limited diagnostic sensitivity.
Making a definitive diagnosis is always challenging as such patients. Therefore, in the present patient, a histological examination via thoracoscopic pleural biopsy was employed for a definitive diagnosis of the pleural effusion. Mycoplasma PCR was performed on the pleural biopsy tissue, and the patient was finally diagnosed with Mycoplasma pneumoniae infection with pleural effusion.
Pleural biopsy has great value in the diagnosis of tuberculosis. Medical thoracoscopy is well documented as a simple procedure with high diagnostic yield and excellent safety for the diagnosis of tuberculous pleural effusion.25 It can be concluded that the use of thoracoscopy under local anesthesia is also highly effective for excluding tuberculous pleurisy. Therefore, even if Mycoplasma PCR is negative, tuberculosis can be eliminated with a high probability by medical thoracoscopy, thus providing great assistance in guiding the therapeutic strategy. Because both diseases are curable, it is important to achieve a definitive diagnosis as early as possible. Early performance of thoracoscopic pleural biopsy is important to distinguish the possibility of tuberculous pleurisy or acute Mycoplasma pneumoniae infection-induced pleural effusion, rather than waiting for the Mycobacteria culture results.
Although our patient had a history of tuberculosis, the TST was negative. This can be affected by the conditions responsible for severe protein malnutrition (albumin level of 29.6/L), which is a common risk factor for false-negative TST results.26
To the best of our knowledge, only a few cases requiring differentiation between tuberculous pleurisy and Mycoplasma pleurisy have been reported from countries without a high burden of tuberculosis.27 The present case is the first report of its kind from an area with a high prevalence of tuberculosis. However, this study has some limitations. A retrospective study involving more cases of previously diagnosed tuberculous pleurisy with Mycoplasma pneumoniae-specific IgM positivity should be conducted to assess the possible misdiagnosis rate, which may help clinicians develop better clinical management strategies.
Conclusion
We have herein reported the clinical course of Mycoplasma pneumoniae-related massive pleural effusion that was initially diagnosed as tuberculous pleurisy. Clinicians must be aware that a high ADA level and lymphocyte predominance in pleural effusion do not always indicate tuberculous pleurisy, and they must closely examine the possibility of Mycoplasma pneumoniae pleurisy before concluding that the pleural effusion has been caused by tuberculous infection, especially in areas with a high prevalence of tuberculosis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Chinese Medicine Science and Technology Development Project of Shandong Province (Grant No. 2019-0525).
ORCID iD
References
- 1.Loens K Goossens H andIeven M.. Acute respiratory infection due to Mycoplasma pneumoniae: current status of diagnostic methods. Eur J Clin Microbiol Infect Dis 2010; 29: 1055–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Chen M, Zhao T, et al. Causative agent distribution and antibiotic therapy assessment among adult patients with community acquired pneumonia in Chinese urban population. BMC Infect Dis 2009; 9: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao B, Ren LL, Zhao F, et al. PL-004 Viral and Mycoplasma pneumoniae community acquired pneumonia and novel clinical outcome evaluation in ambulatory adult patients. Eur J Clin Microbiol Infect Dis 2010; 29: 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada F, Ando YM, Matsumoto S, et al. Chlamydia pneumoniae pneumonia and Mycoplasma pneumoniae pneumonia: comparison of clinical findings and CT findings. J Comput Assist Tomogr 2005; 29: 626–632. [DOI] [PubMed] [Google Scholar]
- 5.Atsushi N, Akitoshi S, Tsutomu A, et al. Chlamydia pneumoniae: comparison with findings of Mycoplasma pneumoniae and Streptococcus pneumoniae at thin-section CT. Radiology 2006; 238: 330. [DOI] [PubMed] [Google Scholar]
- 6.Reittner P, Müller NL, Heyneman L, et al. Mycoplasma pneumoniae pneumonia: radiographic and high-resolution CT features in 28 patients. AJR Am J Roentgenol 2000; 174: 37. [DOI] [PubMed] [Google Scholar]
- 7.Miyashita N, Sugiu T, Kawai Y, et al. Radiographic features of Mycoplasma pneumoniae pneumonia: differential diagnosis and performance timing. BMC Med Imaging 2009; 9: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Light RW. Clinical practice. Pleural effusion. N Engl J Med 2002; 346: 1971. [DOI] [PubMed] [Google Scholar]
- 9.Light RW. Update on tuberculous pleural effusion. Respirology 2010; 15: 451–458. DOI: 10.1111/j.1440-1843.2010.01723.x. [DOI] [PubMed] [Google Scholar]
- 10.Cha SI, Shin KM, Jeon KN, et al. Clinical relevance and characteristics of pleural effusion in patients with Mycoplasma pneumoniae pneumonia. Scand J Infect Dis 2012; 44: 793–797. DOI: 10.3109/00365548.2012.681696. [DOI] [PubMed] [Google Scholar]
- 11.Zumla A, Raviglione M, Hafner R, et al. Tuberculosis. N Engl J Med 2013; 368: 745–755. DOI: 10.1056/NEJMra1200894. [DOI] [PubMed] [Google Scholar]
- 12.Bielsa S, Palma R, Pardina M, et al. Comparison of polymorphonuclear- and lymphocyte-rich tuberculous pleural effusions. Int J Tuberc Lung Dis 2013; 17: 85–89. DOI: 10.5588/ijtld.12.0236. [DOI] [PubMed] [Google Scholar]
- 13.Zemlin AE Burgess LJ andCarstens ME.. The diagnostic utility of adenosine deaminase isoenzymes in tuberculous pleural effusions. Int J Tuberc Lung Dis 2009; 13: 214–220. [PubMed] [Google Scholar]
- 14.Udwadia ZF andSen T.. Pleural tuberculosis: an update. Curr Opin Pulm Med 2010; 16: 399–406. [DOI] [PubMed] [Google Scholar]
- 15.Doosoo J. Tuberculous pleurisy: an update. Tuberc Respir Dis (Seoul) 2014; 76: 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Light RW. Pleural diseases. 6th ed Philadelphia: Lippincott Williams & Wilkins, 2013, pp.1–504. [Google Scholar]
- 17.Levine H Szanto PB andCugell DW.. Tuberculous pleurisy. An acute illness. Arch Intern Med 1968; 122: 329–332. [PubMed] [Google Scholar]
- 18.John SD Ramanathan J andSwischuk LE.. Spectrum of clinical and radiographic findings in pediatric mycoplasma pneumonia. Radiographics 2001; 21: 121. [DOI] [PubMed] [Google Scholar]
- 19.Jayantha UK. Study on pleural effusions due to Mycoplasma pneumoniae infection. Sri Lanka Journal of Child Health 2009; 34: 5–6. DOI: 10.4038/sljch.v34i1.562. [Google Scholar]
- 20.Chiou CC, Liu YC, Lin HH, et al. Mycoplasma pneumoniae infection complicated by lung abscess, pleural effusion, thrombocytopenia and disseminated intravascular coagulation. Pediatr Infect Dis J 1997; 16: 327. [DOI] [PubMed] [Google Scholar]
- 21.Narita M. [ Utility and limitation of the rapid IgM antibody detection test for the diagnosis of Mycoplasma pneumoniae infection]. Kansenshogaku Zasshi 2007; 81: 149–154. [DOI] [PubMed] [Google Scholar]
- 22.Gardiner SJ Gavranich JB andChang AB.. Antibiotics for community-acquired lower respiratory tract infections secondary to Mycoplasma pneumoniae in children. Cochrane Database Syst Rev 2015; 1: CD004875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Totten AH, Leal SM, Jr, Ratliff AE, et al. Evaluation of the ELITe InGenius PCR Platform for detection of Mycoplasma pneumoniae. J Clin Microbiol 2019; 57: pii: e00287-19. DOI: 10.1128/JCM.00287-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brodie D andSchluger NW.. The diagnosis of tuberculosis. Clin Chest Med 2005; 26: 247–271, vi. DOI: 10.1016/j.ccm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Wang Z, Xu LL, Wu YB, et al. Diagnostic value and safety of medical thoracoscopy in tuberculous pleural effusion. Respir Med 2015; 109: 1188–1192. DOI: 10.1016/j.rmed.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 26.Pelly TF, Santillan CF, Gilman RH, et al. Tuberculosis skin testing, anergy and protein malnutrition in Peru. Int J Tuberc Lung Dis 2005; 9: 977–984. [PMC free article] [PubMed] [Google Scholar]
- 27.Kazuyoshi N, Tohru Y, Yuichi Y, et al. [Case of tuberculous pleurisy distinguished from pleurisy caused by Mycoplasma infection]. Kekkaku 2013; 88: 423–427. [PubMed] [Google Scholar]