Abstract
The coronavirus disease-2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) that has significant potential cardiovascular implications for patients. These include myocarditis, acute coronary syndromes, cardiac arrhythmias, cardiomyopathies with heart failure and cardiogenic shock, and venous thromboembolic events. We describe a Caribbean-Black gentleman with COVID-19 infection presenting with atrial arrhythmias, namely, atrial flutter and atrial fibrillation, which resolved with rate and rhythm control strategies, and supportive care.
Keywords: atrial arrhythmias, atrial flutter, atrial fibrillation, severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, coronavirus disease 2019, COVID-19
Introduction
Coronavirus disease-2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2).1,2 The disease was first identified in 2019 in Wuhan, China, and has since spread globally, resulting in the 2019-2020 coronavirus pandemic.1
The World Health Organization declared the 2019-2020 coronavirus outbreak a Public Health Emergency of International Concern on January 30, 2020, and a pandemic on March 11, 2020.3,4
COVID-19 may have deleterious effects on the cardiovascular (CV) system, and patients with preexisting CV disease. Several recent Chinese studies have since demonstrated the sequelae of CV events.5-7 As the pandemic evolves, the emerging literature on CV outcomes are not well characterized, but likely encompass acute coronary syndromes, myocarditis, cardiomyopathies, cardiogenic shock, lethal arrhythmias, and sudden cardiac death.
We describe a case of a middle-aged Caribbean-Black gentleman presenting with COVID-19 infection who experienced atrial arrhythmias, namely, atrial flutter (AFL) and atrial fibrillation (AF), which resolved with rate and rhythm control strategies, and supportive care.
Case Report
A 46-year-old Caribbean-Black male with no significant medical history presented to the emergency department (San Fernando General Hospital, Trinidad) with a symptom complex of fever, cough, and shortness of breath over the preceding 2 days. His vital signs indicated systolic blood pressures of 140 mm Hg, heart rate of 142 beats per minute, and respiratory rate of 28 breaths per minute with an oxygen saturation of 88% on room air. Apart from hypertension, tachycardia, and tachypnea, his physical examination revealed a normal jugular venous pulse, scattered bilateral crackles, and no peripheral edema.
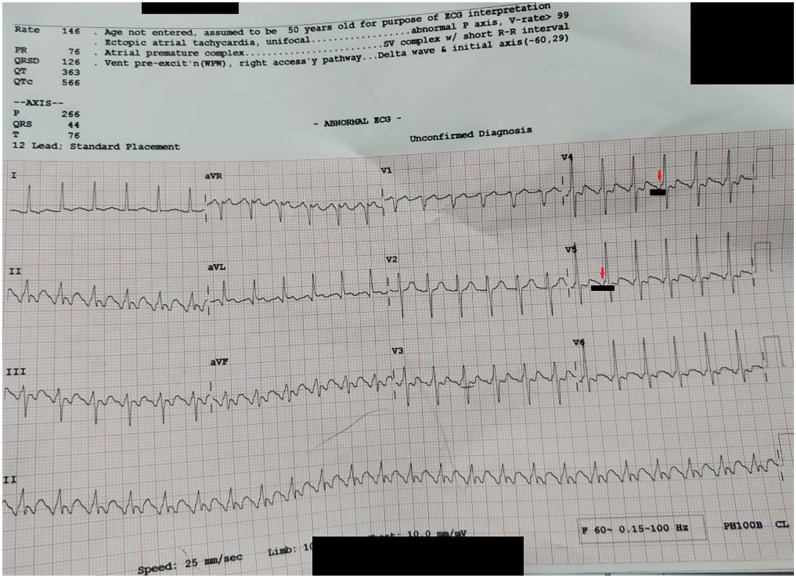
A 12-lead electrocardiogram revealed typical AFL with a 2 to 1 atrioventricular block and rate-related ST-T segment changes (see Figure 1).
Figure 1.
The patient’s electrocardiogram in which the red arrows indicate the typical flutter waves (f-waves) that occur right before the QRS complex, simulating a pseudo-preexcitation pattern. The segment underscored in black indicates the f-waves in series at a rate of approximately 240 beats per minute. The QRS complexes are occurring at 120 to 140 beats per minute, hence the 2:1 atrioventricular block.
A chest radiograph did not reveal any acute cardiopulmonary disease (see Figure 2), while a bedside 2-dimensional transthoracic echocardiogram demonstrated a preserved left ventricular ejection fraction, without any regional wall motion abnormalities. Pertinent diagnostic laboratory investigations included a D-dimer 357 ng/dL (normal ≤500 ng/mL), pro-brain natriuretic peptide 413 pg/mL (normal ≤300 pg/mL), cardiac biomarkers, CK-MB 15 U/L (normal <20 U/L), and troponin I 0.12 ng/mL (normal 0.0-0.15 ng/mL). Other routine investigations are indicated in Table 1. The patient’s arterial blood gas was consistent with mild hypoxia on 24% fractional inspiration of oxygen with an estimated alveolar-arterial gradient of 17 mm Hg.
Figure 2.
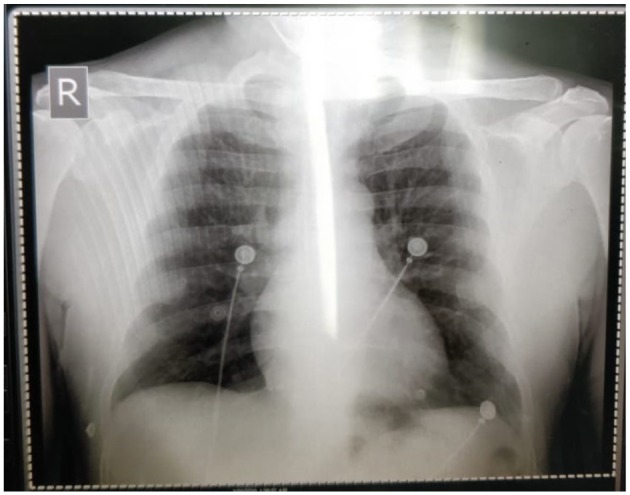
The patient’s chest radiograph does not indicate any airspace disease that would be expected in coronavirus-2019 (COVID-19) infection.
Table 1.
Routine Investigations.
| Tests Performed | Result | Reference Range |
|---|---|---|
| Hemoglobin | 9.4 g/dL | 14.0-17.5 g/dL |
| White blood cell count | 13.2 × 109/L | 4.5-11.0 × 109/L |
| Platelet count | 201 × 103/µL | 156-373 × 103/µL |
| Serum sodium | 134 mmol/L | 135-145 mmol/L |
| Serum potassium | 2.8 mmol/L | 3.5-5.1 mmol/L |
| Serum bicarbonate | 22 mmol/L | 22-26 mmol/L |
| Serum creatinine | 0.5 mg/dL | 0.5-1.2 mg/dL |
| Serum osmolality | 283 mOsm/kg | 275-295 mOsm/kg |
| Blood urea nitrogen | 8 mg/dL | 3-20 mg/dL |
| Fasting blood sugar | 116 mg/dL | 60-120 mg/dL |
| Alanine aminotransferase | 26 IU/L | 20-60 IU/L |
| Aspartate aminotransferase | 68 IU/L | 5-40 IU/L |
| Total bilirubin | 2.2 mg/dL | 0.2-1.2 mg/dL |
| Alkaline phosphatase | 101 IU/L | 40-129 IU/L |
| Albumin | 2.7 g/dL | 3.5-5.5 g/dL |
| Albumin-corrected calcium | 7.3 mg/dL | 9.6-11.2 mg/dL |
| Magnesium | 1.6 mg/dL | 1.6-2.3 mg/dL |
| Phosphorous | 2.3 mg/dL | 2.5-6.5 mg/dL |
| Serum cortisol level | 18.3 µg/dL | 10-20 µg/dL |
| Thyroid-stimulating hormone | 1.44 mU/L | 0.5-5.0 mU/L |
| Urine osmolality | 534 mOsm/kg | 300-900 mOsm/kg |
| Urine sodium | < 20 mEq/L | 40-220 mEq/L |
| Erythrocyte sedimentation rate | 68 mm/h | 0-22 mm/h |
| High-sensitivity C-reactive protein | 83 mg/dL | 0.0-1.0 mg/dL |
| D-dimer | 357 ng/mL | <500 ng/mL |
| pro-brain natriuretic peptide | 413 pg/mL | ≤300 pg/mL |
| Creatine kinase | 873 U/L | 30-170 U/L |
| Creatine kinase MB | 15 U/L | <20 U/L |
| Lactate dehydrogenase | 1717 U/L | 313-618 U/L |
| High-sensitivity troponin I | 0.12 ng/mL | 0.0-0.15 ng/mL |
| Blood cultures | Negative | Positive or negative |
| Urine culture | Negative | Positive or negative |
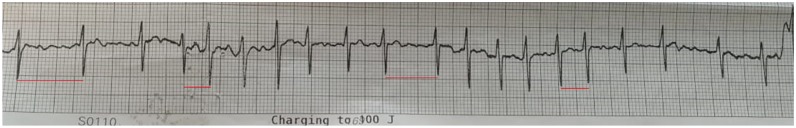
In the designated isolation room, he was initiated on an amiodarone and digoxin bolus, moderate-intensity beta-blockade, and subsequently admitted for further hospitalization (Table 2). Despite these therapies, the patient unsuccessfully underwent cardioversion with 100 J and subsequently transitioned to atrial fibrillation with rapid ventricular (AF RVR) response (see Figure 3). In the interim, the patient’s COVID-19 test (Centers for Disease Control and Prevention’s 2019-nCoV Real-Time RT-PCR Diagnostic Panel, Atlanta, GA) returned positive, and he was transferred to another quarantine facility (Couva Medical and Multi-Training Facility, Trinidad) with intensive care unit (ICU) capabilities for further management.
Table 2.
The Patient’s Individualized Cardiovascular Medicine Regimen for Coronavirus-2019 (COVID-19) Infection and Rationale.
| Drug | Dose | Rationale |
|---|---|---|
| Direct oral anticoagulation (DOAC) | Not utilized | DOAC was not instituted as the patient was in paroxysmal atrial fibrillation with a CHADS-VASc and HAS-BLED score of 0. The patient was discharged to self-quarantine with an outpatient 1-week Holter monitor prior to the follow-up appointment. |
| Atenolol | 50 mg every 8 hours | A lenient rate control strategy with this β-blocker was adopted with the significant advantages being relatively cardioselective and minimal interactions given the patient’s normal renal function.8 |
| Amiodarone | 200 mg every 12 hours | Oral amiodarone after a 48-hour infusion was used synergistically as a rhythm control strategy in addition to a rate control strategy. As the patient’s chest radiograph was normal, it was initiated with increased vigilance for any pneumonitis that could potentially complicate COVID-19 infection.9,10 |
| Digoxin | Not utilized | This drug was discontinued after the initial loading dose.11 |
| Hydroxychloroquine | Not utilized | This was considered, however, ultimately not utilized after a detailed risk-benefit analysis. There was a major concern about its adverse effect profile, including QT prolongation and drug-drug interactions. |
| Azithromycin | Not utilized | This antibiotic, while displaying therapeutic synergy with hydroxychloroquine was deferred due to its arrhythmogenic effects from QT prolongation.12,13 |
| Lopinavir-Ritonavir | Not utilized | This antiretroviral combination was not utilized due to drug-drug interactions and lack of clinical effectiveness in a recent trial.14 |
Figure 3.
The patient’s rhythm strip post-cardioversion, which indicates coarse atrial fibrillation with a rapid ventricular response. The variable RR intervals highlighted by the interspersed red lines.
During the ensuing hospitalization, he was continued on an amiodarone infusion at 1 milligram per minute and atenolol, and his symptoms gradually ameliorated with decreasing oxygen requirements. He reverted to normal sinus rhythm within 48 hours, and as a result, anticoagulation was deferred in light of both CHADS-VASc and HAS-BLED scores of 0 each. The remainder of his hospital course was uneventful, and he was subsequently discharged to home quarantine on oral low dose, twice daily amiodarone with a follow-up visit, and 1-week Holter monitor in 1 month.
Discussion
It has been recently reported that CV compromise is a common complication of patients who are hospitalized with COVID-19 infection and is associated with a higher risk of mortality.15 Cardiac arrhythmias are also frequent clinical manifestations; however, there is a paucity in the emerging literature with regard to the nature and classification of these arrhythmogenic events.
In a recent series, comprising nearly 148 patients, almost one tenth reported palpitations.16 In another recent similarly sized study, arrhythmia was noted in almost one sixth of the patients and frequently occurred within the ICU subgroup of patients with almost half being affected.17 Despite these emerging studies, the characteristics of these arrhythmias are not yet published nor previously described. The development of potentially lethal arrhythmias, especially in the setting of elevated cardiac biomarkers, should herald myocarditis as a differential diagnosis.18,19 The Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with COVID-19 do not currently include guidelines with regard to specific arrhythmia management.20
Arrhythmias are complex and multifactorial in a COVID-19 patient and may result from metabolic derangements, hypoxia, acidosis, intravascular volume imbalances, neurohormonal, and catecholaminergic stress.21,22 Sepsis is characterized by a systemic milieu involving inflammatory cytokines and autonomic dysfunction.23 This maladaptive pathophysiology is a significant trigger for the development of AF, as was illustrated in this patient.24 This likely occurred in our patient as he initially presented with AFL with 2 to 1 atrioventricular block and transitioned to AF with rapid ventricular response in the setting of COVID-19 infection. AF is a common sequela of critical illness, with an estimated prevalence of almost 10% in ICU patients, and several studies report worse outcomes in patients with new-onset AF as compared with their non-AF counterparts.25,26 Sinus rhythm restoration is of high priority as it improves the patient’s hemodynamics. AF may attenuate cardiac output due to impaired left ventricular filling, especially with rapid ventricular response.22,27 Presently, there are no evidence-based guidelines for the use of anticoagulant prophylaxis in these patients.28
Additionally, severe infection induces the sympathetic nervous system (SNS), and there is also a relationship between SNS activity and supraventricular tachyarrhythmia.29 Tachycardia, in itself, is an independent prognosticator or mortality in patients with sepsis.30 Postulated mechanisms of this arrhythmogenesis include SNS-induced calcium entry into cardiac myocytes as well as a spontaneous release of calcium from the sarcoplasmic reticulum.31,32 Our patient illustrated several of the above electrolyte abnormalities, including hypokalemia, hypomagnesemia, and hypophosphatemia, all of which were aggressively repleted. In some cases, it is observed that tachycardia continues despite adequate volume resuscitation.33 Our patient also displayed anemia with mild rhabdomyolysis, which was managed with judicious intravenous crystalloid hydration.
Conclusion
We describe a case of a middle-aged Caribbean-Black gentleman presenting with COVID-19 who experienced atrial arrhythmias, namely, AFL and AF, which resolved with rate and rhythm control strategies, and supportive care. Further observational studies are required to characterize the nature and classification of arrhythmias in this COVID-19 pandemic.
Footnotes
Authors’ Note: All available data can be obtained by contacting the corresponding author.
Author Contributions: RS, RN, SG, MR, KF, KA, NB, and NAS all contributed equally in writing the manuscript. All authors read and approved the final manuscript.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent: The patient has provided verbal informed consent to have the details of his case published.
ORCID iD: Naveen Anand Seecheran  https://orcid.org/0000-0002-7779-0181
https://orcid.org/0000-0002-7779-0181
References
- 1. Hui DS, Azhar EI, Madani TA, et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization. Naming the coronavirus disease (COVID-19) and the virus that causes it. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. Accessed March 24, 2020.
- 3. World Health Organization. WHO Director-General’s opening remarks at the media briefing on COVID-19—11 March 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19—11-march-2020. Accessed March 24, 2020.
- 4. World Health Organization. Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). https://www.who.int/news-room/detail/30-01-2020-statement-on-the-second-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-outbreak-of-novel-coronavirus-(2019-ncov). Accessed March 24, 2020.
- 5. Li B, Yang J, Zhao F, et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China [published online March 11, 2020]. Clin Res Cardiol. doi: 10.1007/s00392-020-01626-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259-260. doi: 10.1038/s41569-020-0360-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054-1062. doi: 10.1016/s0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van Gelder IC, Hobbelt AH, Mulder BA, Rienstra M. Rate control in atrial fibrillation: many questions still unanswered. Circulation. 2015;132:1597-1599. [DOI] [PubMed] [Google Scholar]
- 9. Kelly JP, DeVore AD, Wu J, et al. Rhythm control versus rate control in patients with atrial fibrillation and heart failure with preserved ejection fraction: insights from get with the guidelines-heart failure. J Am Heart Assoc. 2019;8:e011560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Park HS, Kim YN. Adverse effects of long-term amiodarone therapy. Korean J Intern Med. 2014;29:571-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Virgadamo S, Charnigo R, Darrat Y, Morales G, Elayi CS. Digoxin: a systematic review in atrial fibrillation, congestive heart failure and post myocardial infarction. World J Cardiol. 2015;7:808-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial [published online March 20, 2020]. Int J Antimicrob Agents. doi: 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13. Ray WA, Murray KT, Hall K, Arbogast PG, Stein CM. Azithromycin and the risk of cardiovascular death. N Engl J Med. 2012;366:1881-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao B, Wang Y, Wen D, et al. A Trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19 [published online March 18, 2020]. N Engl J Med. doi: 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China [published online March 25, 2020]. JAMA Cardiol. doi: 10.1001/jamacardio.2020.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu K, Fang YY, Deng Y, et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province [published online February 7, 2020]. Chin Med J. doi: 10.1097/CM9.0000000000000744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China [published online February 7, 2020]. JAMA. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published online February 24, 2020]. Lancet Respir Med. doi: 10.1016/s2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen C, Zhou Y, Wang DW. SARS-CoV-2: a potential novel etiology of fulminant myocarditis [published online March 5, 2020]. Herz. doi: 10.1007/s00059-020-04909-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. The Surviving Sepsis Campaign: guidelines on the management of critically ill adults with COVID-19 https://www.sccm.org/getattachment/Disaster/SSC-COVID19-Critical-Care-Guidelines.pdf?lang=en-US. Accessed March 25, 2020.
- 21. Li R, Wang Y, Ma Z, et al. Maresin 1 mitigates inflammatory response and protects mice from sepsis. Mediators Inflamm. 2016;2016:3798465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuipers S, Klouwenberg PMK, Cremer OL. Incidence, risk factors and outcomes of new-onset atrial fibrillation in patients with sepsis: a systematic review. Crit Care. 2014;18:688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goodman S, Weiss Y, Weissman C. Update on cardiac arrhythmias in the ICU. Curr Opin Crit Care. 2008;14:549-554. [DOI] [PubMed] [Google Scholar]
- 24. Aviles RJ, Martin DO, Apperson-Hansen C, et al. Inflammation as a risk factor for atrial fibrillation. Circulation 2003;108:3006-3010. [DOI] [PubMed] [Google Scholar]
- 25. Seguin P, Launey Y. Atrial fibrillation is not just an artefact in the ICU. Crit Care. 2010;14:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306:2248-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Okajima M, Takamura M, Taniguchi T. Landiolol, an ultra-short-acting β1-blocker, is useful for managing supraventricular tachyarrhythmias in sepsis. World J Crit Care Med. 2015;4:251-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Darwish OS, Strube S, Nguyen HM, Tanios MA. Challenges of anticoagulation for atrial fibrillation in patients with severe sepsis. Ann Pharmacother. 2013;47:1266-1271. [DOI] [PubMed] [Google Scholar]
- 29. Otake H, Suzuki H, Honda T, Maruyama Y. Influences of autonomic nervous system on atrial arrhythmogenic substrates and the incidence of atrial fibrillation in diabetic heart. Int Heart J. 2009;50:627-641. [DOI] [PubMed] [Google Scholar]
- 30. Leibovici L, Gafter-Gvili A, Paul M, et al. Relative tachycardia in patients with sepsis: an independent risk factor for mortality. QJM. 2007;100:629-634. [DOI] [PubMed] [Google Scholar]
- 31. Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198-205. [DOI] [PubMed] [Google Scholar]
- 32. Keurs HET, Boyden PA. Calcium and arrhythmogenesis. Physiol Rev. 2007;87:457-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zou L, Feng Y, Chen YJ, et al. Toll-like receptor 2 plays a critical role in cardiac dysfunction during polymicrobial sepsis. Crit Care Med. 2010;38:1335-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]