Abstract
Background
Long regarded as an epicenter of drug-resistant malaria, Southeast Asia continues to provide new challenges to the control of Plasmodium falciparum malaria. Recently, resistance to the artemisinin combination therapy partner drug piperaquine has been observed in multiple locations across Southeast Asia. Genetic studies have identified single nucleotide polymorphisms as well as copy number variations in the plasmepsin 2 and plasmepsin 3 genes, which encode haemoglobin-degrading proteases that associate with clinical and in vitro piperaquine resistance.
Results
To accurately and quickly determine the presence of copy number variations in the plasmepsin 2/3 genes in field isolates, this study developed a quantitative PCR assay using TaqMan probes. Copy number estimates were validated using a separate SYBR green-based quantitative PCR assay as well as a novel PCR-based breakpoint assay to detect the hybrid gene product. Field samples from 2012 to 2015 across three sites in Cambodia were tested using DNA extracted from dried blood spots and whole blood to monitor the extent of plasmepsin 2/3 gene amplifications, as well as amplifications in the multidrug resistance transporter 1 gene (pfmdr1), a marker of mefloquine resistance. This study found high concordance across all methods of copy number detection. For samples derived from dried blood spots, a success rate greater than 80% was found in each assay, with more recent samples performing better. Evidence of extensive plasmepsin 2/3 copy number amplifications was observed in Pursat (94%, 2015) (Western Cambodia) and Preah Vihear (87%, 2014) (Northern Cambodia), and lower levels in Ratanakiri (16%, 2014) (Eastern Cambodia). A shift was observed from two copies of plasmepsin 2 in Pursat in 2013 to three copies in 2014–2015 (25% to 64%). Pfmdr1 amplifications were absent in all samples from Preah Vihear and Ratanakiri in 2014 and absent in Pursat in 2015.
Conclusions
The multiplex TaqMan assay is a robust tool for monitoring both plasmepsin 2/3 and pfmdr1 copy number variations in field isolates, and the SYBR-green and breakpoint assays are useful for monitoring plasmepsin 2/3 amplifications. This study shows increasing levels of plasmepsin 2 copy numbers across Cambodia from 2012 to 2015 and a complete reversion of multicopy pfmdr1 parasites to single copy parasites in all study locations.
Keywords: Malaria, Piperaquine, Plasmepsin, qPCR, Cambodia, Copy number
Background
As malaria endemic countries strive toward malaria elimination, one of the main obstacles is the continued availability of efficacious drugs. In Southeast Asia, drug resistance is wide-spread, with the most recent emergence of resistance to the current first-line treatment, artemisinin-based combination therapy (ACT) [1].
The declining efficacy of ACT in recent years can be largely attributed to rising resistance to artemisinin partner drugs, notably piperaquine. Dihydroartemisinin–piperaquine (DHA–PPQ) was introduced as the first-line treatment for malaria in 2008 in Cambodia, following therapeutic failure of artesunate–mefloquine (AS–MQ), the ACT used prior to 2008. Since 2012–2013, studies in Cambodia have shown declining efficacy to piperaquine in vitro, and subsequent increases in clinical treatment failures [2–7]. Genomic studies carried out in parallel with samples from these clinical efficacy studies have shown that there are multiple signals across the parasite genome that associate with both in vitro piperaquine resistance and clinical treatment failures [8, 9]. Specifically, a gene duplication within the plasmepsin multi-gene cluster on the parasite chromosome 14 and a non-synonymous SNP in a putative exonuclease gene (PF3D7_1362500) on chromosome 13, exo-E415G. Additional work also points to mutations in the chloroquine resistance transporter gene, pfcrt that can confer differing levels of piperaquine resistance in field and laboratory isolates [10–13].
The duplication encompasses plasmepsin 2 and a hybrid of the plasmepsin 1 and 3 genes that was highly correlated (adjusted hazard-ratio 16.7) with parasite recrudescence following adequate drug treatment with DHA–PPQ. This effect holds in the artemisinin resistance-associated kelch13 propeller (k13) domain mutant populations [14] (adjusted hazard-ratio 5.2) suggesting an independent mechanism [8]. Members of the plasmepsin gene family in P. falciparum are involved in the haemoglobin degradation pathway, specifically in the formation of haemozoin [15]. As the parasites digest haemoglobin and release haem, toxic by-products that cause oxidative stress are formed and the conversion of intermediates to inert haemozoin crystals detoxifies the harmful by-products. The plasmepsin enzymes are redundant and other enzymes also facilitate the haemoglobin digestion pathway, including falcilysins and falcipains [16–18]. The main duplication in plasmepsin 2/3 observed in Southeast Asia has a conserved breakpoint within the distal end of plasmepsin 3 that includes complete duplication of the plasmepsin 2 gene. In the same studies, an association was seen between increased copy numbers of the multidrug resistance transporter 1 gene (pfmdr1), a marker of mefloquine resistance, and low piperaquine IC50 values. It is unknown if the association between decreased piperaquine IC50 values and single copy pfmdr1 is due to a drug effect, or is due to the expansion of piperaquine resistance on a mefloquine-sensitive parasite line. Some studies have shown that increased pfmdr1 copy numbers are associated with increased susceptibility to piperaquine [19] and more recent work has suggested that plasmepsin 2/3 amplifications and pfmdr1 deamplifications have created a genetic background that favors pfcrt mutations [20]. With the observation of possible counteracting resistance mechanisms to piperaquine and mefloquine, it has been suggested to re-introduce mefloquine to areas of emerging piperaquine resistance, or to combine mefloquine into a triple ACT (TACT) with piperaquine. Both options are currently being investigated [21, 22].
Monitoring the plasmepsin 2/3 amplification as a marker of piperaquine resistance will enable rapid determination of its frequency in populations using DHA–PPQ as well as identification of the amplification in new parasite populations. This study developed a TaqMan based quantitative PCR (qPCR) to measure the copy number of plasmepsin 2 within the duplicated region, and have also combined it with a TaqMan assay that can detect increased copy numbers of pfmdr1. This single reaction multiplex qPCR can be used to efficiently monitor resistance to both piperaquine and mefloquine, which will be of particular relevance if TACT regimens are adopted in countries such as Cambodia. This study also developed a PCR-based breakpoint assay for detection of the hybrid sequence created as a result of the plasmepsin 2/3 duplication. The breakpoint assay can be used in in conjunction with the qPCR assays or in low-resource settings where qPCR is not feasible.
Methods
Samples
Laboratory isolates used in qPCR validation were obtained from a clinical trial carried out in three sites in Cambodia, Pursat, Preah Vihear, and Ratanakiri, between 2012 and 2013 [2]. Blood samples from this study were taken as whole-blood venous draws following malaria diagnosis and from an initial finger prick dried blood spot (DBS). A subset of DNA samples extracted from the venous blood were whole-genome sequenced and pfmdr1 and plasmepsin 2 copy-numbers were called from sequence data according to Amato et al. [8]. Additional field isolates (after 2013) from clinical trials performed by Amaratunga et al. [2] at the same three sites in Cambodia as above were used to test ongoing copy-number polymorphisms (clinicaltrials.gov ID: NCT01736319).
Quantification of plasmepsin 2, plasmepsin 3, and pfmdr1 by real-time PCR
Primers for both plasmepsin 2 and plasmepsin 3 were designed using GenScript online tool (https://www.genscript.com/tools/) to match the Tm of the previously described pfmdr1 TaqMan assay [23]. Plasmepsin 2 primers (forward-5′-ATGGTGATGCAGAAGTTGGA-3′, reverse-5′-AACATCCTGCAGTTGTACATTTAAC-3′) and plasmepsin 3 primers (forward-5′-CCACTTGTGGTAACACGAAATTA-3′; reverse-5′-TGGTTCAAGGTATTGTTTAGGTTC-3′) were selected to match the optimized reaction conditions of the pfmdr1 primers (forward 5′-TGCATCTATAAAACGATCAGACAAA-3′, reverse 5′-TCGTGTGTTCCATGTGACTGT-3′). For the plasmepsin assay, the same β-tubulin single copy reference primers (forward 5′-TGATGTGCGCAAGTGATCC-3′, reverse 5′-TCCTTTGTGGACATTCTTCCTC-3′) were used as in the pfmdr1 assay. First, the two-probe assay with either plasmepsin 2 (5′Fam-CAGGATCTGCTAATTTATGGGTCCCA-3′BHQ-2) or plasmepsin 3 (5′Fam-CCAACACTCGAATATCGTTCACCAA-3′BHQ-2) and β-tubulin (5′MAX-TAGCACATGCCGTTAAATATCTTCCATGTCT-3′BHQ-1) was validated first using DNA extracted from whole blood and compared with copy number estimates called from whole-genome sequence data. The plasmepsin 2 probe set was then multiplexed with pfmdr1 (5′ Cy5-TTTAATAACCCTGATCGAAATGGAACCTTTG-3′BHQ-2) and then tested using DNA extracted from DBS in the same set of samples. All primers and probes were ordered from Integrated DNA Technologies, Inc. Reactions were carried out in 25 μL volumes in 96 well plates (Star Labs) on a Roche LightCycler 480. For each reaction, 2× Concentrated Roche LightCycler 480 Probes Master (containing FastStart Taq DNA Polymerase), 300 nmol/L of each plasmepsin 2 or plasmepsin 3, pfmdr1, and β-tubulin primers, 100 nmol/L of each probe, and 2–5 μL of sample DNA were used. PCR cycling conditions had an initial step of 95 °C for 10 min followed by 50 cycles of 95 °C for 15 s and 58 °C for 60 s. To calculate the fold-change, the ΔΔCt method, ΔΔCt = (CtTE − CtHE) − (CtTC − CtHC) was used, where T is the test gene (either plasmepsin 2, plasmepsin 3, or pfmdr1), H is the reference gene (β-tubulin), E is the experimental sample, and C is the control sample (for all tests 3D7 was used as the single copy control). Relative expression was calculated as 2−ΔΔCt.
SYBR green validation of plasmepsin 2 copy number using quantitative PCR
PCR primers for estimating plasmepsin 2/3 copy number amplification were designed manually inside the plasmepsin 2 gene (forward-5′-CTTATACTGCTTCAACATTTGATGGTATCCTTGG-3′; reverse-5′-GGTTAAGAATCCTGTATGTTTATCATGTACAGGTAAG-3′). Previously described primers for P. falciparum lactate dehydrogenase (ldh) [24], were used as the control for a single copy gene (forward-5′-AGGACAATATGGACACTCCGAT-3′; reverse-5′-TTTCAGCTATGGCTTCATCAAA-3′). Quantitative PCR (qPCR) reactions were carried out in 20 μL volumes in a 96-well plate (Bio-Rad, Hercules, CA) containing 10 μL SensiFAST SYBR No-ROX mix (2×) (Bioline Inc., Taunton, MA), 300 nM of each primer, and 2 μL genomic DNA. Reactions were performed using a CFX Connect Real-Time PCR Detection System (Bio-Rad) using the following conditions: 5 min at 95 °C, followed by 40 cycles of 10 s at 95 °C, 20 s at 58 °C, and 20 s at 60 °C. Relative copy number was calculated on the basis of the 2−ΔΔCt method for relative quantification. ΔΔCt was calculated as (Ctldh − Ctpfplasmepsin2) − (Ctldh cal − Ctpfplasmepsin2 cal), where cal is the calibration control of genomic 3D7 DNA with one copy of both ldh and plasmepsin 2. DNA from an isolate with two copies of plasmepsin 2/3 (PH1387-C) [2] was used as an internal plate control. All samples were analysed in triplicate and each plate was replicated in triplicate.
Plasmepsin 2/3 duplication breakpoint PCR assay
Whole genome sequencing (WGS) data from P. falciparum genomic DNA collected during field studies in Cambodia was used to detect the plasmepsin 2/3 gene amplification as previously reported [2, 8]. With the available WGS data, the breakpoint of the plasmepsin 2/3 amplification was used to manually design PCR primers to amplify the region surrounding the breakpoint. Primers AF (forward 5′-CCACGATTTATATTGGCAAGTTGATTTAG-3′) and AR (reverse 5′-CATTTCTACTAAAATAGCTTTAGCATCATTCACG-3′) amplify a 623 base pair product surrounding the breakpoint located at the 3′ end of plasmepsin 1. Primers BF (forward-5′-CGTAGAATCTGCAAGTGT TTTCAAAG-3′) and BR (reverse 5′-AATGTTATAAATGCAATATAATCAAACGACATCAC-3′) amplify a 484 base pair product surrounding the breakpoint located at the 3′ end of plasmepsin 3. BF + AR amplify the junction between the breakpoint and produce a 497 base pair product in isolates with plasmepsin 2/3 amplifications. These primers face opposite directions in samples without duplications and are not expected to amplify a product in single copy isolates. Both control (AF + AR; BF + BR) and duplication (BF + AR) primer sets were used with all samples and one copy isolates were only noted if the control primer sets amplified a product and duplication PCR was negative. Two or more copies were annotated as > 1 copy of plasmepsin 2/3 only if both the control and duplication primer sets produced a product. PCR reactions contained 10 μL SapphireAmp Fast PCR Master Mix (Takara Bio USA, Mountain View, CA), 0.3 μL of each primer (10 μM stocks), 1 μL of genomic DNA up to 20 μL final volume with water. PCR conditions were: 92 °C for 2 min, followed by 30 cycles of 92 °C for 30 s, 59 °C for 30 s, 66 °C for 1.5 min, followed by a 1 min extension at 66 °C.
Results
Validation of copy number assays
Assays for plasmepsin 2 and plasmepsin 3 were designed and compared to determine if both genes could serve as markers for the entire duplication. For surveillance purposes the plasmepsin 2 assay was multiplexed with an existing pfmdr1 TaqMan assay. Both reference laboratory strains and culture-adapted field isolates were used to test the repeatability of each assay. The 3D7 isolate has single copies of all genes being tested while the Dd2 parasite has a duplication of the pfmdr1 gene and the field isolates PH1265-C and PH1387-C have duplications of the plasmepsin 2/3 complex. Two field isolates (PH1097-C and PH1310-C) have single copies of all genes and were used as baseline controls. Individual plasmepsin assays and the combined plasmepsin 2–pfmdr1 assay showed high replicability across duplicates (Table 1).
Table 1.
Validation of TaqMan assays with laboratory isolates
| Strain | Assay | Replicates | Plasmepsin Est. FC | MDR-1 Est. FC | ||
|---|---|---|---|---|---|---|
| Avg | SD (range) | Avg | SD (range) | |||
| 3D7 | plasmepsin 2 | 8 | 1.00 | 0.02 (0.97–1.04) | – | – |
| Dd2 | plasmepsin 2 | 8 | 1.06 | 0.03 (1.03–1.12) | – | – |
| PH1097-C | plasmepsin 2 | 6 | 1.06 | 0.02 (1.04–1.09) | – | – |
| PH1265-C | plasmepsin 2 | 6 | 1.89 | 0.02 (1.85–1.93) | – | – |
| PH1310-C | plasmepsin 2 | 6 | 1.06 | 0.03 (1.01–1.13) | – | – |
| PH1387-C | plasmepsin 2 | 6 | 1.93 | 0.09 (1.8–2.04) | – | – |
| 3D7 | plasmepsin 3 | 8 | 1.02 | 0.03 (0.96–1.08) | – | – |
| Dd2 | plasmepsin 3 | 8 | 1.09 | 0.03 (1.05–1.15) | – | – |
| PH1097-C | plasmepsin 3 | 6 | 1.06 | 0.02 (1.03–1.09) | – | – |
| PH1265-C | plasmepsin 3 | 6 | 2.05 | 0.06 (1.93–2.14) | – | – |
| PH1310-C | plasmepsin 3 | 6 | 1.00 | 0.03 (1.02–1.09) | – | – |
| PH1387-C | plasmepsin 3 | 6 | 2.29 | 0.07 (2.19–2.38) | – | – |
| 3D7 | plasmepsin 2/pfmdr1 | 8 | 1.01 | 0.02 (0.97–1.04) | 1.00 | 0.02 (0.96–1.02) |
| Dd2 | plasmepsin 2/pfmdr1 | 8 | 1.10 | 0.04 (1.04–1.17) | 1.83 | 0.06 (1.77–1.92) |
| PH1097-C | plasmepsin 2/pfmdr1 | 6 | 1.07 | 0.05 (1–1.13) | 1.06 | 0.05 (1.01–1.11) |
| PH1265-C | plasmepsin 2/pfmdr1 | 6 | 1.80 | 0.03 (1.75–1.84) | 0.96 | 0.02 (0.93–0.99) |
| PH1310-C | plasmepsin 2/pfmdr1 | 6 | 1.02 | 0.02 (1–1.06) | 1.01 | 0.02 (0.98–1.05) |
| PH1387-C | plasmepsin 2/pfmdr1 | 6 | 1.89 | 0.05 (1.84–2) | 0.93 | 0.03 (0.91–0.99) |
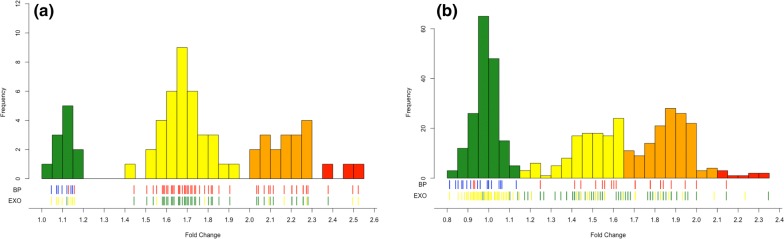
The TaqMan qPCR and SYBR qPCR assays were then compared using DNA extracted from whole blood for 67 patient samples. Copy number estimates from both the plasmepsin 2 and plasmepsin 3 assays were compared and were in 100% concordance across all samples extracted from venous blood. Fold-change values were comparable across both assays. Fifty-six of the 67 tested samples had WGS data available and had plasmepsin 2/3 copy-number estimates available. Fifty-three of the 56 samples with copy-number estimates from WGS had the same estimate from qPCR, with the discrepancies being 1 sample called 2 copies by qPCR and 3 copies by WGS and 2 samples with 4 copies by qPCR and 3 copies by WGS. WGS estimates were used to define the relative expression boundaries between copy number estimates, and relative-expression values were lower in whole-blood extracted samples than in cultured laboratory isolates (Fig. 1a, Additional file 1). A high proportion of the exo-E415G mutation was observed among plasmepsin 2 multi-copy parasites (Fig. 1, Additional file 1). Exo-415 sequencing was determined from samples with available WGS data [2, 8].
Fig. 1.
Distribution of fold-changes of samples from whole-blood (a) or dried blood spots (b) as measured by the plasmepsin 2–pfmdr1 TaqMan qPCR assay. Tick marks underneath the bar graphs indicate individual sample status for the breakpoint PCR assay (BP, top line), with blue indicating no breakpoint detected and red containing the breakpoint, or exo-E415G SNP (EXO, bottom line), where yellow represents the wildtype E amino acid and green is the mutant G amino acid. Bars are coloured by their predicted plasmepsin 2 copy number of either 1, 2, 3, or 4+ (green, yellow, orange, and red, respectively)
To validate the copy number estimates, available samples were tested at the National Institutes of Health (NIH) in the Laboratory of Malaria and Vector Research (LMVR) in Bethesda, MD, USA using a separately developed SYBR-green based approach. Of the 31 samples available at both laboratories, 29 samples matched in plasmepsin 2 copy number estimate with one sample being called 2 copies by TaqMan and 3 copies by SYBR-green, and one sample being called 3 copies by TaqMan and 2 copies by SYBR-green. All dual-tested samples that had copy number estimates from both the SYBR-green method and WGS were in 100% concordance.
Duplication breakpoint assay validation
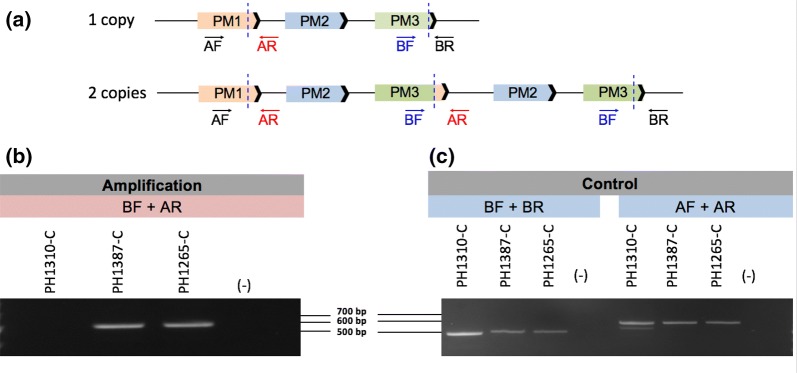
PCR primers that amplify the breakpoint of the plasmepsin 2/3 amplification observed in Cambodian isolates were designed (Fig. 2a). The breakpoint assay identifies copy number amplification in isolates that contain 2 or greater copies of plasmepsin 2 and 3 (Fig. 2b). As expected, no PCR products were observed in samples with a single copy of plasmepsin 2/3 (Fig. 2b). Control primers confirmed that the regions surrounding the plasmepsin 2/3 amplification were present in all isolates (Fig. 2c).
Fig. 2.
Schematic of plasmepsin 2/3 gene duplication. a Gene model depicting the plasmepsin 2/3 breakpoint (dashed blue lines) observed in Cambodian isolates. Primer positions are labeled in the single copy (top) and multi-copy (bottom) isolates. b Amplification primer set BF + AR amplifies a product in an isolate with two copies (PH1387-C) and three copies (PH1265-C) of plasmepsin 2/3. No product is observed for the single copy (PH1310-C) isolate or in the DNA-negative control (−). c Control primers amplify a product in the single copy (PH1310-C) and multi-copy isolates (PH1387-C; PH1265-C). No product is observed in the DNA-negative control (−)
Further verification of the breakpoint assay was performed via sequence analysis of the BF + AR PCR product. Sequence data revealed the same breakpoint location as observed by WGS [8]. All Cambodian samples that were positive for plasmepsin 2/3 amplification as detected by the breakpoint assay (> 1 copy) were in 100% concordance with qPCR and WGS data that called 2 or more copies of plasmepsin 2. Sequencing chromatogram review of PCRs representing 2 and 3 copy samples showed that the breakpoints for representative 2 and 3 copy samples were identical. These sequencing results combined with the identical PCR sizes for all isolates indicates that the breakpoint is identical in all Cambodian isolates tested to date.
To test the utility of using the breakpoint assay for rapid surveillance of large sample sizes for which WGS data is not yet completed or available the presence of amplification in an additional 99 samples was analysed. The results showed that 93/99 (94%) samples tested with the breakpoint PCR assay matched qPCR data for the same samples (Additional file 1). The six non-concordant samples were repeated, always producing the same results with the breakpoint detecting a copy-number increase and the qPCR single-copy. These results suggest that the breakpoint PCR assay is more sensitive than the qPCR assay for detecting minor clones containing the duplication in field isolates.
Copy number surveillance in Cambodia
To test the feasibility of using this assay as a surveillance tool, the plasmepsin 2–pfmdr1 TaqMan assay was performed on 524 samples extracted from DBS collected in drug efficacy studies from 3 field sites within Cambodia (Pursat, Preah Vihear and Ratanakiri) from 2012 to 2015 (Additional file 1). There was an 84% (success rate across all samples, but success rate improved with time since collection (2012, 68%; 2013, 72%; 2014, 94%; 2015, 100%). Assays run on samples from DBS were checked against SYBR-green results run on whole-blood extracted samples analysed at the NIH LMVR. A total of 171 samples were available for analysis using both qPCR assays and had an 88% overall concordance, with only 6 (3%) samples having a discrepancy between calling a single versus multi-copy parasite. Most differences were between calling 2 versus 3 copies in either assay. Additionally, 71 samples that were either unavailable for DBS extraction or failed TaqMan qPCR were able to be typed by the SYBR-green method, giving copy-number estimates for 509 total samples. Samples with qPCR estimates in multiple sample types (whole-blood and DBS) were used to define relative expression limits between number of copies. A drop in relative expression in samples from DBS compared to whole-blood samples was noted, but confirmation of multiple copies was performed by the breakpoint assay (Fig. 1b).
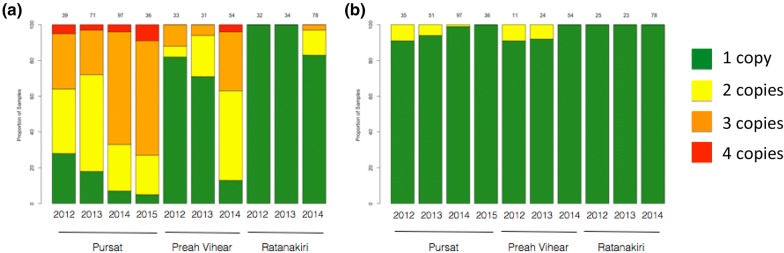
An increase in multi-copy plasmepsin 2 each year was observed in both Pursat and Preah Vihear, but multi-copy parasites were not observed in Ratanakiri until 2014 when they were found in 17% of parasites analysed. An increase in parasites containing 3 copies of plasmepsin 2 from 2012–2013 to 2014–2015 to where they are now the majority (64%) was observed. There was also a large increase in multi-copy containing parasites in Preah Vihear from 2013 (29%) to 2014 (87%) (Fig. 3a). Among all 438 samples with pfmdr1 copy-number estimates, only 10 (2%) had multiple copies and no parasites with duplications were seen in the most recently sampled year for each site (Fig. 3b).
Fig. 3.
Proportion of samples by copy-number of plasmepsin 2 (a) and pfmdr1 (b). Bars represent proportion of samples by site and year for assays of dried-blood spot derived samples, and are coloured by number of copies detected
Discussion
This newly developed TaqMan assay accurately determines plasmepsin 2 and pfmdr1 gene amplifications in samples from DBS and can be performed using standard lab equipment and a suitable qPCR machine. The specificity of TaqMan probes combined with the different absorbance spectra of labelling dyes create a system for typing the reference gene simultaneously with one or more experimental genes, making it higher throughput than SYBR-green based methods. This method shows high typeability in samples collected on dried filter paper blood spots making it ideal for surveillance of populations across wide areas. The breakpoint PCR assay also effectively determines increased plasmepsin 2/3 copy number in field isolates and can be used in areas where qPCR is infeasible. The sensitivity of this assay enables the detection of minor clones, which proves advantageous, given the potential for polyclonal infections. However, a notable disadvantage of this method is the inability to distinguish the relative copy number (2, 3, or 4+ copies vs. > 1) in comparison with qPCR. The breakpoint assay was also designed with primers specific to the plasmepsin 2/3 breakpoint observed in Cambodian field isolates [8]. The PCR primers used to detect the Cambodian breakpoint observed to date may not amplify a product in samples that contain different breakpoints. As previous studies have suggested [25, 26], copy number variations in the same gene can arise independently on different genetic backgrounds and result in distinct molecular breakpoints of amplification. Since qPCR methods do not rely on the location of primers in reference to an estimated breakpoint, they can be used broadly. Furthermore, the high-throughput potential of the qPCR assay to type multiple genes in the same assay while providing actual copy number estimates makes the TaqMan assay the preferred method in areas where qPCR is possible.
Since the first Plasmodium falciparum genome was sequenced in 2002 [27], nearly 10,000 additional genomes have been sequenced from parasites collected around the endemic world. While the first genome may not have made great strides to discover new drug or vaccine targets or point towards complicated mechanisms of disease, it was the first step in understanding the great complexity of the Plasmodium genomic landscape. It is this understanding that has led to great advances in determining new molecular markers of drug resistance. Historically, it has taken decades to determine a molecular cause of drug resistance, as was the case with chloroquine [28]. The availability of new methods and a catalogue of genomic variation allowed for rapid discovery and publishing of a candidate molecular marker of artemisinin resistance in 2014 [14], shortly after reports of suspected drug resistance to artemisinin in Southeast Asia were published in 2008 [29] and 2009 [30].
In 2014, the first report of DHA–PPQ treatment failures was published [7]. Because of an ever-increasing catalogue of sequenced samples and clinical and laboratory studies, a putative marker of drug resistance was published less than 2 years after the first reports of resistance [8, 9], with additional studies identifying new markers in pfcrt shortly after [10, 11]. Being able to extensively type variation by WGS made it possible to identify the association of increased piperaquine IC50 values with both SNPs and a copy-number variation. The copy-number variation at the plasmepsin 2/3 locus showed high correlation with the phenotype and the new assays for detection will assist in monitoring its frequency in established populations, and can also monitor unaffected populations to check for new emergence or spread.
For a molecular marker to be effective in surveillance, it must be easily typed in field-derived samples. Most molecular markers of P. falciparum drug resistance are SNPs and can be typed by simple PCR assays [31–36], although copy-number markers exist for mefloquine [23] and antifolates [37], they are more difficult to type. Quantitative PCR makes for an easy and inexpensive method to determine copy number in samples, and unlike breakpoint assays, qPCR can determine the number of copies. It is not known if more than 2 copies of plasmepsin 2/3 have a phenotypic effect but the increase in samples with 3 or more copies suggest some sort of selective mechanism. It is possible that a third copy of plasmepsin 2/3 prevents a loss of resistance if the duplication is unstable and an extra copy is lost, therefore the third copy would act as a “buffer.”
Recent studies in Plasmodium have provided insight into the functional role of the plasmepsins in response to piperaquine pressure. Loesbanluechai et al. [38] showed that overexpression of plasmepsin 2 and plasmepsin 3 in the 3D7 parasite background did not change parasite susceptibility to piperaquine, artemisinin, or chloroquine. Other studies have suggested that plasmepsin 2 and plasmepsin 3 knockouts in the same 3D7 background showed decreased piperaquine survival as measured by IC50 values [39]. More recent studies by Silva et al. [20] using transgenic parasites with copy number variations in plasmepsin 2/3 showed that pfmdr1 deamplified in the presence of the plasmepsin 2/3 amplifications. Thus, additional functional work is needed to fully understand the relevance of the plasmepsin 2/3 gene amplification in conferring any survival or fitness advantages in response to PPQ pressure.
The importance of examining the role of the plasmepsin 2/3 amplification in piperaquine resistance has been further demonstrated by studies that have detected plasmepsin 2 amplifications in several African countries. Inoue et al., Gupta et al., and Leroy et al. [40–42] have reported plasmepsin 2 duplications in Mozambique, Mali, Gabon, Burkina Faso, and Uganda where DHA–PPQ is currently used and remains effective. It is possible that plasmepsin 2/3 amplifications will be found in other regions where piperaquine has been used as a partner drug and thus continued surveillance of plasmepsin 2/3 copy numbers will prove necessary.
Mefloquine is now being re-introduced as a partner drug for artemisinin, either as a dual or triple combination therapy, in areas where DHA–PPQ is no longer effective [21]. It was removed as the first-line treatment following widespread resistance via copy number amplifications of the pfmdr1 gene. Since mefloquine’s removal as a nationally-recommended treatment the levels of pfmdr1 multi-copy number parasites has decreased and now is no longer detected in the samples from three distant sites in Cambodia (Fig. 3, Additional file 1). It has been suggested that a counter-acting mechanism of action has selected against multiple copies of pfmdr1 in parasites subjected to piperaquine. This is feasible but will require confirmation, while another possibility is that the plasmepsin 2/3 multi-copy parasites emerged on a pfmdr1 single copy parasite background and it is this lineage(s) that have expanded.
In order to effectively monitor the spread of anti-malarial drug resistance, it is imperative to have robust, high-throughput methods for detecting genetic markers of resistance. Accurate and timely surveillance of drug resistance markers aids in maintaining and prolonging the efficacy of the limited selection of anti-malarial drugs available. The TaqMan qPCR, SYBR qPCR, and breakpoint assays described here provide a way of typing plasmepsin 2/3 amplification, that can be readily combined with TaqMan typing of pfmdr1 amplifications, to monitor genetic markers of mefloquine and piperaquine resistance in areas where these important ACT partner drugs are used as frontline treatment for malaria.
Conclusions
The emergence of DHA–PPQ resistance greatly threatens the efficacy of the remaining ACTs worldwide. With the availability of plasmepsin 2/3 amplifications as molecular markers of piperaquine resistance, it is necessary to have robust assays that can be used to monitor the presence and frequency of these markers in contemporary parasite isolates across endemic regions. This study has developed a multiplex TaqMan qPCR assay that measures both plasmepsin 2 and pfmdr1 copy number, a marker of mefloquine resistance, and a SYBR-green qPCR assay for monitoring plasmepsin 2 copy number in areas where multiplex qPCR is not possible. A PCR-based breakpoint assay was also developed to detect the presence of the plasmepsin 2/3 amplification breakpoint reported in Cambodia. Using these methods, this study shows increasing levels of plasmepsin 2 copy numbers across Cambodia from 2012 to 2015 and a reversion of multicopy pfmdr1 parasites to single copy isolates in 2014–2015. The tools developed by this study will enable continued surveillance of plasmepsin 2–pfmdr1 amplifications in regions where piperaquine and mefloquine are used as ACT partner drugs.
Supplementary information
Additional file 1. The additional data table includes plasmepsin 2/3 copy number estimates using the various methods tested in this manuscript (SYBR qPCR, TaqMan qPCR, and breakpoint PCR) compared to whole genome sequencing (WGS) data, when available. Parasite density is listed at time 0 (upon enrollment) when the samples were collected as dried blood spots (DBS) or whole blood (WB), from which gDNA was later extracted.
Acknowledgements
The authors wish to thank the members of the Lee lab at the Sanger Institute for contributing DNA from laboratory isolates for experimental controls.
Abbreviations
- ACT
Artemisinin-based combination therapy
- DBS
Dried blood spot
- DHA–PPQ
Dihydroartemisinin–piperaquine
- IC50
50% inhibitory concentration
- LMVR
Laboratory of Malaria and Vector Research
- NIH
National Institutes of Health
- qPCR
Quantitative polymerase chain reaction
- WGS
Whole-genome sequencing
Authors’ contributions
MRA designed SYBR-green and breakpoint assays and analysed data. CGJ designed TaqMan assays and analysed data. RA and OM provided CNV estimates from WGS data. MK, RV and MD processed DBS samples. CA, SSr, SSu and RMF conducted the clinical trials and collected the samples for this study. CGJ and MRA wrote the manuscript and prepared figures. RMF, TEW and DPK edited figures and manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the Wellcome Trust (090770/Z/09/Z; 098051, 206194), the Medical Research Council UK and the Department for International Development (DFID) (G0600718 and MR/M005212/1), the Intramural Research Program of the NIAID, and the Bill & Melinda Gates Foundation (OPP1118166).
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.
Ethics approval and consent to participate
All participants or guardians provided written consent and samples were collected under approval from the Cambodian National Ethics Committee for Health Research and the National Institute of Allergy and Infectious Diseases Institutional Review board.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Megan R. Ansbro and Christopher G. Jacob contributed equally to this work
Contributor Information
Megan R. Ansbro, Email: ma20@sanger.ac.uk
Christopher G. Jacob, Email: cj9@sanger.ac.uk
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12936-020-03249-x.
References
- 1.WHO . World malaria report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 2.Amaratunga C, Lim P, Suon S, Sreng S, Mao S, Sopha C, et al. Dihydroartemisinin–piperaquine resistance in Plasmodium falciparum malaria in Cambodia: a multisite prospective cohort study. Lancet Infect Dis. 2016;16:357–365. doi: 10.1016/S1473-3099(15)00487-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaorattanakawee S, Saunders DL, Sea D, Chanarat N, Yingyuen K, Sundrakes S, et al. Ex vivo drug susceptibility testing and molecular profiling of clinical Plasmodium falciparum isolates from Cambodia from 2008 to 2013 suggest emerging piperaquine resistance. Antimicrob Agents Chemother. 2015;59:4631–4643. doi: 10.1128/AAC.00366-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spring MD, Lin JT, Manning JE, Vanachayangkul P, Somethy S, Bun R, et al. Dihydroartemisinin–piperaquine failure associated with a triple mutant including kelch13 C580Y in Cambodia: an observational cohort study. Lancet Infect Dis. 2015;15:683–691. doi: 10.1016/S1473-3099(15)70049-6. [DOI] [PubMed] [Google Scholar]
- 5.Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, et al. Efficacy of dihydroartemisinin–piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob Agents Chemother. 2013;57:818–826. doi: 10.1128/AAC.00686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leang R, Taylor WR, Bouth DM, Song L, Tarning J, Char MC, et al. Evidence of Plasmodium falciparum malaria multidrug resistance to artemisinin and piperaquine in Western Cambodia: dihydroartemisinin–piperaquine open-label multicenter clinical assessment. Antimicrob Agents Chemother. 2015;59:4719–4726. doi: 10.1128/AAC.00835-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saunders DL, Vanachayangkul P, Lon C, US Army Military Malaria Research Program. National Center for Parasitology, Entomology and Malaria Control. Royal Cambodian Armed Forces Dihydroartemisinin–piperaquine failure in Cambodia. N Engl J Med. 2014;371:484–485. doi: 10.1056/NEJMc1403007. [DOI] [PubMed] [Google Scholar]
- 8.Amato R, Lim P, Miotto O, Amaratunga C, Dek D, Pearson RD, et al. Genetic markers associated with dihydroartemisinin–piperaquine failure in Plasmodium falciparum malaria in Cambodia: a genotype–phenotype association study. Lancet Infect Dis. 2017;17:164–173. doi: 10.1016/S1473-3099(16)30409-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkowski B, Duru V, Khim N, Ross LS, Saintpierre B, Beghain J, et al. A surrogate marker of piperaquine-resistant Plasmodium falciparum malaria: a phenotype–genotype association study. Lancet Infect Dis. 2017;17:174–183. doi: 10.1016/S1473-3099(16)30415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross LS, Dhingra SK, Mok S, Yeo T, Wicht KJ, Kumpornsin K, et al. Emerging Southeast Asian PfCRT mutations confer Plasmodium falciparum resistance to the first-line antimalarial piperaquine. Nat Commun. 2018;9:3314. doi: 10.1038/s41467-018-05652-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agrawal S, Moser KA, Morton L, Cummings MP, Parihar A, Dwivedi A, et al. Association of a novel mutation in the Plasmodium falciparum chloroquine resistance transporter with decreased piperaquine sensitivity. J Infect Dis. 2017;216:468–476. doi: 10.1093/infdis/jix334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, et al. Determinants of dihydroartemisinin–piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis. 2019;19(9):952–961. doi: 10.1016/S1473-3099(19)30391-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton WL, Amato R, van der Pluijm RW, Jacob CG, Quang HH, Thuy-Nhien NT, et al. Evolution and expansion of multidrug-resistant malaria in southeast Asia: a genomic epidemiology study. Lancet Infect Dis. 2019;19:952–961. doi: 10.1016/S1473-3099(19)30392-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banerjee R, Liu J, Beatty W, Pelosof L, Klemba M, Goldberg DE. Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine. Proc Natl Acad Sci USA. 2002;99:990–995. doi: 10.1073/pnas.022630099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldberg DE. Hemoglobin degradation. Curr Top Microbiol Immunol. 2005;295:275–291. doi: 10.1007/3-540-29088-5_11. [DOI] [PubMed] [Google Scholar]
- 17.Wunderlich J, Rohrbach P, Dalton JP. The malaria digestive vacuole. Front Biosci. 2012;4:1424–1448. doi: 10.2741/s344. [DOI] [PubMed] [Google Scholar]
- 18.Moura PA, Dame JB, Fidock DA. Role of Plasmodium falciparum digestive vacuole plasmepsins in the specificity and antimalarial mode of action of cysteine and aspartic protease inhibitors. Antimicrob Agents Chemother. 2009;53:4968–4978. doi: 10.1128/AAC.00882-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veiga MI, Ferreira PE, Malmberg M, Jornhagen L, Bjorkman A, Nosten F, et al. pfmdr1 amplification is related to increased Plasmodium falciparum in vitro sensitivity to the bisquinoline piperaquine. Antimicrob Agents Chemother. 2012;56:3615–3619. doi: 10.1128/AAC.06350-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Silva M, Calcada C, Teixeira M, Veiga MI, Ferreira PE. Multigenic architecture of piperaquine resistance trait in Plasmodium falciparum. Lancet Infect Dis. 2020;20:26–27. doi: 10.1016/S1473-3099(19)30689-9. [DOI] [PubMed] [Google Scholar]
- 21.Maxmen A. Back on TRAC: new trial launched in bid to outpace multidrug-resistant malaria. Nat Med. 2016;22:220–221. doi: 10.1038/nm0316-220. [DOI] [PubMed] [Google Scholar]
- 22.White NJ. Triple artemisinin-containing combination anti-malarial treatments should be implemented now to delay the emergence of resistance. Malar J. 2019;18:338. doi: 10.1186/s12936-019-2955-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, Phaipun L, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim P, Dek D, Try V, Eastman RT, Chy S, Sreng S, et al. Ex vivo susceptibility of Plasmodium falciparum to antimalarial drugs in western, northern, and eastern Cambodia, 2011–2012: association with molecular markers. Antimicrob Agents Chemother. 2013;57:5277–5283. doi: 10.1128/AAC.00687-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hostetler JB, Lo E, Kanjee U, Amaratunga C, Suon S, Sreng S, et al. Independent origin and global distribution of distinct Plasmodium vivax Duffy binding protein gene duplications. PLoS Negl Trop Dis. 2016;10:e0005091. doi: 10.1371/journal.pntd.0005091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ménard D, Chan ER, Benedet C, Ratsimbasoa A, Kim S, Chim P, et al. Whole genome sequencing of field isolates reveals a common duplication of the Duffy binding protein gene in Malagasy Plasmodium vivax strains. PLoS Negl Trop Dis. 2013;7:e2489. doi: 10.1371/journal.pntd.0002489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardner MJ, Hall N, Fung E, White O, Berriman M, Hyman RW, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plowe CV. The evolution of drug-resistant malaria. Trans R Soc Trop Med Hyg. 2009;103(Suppl 1):S11–S14. doi: 10.1016/j.trstmh.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noedl H, Se Y, Schaecher K, Smith BL, Socheat D, Fukuda MM, et al. Evidence of artemisinin-resistant malaria in western Cambodia. N Engl J Med. 2008;359:2619–2620. doi: 10.1056/NEJMc0805011. [DOI] [PubMed] [Google Scholar]
- 30.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2009;361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Djimdé A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, et al. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med. 2001;344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 32.Plowe CV, Djimdé A, Bouare M, Doumbo O, Wellems TE. Pyrimethamine and proguanil resistance-conferring mutations in Plasmodium falciparum dihydrofolate reductase: polymerase chain reaction methods for surveillance in Africa. Am J Trop Med Hyg. 1995;52:565–568. doi: 10.4269/ajtmh.1995.52.565. [DOI] [PubMed] [Google Scholar]
- 33.Gyang FN, Peterson DS, Wellems TE. Plasmodium falciparum: rapid detection of dihydrofolate reductase mutations that confer resistance to cycloguanil and pyrimethamine. Exp Parasitol. 1992;74:470–472. doi: 10.1016/0014-4894(92)90209-S. [DOI] [PubMed] [Google Scholar]
- 34.Wang P, Brooks DR, Sims PF, Hyde JE. A mutation-specific PCR system to detect sequence variation in the dihydropteroate synthetase gene of Plasmodium falciparum. Mol Biochem Parasitol. 1995;71:115–125. doi: 10.1016/0166-6851(95)00041-X. [DOI] [PubMed] [Google Scholar]
- 35.Khan B, Omar S, Kanyara JN, Warren-Perry M, Nyalwidhe J, Peterson DS, et al. Antifolate drug resistance and point mutations in Plasmodium falciparum in Kenya. Trans R Soc Trop Med Hyg. 1997;91:456–460. doi: 10.1016/S0035-9203(97)90284-4. [DOI] [PubMed] [Google Scholar]
- 36.Plowe CV, Cortese JF, Djimdé A, Nwanyanwu OC, Watkins WM, Winstanley PA, et al. Mutations in Plasmodium falciparum dihydrofolate reductase and dihydropteroate synthase and epidemiologic patterns of pyrimethamine–sulfadoxine use and resistance. J Infect Dis. 1997;176:1590–1596. doi: 10.1086/514159. [DOI] [PubMed] [Google Scholar]
- 37.Nair S, Miller B, Barends M, Jaidee A, Patel J, Mayxay M, et al. Adaptive copy number evolution in malaria parasites. PLoS Genet. 2008;4:e1000243. doi: 10.1371/journal.pgen.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loesbanluechai D, Kotanan N, de Cozar C, Kochakarn T, Ansbro MR, Chotivanich K, et al. Overexpression of plasmepsin II and plasmepsin III does not directly cause reduction in Plasmodium falciparum sensitivity to artesunate, chloroquine and piperaquine. Int J Parasitol Drugs Drug Resist. 2019;9:16–22. doi: 10.1016/j.ijpddr.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mukherjee A, Gagnon D, Wirth DF, Richard D. Inactivation of plasmepsins 2 and 3 sensitizes Plasmodium falciparum to the antimalarial drug piperaquine. Antimicrob Agents Chemother. 2018;62:e02309–e02317. doi: 10.1128/AAC.02309-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inoue J, Silva M, Fofana B, Sanogo K, Martensson A, Sagara I, et al. Plasmodium falciparum plasmepsin 2 duplications, West Africa. Emerg Infect Dis. 2018;24:1591–1593. doi: 10.3201/eid2408.180370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta H, Macete E, Bulo H, Salvador C, Warsame M, Carvalho E, et al. Drug-resistant polymorphisms and copy numbers in Plasmodium falciparum, Mozambique, 2015. Emerg Infect Dis. 2018;24:40–48. doi: 10.3201/eid2401.170864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leroy D, Macintyre F, Adoke Y, Ouoba S, Barry A, Mombo-Ngoma G, et al. African isolates show a high proportion of multiple copies of the Plasmodium falciparum plasmepsin-2 gene, a piperaquine resistance marker. Malar J. 2019;18:126. doi: 10.1186/s12936-019-2756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. The additional data table includes plasmepsin 2/3 copy number estimates using the various methods tested in this manuscript (SYBR qPCR, TaqMan qPCR, and breakpoint PCR) compared to whole genome sequencing (WGS) data, when available. Parasite density is listed at time 0 (upon enrollment) when the samples were collected as dried blood spots (DBS) or whole blood (WB), from which gDNA was later extracted.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study.