Abstract
Patients with heart failure (HF) undergoing cardiac resynchronization therapy (CRT) who exhibit above-expected improvement are known as super-responders. We assessed the rate of super-responders in a population with left bundle branch block (LBBB) > 150 ms in the absence of scar tissue in the left ventricular posterolateral wall as well as prognostic variables. In this prospective observational cohort study (n=20) an electrocardiogram (ECG) was performed pre- and post-CRT. The classic and Strauss LBBB criteria were adopted (> 150 ms). The percent (%) reduction of the QRS was calculated after implantation. All patients responded to the Minnesota Living with Heart Failure questionnaire and underwent an echocardiogram to measure left ventricular ejection function (LVEF), left atrium (LA) diameter, left ventricular end-systolic volume (LVEDV), left ventricular end-diastolic volume (LVESV), and left ventricular end-diastolic diameter (LVEDD) pre- and 6 months post-CRT. Cardiac magnetic resonance imaging (MRI) measured the presence of scar tissue in the posterolateral LV wall and the total scar burden (% LV mass). Fisher’s exact test and the Mann-Whitney test were performed to evaluate possible prognostic variables. The mean age was 58.20±8.79 years old, 60% female, with a mean LVEF of 28.15±5.10%, ECG with LBBB mean QRS of 162.15±7.86 ms, LBBB > 150 ms with Strauss standard in 90% of cases, and 90% with non-ischemic cardiomyopathy. Twelve cases (60%) of super-responders (reduction > 30% LVESV after 6 months) were observed. Super-responders did not present a difference in response in sex (12 vs 8 P=0.67), age (58.67 vs 57.7 P=087), Minnesota quality of life (55.50 vs 67.70 P=0.2), % initial QRS reduction (21.16 vs 18.69 P=0.21), LVEF (29.25 vs 26.5 P=0.38), LVEDD (66.33 vs 67.67 P=0.83), LVEDV (211.16 vs 228.53 P=0.75), LVESV (145.83 vs 167.00 P=0.75), or LA diameter (41.58 vs 43.63 P=0.45). The presence of LBBB > 150 ms, using the Strauss standard (90%) and the absence of scar in the posterolateral wall may account for these positive results. Super-responders benefit the most from CRT, and the results of this study can contribute to a better selection of CRT candidates.
Keywords: Congestive heart failure, cardiac resynchronization therapy, left bundle branch block, myocardial scar, super-responders
Introduction
Cardiac resynchronization therapy (CRT) has shown positive results in the treatment of congestive heart failure (HF) in patients with conduction disorder, marked left ventricular dysfunction, and New York Heart Association (NYHA) outpatient class II, III, and IV. It is considered a class I treatment and attained the highest level of scientific evidence among various Cardiac Resynchronization Therapy Guidelines [1,2].
Around 30% of patients, called non-responders, do not have a positive outcome after CRT [3]. The classification of responders and non-responders is heterogeneous throughout several studies [4,5]. Various authors have described groups of patients who experience higher than expected responses including marked improvement in left ventricular function, NYHA class, and echocardiographic parameters. This population is referred to as super-responders and range from 10 to 38% of study participants [6-16].
After analysis and follow-up of large studies on cardiac resynchronization, the presence of left bundle branch block (LBBB) > 150 ms is considered an important prognostic variable in the choice of candidates for CRT. A meta-analysis reported that LBBB-associated dyssynchrony with a QRS duration > 150 ms was considered the most important variable in the selection of CRT candidates [17]. Another study showed the benefit of CRT was associated with QRS patients with LBBB morphology lasting more than 150 ms [18]. New criteria for LBBB, designated as the Strauss criteria, were defined in 2011 and are promising in diagnosing true cardiac desynchrony and consequently a better resynchronization response [19].
The absence of scar in the left ventricular (LV) posterolateral wall as well as the scar burden (%) evaluated by the delayed magnetic resonance (MRI) enhancement technique was also associated with an increased response rate to CRT. Fibrosis > 50% of the lateral or posterolateral LV segment has been associated with a lower response rate to cardiac resynchronization [20]. In a systematic review the presence of significant scarring in the LV posterolateral wall was related to a 46% reduction by echocardiographic criteria and a 67% reduction by clinical criteria to CRT [21].
The rate of CRT super-response as well as possible prognostic variables is not known in this new scenario (LBBB > 150 ms, Strauss-defined LBBB, absence of scar tissue in the LV posterolateral wall). This study estimates the rate of super-responders as well as potential prognostic variables in this population.
Material and methods
Patient selection
From February 2015 to December 2016, 24 patients followed at the Dante Pazzanese Institute of Cardiology (IDPC), a tertiary cardiac hospital in São Paulo, Brazil, were selected for resynchronization pacemaker implantation according to the criteria for inclusion and exclusion below.
Inclusion criteria: 1) patients with HF LVEF < 35% of idiopathic ischemic dilated etiology, 2) LBBB > 150 ms, 3) optimized HF treatment, 4) NYHA CLASS II, III, IV (outpatient), and 5) sinus rhythm.
Exclusion criteria: 1) patient refusal, 2) recent myocardial infarction (< 6 months), 3) presence of atrial fibrillation, 4) patients with a prosthesis that alters the scar burden evaluation by MRI/artifacts (Ex: stents, metal valves), 5) patients with creatinine clearance < 40 ml/min (contraindicated for use of gadolinium for MRI fibrosis assessment technique), 6) presence of cardiac pacemaker, 7) patients with HF using vasoactive drugs, 8) presence of delayed myocardial enhancement MRI in the LV posterolateral region, or 9) comorbidities with life expectation of < 12 months.
Patients with ischemic and nonischemic HF were included. Ischemic HF patients where considered those to have reported a previous infarction, who underwent angioplasty or saphenous/mammary bridge, or those with coronary angiography lesions > 50%.
Electrocardiogram analysis
All 20 patients underwent 12-lead ECG for evaluation of QRS morphology and duration pre- and post-implant. QRS duration measurement should be measured from its inception to the point where the complex returns to baseline. The value selected should be where the QRS has the longest duration in any of the 12 leads. The classic LBBB criteria as well as that suggested by Strauss were adopted [19]. The duration of QRS pre- and post-implantation were analyzed, as well as the percentage of reduction of final QRS in relation to the initial measurement.
Echocardiogram analysis
All 20 patients underwent echocardiographic parameter measurements pre- (< 1 month) and post-implantation (> 6 months). We measured the LVEF, LVESV, LSEDV, LA diameter, and LVEDD pre- and post-CRT according to the recommendations of the American Society of Echocardiography (ASE) [22]. To standardize pre- and post-implant analysis, the echocardiogram was always performed by the same physician with extensive experience in echocardiography. The echocardiogram machine used was the GE model E9.
Myocardial MRI analysis
All 20 patients underwent cardiac MRI, which was considered safe and noninvasive, to assess LVEF, scar burden (%), and its presence or absence in the LV posterolateral wall according to the recommendations of the American consensus statement on cardiac MRI [23]. The examinations were performed using the Philips Ingenia 3.0 Tesla resonance imaging device by a team of IDPC physicians with extensive experience in cardiac MRI analysis.
CRT super-response criteria and possible prognostic variables
Although there are several parameters used in the evaluation of cardiac resynchronization super-response rate, we used a reduction of > 30% LVESV as a defining variable of CRT super-response. From the possible prognostic variables related to super-response rate [6-16], age, gender, Minnesota Quality of Life score, echocardiographic parameters (LA, LVESV, LVEDV, LVEDD, LVEF), and electrocardiographic recordings (% reduction in initial QRS) were analyzed.
The Minnesota Quality of Life Questionnaire is comprised of 21 questions about physical, emotional, and financial considerations and lifestyle issues related to HF. The score on each question ranges from 0 corresponding to “without limitations” to 5 referring to “maximum limitation”. The total score can range from 0 to 105 points where the lowest score represents a better quality of life (good quality of life < 26 points, moderate quality 26-45 points, and poor quality of life > 45 points) [24]. A reduction of 10 points is considered a good response to CRT [1].
Surgical technique, material used, cardiac resynchronization programming
All 20 patients underwent the conventional resynchronization pacemaker implant technique. Whenever possible, the LV electrode was positioned in the posterolateral wall through the coronary sinus. If necessary, a left lateral mini-thoracotomy could be performed for epicardial LV lead implantation.
The brands of devices used for the implants were the 4 available in the Brazilian market (Biotronik, Boston, Medtronic, St Jude).
At 1 month after implantation, the 20 patients underwent the first technical evaluation of the CRT through telemetry. In telemetry, the atrioventricular interval (standard AV-sense 120 ms) was adopted [25]. It was ensured that ventricular capture was > 90% (ideally > 97%). The best bi-ventricular pacing (VV) intervals were defined as producing the best (narrowest) surface QRS (LV < VD between -40 to 0 ms) [26,27]. In addition, by means of chest x-ray, we confirmed the correct positioning of the electrodes (LV posterolateral).
Follow up
All 20 patients completed the 6-month follow-up for the final assessment of CRT super-response rate. The patient was offered access to the hospital’s emergency care service as well as the institution’s pacemaker outpatient clinic for any necessary evaluation. They signed a consent form previously accepted by the Institutional Teaching and Research Committee of the IDPC, informing the risks and benefits of the intervention.
During the selection process, four patients were excluded for stroke, sudden death, morbid obesity associated with claustrophobia contraindicating the MRI assessment, and heart transplantation. The study design is presented in Figure 1.
Figure 1.
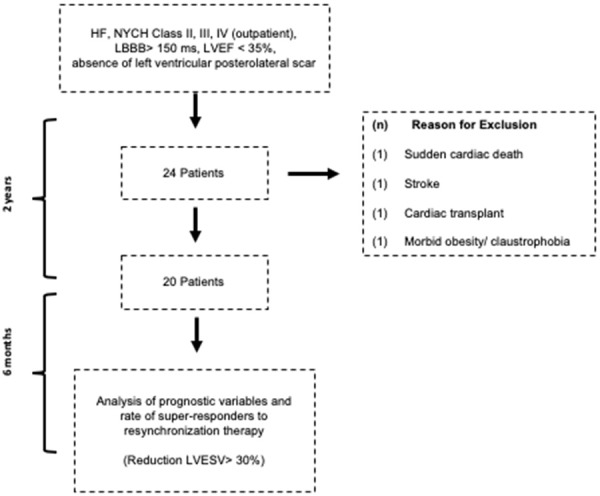
Prospective observational study flowchart.
Statistical analysis
The descriptive outcomes of the quantitative variables of our population are presented as the mean and standard deviation. In the evaluation of possible associations among qualitative variables for super-response, the Fisher’s Test was used. To evaluate the possible association of quantitative variables for super-response, the Mann Whitney Test was used. The significance level adopted was 5%. Data were analyzed using SPSS Version 19 and R core.
Results
The 20 included patients had a mean follow-up of 237 days. Table 1 presents the description of the included population.
Table 1.
Sociodemographic and clinical patient characteristics
| Average (SD) | |
|---|---|
| Age/Years | 58.20±8.79 |
| Sex F/M | 60%/40% |
| Etiology | |
| Ischemic heart failure | 10% |
| Idiopathic dilated cardiomyopathy | 90% |
| Drugs | |
| Beta Blocker | 100% |
| ACE inhibitors/ARBs | 95 |
| Aldosterone | 80% |
| NYHA Classification | |
| Class II | 75% |
| Class III | 25% |
| Quality of Life (Minnesota) | 56.6±18.8 |
| ECG | |
| LBBB | 100% |
| LBBB Strauss + | 90% |
| QRS Duration (ms) | 162.15±7.86 |
| Echocardiogram | |
| LVEF (%) | 28.15±5.10 |
| LVEDD | 67.67±12.37 |
| LVESV (ml) | 154.3±59.10 |
| LVEDV (ml) | 160.5±69.87 |
| LA diameter (mm) | 42.40±4.63 |
| Myocardial MRI | |
| LVEF % | 23.15%±5.81 |
| Myocardial fibrosis MRI (%) | 1.97%±4.31 |
HF: heart failure; ACE inhibitors: angiotensin-converting enzyme; ARBs: angiotensin receptor blocker; LVEF: Left ventricular ejection fraction; LVEDD: left ventricle end-diastolic diameter; LVESV: End-systolic volume; EDV: End-diastolic volume; ECG: Electrocardiogram; LBBB: Left bundle branch block; LA: Left atrium.
The rate of CRT super-response was 60% (Figure 2) when we used the ventricular remodeling criteria (> 30% decrease in LVESV).
Figure 2.

CRT super-responder rate analysis of the 20 patients before and after CRT.
The results for LVEF, LVEDV, LVESV, QRS duration, and Minnesota quality of life test of the 20 included patients before and after CRT are shown in Figures 3, 4, 5, 6 and 7.
Figure 3.

LVEF analysis of the 20 patients before and after CRT.
Figure 4.

LVEDV analysis of the 20 patients before and after CRT.
Figure 5.

LVESV analysis of the 20 patients before and after CRT.
Figure 6.

QRS duration analysis of the 20 patients before and after CRT.
Figure 7.

Minnesota test analysis of the 20 patients before and after CRT.
In the analysis of these variables after CRT, we observed a relative increase of 48.52% in LVEF (Figure 3), a relative reduction of 35.13% in LVEDV (Figure 4), a relative reduction of 28.47% in LVESV (Figure 5), a relative reduction of 20.17% in QRS duration (Figure 6), and a relative reduction of 33.83% on the Minnesota test (Figure 7).
Of the total patients with LBBB > 150 ms, 90% met the Strauss criteria. The improvement in the NYHA class of the study patients ranged from 75% initial class II and 25% class III to 65% final class I and 35% class II. The percentage of ventricular capture in the telemetry assessment averaged 98.5%. All 20 patients had the LV electrode implanted in the LV posterolateral region.
The analysis of possible variables related to super-response (reduction > 30% LVESV) are presented in Table 2.
Table 2.
Possible predictors of CRT super-response
| Reduction > 30% LVESV | Reduction < 30% LVESV | p-value | |
|---|---|---|---|
| Patient characteristics | |||
| Sex M/F (n=20) | 4/8 (n=12) | 4/4 (n=8) | 0.64 |
| Age (years) | 58.67±9.99 | 57.5±7.19 | 0.87 |
| Quality of Life (Minnesota) | 55.50±16.54 | 67.50±19.63 | 0.20 |
| ECG | |||
| QRS Reduction % | 21.16±3.74 | 18.69±4.77 | 0.21 |
| Echocardiogram | |||
| LVEF % | 29.25±3.64 | 26.5±6.67 | 0.38 |
| LVESV (ml) | 145.83±43.35 | 167.00±78.94 | 0.75 |
| LVEDV (ml) | 211.33±54.20 | 229.63±75.19 | 0.75 |
| LVEDD | 66.33±5.10 | 67.67±12.34 | 0.82 |
| LA (mm) | 41.58±3.87 | 43.63±5.65 | 0.45 |
| Myocardial MRI | |||
| LVEF % | 23.42±5.4 | 22.75±6.75 | 0.58 |
| Fibrosis % | 1.61±2.58 | 2.5±6.27 | 0.69 |
| Ventricular stimulation | |||
| Load of ventricular stimulation (%) | 98.25±1.31 | 97.63±3.15 | 0.34 |
LVEF: Left ventricular ejection fraction; LVEDD: left ventricle end-diastolic diameter; LVESV: End-systolic volume; LVEDV: End-diastolic volume; ECG: Electrocardiogram; LA: Left atrium.
In the graphical representation of chosen variables presented in Table 2, we note that the super responders show a greater variability in their improvement of LVEF, a decrease in LVEDF, as well as a reduction of the percentage of initial QRS duration while demonstrating the best performance in the Minnesota test, although these differences did not reach statistical significance when compared to non superresponders.
Of the complications observed, we had 5 cases with chronically high stimulation thresholds (> 2.5/0.4 mv), 1 case of phrenic stimulation, 1 case of pain score > 7 points by numerical rating scale, 2 cases of LV electrodes implanted in the epicardium, and 1 case of cardiac tamponade diagnosed intraoperatively with a favorable outcome.
Discussion
In our study, based on contemporary CRT selection criteria, we observed a 60% super-response rate, distinct from the results presented to date, where the super-response rate varies between 10-38% [6-16]. Contrary to the classical resynchronization studies, in our study, the presence of LBBB with QRS > 150 ms was part of the selection criteria and most of the patients with LBBB met the Strauss criteria [19] (90%). We also excluded the presence of fibrosis in the LV posterolateral wall [10,21] as well as maintained, on average, the bi-ventricular pacing load of 98.5% [26,27]. In assessing the success of CRT, these aspects represent major advances in modern cardiac resynchronization [1,2].
Our rate of adherence to HF drug treatment was about 91.6% when considering the use of the triad of beta-blockers, ACEI/ARBs, and aldosterone antagonists. In the COMPANION, CARE-HF, REVERSE, MADIT-CRT, and RAFT studies, an average adherence of 70.90% was reported (it would be 96% if we consider the use of BB, ACEI/ARBs) [17,18]. This is likely because our patients were followed with a group of cardiomyopathies that reinforce the maintenance of medications even after CRT.
During the selection of CRT candidates, we included exclusively patients who presented LBBB > 150 ms as a marker of true cardiac dyssynchrony. Unlike the vast majority of cardiac resynchronization studies that included LBBB with QRS > 120 ms and up to 30% of those selected with non-LBBB conduction disorder [17,18]. Two meta-analyses previously evaluated the importance of LBBB with QRS > 150 ms as an important prognostic variable in the success of CRT [17,18]. This contributed to the selection of patients with true cardiac dyssynchrony for whom CRT has the potential to be extremely beneficial.
The surgical technique was rigorously followed and the LV electrode was placed in the posterolateral wall, via endocardial or epicardial route in all 20 patients. This is fundamental, as this is where the conduction delay caused by the LBBB is found [28]. We know that an electrode implanted outside this region may be associated with a poorer prognosis [28].
A previous study showed that a scar burden > 50% in the posterolateral segment is associated with a lower response rate to cardiac resynchronization [20]. A systematic review conducted in 2015 also observed that the presence of significant fibrosis in the LV posterolateral wall is related to a 46% reduction in the response rate by echocardiographic criteria and 67% by clinical criteria to CRT [21].
In addition, correct resynchronizer programming ensured the necessary ventricular capture as well as prompt correction of potential factors that could be related to non-response to CRT. AV and VV interval adjustment were performed to ensure maximum LV capture as well as greater surface QRS narrowing [25-27]. We optimized the resynchronization program parameters according to the best available evidence [1,2]. In the vast majority of CRT-related studies, LV lead implant site data and ventricular capture percentage are not explicitly described.
We know that the rate of CRT super-response in previous studies is between 10% and 38% of patients eligible for CRT [6-16]. Using echocardiographic criteria in the analysis of CRT super-response (LVESV reduction > 30%), we obtained a rate of 60% of super-responders. A previous study of 8 patients presenting with LBBB (QRS 120-160 ms) for over 5 years, with initial LVEF > 50%, who evolved to HF of electrical etiology, LVEF < 40% at follow-up, reported that of these 8 patients, 6 had super-response (LVEF > 45% and improvement of 1 more NYHA class in 12 months) [29]. This corresponds to a 75% rate of CRT super-response. Although we use different criteria for super-response (in our study LVESV > 30%), we do not consider LBBB to be the cause of HF, but a component that could have caused or aggravated HF. Our results are consistent with these higher super-response rate findings.
We did not obtain a significant association between other possible variables associated with this increase in the super-response rate (LVEF, LVEDV, LVESV, LVEDD, LA size, % fibrosis, and gender) with LBBB > 150 ms. This may mean that using the current class I indications for cardiac resynchronization (LVEF < 35%, LBBB > 150 ms), associated with Strauss-defined LBBB, such as absence of scarring in the LV posterolateral wall and degree of ventricular capture > 97%, were not predictors of CRT super-response.
In a previous study describing a 38% super-responder rate (> 30% reduction in LVESV, the same as in this study), female gender, QRS duration, and nonischemic etiology were found to be prognostic variables [10]. The inclusion criteria were patients with QRS > 130 ms regardless of the type of block. We did not obtain the same results because we included in our selection LBBB > 150 ms (range 150-180 ms), the variable most associated with super-response [17,18].
Another study observed that in patients with heart failure (NYHA class I and II), female gender, non-ischemic heart disease, LBBB, QRS > 150 ms, BMI < 30 kg/m2, and reduced LA size were predictors of super-response (criterion used was > 14.5% LVEF) [16]. In our study, based on the current evidence of Class I symptoms, LBBB > 150 ms was already part of the inclusion criteria. This may be the great prognostic variable associated with CRT super-response since we did not obtain a difference (we used a different criterion; > 30% reduction in LVESV) when considering gender and size of the left atrium. We did not consider the etiology of HF as a predictor variable in our study because only 10% of our patients had ischemic HF.
Female gender was previously cited as one of the variables related to the rate of super-response [30] perhaps because it is related to nonischemic etiology and true LBBB (Strauss) [19]. Women with LBBB < 150 ms tend to respond to CRT while men do not [31-33]. In a study assessing the difference in CRT response between genders by analyzing QRS duration, women with LBBB and QRS > 180 ms and men with QRS > 200 ms tended to have a decreased response to cardiac resynchronization [34]. We believe that we did not find this difference between genders in the evaluation of CRT super-response rate because the duration of LBBB in our study varied from 150-180 ms and in this range there seems to be no difference between genders.
One of the possible limitations of our study is the size of our sample. We could not obtain a statistically significant association between the analyzed variables. Noting the clinical significance between the super-responders and non-super-responders, we also found no clinically relevant difference between the predictor variables analyzed. This may mean the statistical analysis in our sample accurately represents these results.
An attempt to correlate the percentage of scar tissue to the resynchronization response rate is recent. The cutoff limit used to separate responders or not to CRT ranged from 15 to 35% of the total scar burden [35-37]. In our study, the analysis was impaired by the low percentage of scarring between the compared groups. The percentage of scar burden ranged from 0 to 18% with an average of 1.96%. In a previous evaluation of a series of 20 cases, with an average LVEF of 17%, mean QRS of 164 ms, and percent scar burden of 17.2% showed an increase in the super-response rate (> 25% reduction in LVESV) when the LV electrode was implanted outside the fibrosis (posterolateral) region [38]. Surprisingly, the extent of scarring was not associated with super-response (higher on average than our study, 17.2%).
In addition, we had a low representation of patients with ischemic HF (10%) in our study. Nonischemic dilated heart disease is more likely to be associated with primary electrical heart disease (LBBB) than ischemic heart disease (as it has a defined etiology). In this sense, reverse remodeling associated with ischemic etiology should be smaller [39]. Yet in our study this cannot be evaluated. Interestingly, a previous study observed that a slight improvement in reverse remodeling in patients with ischemic heart disease could yield good clinical results at 1 year. Where, in patients with nonischemic/dilated heart disease, these same results appear with a more discernible improvement in reverse remodeling [39].
Conclusion
In this study, the rate of cardiac resynchronization super-response was 60%, clearly higher than most studies in which, with rare exceptions, the rate does not exceed 38%. The detailed analysis of these patients showed that the reason for this differentiated result is because, unlike previous cardiac resynchronization studies, we included only candidates with QRS duration > 150 ms and almost all reaching LBBB criteria according to Strauss. We also added a cardiac MRI to rule out the presence of fibrosis in the LV posterolateral wall and sought to maintain the mean biventricular pacing load of 98.5%. Adding these inclusion factors to the current class I indications from the 2012 American and 2013 European guidelines, we increased the rate of CRT super-responders. In this new context, no other variable analyzed was associated with the rate of super-response as shown in earlier studies. Careful analysis of these cases is essential, as through them we can improve the indication and technique of CRT, thus improving the cost-effectiveness of this treatment modality for heart failure as well as the relevant clinical outcomes.
Acknowledgements
I thank my advisor for the opportunity to conduct this study and the Dante Pazzanse Cardiology Hospital for supplying the necessary structure and materials for the progress of our study.
Disclosure of conflict of interest
None.
References
- 1.Tracy CM, Epstein AE, Darbar D, Dimarco JP, Dunbar SB, Estes NA 3rd, Ferguson TB Jr, Hammill SC, Karasik PE, Link MS, Marine JE, Schoenfeld MH, Shanker AJ, Silka MJ, Stevenson LW, Stevenson WG, Varosy PD. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2012;60:1297–1313. doi: 10.1016/j.jacc.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 2.European Society of Cardiology (ESC); European Heart Rhythm Association (EHRA) Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM, Gorenek B, Israel CW, Leclercq C, Linde C, Mont L, Padeletti L, Sutton R, Vardas PE. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA) Europace. 2013;15:1070–1118. doi: 10.1093/europace/eut206. [DOI] [PubMed] [Google Scholar]
- 3.Van Hemel N, Scheffer M. Cardiac resynchronization therapy in daily practice and loss of confidence in predictive techniques to response. Neth Heart J. 2009;17:4–5. doi: 10.1007/BF03086206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, Chan YS, Kong SL, Bax JJ. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112:1580–1586. doi: 10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 5.Rao RK, Kumar UN, Schafer J, Viloria E, De Lurgio D, Foster E. Reduced ventricular volumes and improved systolic function with cardiac resynchronization therapy: a randomized trial comparing simultaneous biventricular pacing, sequential biventricular pacing, and left ventricular pacing. Circulation. 2007;115:2136–2144. doi: 10.1161/CIRCULATIONAHA.106.634444. [DOI] [PubMed] [Google Scholar]
- 6.António N, Teixeira R, Coelho L, Lourenço C, Monteiro P, Ventura M, Cristóvão J, Elvas L, Gonçalves L, Providência LA. Identification of “superresponders” to cardiac resynchronization therapy: the importance of symptom duration and left ventricular geometry. Europace. 2009;11:343–349. doi: 10.1093/europace/eup038. [DOI] [PubMed] [Google Scholar]
- 7.Castellant P, Fatemi M, Bertault-Valls V, Etienne Y, Blanc J. Cardiac resynchronization therapy: “nonresponders” and “hyperresponders”. Heart Rhythm. 2008;5:193–197. doi: 10.1016/j.hrthm.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 8.Gasparini M, Regoli F, Ceriotti C, Galimberti P, Bragato R, De Vita S, Pini D, Andreuzzi B, Mangiavacchi M, Klersy C. Remission of left ventricular systolic dysfunction and of heart failure symptoms after cardiac resynchronization therapy: temporal pattern and clinical predictors. Am Heart J. 2008;155:507–514. doi: 10.1016/j.ahj.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 9.Castellant P, Fatemi M, Orhan E, Etienne Y, Blanc JJ. Patients with non-ischaemic dilated cardiomyopathy and hyper-responders to cardiac resynchronization therapy: characteristics and long-term evolution. Europace. 2009;11:350–355. doi: 10.1093/europace/eup035. [DOI] [PubMed] [Google Scholar]
- 10.van Bommel RJ, Bax JJ, Abraham WT, Chung ES, Pires LA, Tavazzi L, Zimetbaum PJ, Gerritse B, Kristiansen N, Ghio S. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: a PROSPECT (Predictors of Response to CRT) sub-analysis. Eur Heart J. 2009;30:2470–2477. doi: 10.1093/eurheartj/ehp368. [DOI] [PubMed] [Google Scholar]
- 11.Reant P, Zaroui A, Donal E, Mignot A, Bordachar P, Deplagne A, Solnon A, Ritter P, Daubert JC, Clementy J, Leclercq C, Roudaut R, Habib G, Lafitte S. Identification and characterization of super-responders after cardiac resynchronization therapy. Am J Cardiol. 2010;105:1327–1335. doi: 10.1016/j.amjcard.2009.12.058. [DOI] [PubMed] [Google Scholar]
- 12.Rickard J, Kumbhani DJ, Popovic Z, Verhaert D, Manne M, Sraow D, Baranowski B, Martin DO, Lindsay BD, Grimm RA, Wilkoff BL, Tchou P. Characterization of super-response to cardiac resynchronization therapy. Heart Rhythm. 2010;7:885–889. doi: 10.1016/j.hrthm.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Adelstein E, Schwartzman D, Gorcsan J 3rd, Saba S. Predicting hyperresponse among pacemaker-dependent nonischemic cardiomyopathy patients upgraded to cardiac resynchronization. J Cardiovasc Electrophysiol. 2011;22:905–911. doi: 10.1111/j.1540-8167.2011.02018.x. [DOI] [PubMed] [Google Scholar]
- 14.Qing Q, Ding LG, Hua W, Chen KP, Wang FZ, Zhang S. Potential predictors of non-response and super-response to cardiac resynchronization therapy. Chin Med J (Engl) 2011;124:1338–1441. [PubMed] [Google Scholar]
- 15.Serdoz LV, Daleffe E, Merlo M, Zecchin M, Barbati G, Pecora D, Pinamonti B, Fantoni C, Lupo P, Di Lenarda A, Sinagra G, Cappato R. Predictors for restoration of normal left ventricular function in response to cardiac resynchronization therapy measured at time of implantation. Am J Cardiol. 2011;108:75–80. doi: 10.1016/j.amjcard.2011.02.347. [DOI] [PubMed] [Google Scholar]
- 16.Hsu JC, Solomon SD, Bourgoun M, McNitt S, Goldenberg I, Klein H, Moss AJ, Foster E MADIT-CRT Executive Committee. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome: the MADIT-CRT (multicenter automatic defibrillator implantation trial with cardiac resynchronization therapy) study. J Am Coll Cardiol. 2012;59:2366–2373. doi: 10.1016/j.jacc.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 17.Sipahi I, Carrigan TP, Rowland DY, Stambler BS, Fang JC. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch Intern Med. 2011;171:1454–1462. doi: 10.1001/archinternmed.2011.247. [DOI] [PubMed] [Google Scholar]
- 18.Stravos S, Lazzara R, Thadani U. The benefit of cardiac resynchronization therapy and QRS duration: a meta-analysis. J Cardiovasc Electrophysiol. 2012;23:163–168. doi: 10.1111/j.1540-8167.2011.02144.x. [DOI] [PubMed] [Google Scholar]
- 19.Strauss DG, Selvester RH, Wagner GS. Defining left bundle branch block in the era of cardiac resynchronization therapy. Am J Cardiol. 2011;107:927–934. doi: 10.1016/j.amjcard.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 20.Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, van der Wall EE, Schalij MJ, Bax JJ. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–976. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 21.Daoulah A, Alsheikh-Ali AA, Al-Faifi SM, Ocheltree SR, Haq E, Asrar FM, Fathey A, Haneef AA, Al Mousily F, O el-S, Lotfi A. Cardiac resynchronization therapy in patients with postero-lateral scar by cardiac magnetic resonance: a systematic review and meta-analysis. J Electrocardiol. 2015;48:783–790. doi: 10.1016/j.jelectrocard.2015.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, Solomon SD, Spencer KT, Sutton MS, Stewart WJ Chamber Quantification Writing Group; American Society of Echocardiography’s Guideline and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.American College of Cardiology Foundation Task Force on Expert Consensus Documents. Hundley WG, Bluemke DA, Finn JP, Flamm SD, Fogel MA, Friedrich MG, Ho VB, Jerosch-Herold M, Kramer CM, Manning WJ, Patel M, Pohost GM, Stillman AE, White RD, Woodard PK. ACCF/ACR/AHA/NASCI/SCMR 2010 expert consensus document on cardiovascular magnetic resonance: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents. Circulation. 2010;121:2462–2508. doi: 10.1161/CIR.0b013e3181d44a8f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behlouli H, Feldman DE, Ducharme A, Frenette M, Giannetti N, Grondin F, Michel C, Sheppard R, Pilote L. Identifying relative cutoff scores with neural networks for interpretation of the Minnesota living with heart failure questionnaire. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:6242–6246. doi: 10.1109/IEMBS.2009.5334659. [DOI] [PubMed] [Google Scholar]
- 25.Brenyo A, Kutyifa V, Moss AJ, Mathias A, Barsheshet A, Pouleur AC, Knappe D, McNitt S, Polonsky B, Huang DT, Solomon SD, Zareba W, Goldenberg I. Atrioventricular delay programming and the benefit of cardiac resynchronization therapy in MADIT-CRT. Heart Rhythm. 2013;10:1136–1143. doi: 10.1016/j.hrthm.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Bogaard MD, Meine M, Tuinenburg AE, Maskara B, Loh P, Doevendans PA. Cardiac resynchronization therapy beyond nominal settings: who needs individual programming of the atrioventricular and interventricular delay? Europace. 2012;14:1746–1753. doi: 10.1093/europace/eus170. [DOI] [PubMed] [Google Scholar]
- 27.Ruwald AC, Kutyifa V, Ruwald MH, Solomon S, Daubert JP, Jons C, Brenyo A, McNitt S, Do D, Tanabe K, Al-Ahmad A, Wang P, Moss AJ, Zareba W. The association between biventricular pacing and cardiac resynchronization therapy-defibrillator efficacy when compared with implantable cardioverter defibrillator on outcomes and reverse remodelling. Eur Heart J. 2015;36:440–448. doi: 10.1093/eurheartj/ehu294. [DOI] [PubMed] [Google Scholar]
- 28.Singh JP, Klein HU, Huang DT, Reek S, Kuniss M, Quesada A, Barsheshet A, Cannom D, Goldenberg I, McNitt S, Daubert JP, Zareba W, Moss AJ. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation. 2011;123:1159–1166. doi: 10.1161/CIRCULATIONAHA.110.000646. [DOI] [PubMed] [Google Scholar]
- 29.Vaillant C, Martins RP, Donal E, Leclercq C, Thébault C, Behar N, Mabo P, Daubert JC. Resolution of left bundle branch block-induced cardiomyopathy by cardiac resynchronization therapy. J Am Coll Cardiol. 2013;61:1089–1095. doi: 10.1016/j.jacc.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 30.Varma N, Mittal S, Prillinger JB, Snell J, Dalal N, Piccini JP. Survival in women versus men following implantation of pacemakers, defibrillators, and cardiac resynchronization therapy devices in a large, nationwide cohort. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.116.005031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Varma N, Manne M, Nguyen D, He J, Niebauer M, Tchou P. Probability and magnitude of response to cardiac resynchronization therapy according to QRS duration and gender in nonischemic cardiomyopathy and LBBB. Heart Rhythm. 2014;11:1139–1147. doi: 10.1016/j.hrthm.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Zusterzeel R, Selzman KA, Sanders WE, Caños DA, O’Callaghan KM, Carpenter JL, Piña IL, Strauss DG. Cardiac resynchronization therapy in women: US food and drug administration meta-analysis of patient-level data. JAMA Intern Med. 2014;174:1340–1348. doi: 10.1001/jamainternmed.2014.2717. [DOI] [PubMed] [Google Scholar]
- 33.Zusterzeel R, Curtis JP, Caños DA, Sanders WE, Selzman KA, Piña IL, Spatz ES, Bao H, Ponirakis A, Varosy PD, Masoudi FA, Strauss DG. Sex-specific mortality risk by QRS morphology and duration in patients receiving CRT: results from the NCDR. J Am Coll Cardiol. 2014;64:887–894. doi: 10.1016/j.jacc.2014.06.1162. [DOI] [PubMed] [Google Scholar]
- 34.Varma N, Lappe J, He J, Niebauer M, Manne M, Tchou P. Sex-specific response to cardiac resynchronization therapy effect of left ventricular size and QRS duration in left bundle branch block. JACC Clin Electrophysiol. 2017;3:844–853. doi: 10.1016/j.jacep.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 35.White JA, Yee R, Yuan X, Krahn A, Skanes A, Parker M, Klein G, Drangova M. Delayed enhancement magnetic resonance imaging predicts response to cardiac resynchronization therapy in patients with intraventricular dyssynchrony. J Am Coll Cardiol. 2006;48:1953–1960. doi: 10.1016/j.jacc.2006.07.046. [DOI] [PubMed] [Google Scholar]
- 36.Chalil S, Foley PW, Muyhaldeen SA, Patel KC, Yousef ZR, Smith RE, Frenneaux MP, Leyva F. Late gadolinium enhancement-cardiovascular magnetic resonance as a predictor of response to cardiac resynchronization therapy in patients with ischaemic cardiomyopathy. Europace. 2007;9:1031–1037. doi: 10.1093/europace/eum133. [DOI] [PubMed] [Google Scholar]
- 37.Marsan NA, Westenberg JJ, Ypenburg C, van Bommel RJ, Roes S, Delgado V, Tops LF, van der Geest RJ, Boersma E, de Roos A, Schalij MJ, Bax JJ. Magnetic resonance imaging and response to cardiac resynchronization therapy: relative merits of left ventricular dyssynchrony and scar tissue. Eur Heart J. 2009;30:2360–2367. doi: 10.1093/eurheartj/ehp280. [DOI] [PubMed] [Google Scholar]
- 38.Wassef A, Yee R, Wong J, Scholl D, Gula L, McCarty D. A case series examination of twenty “super responders” to cardiac resynchronization therapy. Canadian J Cardiol. 2012;28:S265–S266. [Google Scholar]
- 39.Martens P, Nijst P, Verbrugge FH, Dupont M, Tang WHW, Mullens W. Profound differences in prognostic impact of left ventricular reverse remodeling after cardiac resynchronization therapy relate to heart failure etiology. Heart Rhythm. 2018;15:130–136. doi: 10.1016/j.hrthm.2017.08.021. [DOI] [PubMed] [Google Scholar]


