INTRODUCTION
Diabetic nephropathy is the major cause of end-stage renal failure and the main contributing factor, together with cardiovascular disease, of increased morbidity and mortality in both type 1 and type 2 diabetes (1,2). Diabetic nephropathy is usually associated with macroalbuminuria (3) but, in some patients, reduced glomerular filtration rate (eGFR) without increased albuminuria and macroalbuminuria without reduced eGFR may be present.
Diabetic nephropathy is secondary to a series of alterations in hemodynamic and metabolic pathways. Abnormalities, in the lipid/lipoprotein pathway, are considered strongly involved in the development of nephropathy in diabetes (4). Although many of the initial studies on lipid abnormalities focused on abnormalities of cholesterol and triglycerides, sphingolipids and glycosphingolipids have recently started to emerge as important players in diabetic nephropathy (4, 5).
We previously described reduced levels of very long chain ceramides at baseline in type 1 diabetes patients from the DCCT/EDIC cohort who subsequently developed macroalbuminuria during follow-up (6). In this prior report,sphingolipids were measured in serum collected from patients at entry into the study (baseline samples) when the patients were devoid of any complaints or signs of kidney disease and their albumin excretion rate was less than 40mg/24 hours and the patients were followed until EDIC year 18.
Glycosphingolipids (GSLs), the most abundant sphingolipids in circulating lipoproteins after sphingomyelin, have been recently postulated as being important contributors to the development of diabetic nephropathy. Glycosphingolipids (GSLs) are a heterogenous class of lipids within the sphingolipid family. A simple subclass of GSLs is glucosylceramide, which is generated from ceramides after glucosylceramide synthase-mediated incorporation of glucose (7). Addition of galactose to glucosylceramide leads to the formation of lactosylceramide. Lactosylceramide is the main precursor of complex glycosphingolipids.
Glycosphingolipids have been shown in several studies to regulate a number of cellular processes including cell proliferation, apoptosis, inflammation and cellular signaling (8–12). Glycosphingolipids are particularly abundant in the kidney specifically in podocytes, mesangial cells and in tubular epithelial cells and are thought to play critical roles in kidney metabolism (7).
Glycosphingolipids played a role in the development of glomerular hypertrophy in streptozotocin-induced type 1 diabetes in rats that was reversed by the administration of PDMP (15), a ceramide analog, which prevents lactoceramide synthesis. Also, studies performed in the db/db mouse model of type II diabetes and in vitro in cultured mesangial cells have shown an elevation of GSLs in the kidney of diabetic db/db mice. Interestingly, inhibition of glycosphingolipids synthesis reversed hyperglycemia-induced mesangial cell hypertrophy by decreasing phosphorylated (p)Smad3 and pAkt signaling (16). Human studies investigating the role of sphingolipids in diabetic nephropathy are very limited. Besides our studies on the association of plasma ceramides in the DCCT/EDIC cohort with the development of macroalbuminuria (6), a cross sectional study of 326 type 1 diabetes patients enrolled in the Finnish Diabetic Nephropathy study (FinnDiane) found that plasma sphingomyelin levels emerged as a biochemical covariate of urinary albumin excretion rate (17).
Therefore, in the current study we examined whether glycosphingolipid levels are associated with the development of diabetic nephropathy in type 1 diabetes. Our main goal was to determine whether or not plasma sphingolipid changes could identify patients at high risk to develop nephropathy in the early phases of disease, using both macroalbuminuria and reduced eGFR to define diabetic nephropathy. Specifically, we examined whether plasma levels of glycosphingolipid species measured at DCCT Baseline would be associated with the development of abnormal albuminuria or chronic kidney disease during 21–28 years of disease progression.
METHODS
Research Design and Study Participants
The DCCT was a randomized controlled trial of 1,441 patients who were 13–39 years of age and had type 1 diabetes for 1–15 years at study entry [18]. Participants were randomly assigned to a treatment assignment from two baseline study cohorts. Participants in the primary prevention study cohort had no retinopathy (based on fundus photography), diabetes for 1–5 years, and no microalbuminuria (AER <40 mg/24 h). Participants in the secondary intervention cohort had mild to moderate non-proliferative diabetic retinopathy (≥ 1 microaneurysm in either eye), diabetes for 1–15 years and AER < 200 mg/24 h. Additionally, none of the study participants had eGFR >60 ml/1.73 m2, hypertension (≥140/90 mmHG), or total cholesterol > 200 mg/dl and/or LDL cholesterol > 160 mg/dl at study entry. At the baseline DCCT examination, each participant received a complete physical examination which included a medical history, an electrocardiogram, and routine laboratory analyses to determine serum creatinine, lipid profile, and HbA1c levels [18]. The participants were then randomized into groups that received either intensive or conventional insulin therapy and were followed for an average of 6.5 years. The study was terminated in 1993 because of the observed major beneficial effect of intensive therapy on retinal, renal, and neurologic complications. In 1994, approximately 95% of the DCCT participants were enrolled into an observational study, the Epidemiology of Diabetes Interventions and Complications (EDIC) study. The goal of the EDIC study was to assess the long-term effects of prior separation of glycemic levels on micro- and macrovascular outcomes in type 1 diabetes [19]. During EDIC, all patients were under the care of their personal physicians and encouraged to practice intensive insulin therapy.
The present analysis was performed on a subgroup of 432 patients representative of the whole DCCT/EDIC cohort. Glycosphinglolipids (hexosyl and lactosyl ceramides) were assayed in plasma samples collected at the time of their entry into the DCCT (Baseline) before they were randomized into one of the two study treatment arms.
Blood Sample Collection for Plasma Sphingolipid Analysis
Fasting plasma samples collected in EDTA and obtained at DCCT Baseline were sent at the time of collection to the DCCT/ EDIC central laboratory for standard lipid analysis. Aliquots of these samples were archived for future research purposes. In 1999–2000, as part of a NIH/JDF Program Project Grant awarded to the Medical University of South Carolina, plasma samples collected during DCCT were provided by the DCCT/EDIC Coordinating Center and NIDDK to complement studies conducted during the Program Project. These plasma samples were stored at −70 °C and refreezing effects were minimized by preparing aliquots of the plasma when thawed for the first time and by using a new, frozen aliquot for the assay of plasma sphingolipids. The IRB at Medical University of South Carolina and all participating DCCT/EDIC centers approved the sample collection procedures. Written informed consent was obtained from all participants.
Sphingolipid Extraction and Analysis
Analyses of plasma levels of sphingolipid species were conducted in the Lipidomics Core Facility at the Medical University of South Carolina as previously described (20–22). Briefly, 100 μl of plasma from each patient was spiked with internal standards and the sphingolipid complement in each sample was quantitatively extracted. The sphingolipids in plasma extracts were separated and their masses quantitated using high performance liquid chromatography-tandem mass spectrometry (LC-ESIMS/ MS) as described previously (20–22). Lipids eluted during chromatography were detected and quantitated using a Thermo Scientific Quantum Access triple quadruple mass spectrometer equipped with an electrospray ion source (ESI) operating in multiple reaction monitoring (MRM) positive ion mode. Chromatographic separations were obtained under a gradient elution of a Peeke Scientific (Redwood City, CA), Spectra C8SR 150×3.0 mm; 3-μm particle size column. Quantitative analyses were based on calibration curves generated by injecting known amounts of the target analytes and an equal amount of the internal standards. A listing of the internal standards used and of the sphingolipids with available calibration standards was previously published by our group (20). The calibration standards were obtained predominately from the MUSC Lipidomics Share Resource facility, and from commercially available sources, Avanti Polar Lipids Inc. and Matreya LLC. The final concentrations of analytes in samples were determined using the appropriate corrections for sample loss based on internal standard recovery calculations. The resulting data were then normalized to the volume of sample analyzed. Final results are reported as the nanomolar concentration in plasma.
Outcomes
The two primary kidney endpoints for each participant were individually characterized from the baseline DCCT visit (1983–1989) up to year 18 of the EDIC study (2012). Four-hour urine collections for measurement of albumin excretion rate (AER) and creatinine clearance were also obtained during EDIC on alternate years (19); estimated glomerular filtration rate (eGFR) was calculated based on annual serum creatinine levels measured during DCCT and EDIC. Levels of estimated glomerular filtration rate were used to define progression to chronic kidney disease (CKD) and albumin excretion rate defined progression to macroalbuminuria (MA). Although, AER is usually associated with progression to CKD, with increases in AER generally preceding declines in measured eGFR, renal failure can occur in some patients with concurrent normal AER levels. Thus, each outcome was analyzed independent of the progression of the other. Participants had eGFR values calculated annually from serum creatinine levels using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation (23) during both DCCT and EDIC. CKD was defined as any estimated GFR measures <60 ml/1.73 m2. Time to CKD was defined as the time measured from DCCT baseline to the date of the first low eGFR value. Incident macroalbuminuria (MA) was defined as having an AER ≥300 mg/24 h at any time during the study. Time to MA was defined as the time measured from DCCT baseline to the date of the first elevated AER value.
Statistical Analysis
Demographic, clinical and biomarker measurements taken at DCCT baseline and closeout visits are tabulated for the entire cohort as well as stratified by disease outcome group. Baseline covariates for the analysis were obtained from baseline history, physical examinations and laboratory data (lipids and renal function). Standard descriptive statistics were used to summarize the general demographic and clinical data. The Kruskal-Wallis test was used to evaluate continuous baseline demographic and clinical measures across kidney dysfunction outcomes; the Pearson chi-square test was used to assess the association for categorical variables. Concentrations of glycated sphingolipids were measured at the DCCT baseline and used to determine the association with odds of progression to kidney dysfunction during DCCT and 18 years of EDIC follow up. Prior to final analysis, model residuals were assessed for normality through the use of histograms and quantile-quantile (Q-Q) plots and transformed when necessary. Results show that all the measured sphingolipids were positively skewed; therefore, natural logarithm transformations were applied which resulted in normally distributed biomarkers. Following data normalization, all levels were standardized (z-scores), and the analysis results represent the association between a difference of 1 standard deviation in each biomarker and the instantaneous risk of progression to kidney dysfunction. Cox Proportional Hazards regression analysis was used to quantify the association of increased sphingolipid levels at baseline with the time to subsequent development of kidney dysfunction outcomes. The primary parameter of interest in the hazard regression models was the ratio of the hazards for a 1 standard unit increase in sphingolipid levels (and associated 95% confidence interval) for the progression to each diabetic nephropathy outcome as compared to those without that outcome. Modifying effects DCCT treatment group assignment and baseline disease status on the effects of the association between sphingolipid levels and progression to kidney disease were examined for all models using appropriate interactions terms added to the final adjusted models. All statistical analyses were performed using the SAS system version 9.4 (SAS Institute, Cary NC, USA). Significance for all planned comparisons was set at a 2-sided p-value of 0.05 and no correction for multiple testing has been applied to reported p-values.
RESULTS
The concentrations of glycosylated sphingolipid species were measured in plasma samples from 432 DCCT/EDIC participants who did not have elevated AER (all AER<200 mg/24 h) or abnormal eGFR (>60 ml/1.73 m2) values at DCCT Baseline. There were some significant differences between the demographic and clinical characteristics of the subgroup who participate in this study and the remaining DCCT/EDIC cohort that did not (see table S1). Demographic and clinical differences at DCCT baseline between study subjects who progressed to diabetic nephropathy and those who remained disease free are summarized in table 1. AT DCCT baseline, the mean age was 27.0 ± 7.4 years with an average duration of diabetes of 6.1 ± 4.3 years; 209 (48.4%) of participants were male and 220 (50.9%) were assigned to the DCCT intensive treatment group. Although participants included in the study were similarly distributed into both DCCT treatment arms, participants that ultimately progressed to either MA or CKD were less likely to be in the intensive treatment group as compared to those that did not progress to kidney dysfunction. Participants that progressed to kidney dysfunction also had higher baseline levels of HbA1c %, AER, and triglycerides as compared to those did not progress (all p<0.05).
Table 1.
Baseline Demographics and Clinical Characteristics by disease progression status.
| Characteristic | Disease Progression* | |||
|---|---|---|---|---|
| Persistently Normal (n=354) | Macroalbuminuria (n=69) | Chronic Kidney Disease (n=36) | MA and CKD (n=27) | |
| Age (years) | 27.3 (7.2) | 24.5 (7.5)Ɨ | 27.8 (8.2) | 26.3 (8.0) |
| Diabetes Duration (years) | 5.9 (4.3) | 7.1 (4.2)Ɨ | 7.3 (4.4) | 7.7 (4.5)Ɨ |
| Female %(n) | 53.4 (189) | 42.0 (29) | 50.0 (18) | 48.2 (13) |
| Intensive Trt assignment %(n) | 55.7 (197) | 30.4 (21)Ɨ | 25.0 (9)Ɨ | 25.9 (7)Ɨ |
| Primary Prevention Cohort %(n) | 52.5 (186) | 34.8 (24)Ɨ | 44.4 (16) | 40.7 (11) |
| HbA1c % | 8.8 (1.5) | 10.3 (1.7)Ɨ | 10.2 (2.0)Ɨ | 10.7 (1.9)Ɨ |
| AER | 12.6 (8.5) | 32.2 (39.6)Ɨ | 26.4 (35.2)Ɨ | 31.3 (39.4)Ɨ |
| eGFR | 125.7 (13.7) | 130.8 (13.6)Ɨ | 123.7 (15.0) | 127.3 (15.0) |
| Total Cholesterol | 180.3 (34.3) | 181.1 (30.3) | 190.6 (33.7) | 186.8 (29.0) |
| LDL Cholesterol | 112.8 (29.3) | 113.0 (28.0) | 120.6 (30.7) | 118.1 (26.2) |
| HDL Cholesterol | 51.4 (12.3) | 47.6 (12.8)Ɨ | 48.6 (13.2) | 46.3 (11.6)Ɨ |
| Triglycerides | 80.4 (47.9) | 105.4 (54.8)Ɨ | 106.8 (49.6)Ɨ | 111.8 (53.1)Ɨ |
| Systolic Blood Pressure | 113 (11) | 115 (10) | 114 (10) | 114 (8) |
| Diastolic Blood Pressure | 71 (9) | 73 (9.4)Ɨ | 72 (9) | 74 (8)Ɨ |
| Any ACE/ARB use Prior %(n)ǂ | 62.2 (220) | 34.8 (24)Ɨ | 86.1 (31)Ɨ | 81.5 (22)Ɨ |
Disease progression groups are not mutually exclusive; of the 432 participants with sphingolipid data, 78 participants progressed to either MA or CKD: 27 Progressed to Both MA and CDK while 42 progressed to only MA and 9 progressed to only CKD. Continuous data shown as unadjusted mean (standard deviation) and categorical data shown as % (n).
Any ACE/ARB use prior to disease progression (or end of study follow up in Persistently normal cohort).
P<0.05 as compared to persistently normal participants (From Kruskal-Wallis test statistic).
The unadjusted concentrations of plasma glycosylated sphingolipids measured at DCCT baseline are summarized across disease progression cohorts in table 2. Sixty-nine of the 432 Participants (16.0%) progress to MA, 36 progressed to CKD (8.3%) and 27 progressed to both MA and CKD (6.3%) during DCCT or the first 18 years of EDIC follow up. In those that progress to MA, the median time to progression from DCCT baseline was 10.8 (IQR= 4.3–14.9) years. In those that progress to CKD, the median time to progression was 19.3 (IQR= 14.4–21.2) years. Many of the unadjusted baseline hexosyl sphingolipid species showed increased levels when either or both kidney dysfunction outcome occur during the study treatment period or follow up (C18-H - C22-H, C26-H). Conversely, the lactosyl sphingolipid species levels were lower when either or both kidney dysfunction outcomes occured during the study treatment period of follow up (C14-L - C18-L, C24-L - C26-L).
Table 2.
Baseline Gylcated Sphingolipids by disease progression status.
| Sphingolipid | Disease Progression* | |||
|---|---|---|---|---|
| Persistently Normal (n=354) | Macroalbuminuria (n=69) | Chronic Kidney Disease (n=36) | MA and CKD (n=27) | |
| Hexosyl- | ||||
| C14 | 10.6 (6.7) | 9.0 (3.6) | 9.4 (4.6) | 9.0 (4.4) |
| C16 | 1895.7 (651.8) | 1630.5 (570.5)Ɨ | 1765.4 (543.4) | 1686.2 (535.4) |
| C18 | 100.7 (91.9) | 103.7 (39.0) | 110.3 (39.3)Ɨ | 114.0 (43.4)Ɨ |
| C18:1 | 36.8 (37.4) | 51.6 (30.3)Ɨ | 51.4 (25.5)Ɨ | 54.5 (28.0)Ɨ |
| C20 | 83.4 (36.1) | 99.6 (42.9)Ɨ | 99.7 (43.9)Ɨ | 103.2 (46.2)Ɨ |
| C20:1 | 15.3 (20.7) | 17.6 (8.5)Ɨ | 18.6 (8.6)Ɨ | 18.8 (8.9)Ɨ |
| C22 | 1131.9 (456.5) | 1036.3 (352.9) | 1105.2 (382.0) | 1075.4 (357.1) |
| C22:1 | 107.8 (185.5) | 87.4 (30.3) | 89.9 (28.8) | 88.7 (28.7) |
| C24 | 1264.7 (499.8) | 1143.9 (3353.8) | 1199.7 (441.5) | 1163.4 (363.8) |
| C24:1 | 446.7 (476.6) | 413.0 (168.1) | 406.8 (168.3) | 407.9 (182.2) |
| C26 | 10.1 (4.3) | 12.8 (6.3)Ɨ | 12.9 (6.7)Ɨ | 13.2 (6.5)Ɨ |
| C26:1 | 6.6 (7.2) | 7.5 (4.2)Ɨ | 7.2 (3.9) | 7.3 (4.0) |
| Lactosyl- | ||||
| C14 | 129.8 (47.5) | 91.7 (36.1)Ɨ | 104.3 (43.9)Ɨ | 97.7 (44.5)Ɨ |
| C16 | 3979.9 (1454.4) | 2963.7 (1329.4)Ɨ | 3298.1 (1433.9)Ɨ | 3163.4 (1561.6)Ɨ |
| C18 | 183.3 (62.1) | 134.7 (76.6)Ɨ | 165.0 (97.7)Ɨ | 155.5 (100.4)Ɨ |
| C18:1 | 93.9 (37.3) | 90.2 (25.1) | 102.8 (32.7) | 98.7 (27.9) |
| C20 | 44.5 (22.6) | 45.5 (23.9) | 51.8 (32.3) | 46.8 (26.3) |
| C20:1 | 23.0 (11.9) | 20.2 (9.7) | 23.6 (10.9) | 21.9 (10.8) |
| C22 | 113.1 (52.6) | 102.7 (46.5) | 112.0 (56.9) | 103.4 (46.3) |
| C22:1 | 17.1 (9.2) | 29.3 (24.2)Ɨ | 30.8 (26.7)Ɨ | 31.8 (26.4)Ɨ |
| C24 | 206.5 (77.0) | 146.3 (76.8)Ɨ | 165.9 (89.1)Ɨ | 150.0 (86.7)Ɨ |
| C24:1 | 260.6 (107.1) | 217.6 (76.6)Ɨ | 227.0 (82.5) | 217.7 (83.3) |
| C26 | 3.9 (12.7) | 2.1 (2.3)Ɨ | 2.1 (1.9)Ɨ | 2.0 (2.0)Ɨ |
| C26:1 | 5.3 (2.5) | 3.7 (2.1)Ɨ | 4.0 (1.9)Ɨ | 3.5 (1.9)Ɨ |
Disease progression groups are not mutually exclusive; of the 432 participants with sphingolipid data, 78 participants progressed to either MA or CKD: 27 Progressed to Both MA and CDK while 42 progressed to only MA and 9 progressed to only CKD. Data shown as unadjusted means (standard deviation).
P<0.05 as compared to persistently normal participants.
Statistical models assessing the risk of progressing to kidney dysfunction were adjusted for DCCT treatment assignment, baseline retinopathy cohort, gender, baseline levels of eGFR, AER, HbA1c %, LDL cholesterol, triglycerides, systolic blood pressure, age, and a variable indicating the use of ACE/ARB drugs prior to disease progression or censoring of subject study data. In adjusted models, the association between a one standard deviation unit increase in hexosyl sphingolipids and risk of kidney outcomes are shown in figure 1. Increases in selected species of long and very long hexosyl sphingolipids were significantly associated with increased risk to progress to CKD (C18-H, C18:1-H, C20-H, C20:1-H, C26-H). The association between hexosyl sphingolipids and the progression to MA was similar to their association with CKD but, in general, it was far less pronounced in magnitude.The increases for high levels of C18:1-H, C20-H, C26-H and C26:1-H were significantly associated with progression to MA. The association between a one standard deviation unit increase in lactosyl sphingolipids and kidney outcomes are shown in figure 2. Contrary to the associations between hexosyl sphingolipids and kidney disease outcomes, increases in measured levels of lactosyl sphingolipid species were, for the most part, associated with decreased risk of progress to CKD (C14-L, C-16-L, C18-L, C24-L, C26-L and C26:1-L) indicating that low levels of these lactosyl sphingolipid species were associated with increased risk to progress to CKD. Similar results were observed when MA instead of CKD was used as the outcome. Interestingly and in contrast to hexosylceramides, the association of low levels of some of the lactosylceramide sub-species and MA was more pronounced in magnitude than that observed with progression to CKD. A notable exception for both CKD and MA was a positive association between increased levels of C22:1-L and progression to these two outcomes. Additionally, the possible modifying effects of DCCT treatment assignment on the relationships between plasma sphingolipid levels and progression to kidney disease outcomes was insignificant in all cases (p>0.05).
Figure 1.
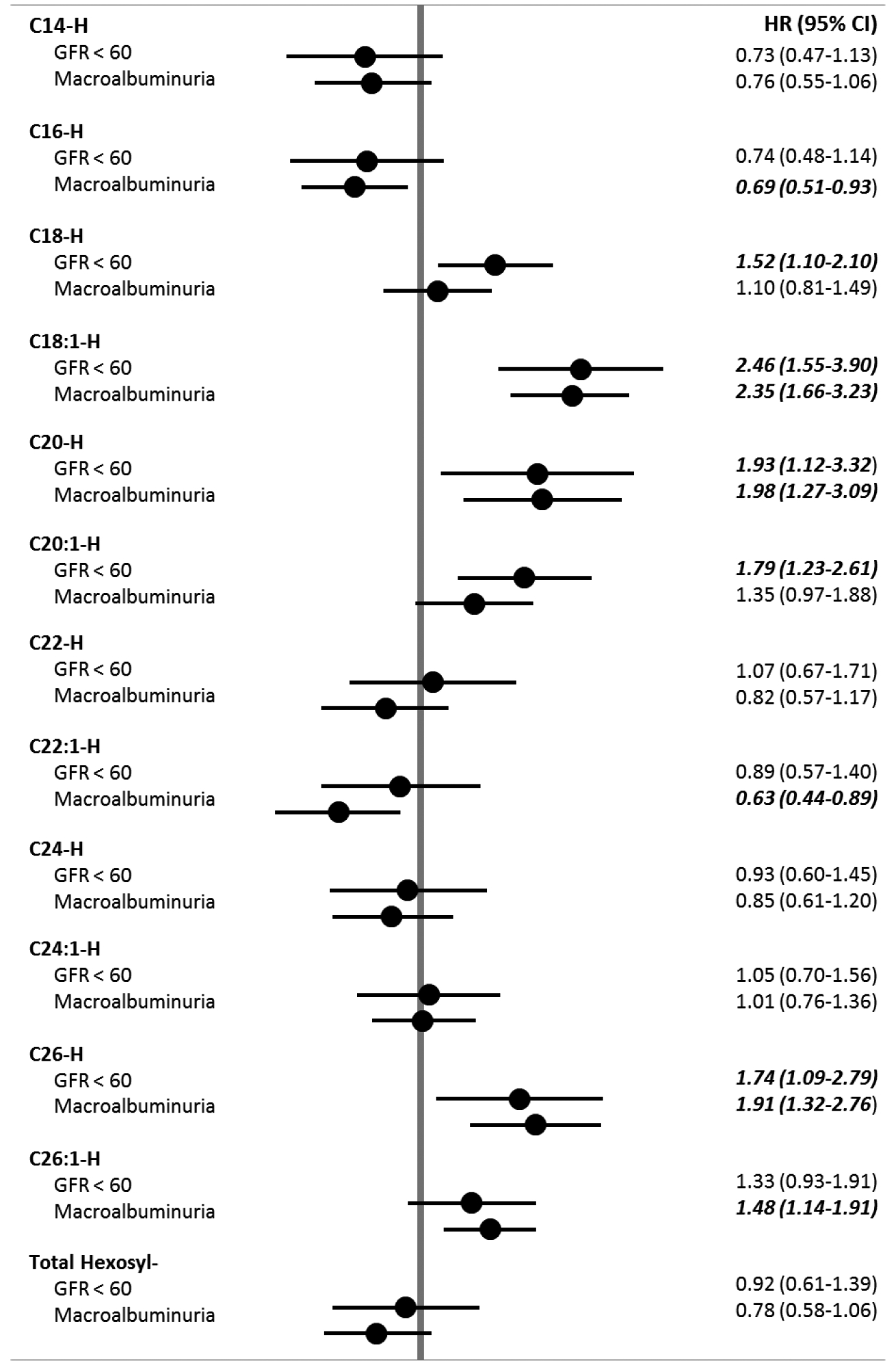
Baseline Hexosyl-Sphingolipids associations with kidney dysfunction outcomes.
Data are shown as hazard ratios (95% CI) adjusted for DCCT treatment assignment, baseline retinopathy cohort, gender, baseline levels of eGFR, AER, HbA1c %, LDL cholesterol, triglycerides, systolic blood pressure, age, and a variable indicating the use of ACE/ARB drugs prior to disease progression of censoring of subject study data. Total Hexosyl species levels calculated as the sum of each measured hexosyl species level. P<0.05
Figure 2.
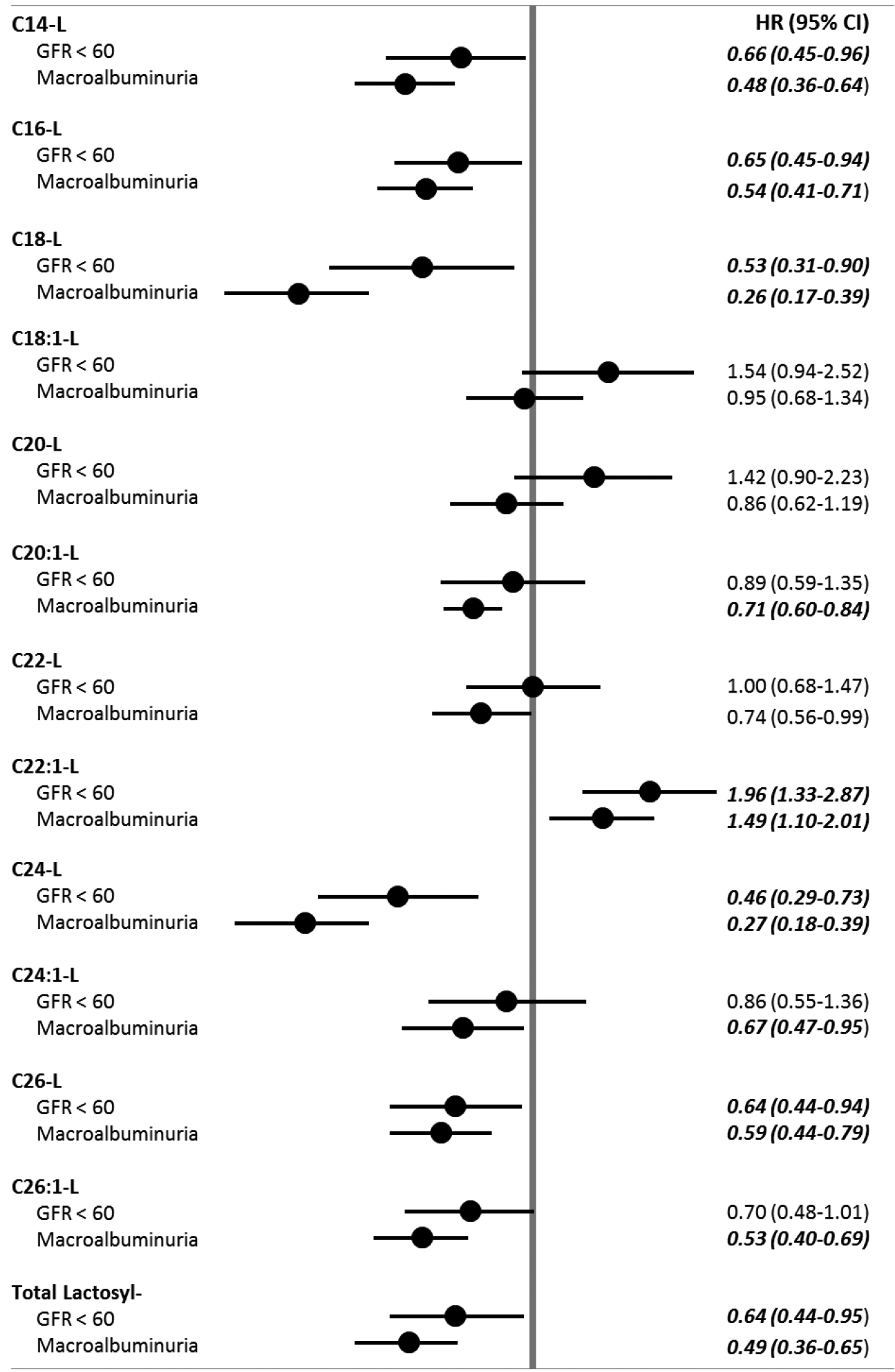
Baseline Lactosyl-Sphingolipids associations with kidney dysfunction outcomes.
Data are shown as hazard ratios (95% CI) adjusted for DCCT treatment assignment, baseline retinopathy cohort, gender, baseline levels of eGFR, AER, HbA1c %, LDL cholesterol, triglycerides, systolic blood pressure, age, and a variable indicating the use of ACE/ARB drugs prior to disease progression of censoring of subject study data. Total lactosyl species levels calculated as the sum of each measured lactosyl species level. P<0.05
DISCUSSION
Although previous publications clearly show, mainly in animal models, an association between elevated levels of glycosphingolipids and diabetic nephropathy (14–16, 24), no studies to date have examined the predictive value of plasma glycosphingolipids towards the development of diabetic nephropathy. The present study was designed to determine whether plasma glycosphingolipids were associated with the development of nephropathy in a sub-cohort of patients with type 1 diabetes from the DCCT/EDIC study and to investigate whether or not these levels were able to identify patients at high risk to develop nephropathy, even when measured in the initial phases of the disease when no clinical/laboratorial indication of kidney dysfunction was present. All patients enrolled in the present study had normal serum creatinine, normal AER and normal eGFR when the samples obtained to perform the study were collected.
We studied two classes of glycosphingolipids: hexosylceramides and lactosylceramides and examined the possible relationship between the levels of the different species of these two glycosphingolipids and the development of nephropathy over a period of more than 20 years of DCCT/EDIC follow-up. We used both macroalbuminuria and reduced glomerular filtration rate as the outcomes to define diabetic nephropathy. Twenty seven of the 69 patients who progressed to macroalbuminuria during DCCT/EDIC follow-up had reduced eGFR. Macroalbuminuria was not present in 9 of the 36 patients who progressed to reduced eGFR during the follow-up period. As expected the median time of progression to develop macroalbuminuria was approximately 11 years but the period to progress to reduced GFR was longer and approximately 19 years confirming that macroalbuminuria and reduced GFR, while they generally co-exist, do not progress in a parallel time frame.
Interestingly, the hazard ratios for hexosylceramide and lactosylceramide in relationship to development of reduced eGFR and macroalbuminuria, although following very similar trends have marked differences. Increased levels of long (C18-, C18:1-, C20-, C20:1) and very long (C26-) hexosylceramides were significantly associated with future development of reduced eGFR but not all of them were significantly associated with the development of MA.
In contrast, high levels of lactosylceramides were not observed in patients who progressed to either macroalbuminuria or reduced eGFR during follow-up. Only increased levels of C22:1 lactosylceramide were associated with progression to reduced eGFR and macroalbuminuria. However, low levels of long (C14-, C16- and C18-) and very long (C22:1-, C24-, C24:1, C26- and C26:1) lactosylceramides were significantly associated with the development of macroalbuminuria and reduced eGFR.
It is also interesting to note that only hexosylceramides with relatively small concentrations in plasma were significantly increased in patients who developed reduced eGFR or MA during follow-up. In contrast, the significantly reduced levels of lactosylceramides associated with development of both eGFR and macroalbuminuria were observed in species with the highest concentrations in plasma, specifically C16 lactosylceramide. Our present findings for lactosylceramides are similar to those previously published by our group on ceramides, which clearly showed that low levels of long and very long ceramides were associated with increased risk to develop macroalbuminuria. (6)
Studies examining the association of sphingolipids and glycosphingolipids to nephropathy in both type 1 and type 2 diabetic animal models show increased accumulation of ceramides and sphingolipids on their kidney cortices associated with increased urinary levels of sphingolipids (16). The studies in these animal models did not include measurement of plasma levels of sphingolipids.
Studies conducted in streptozotocin-treated diabetic mice, a model for type 1 diabetes, showed increases in glycosphingolipid expression in association with renal hypertrophy and the authors postulated that kidney deposition of glycosphingolipids was responsible for the renal hypertrophy. (15) They also postulated that the formation and deposition of glycosphingolipids was dependent on the degree of hyperglycemia, thus creating a link between glucose levels and the development of nephropathy in diabetes. Recent studies (16, 25) performed in the leptin receptor deficient diabetic db/db mice, a model for type 2 diabetes, showed that glomerular hypertrophy and tubular vacuolization were observed in the animals, both at an earlier stage and late stage of diabetic nephropathy, and elevated levels of hexosylceramides and lactosylceramides in the kidney cortices were also observed. To further evaluate the mechanisms by which glucose leads to increased accumulation of glycosphingolipids in the kidney, the authors performed studies using mesangial cells that clearly showed that exposure of these cells to glucose levels similar to those found in diabetes led to increased levels of cell hexosylceramides, cellular hypertrophy and deposition of extracellular matrix proteins (16). They also reported that in the presence of a glucosylceramide inhibitor or lowering of glucose levels, mesangial cell hypertrophy was reversed. Both HexCer and LacCer are well established regulators of biological processes such as cell proliferation, apoptosis and inflammation (8–10, 14, 25, 26). Studies examining possible mechanisms by which elevated glucose levels led to extracellular matrix accumulation suggested that activation of the signaling pathways Smad3 and Akt was involved (16).
Human studies examining ceramides and glycosphingolipids in patients with established clinical disease have not been performed but moderately increased sphingomyelin levels associated with kidney disease have been reported in a cross sectional study of 326 type 1 patients with diabetes as part of the Finnish Diabetic Nephropathy study (17). In this study sphingomyelin emerged as the strongest biochemical covariate of albumin excretion rate followed by very large and large VLDL particles.
The studies using animal models, mentioned above, were mainly focused on the accumulation of tissue glycosphingolipids (kidney) from animals with established kidney disease and in glycosphingolipid measurements in urine. Therefore, they studied cross-sectional associations between glycosphingolipids and established kidney disease not the value of sphingolipids to identify patients at high risk to progress to kidney disease. Our present study revealed a marked decrease in the major circulating species of lactoceramides in patients who over a long follow-up period (18–22 years) developed macroalbuminuria and reduced GFR. Whether the marked decrease in lactoceramides and ceramides, as shown in our previous study (6) is associated both with deposition of ceramides and lactoceramides in the kidney of these patients needs to be investigated. Accumulation of ceramides and sphingolipids is clearly associated with kidney damage in animal models. However accumulation of these compounds in the kidney would not necessarily explain the significant decrease in the circulating levels of these compounds. The more likely explanation is that these compounds, besides being excessively accumulated in the kidney, are also lost in the urine in the initial stages on the disease. In type 1 diabetes hyperfiltration and markedly increased GFR are observed in the initial phases of the disease in some but not all patients. The patients studied at baseline had normal function using the conventional clinical parameters but hyperfiltration, if present, is not detectable by conventional clinical assessment. We postulate that the reduction of ceramides (6) and lactoceramides in the patients with type 1 diabetes are a very earlier indicator of kidney hyperfiltration, which, in turn, is associated with accelerated progression to diabetic nephropathy.
Although glucose is needed for the formation of glycosphingolipids and elevated glucose levels enhance the accumulation of GSLs in the kidney, elevated glucose is not the only possible mediator leading to increased GSLs accumulation in kidney disease. Studies performed in lupus nephritis show a similar pattern of kidney disease in animal models of lupus (24), and increased accumulation of lactoceramides. A possible mechanism postulated as possibly involved in the production of GSLs is the generation of reactive oxygen species (27,30–32). ROS generation is elevated in diabetes as well as in other diseases in which inflammation is a predominant component of the disease including lupus nephritis. ROS are known to regulate neutral sphingomyelinase (nSMase) 2 activity which therefore impacts GSL synthesis/breakdown (27,28). ROS may also affect other sphingolipid regulatory enzymes such as nSMase2, ceramidase, and SM synthase, which are known to be associated with aging-associated inflammation (33) and are involved in the formation of GSLs. Enhanced breakdown of complex glycosphingolipids cannot be excluded as another possible mechanism contributing to accumulations in HexCer/LacCer in the kidney.
Alterations in sphingolipids by several possible different mechanisms or a combination of mechanisms may contribute to complications of diabetes specifically to the development of diabetic nephropathy. Until recently most investigators postulated that HexCer and LacCer are synthesized within the kidney and afterwards accumulated leading to kidney damage (4,7,16) and excluded alterations in circulating sphingolipids as a possible cause for the development of nephropathy (34). However recent clinical studies (35) associated altered levels of circulating sphingolipids specifically ceramides and hexosylceramides with systemic lupus erythematous in females. This study like our own data strongly suggests that altered circulating sphingolipids are associated with and play a role in the development of disease.
Our findings in a large cohort of type 1 diabetes opens the hypothesis that circulating plasma sphingolipids may play an important role in the development of diabetic nephropathy. That significantly low levels of plasma lactosylceramides on patients with type 1 diabetes, without any obvious marker of kidney disease when the blood was collected (DCCT baseline), could identify patients who later progress to nephropathy strongly suggests that both deposition of these compounds in the kidney and possibly an increased loss of lactosylceramides in urine may occur in the very initial phases of the disease, likely due to glomerular hyperfiltration.
Our data opens a series of questions in this new field of research which will need to be addressed by examining both plasma levels and urine levels in the same individuals before and after development of nephropathy. Whether glomerular hyperfiltration contributes to accelerated development of nephropathy, by enhancing accumulation of glycosphingolipids in the kidney, needs to be further investigated since it may change the paradigm of intervention to prevent/minimize development/progression of nephropathy. Possible interventions that seem to have worked in the past to delay development of nephropathy in animal models of diabetes and aging have been inhibition of enzymes involved in the generation of GSLs, inhibitors of ROS such as pyridoxamine and caloric restriction which will strongly influence the lipoprotein GSL composition and subsequent delivery.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the grant R01 DK081352 funded by NIH/NIDDK. The work was also supported by the Research Service of the Ralph H.Johnson Department of the Veterans Affairs Medical Center. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government. The DCCT/EDIC was sponsored through research contracts from the Division of Diabetes, Endocrinology and Metabolic Diseases (NIDDK) of the NIH. Additional support was provided by the National Center for Research Resources through the GCRC program and by Genentech Inc through a Cooperative Research and Development Agreement with the NIDDK. MLV researched the data, wrote, reviewed, and edited the manuscript. NLB and KJH performed statistical analysis, wrote, reviewed, and edited the manuscript. RK and SH researched the data, reviewed and edited the manuscript. GV researched the data, wrote, reviewed, and edited the manuscript. DCCT/EDIC Research Group researched the data, reviewed, and edited the manuscript.
REFERENCES
- 1.National Kidney Foundation: Clinical practice guidelines for diabetes and CKD: 2012 update Am J Kidney Dis, 60 (2012), pp. 850–886 [DOI] [PubMed] [Google Scholar]
- 2.Gerstein HC, Mann JF, Yi Q, et al. : Albuminuria and risk of cardiovascular events, death, and heart failure in diabetic and nondiabetic individuals J Am Med Assoc, 286 (2001), pp. 421–426 [DOI] [PubMed] [Google Scholar]
- 3.Mogensen CE: Microalbuminuria as a predictor of clinical diabetic nephropathy Kidney Int, 31 (1987), pp. 673–689 [DOI] [PubMed] [Google Scholar]
- 4.Fox TE, Kester M: Therapeutic strategies for diabetes and complications: a role for sphingolipids. Adv Exp Med Biol, 688 (2010), pp. 206–216 [DOI] [PubMed] [Google Scholar]
- 5.Grove KJ, Voziyan PA, Spraggins JM, Wang S, Paueksakon P, Harris RC, Hudson BG, Caprioli RM. Diabetic nephropathy induces alterations in the glomerular and tubule lipid profiles. J Lipid Res. 2014. July;55(7):1375–85. doi: 10.1194/jlr.M049189. Epub 2014 May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein RL, Hammad SM, Baker NL, Hunt KJ, AlGadban MM, Cleary P, Virella G and Lopes-Virella MF and the DCCT Group of Investigators: decreased plasma levels of select verylong chain ceramides species are associated with the development of nephropathy in type 1 diabetes. Metabolism 2014, 63:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mather AR, Siskind LJ. Glycosphingolipids and kidney disease. Adv Exp Med Biol 721: 121–138, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Chatterjee S, Wei H. Roles of glycosphingolipids in cell signaling: adhesion, migration, and proliferation. Methods Enzymol 363: 300–312, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Morales A, Colell A, Mari M, Garcia-Ruiz C, Fernandez-Checa JC. Glycosphingolipids and mitochondria: role in apoptosis and disease. Glycoconj J 20: 579–588, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Wennekes T, van den Berg RJ, Boot RG, van der Mar el GA, Overkleeft HS, Aerts JM. Glycosphingolipids–nature, function, and pharmacological modulation. Angew Chem Int Ed Engl 48: 8848–8869, 2009. [DOI] [PubMed] [Google Scholar]
- 11.Summers SA: Sphingolipids and insulin resistance: the five Ws. Curr Opin Lipidol, 21 (2010), pp. 128–135 [DOI] [PubMed] [Google Scholar]
- 12.Bikman BT, Summers SA: Ceramides as modulators of cellular and whole-body metabolism. J Clin Invest, 121 (2011), pp. 4222–4230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langeveld M, Aer ts JM. Glycosphingolipids and insulin resistance. Prog Lipid Res 48: 196–205, 2009. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Kiechle FL. Review: Glycosphingolipids in health and disease. Ann Clin Lab Sci 34: 3–13, 2004. [PubMed] [Google Scholar]
- 15.Zador IZ, Deshmukh GD, Kunkel R, Johnson K, Radin NS, Shayman JA. A role for glycosphingolipid accumulation in the renal hypertrophy of streptozotocin-induced diabetes mellitus. J Clin Invest 91: 797–803, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subathra M, Korrapati M, Howell LA, Arthur JM, Shayman JA, Schnellmann RG, and Siskind LJ: Kidney glycosphingolipids are elevated early in diabetic nephropathy and mediate hypertrophy of mesangial cells. Am J Physiol Renal Physiol 309: F204–F215, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mäkinen VP, Tynkkynen T, Soininen P, et al. Sphingomyelin is associated with kidney disease in type 1 diabetes (The FinnDiane Study) Metabolomics, 8 (2012), pp. 369–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Diabetes Control and Complications Group. The effect ofintensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–86. [DOI] [PubMed] [Google Scholar]
- 19.Epidemiology of Diabetes Interventions and Complications (EDIC). Design, implementation, and preliminary results of a long-term follow-up of the Diabetes Control and Complications Trial cohort. Diabetes Care 1999;22:99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammad SM, Pierce JS, Soodavar F, et al. Blood sphingolipidomics in healthy humans: impact of sample collectionmethodology. J Lipid Res 2010;51:3074–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee MH, Hammad SM, Semler AJ, et al. HDL3, but not HDL2, stimulates plasminogen activator inhibitor-1 release from adipocytes: the role of sphingosine-1-phosphate. J Lipid Res 2010;51:2619–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hammad SM, Al Gadban MM, Semler AJ, et al. Sphingosine 1-phosphate distribution in plasma: associations with atypical lipoprotein profiles. J Lipids 2012;2012:180705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowling TK, Mather AR, Thiyagar ajan T, Hernandez-Corbacho MJ, Power s TW, Jones EE, Snider AJ, Oates JC, Dr ake RR, Siskind LJ. Renal glycosphingolipid metabolism is dysfunctional in lupus nephritis. J Am Soc Nephrol 2015; 26: 1402–1413, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Korrapati MC, Howell LH, Shaner BE, Megyesi JK, Siskind LJ, Schnellmann RG. Suramin: a potential therapy for diabetic nephropathy. PLos One 8: e73655, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mather AR, Siskind LJ. Glycosphingolipids and kidney disease. Adv Exp Med Biol 2011;721: 121–138. [DOI] [PubMed] [Google Scholar]
- 27.Rutkute K, Asmis RH, Nikolova-Karakashian MN (2007) Regulation of neutral sphingomyelinase-2 by GSH: a new insight to the role of oxidative stress in aging-associated inflammation. J Lipid Res 48: 2443–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolova-Karakashian M, Karakashian A, Rutkute K (2008) Role of neutral sphingomyelinases in aging and inflammation. Subcell Biochem 49: 469–486. [DOI] [PubMed] [Google Scholar]
- 30.Alessenko AV, Bugrova AE, Dudnik LB (2004) Connection of lipid peroxide oxidation with the sphingomyelin pathway in the development of Alzheimer’s disease. Biochem Soc Trans 32: 144–146. [DOI] [PubMed] [Google Scholar]
- 31.Ogiso M, Hoshi M, Nishigori H (1999) Neutral and acidic glycosphingolipids in glucocorticoid-induced cataract in chick lens. Exp Eye Res 68: 229–236. [DOI] [PubMed] [Google Scholar]
- 32.Zager RA, Conrad DS, Burkhart K (1998) Ceramide accumulation during oxidant renal tubular injury: mechanisms and potential consequences. J Am Soc Nephrol 9: 1670–1680. [DOI] [PubMed] [Google Scholar]
- 33.Sacket SJ, Chung HY, Okajima F, Im DS (2009) Increase in sphingolipid catabolic enzyme activity during aging. Acta Pharmacol Sin 30: 1454–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hernandez-Corbacho MJ, Jenkins RW, Clarke CJ, Hannun YA, Obeid LM, Snider AJ and Siskind LJ. Accumulation of long-chain glycosphingolipids during aging is prevented by caloric restriction. PLos One 6: e20411, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Checa A, Idborg H, Zandian A, Sar DG, Surowiec I, Trygg J, Svenungsson E, Jakobsson PJ, Nilsson P, Gunnarsson I, Wheelock CE (2017). Dysregulations in circulating sphingolipids associate with disease activity indices in female patients with systemic lupus erythematous: a cross-sectional study. Lupus 26:1023–1033 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


