Introduction
Complete TYK2 deficiency (IMMUNODEFICIENCY 35 OMIM (611521)) is a rare disorder, inherited as an autosomal recessive (AR) trait, which has been previously described in nine patients from seven unrelated kindreds (1-3). It was first reported in a Japanese patient with mycobacterial and viral disease associated with hyper IgE syndrome (1). Later, investigation of five more families with complete AR TYK2 deficiency showed that the main clinical phenotype of the patients was mycobacterial and/or viral disease without hyper IgE syndrome (2). In addition, another patient with some features of hyper IgE (eczema, skin abscesses, respiratory infections and IgE levels >1000 IU/ml) and TYK2 deficiency was described (3). TYK2 is a member of the Janus kinase family (JAK1, 2, 3, and TYK2) that plays a significant role in signaling receptors of group 1 and 2 of cytokines, namely IL-10, IL-12, IL-23, and IFN-α/β. Briefly, attachment of the ligand to the cytokine receptor induces conformational changes and activation of the JAKs kinases via phosphorylation. The JAKs then phosphorylate the intracellular part of the receptor which create a docking site for the signal transducer and activator of transcriptions (STATs) molecules. STATs are subsequently phosphorylated and translocated to the nucleus to activate the transcription of target genes (4). TYK2 protein is essential for mediating cytokine signaling and its defect will interfere with IFN-α, IFN-β, IL-12, IL-23 and IL-10 pathways (4). Attachment of IL-12 to IL-12 receptor (IL-12Rβ1 and IL-12Rβ2) and IL-23 to IL-23R (IL-12Rβ1 and IL-23R) induce the transcription of IFN-γ in the nucleus, which is crucial for anti-mycobacterial immunity (5). IFN-α/β signals are also essential for transcription of various immune factors that help to defend against viruses (6). As a result, patients with complete AR TYK2 deficiency suffered from intracellular bacterial infections such as Mycobacterium, Salmonella, Brucella and various viral infections like Herpes Simplex Virus (HSV) group (2, 6). This is referred to as syndromic, opposed to isolated, Mendelian susceptibility to mycobacterial disease (MSMD) (7). TYK2 has four main domains including 4.1, ezrin, radixin, moesin (FERM), SH2-like, pseudokinase and kinase. Patients with complete AR TYK2 deficiency display deleterious loss-of-function (LOF) mutations in the FERM (S50Hfs*1, C70Hfs*21, E154* and P216Hfs*14), pseudokinase (R638* and L767*) and kinase domains (T1106Hfs*4). They are all LOF and lead to the non-expression of TYK2 (1-3) On the other hand, isolated mycobacterial infection, including MSMD or tuberculosis in particular, can be caused by homozygosity for the TYK2 P1104A common variant (8). In this study, we investigated a patient with mycobacterial and viral infections having a mutation in TYK2 exon 7 encoding the FERM domain of the TYK2 protein.
Case presentation:
The patient lives in Iran and is born to first-degree consanguineous Persian-Turks parents. He is the second child of the family (Figure 1A). His brother is healthy but there was a history of unknown deaths that occurred in his two uncles (they died at the ages of 1 and 3 years old). They both presented with lymphadenopathies and fever with no specific diagnosis in their medical history.
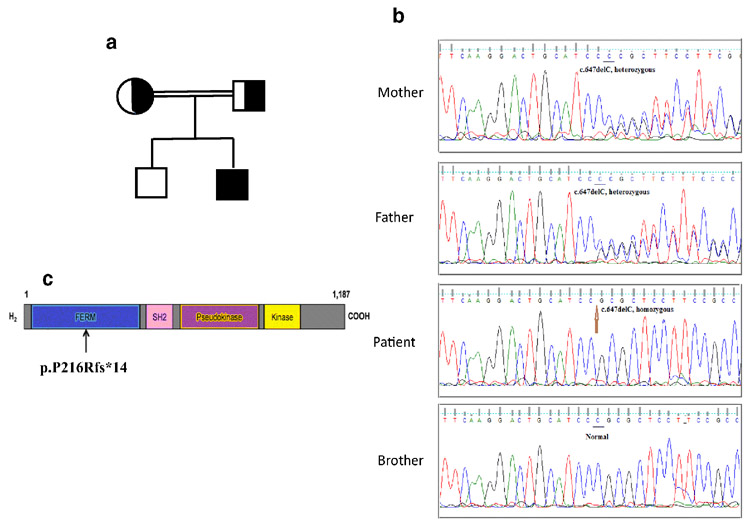
Figure 1-. identification of TYK2 homozygous mutation.
(a) Pedigree of a family in which a homozygous mutation in TYK2 was identified in the index case. Squares and circles denote male (father) and female (mother), respectively.
Half-filled square and circle symbolize heterozygous carriers of c.647delC mutation. Living affected and unaffected males show in black and clear squares, respectively.
(b) Validation by Sanger sequencing of the TYK2 mutation in the patient, brother and their parents. PCR followed by Sanger sequencing confirmed the deletion of a C in exon 7 (c.647delC) leading to a frameshift and the substitution at position 216 causing proline to be converted to arginine followed by a stop codon 13 amino acids after (p.P216Rfs*14). (c) Schematic representation of the TYK2 protein with the position of mutation reported here.
Our patients’ complications related to Bacillus Calmette–Guérin (BCG) vaccination at birth were started when he was 7 months old. He was referred to Immunology, Asthma and Allergy Research Institute (IAARI) for immunological evaluations. At that time, he had fever, bilateral axillary and cervical lymphadenitis, and ulcers at the site of injection (1 × 1 cm) as well as in axillary area (2.5 × 2.5). In addition, he had hepatosplenomegaly in his imaging evaluation. Biopsies were taken from his ulcerous axillary lymph nodes and were evaluated by Ziehl-Neelsen stain method and culturing techniques, the result of which revealed the existence of Mycobacterium bovis-BCG. Similar result was obtained in the sample taken from his gastric secretion. No bacterial growth was found in blood culture testing. In his hematological and serological evaluations, he had microcytic anemia and serum IgE level was in normal range (62.5 IU/ml). The patient did not have atopic dermatitis, staphylococcal diseases, or any other clinical features of hyper IgE syndrome. Increased erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level were also detected. His nitroblue tetrazolium (NBT) and dihydrorhodamine (DHR) tests results were normal. The immunoglobulins levels and cluster of differentiation (CD) markers were in normal range.
Later, he had two episodes of herpes simplex infection (confirmed by PCR) at the age of 3 and 7 years old, presented as fever and herpetic gingivostomatitis. He was also hospitalized due to aseptic meningitis (the cerebrospinal fluid findings were lymphocytic pleocytosis, normal glucose level, and a slightly elevated protein level that resolved spontaneously) at the age of 6 years old and chicken pox (VZV) infection six months later.
Immunologically, Lymphocyte Transformation Test (LTT) and cytokine assay following whole blood stimulation were performed when he was 7 months old and repeated when he was referred at 6 years old for infection complications; the results were comparable. Results of LTT assay showed decreased response to both phytohemagglutinin (PHA) and BCG (Table 1) in terms of stimulation index (SI) calculated by dividing the optic density (OD) of stimulated to un-stimulated cells. Considerable decreased response in the presence of BCG along with the physical examinations could be a sign of possible mutation in the IL-12/IFN-γ loop which might result to specific manifestations of MSMD. For cytokine assay, we evaluated both IL-12p40 production in whole blood after stimulating for 18 hours in the presence of BCG or BCG plus IFN-γ and IFN-γ production in a 48-hour culture setting in the presence of BCG or BCG plus IL-12 Age and sex matched healthy control who had received the BCG vaccine was tested simultaneously (Table 1). Induction of IFN-γ secretion in response to IL-12 was considerably decreased during stimulation of patient’s whole blood sample (ratio: 1.4 BCG+IL-12/BCG). By contrast, the secretion of IL-12p40 in BCG+IFN-γ setting was comparable to the amount obtained by healthy control sample. These findings led us to evaluate the patient for a defect in IL-12 signaling pathway.
Table 1-.
Laboratory findings of the TYK2 deficient patient
| Laboratory results | Patient | NR* |
|---|---|---|
| WBC (× 103) | 8.46 | 6-17.5 |
| Lymphocytes (× 103) | 6.21 | 4-10.5 |
| Neutrophils (× 103) | 1.26 | 1.5-8.5 |
| CD3 | 3.7 | 1.9-5.9 |
| CD4 | 1.9 | 1.4-4.3 |
| CD8 | 1.9 | 0.50-1.7 |
| CD19 | 1.3 | 0.02-2.3 |
| CD4/CD8 | 1 | >1 |
| IgA mg/dl | 24 | 11-90 |
| IgG mg/dl | 1234 | 217–904 |
| IgM mg/dl | 55 | 34-126 |
| IgE IU/ml | 62.5 | 0.98-570 |
| CH50 | 146 | 70-160 |
| Cell culture tests | Patient | Healthy control(s) |
| LTT with PHA (SI) | 2.2 | 3.5 |
| LTT with BCG (SI) | 1.6 | 2.5 |
| IL-12 p40 (R1) | 8.2 | 14 |
| IFN-γ (R2) | 1.4 | 5.6 |
NR: Normal range, Age-related reference range from The HARRIET LANE HANDBOOK, Jason W. Custer and Rachel E. Rau, Twenty-first Edition.
WBC: white blood cells; CH50: 50% haemolytic complement; LTT: Lymphocyte Transformation Test; PHA: Phytohemoglutin; SI: stimulation index (OD of stimulated cells with PHA or Bacillus Calmette–Guérin (BCG)/OD of un-stimulated cells); R1: Ratio of IL-12 p40 production by dividing cells stimulated with BCG+ recombinant human interferon- γ (rhIFN-γ) to the cells stimulated with BCG; R2: Ratio of IFN-γ production by dividing cells stimulated with BCG+ recombinant human interleukin-12 (rhIL-12) to BCG
Lately, we performed whole exome sequencing (WES) and identified a homozygous deletion of one base pair in exon 7 of TYK2. PCR followed by Sanger sequencing confirmed the deletion of a C in exon 7 (c.647delC) leading to a frameshift and the substitution at position 216 causing proline to be converted to arginine followed by a stop codon 14 amino acids after (p.P216Rfs*14)(Figure 1B, 1C). We also performed the familial segregation of the mutation. Both parents were heterozygous and healthy and the younger brother was wild type (WT/WT) and healthy (Figure 1B). This mutation has been reported in another patient and was showed to be loss-of-expression (3).
Discussion
Following mandatory BCG vaccination at birth in Iran and some other countries, patients with certain inborn errors of immunity may develop BCG disease that usually appears 3 months after the vaccination (9-12). These manifestations are seen in patients with syndromic or isolated MSMD, chronic granulomatous disease (CGD), or severe combined immunodeficiency (SCID) which are usually accompanied with high mortality rate (80-84%)(13). In Iran, due to the lack of awareness of individuals and the lack of proper and timely diagnosis of the disease, mortality may be consistent with this range. Early diagnosis is a fundamental aspect for prevention of generalized BCG infectious disease and growth of intracellular microbes (14). Local and generalized complications of BCG vaccine have been observed in patients with complete AR TYK2 deficiency, often associated with other clinical manifestations such as Brucella, Salmonella, and Herpes infection (2).
The absence of TYK2 protein was shown to impair but not abolish IL-12 and IL-23 signaling, thereby diminishing the production of IFN-γ. These results are in accordance with the sensitivity to mycobacteria, BCG vaccine and Mycobacterium tuberculosis, as well as other intra-macrophagic microbes. This aspect was proven in our patient, in whom we showed an impaired secretion of IFN-γ following stimulation by BCG plus IL-12 and a close to healthy control amount of IL-12p40 secretion against BCG plus IFN-γ. In line with other patients with complete AR TYK2 deficiency, our patient also suffers from viral disease, particularly with herpes viruses, attributable to an impaired IFN-α-mediated immunity (2, 6).
The results of WES identified a homozygous mutation in TYK2 gene. The frameshift mutation (p.P216Rfs*14) affects all four domains of the molecule by truncating the protein in the first domain (FERM). The exact same mutation has already been described in 2016 in a Kurdish patient (3) that the mutation completely abrogates the expression of TYK2 in the patient’s cells. This patient, who was not BCG vaccinated, did not suffer from mycobacterial infection, nor from clearly identified viral disease, which is compatible with the low clinical penetrance of complete AR TYK2 deficiency for mycobacterial and viral diseases (2). However, he displayed the characteristics of hyper-IgE syndrome (eczema, skin abscesses, respiratory infections and high IgE levels), only observed in the first Japanese patient explained (1). Importantly, both our patient and the patient reported in 2016 by Fuchs et al. carry the same TYK2 mutation and we thus infer that our patient would show a lack of TYK2 expression despite never tested. Regardless of identical genotype at the TYK2 locus, their clinical phenotypes are different, which might be due to variations in gene modifiers acting post-transcriptionally or in unrelated genes. More studies are needed to fully understand the clinical phenotype of “hyperIgE” and the role if any of TYK2. Patients with syndromic forms of MSMD including viral infections should be considered for complete AR TYK2 deficiency.
Acknowledgment:
We would like to thank the patient and his family. We would like to appreciate Dr Leila Moradi for her scientific suggestions and Dr Nastaran Sabetkish for English editing of the manuscript. This project has been granted and supported by Immunology, Asthma and Allergy Research Institute under the supervision of Tehran University of Medical Sciences (grant number: 89-33-1/253-1), the National Institutes of Health (R37AI095983), the Integrative Biology of Emerging Infectious Diseases Laboratory of Excellence (ANR-10-LABX-62-IBEID), the French National Research Agency under the “Investments for the future” program (ANR-10-IAHU-01), ANR-GENMSMD (ANR-16-CE17-0005-01), the St. Giles Foundation, the Rockefeller University, Howard Hughes Medical Institute, Institut National de la Santé et de la Recherche Médicale (INSERM), and Paris Descartes University.
Financial Disclosure
The authors have no financial relationships relevant to this article to disclose
Footnotes
Conflict of interest:
Authors declare no conflict of interest.
Research involving human participants. Informed consent for participation in this study was obtained in accordance with local regulations, with approval from the IRB.
Informed consent. Written informed consent was obtained from the guardians of the patient.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References:
- 1.Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. 2006;25(5):745–55. [DOI] [PubMed] [Google Scholar]
- 2.Kreins AY, Ciancanelli MJ, Okada S, Kong XF, Ramirez-Alejo N, Kilic SS, et al. Human TYK2 deficiency: Mycobacterial and viral infections without hyper-IgE syndrome. The Journal of experimental medicine. 2015;212(10):1641–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuchs S, Kaiser-Labusch P, Bank J, Ammann S, Kolb-Kokocinski A, Edelbusch C, et al. Tyrosine kinase 2 is not limiting human antiviral type III interferon responses. European journal of immunology. 2016;46(11):2639–49. [DOI] [PubMed] [Google Scholar]
- 4.Watford WT, O'Shea JJ. Human tyk2 kinase deficiency: another primary immunodeficiency syndrome. Immunity. 2006;25(5):695–7. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Barricarte R, Markle JG. Human IFN-gamma immunity to mycobacteria is governed by both IL-12 and IL-23. 2018;3(30). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang SY, Boisson-Dupuis S, Chapgier A, Yang K, Bustamante J, Puel A, et al. Inborn errors of interferon (IFN)-mediated immunity in humans: insights into the respective roles of IFN-alpha/beta, IFN-gamma, and IFN-lambda in host defense. Immunological reviews. 2008;226:29–40. [DOI] [PubMed] [Google Scholar]
- 7.Rosain J, Kong XF, Martinez-Barricarte R, Oleaga-Quintas C, Ramirez-Alejo N, Markle J, et al. Mendelian susceptibility to mycobacterial disease: 2014-2018 update. Immunology and cell biology. 2019;97(4):360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boisson-Dupuis S, Ramirez-Alejo N. Tuberculosis and impaired IL-23-dependent IFN-gamma immunity in humans homozygous for a common TYK2 missense variant. 2018;3(30). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khotaei GT, Sedighipour L, Fattahi F, Pourpak Z. Osteomyelitis as a late complication of Bacille Calmette-Guerin vaccination. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2006;39(2):169–72. [PubMed] [Google Scholar]
- 10.Sarrafzadeh SA, Mahloojirad M, Nourizadeh M, Casanova JL, Pourpak Z, Bustamante J, et al. Mendelian Susceptibility to Mycobacterial Disease due to IL-12Rbeta1 Deficiency in Three Iranian Children. Iranian journal of public health. 2016;45(3):370–5. [PMC free article] [PubMed] [Google Scholar]
- 11.Casanova JL, Jouanguy E, Lamhamedi S, Blanche S, Fischer A. Immunological conditions of children with BCG disseminated infection. Lancet (London, England). 1995;346(8974):581. [DOI] [PubMed] [Google Scholar]
- 12.Casanova JL, Blanche S, Emile JF, Jouanguy E, Lamhamedi S, Altare F, et al. Idiopathic disseminated bacillus Calmette-Guerin infection: a French national retrospective study. Pediatrics. 1996;98(4 Pt 1):774–8. [PubMed] [Google Scholar]
- 13.Poyhonen L, Bustamante J, Casanova JL, Jouanguy E, Zhang Q. Life-Threatening Infections Due to Live-Attenuated Vaccines: Early Manifestations of Inborn Errors of Immunity. Journal of clinical immunology. 2019;39(4):376–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amanati A, Pouladfar G, Kadivar MR, Sanaei Dashti A, Jafarpour Z, Haghpanah S, et al. A 25-year surveillance of disseminated Bacillus Calmette-Guerin disease treatment in children in Southern Iran. Medicine. 2017;96(52):e9035. [DOI] [PMC free article] [PubMed] [Google Scholar]