Abstract
Background: Burn is one of the major global health problem causing trauma and stress. A burn injury can cause functional impairment and psychosocial burdens affecting the quality of life. The purpose of this study was to evaluate the life quality after skin burn and patients’ satisfaction on skin grafting outcomes in Saudi Arabia. Methods: In this cross-sectional community-based survey included 166 participants. Each participant was interviewed using a purposeful questionnaire. Results: The highest incidence of skin burn cases were recorded among female participants (n=133) compared to males (n=33). The highest cause of burn injury was scald (46.4%) and the highest injured parts were hands across all age groups (n=89). Multiple logistic regression models for different parts of skin burn patients showed highly significant values for hands (p < 0.001), CI, 0.181 (0.103-0.259). The maximum injury took place at home (88%). The majority of burns were scald and flame injuries (46.4% and 36.1%). Majority of patients had burns of 0-10% TBSA which is about 62.0% (p < 0.002, R 2=0.289). The mean DLQI scores ranged from < 1 to 27. Conclusion: Skin burns are common in Saudi Arabia and more prevalent among females. Most skin burns occur at home and the most causative agent is hot water predominantly affecting hands. The majority of burns are treated using topical creams, and only some of them undergo skin grafting. There is a relatively higher degree of acceptance of skin burns treatment outcomes among the Saudi population.
Keywords: Burns, dermatology quality of life, scald, interpersonal relationships
Introduction
Burns are a common health problem worldwide. According to the Health World Organization (WHO), burns cause 180000 deaths each year worldwide [1]. However, and to the best of our knowledge, no epidemiological report showing the epidemiology of skin burns in Saudi Arabia. Only localized studies are available showing a series of patients group [2,3].
Skin burn injuries causing complex problems including scar marks, psychological effects and overall life’s quality (LQ) of the affected person [4]. Burns at the exposed areas of the body were more likely to cause emotional and social distress. Several studies have been done on the scar specific quality of life from the patient’s perspectives [5]. There may be different distressing factors associated with psychological problems in patients with burns [6].
The most common cause of burns is scalding. Scald injuries are commonly occurred due to various indoor activities. Females are more prone to scald injury as they are mostly associated with domestic activities like cooking and handling hot liquids [7]. Other common causes are chemical, electrical, fireworks and contact with a hot surface. Burn injuries are also related to socioeconomic status, age factors, as well as educational status [8].
Though significant advancement in the medical care of burns has been implemented in recent years, post-traumatic stress is still a major concern. Patients with complicated burn injuries often require a multidisciplinary approach for early recovery [9].
Skin grafting is a closure procedure broadly used in plastic surgery for the burnt wounds that fail to heal [10-12]. However, the outcomes of skin burns management (initial presentation to plastic performance) differ according to the existing medical facilities and skin grafting technique engaged. Thus, the present study aimed to evaluate the life quality after skin burn and patients’ satisfaction on skin grafting outcomes in Saudi Arabia.
Materials and methods
This is a descriptive cross-sectional study included 166 persons with a history of previous skin burns. Study subjects were reached in 17 regions/cities of Saudi Arabia during the period from January 2018 to June 2018. A standard purposeful questionnaire about skin burns was used. The variables included; age, gender, qualification, cause of injury, place of injury, the time elapsed since injury, affected parts, percent body surface area burnt and dermatology life quality including patient’s satisfaction on skin grafting procedure. The total body surface area burnt (TBSA) was calculated using “Rule of Nine”. The dermatology life quality index tool (DLQI) was carried out to cover the essential aspects of the present study. The DLQI consists of 10 questions classified to subscales like-symptoms and feelings, daily activities, leisure, personal relationships, work, and school and treatment. The DLQI scores were calculated by summing the scores of 10 questions, with a maximum score of 30 and a minimum of 0. The higher score implies poor quality of life.
Statistical analysis
The obtained data were analyzed using IBM SPSS software, version 25.0 (Chicago, IL, USA). One-way ANOVA was computed and p-value < 0.05 was accepted as the level of the significance for interpreting the presented results. The odds ratio (OR) and their relative 95% confidence intervals (CIs) were computed through uni- and multivariate logistic regression models to evaluate the significance of the included variables. Statistical analysis like Cronbach’s α coefficient and component score matric was used to assess the validity and reliability of the questionnaire and DLQI score.
Results
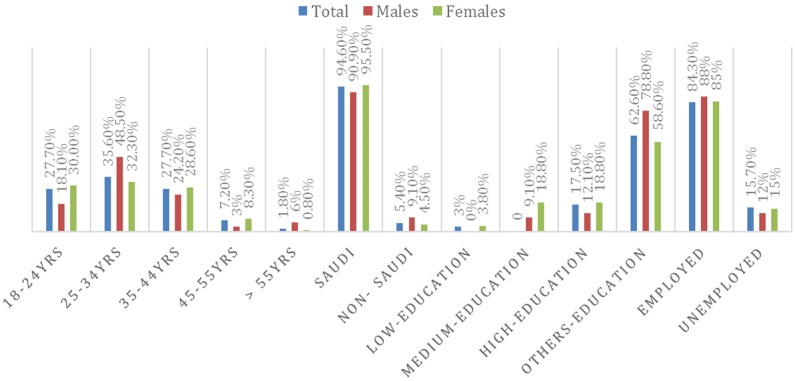
The present study included 166 individuals with skin burns, their ages ranging from 18 to 60 with a mean age of 35 years. The socio-demographic characteristics of the study population were summarized in Table 1. The highest incidence of skin burn cases was recorded among females 133/166 (80%) compared to males 33/166 (20%). Most participants were aged 25-24 years followed by 18-24 and 35-44 years, respectively, as indicated in Table 1, Figure 1. Among the participants, 157 were Saudi nationals and only 9 were non-Saudi.
Table 1.
Socio-demographic characteristic of individuals with Skin burns
| Socio-demographic | Gender | p-value* | R 2 | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Total | Male | Female | ||||||
|
|
|
|
||||||
| N | % | N | % | N | % | |||
| Number | 166 | 100 | 33 | 17.7 | 133 | 82.3 | ||
| Age (years) | ||||||||
| 18-24 | 46 | 27.7 | 6 | 18.1 | 40 | 30.0 | 0.057 | 0.382 |
| 25-34 | 59 | 35.6 | 16 | 48.5 | 43 | 32.3 | ||
| 35-44 | 46 | 27.7 | 8 | 24.2 | 38 | 28.6 | ||
| 45-55 | 12 | 7.2 | 1 | 3.0 | 11 | 8.3 | ||
| > 55 | 3 | 1.8 | 2 | 6.0 | 1 | 0.8 | ||
| Nationality | ||||||||
| Saudi | 157 | 94.6 | 30 | 90.9 | 127 | 95.5 | 0.505 | 0.245 |
| Non-Saudi | 9 | 5.4 | 3 | 9.1 | 6 | 4.5 | ||
| Education | ||||||||
| Low | 5 | 3.0 | 0 | 0 | 5 | 3.8 | 0.186 | 0.271 |
| Medium | 28 | 16.9 | 3 | 9.1 | 25 | 18.8 | ||
| High | 29 | 17.5 | 4 | 12.1 | 25 | 18.8 | ||
| Others | 104 | 62.6 | 26 | 78.8 | 78 | 58.6 | ||
| Employment | ||||||||
| Employed | 140 | 84.3 | 29 | 88.0 | 113 | 85.0 | 0.004 | 0.375 |
| Unemployed | 26 | 15.7 | 4 | 12.0 | 20 | 15.0 | ||
Low-Primary education or lower; Medium-Middle-High School; High-Postgraduate/University degree; Others-Other academic qualification.
p-value indicates a comparison between males and females through linear regression.
Figure 1.
Socio-demographic characteristic of individuals with Skin burns.
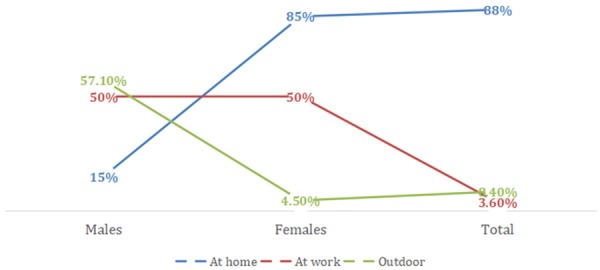
Table 2; Figure 2, described the place of burn injury according to gender. The majority of burns injuries occurred at home (88.0%) compared to at work (6%) and outdoor environment (14%). Females sustained more burn injuries (85.0%) compared to males (15.0%) at home. While in the work environment both males and females were equally affected (50.0% each). In an outdoor environment, males sustained more burn injuries (57.1%) compared to females (42.9%).
Table 2.
Place of injury according to gender
| Place of injury | Males | Females | Total of Patients N (%) |
|---|---|---|---|
| At home | 22 (15.0%) | 124 (85.0%) | 146 (88.0) |
| At work | 3 (50.0%) | 3 (50.0%) | 6 (3.6) |
| Outdoor | 8 (57.1%) | 6 (4.5%) | 14 (8.4) |
Figure 2.
Place of injury according to gender.
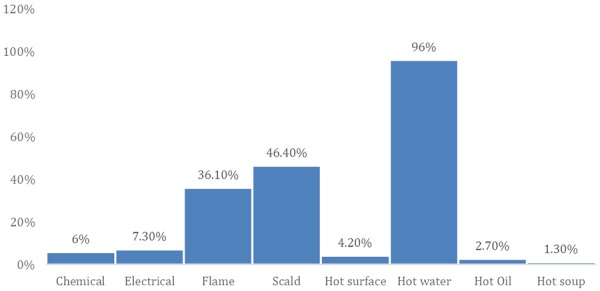
The distribution of causes of burns is shown in Table 3, Figure 3. Various causes of burns were observed in the present study. The majority of burns were scald and flame injuries with 77 cases (46.4%) sustaining scald injuries and 60 cases (36.1%) with flame injuries. These were followed by burns injuries due to an electrical fault (7.3%), chemical (6.0%) and due to contact with hot surfaces (4.2%).
Table 3.
Distribution of causes of Skin burns injuries
| Causes of Injury | N (%) |
|---|---|
| Chemical | 10 (6.0%) |
| Electrical | 12 (7.3%) |
| Flame | 60 (36.1%) |
| Scald | 77 (46.4%) |
| Hot surface | 7 (4.2%) |
Figure 3.
Causes of skin burns injuries.
Table 4; Figure 3, described the frequency of different liquids causing scald injury. Among the scald injury, the highest causes were due to hot water (96.0%) and only 1.3 to 2.7% due to hot soup and hot oil. Most scald and flame injuries occurred in females.
Table 4.
Liquid causing scald injury
| Type of liquid | No (%) |
|---|---|
| Hot water | 72 (96.0) |
| Hot Oil | 2 (2.7) |
| Hot soup | 1 (1.3) |
| Total | 75 (100) |
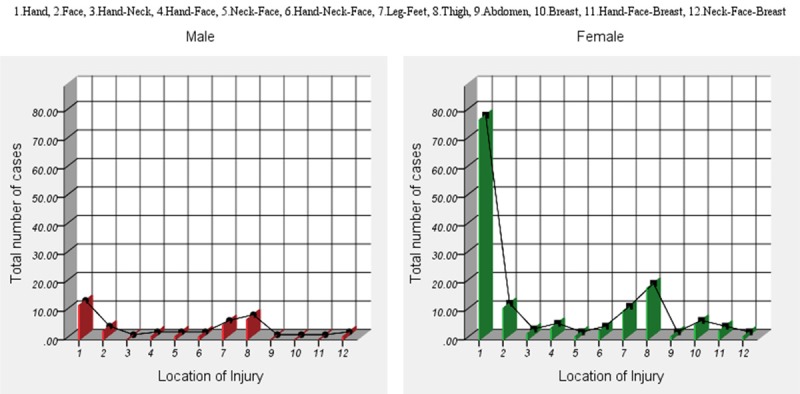
Table 5; Figure 4, summarized the distribution of burned body parts and gender. The highest injured body part was hand 89 (82%) across all age groups with females sustained more injury compared to males with a males’ females ratio of 1:00:25:00 (Tables 5, 6, 7 and Figure 1). This was followed by thigh 25 (15%), Leg and feet 15 (9%) and face 14 (8.4%). The outcomes of the burned cases were found to be dependent on gender (p=.011 and (p < 0.001). Multiple logistic regression model for different parts of burned skin showed highly significant values for hands (p < 0.001), CI, 0.181 (0.103-0.259) and face (p < 0.001), CI, 0.185 (0.112-0.258).
Table 5.
Distribution of skin burn-injured part (s) among the surveyed population
| Age | H | F | HN | HF | NF | HNF | LF | T | A | B | HFB | NFB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 18-24 | 22 | 2 | 1 | 3 | 0 | 3 | 3 | 9 | 1 | 3 | 0 | 0 |
| M | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 0 | 0 | 0 | 0 |
| F | 21 | 2 | 1 | 3 | 0 | 2 | 3 | 6 | 1 | 3 | 0 | 0 |
| 25-34 | 32 | 8 | 0 | 0 | 1 | 1 | 7 | 6 | 0 | 2 | 1 | 1 |
| M | 7 | 2 | 0 | 0 | 0 | 0 | 3 | 2 | 0 | 0 | 0 | 1 |
| F | 25 | 6 | 0 | 0 | 1 | 1 | 4 | 4 | 0 | 2 | 1 | 0 |
| 35-44 | 28 | 3 | 1 | 2 | 0 | 0 | 2 | 8 | 0 | 0 | 1 | 1 |
| M | 3 | 1 | 0 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 |
| F | 25 | 2 | 1 | 1 | 0 | 0 | 1 | 6 | 0 | 0 | 1 | 1 |
| 45-55 | 7 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 0 |
| M | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| F | 6 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | 1 | 0 |
| > 55 | 0 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| M | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| F | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N | 89 | 14 | 2 | 5 | 2 | 4 | 15 | 25 | 1 | 5 | 3 | 2 |
| (%) | (82.0) | (8.4%) | (1.2%) | (3.0%) | (1.2%) | (2.4%) | (9.0%) | (15.0) | (0.6%) | (3.0%) | (1.8%) | (1.2%) |
H-Hands; F-Face; HN-Hand-Neck; HF-Hand-Face; NF-Neck-Face; HNF-Hand-Neck-Face; LF-Leg-Feet; T-Thigh; A-Abdomen; B-Breast; HFB-Hand-Face-Breast; NFB-Neck-Face-Breast.
Figure 4.
Gender-wise variations of skin burn injuries in different parts of the body.
Table 6.
Analysis of variance between the number of cases of skin burn injuries and gender
| Sum of Squares | df | Mean Square | F | Sig. | ||
|---|---|---|---|---|---|---|
| Males | Between Groups | 30.183 | 11 | 2.744 | 2.593 | .011 |
| Within Groups | 50.800 | 48 | 1.058 | |||
| Total | 80.983 | 59 | ||||
| Females | Between Groups | 999.733 | 11 | 90.885 | 7.014 | .001 |
| Within Groups | 622.000 | 48 | 12.958 | |||
| Total | 1621.733 | 59 | ||||
Table 7.
Independent variables for different parts of skin burn patients: multiple logistic regression model
| Variables | 95% CI | p-value |
|---|---|---|
| Hand | 0.181 (0.103-0.259) | .001 |
| Face | 0.185 (0.112-0.258) | .000 |
| Hand-Neck | 0.184 (0.112-0.257) | .407 |
| Hand-Face | 0.185 (0.114-0.256) | .534 |
| Neck-Face | 0.188 (0.118-0.276) | .407 |
| Hand-Neck-Face | 0.190 (0.120-0.259) | .489 |
| Leg-Feet | 0.201 (0.154-0.289) | .945 |
| Thigh | 0.211 (0.134-0.267) | .447 |
| Abdomen | 0.211 (0.149-0.273) | .369 |
| Breast | 0.202 (0.144-0.262) | .534 |
| Hand-Face-Breast | 0.198 (0.142-0.258) | .447 |
| Neck-Face-Breast | 0.199 (0.141-0.257) | .407 |
Table 8, showed the percentage of the total burned surface area according to age and gender. The majority of patients were burned in a rage of 0-10% TBSA which is about 62.0% (p < 0.002, R 2=0.289) of the total participants, with a male-female ratio of 1:4. On the other hand, only 24.7% (p < 0.004, R 2=0.303) of the participants had 11-20% TBSA with male-female ratio of 1:3 and 13.3% (p < 0.005, R 2=0.388) of the participants had 21-30% TBSA, with male-female ratio of 1:3. Most of the participants with 10-30% TBSA were identified within the age group of 18-34 years and females sustained more injuries than men. The differences between men and women concerning TBSA percentages were found statistically significant. There was no participant has burned with more than 30% TBSA. Approximately, 76.5% of the participants have mixed degrees of burns and were cured and discharged using topical creams and skin patching and only 23.5% have endured extensive treatments including skin grafting.
Table 8.
Percentage of the total burned surface area (TBSA) according to age and gender
| Age | Low (0-10% and less) | Medium (11-20%) | High (21-30%) |
|---|---|---|---|
| 18-24 | 30 | 11 | 5 |
| M | 3 | 3 | 0 |
| F | 27 | 8 | 5 |
| 25-34 | 39 | 14 | 6 |
| M | 13 | 4 | 1 |
| F | 26 | 10 | 5 |
| 35-44 | 29 | 12 | 6 |
| M | 5 | 2 | 3 |
| F | 24 | 10 | 3 |
| 45-55 | 5 | 3 | 3 |
| M | 2 | 0 | 0 |
| F | 3 | 3 | 3 |
| > 55 | 0 | 1 | 2 |
| M | 0 | 1 | 1 |
| F | 0 | 0 | 1 |
| Total | 103 | 41 | 22 |
| p-value* | 0.002 | 0.004 | 0.005 |
| R 2 | 0.289 | 0.303 | 0.388 |
p-value indicates a comparison between males and females through linear regression.
Dermatology quality of life (DQL) after burns was accessed covering several parameters using standardized scales. The scopes examined were emotional aspects, daily activities, sports, social and leisure activities, work status, interpersonal relationship and treatment, and after-care. For all the examined parameters maximum response was (96.6%) gave positive comments about their active engagement in work and study-related activities after injury. Only 2.4% has either taken retirement from the previous job or has left completely. 21.7% of the respondents were of opinion that they do not feel any embarrassing or emotional feeling post burn injury, while, 13.2% have some minor embarrassing consequences. About 85% to 95% of the respondents thought that burn injury has no major impact on their social, sport or leisure activities. Their social activity was almost similar to that of healthy individuals.
Concerning treatment, an equal number of the respondents (14.5%) donated both positive and negative opinions on post-burn treatments. The recovery of burn patients was almost 100%. The post-recovery follow-up with doctors also showed positive results. On the other hand, 13.3% of the respondents who have 21-30% TBSA, also did not show any major physical disabilities or dependency on assistance for their daily activities. More than 98% of the respondents offered their outlooks that they have the same family relationship as in the pre-burn period.
The validity and reliability of the questionnaire were statistically analyzed using Cronbach’s reliability analysis which indicated excellent results in all the study subjects (Table 9). Significant correlations were observed between daily activities and sports with that of education. Similar correlations were also observed between social and leisure activities with interpersonal relationships. The mean DLQI scores and Cronbach’s coefficients based on gender, marital status and burn index (BI) are shown in Table 10. The mean DLQI scores ranged from < 1 to 27. A strong correlation was observed between daily activities scale and DLQI score with gender. However, no significant differences were observed between SE (symptoms and emotions), SLA (social and leisure activities, S (sports), IPR (interpersonal relationships) and DLQI scores with respect to the degree of burns. Cronbach’s coefficients values were statistically significant for SE, SLA, WS (work and study), IPR and treatment as the values are more than Cronbach’s ‘α’ coefficient. Patients with burn scars were further cross-checked for their physical appearance, daily and social activities using component score coefficient matrix to trace the reliability of DLQI scores (Table 11). Significant component scores were obtained for DLQ variables of physical appearance with DA, S, and IPR; daily activities with that of IPR and social activities with work and study.
Table 9.
Dermatology Life Quality Index scales
| Parameter Scale (Q) | parameters per scale | Range of correlation | Squared multiple correlations | Cronbach’s α |
|---|---|---|---|---|
| SE | 2 | 0.51-0.90 | 0.86 | 0.80 |
| DA | 1 | 1.00 | 0.28 | 0.78 |
| SLA | 2 | 0.64-0.82 | 0.94 | 0.82 |
| S | 1 | 1.00 | 0.17 | 0.87 |
| WS | 2 | 0.59-0.72 | 0.81 | 0.98 |
| IPR | 1 | 1.00 | 0.24 | 0.88 |
| T | 1 | 1.00 | 0.11 | 0.79 |
SE-Symptoms & Emotions; DA-Daily Activities; SLA-Social & Leisure Activities; S-Sports; WS-Work & Study; IPR-Interpersonal relationships; T-Treatment.
Table 10.
Cronbach’s coefficients and Dermatology Life Quality Index (DLQI) score (Mean ± SD) based on gender, marital status, and Burn Index (BI)
| Variables | Cronbach’s Coefficients | DLQI scores | p-value |
|---|---|---|---|
| Gender | |||
| Male | 6.6 (5.9) | 0.006 | |
| Female | 0.78 | 26.6 (19.2) | |
| Marital Status | |||
| Single | 0.85 | 13.2 (17.4) | 0.040 |
| Married | 0.84 | 18.0 (16.0) | |
| Widow | 0.86 | 0.6 (0.5) | |
| Divorced | 0.87 | 1.4 (1.5) | |
| Burn Index (%) | |||
| 0-10 | 0.77 | 20.6 (17.0) | 0.40 |
| 10-20 | 0.82 | 8.2 (5.8) | |
| 20-30 | 0.85 | 4.4 (1.8) |
Table 11.
Factor analysis-Component score coefficient matrix*
| DLQ variables | Components | ||
|---|---|---|---|
|
| |||
| Physical appearance | Daily activities | Social activities | |
| SE | -0.171 | 0.422** | -0.310 |
| DA | 0.179** | 0.407** | 0.029** |
| SLA | -0.038 | 0.409 | 0.126** |
| S | 0.459** | -0.050 | -0.061 |
| WS | 0.105 | 0.092 | 0.474** |
| IPR | 0.462** | 0.026** | -0.068 |
| T | -0.138 | -0.044 | 0.485 |
Rotation method: Promax with Kaiser Normalization;
Statistically significant.
Table 12 shows the perception level of post-burn patients on skin grafting. A total of 26 participants have undergone skin grafting procedures out of which 6 are males and 20 females. The maximum grafting procedure was undertaken by participants belonging to the age group 25-34 (n=10) and 18-24 (n=7). The satisfactory level for males was 83.3%, whereas, for females was 55%.
Table 12.
Patient’s perception of post-burn skin grafting outcomes
| Age (years) | Total | No of patients undergone skin grafting | Satisfied | Not-satisfied | |||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Males | Females | Males | Females | Males | Females | ||
| 18-24 | 7 | 1 | 6 | 1 | 3 | 0 | 3 |
| 25-34 | 10 | 2 | 8 | 2 | 6 | 0 | 2 |
| 35-44 | 5 | 1 | 4 | 1 | 1 | 0 | 3 |
| 45-55 | 2 | 0 | 2 | 0 | 1 | 0 | 1 |
| > 55 | 2 | 2 | 0 | 1 | 0 | 1 | 0 |
Table 13 shows the correlation between the time of skin grafting procedure & patients’ satisfaction with skin grafting, a total of 16 satisfied participants with early excision and grafting and 10 non-satisfied participants with delayed excision and grafting.
Table 13.
Showing the correlation between the time of skin grafting procedure & patients satisfaction with skin grafting
| Time of skin grafting procedure | Satisfied | Not-satisfied |
|---|---|---|
| Early excision and grafting | 15 | 2 |
| Delayed Excision and grafting | 1 | 8 |
| Total | 16 | 10 |
Discussion
The present study evaluated the dermatology quality of post-burn patients and their emotional or psychological distress due to varied factors. Burn disrupts the integrity of skin which may be superficial or deep sheathed. Although there is a substantial improvement in medical care facilities for the management of skin burns, the remains of scar marks are still the primary concern of the burn care specialist [11].
In the present study, the great majority of cases of skin burns were due to scalding. The number is high among women aged 18-34 years, which was in line with published literature [7]. This may be attributed to kitchen hazard, cultural and social bindings as women are more confined to the home and engaged in cooking [14,15]. The present study revealed several burn cases at home than at outdoor or workplace. The results also agree for different injured body parts in which the highest number of cases showed the burning of hands. Burn injuries of hand were mainly due to the spilling of hot liquids or from contact with hot surfaces [8]. Most of the burned cases in the present study were within the range of 10-20% TBSA. About 26% of the affected individuals have undergone skin grafting with only one surgical intervention.
In the present study, though most of the respondents used cooled water or honey as post-burn therapy, studies showed that it could not effectively reduce the extent of burn of the affected tissue. Timely and appropriate treatments are the best options for early healing of burn wounds and speedy recovery [16]. In most of the topical burn management, silver sulfadiazine is used as antimicrobial agents. However, recently nanocrystalline silver dressings are used which have enhanced antimicrobial and faster healing properties [17]. Recently, it has been reported that silver sulfadiazine along with honey is effective in burn wound healing [18].
The present study explored the time since burning acquired considering the period from 1 month to more than 20 years to understand the time taken by the burn’s scar to attain maturity. Depending on the severity of the injury, burn treatment may require several days to months for complete healing. The time elapsed since the injury has a major impact on psychological outcomes [19].
Patients sustaining burns injury often face difficulty in adapting to social life due to stigmatization, thereby affecting the quality of life. This impairment in quality of life is mainly due to skin scar marks in the uncovered areas of the body or due to physical disfigurement. Many studies elaborated on the psychological, physical and social consequences of burn affected patients [20]. The main reason behind these is the change in body image and their negative perception of social unacceptance [21]. Most of the post-burn survivors exhibit high rates of depression. However, in the present study, several respondents showed their positive social activities in spite of their facial disfigurement.
The DLQI is one of the frequently used tools to assess the dermatology quality of life [5]. Although there are different measurement tools to assess the dermatology quality of life, the present evaluations showed positive outcomes for the skin burn patients. Accurate measurement of the percent of the total body surface area (%TBSA) burn is crucial in the management of burned patients [22]. The DLQI score is within the range of < 1 to 27. Similar findings were reported earlier where the DLQI scores were reported between 4 to 28 [4]. Burns injuries are considered one of the most traumatic injuries leading to post-traumatic stress disorders (PTSD). However, no significant post-traumatic stress disorder (PTSD) among the respondents was noticed in the present study.
Reconstruction of burn scars is one of the positive initiatives of burned patients for the development of good body image [23]. In the present study, only 16% of the total participants have undergone skin grafting. The satisfactory level for males was 83.3%, whereas, for females was 55%. Some burned areas may be concealed but the scar marks remain, while some scar marks may be deep sheathed and adhere to the fat layer. For deep sheathed burn injury autologous fat grafting is one of the possible remedies [24]. Debridement, skin grafting surgery and burn reconstructive surgery has considerable benefits for patients with a significant percentage of TBSA of deep burns [17]. Early excision and grafting helps in early recovery and also have lots of positive outcomes [25]. In hypertrophic burn scarring, scar massage is effective in decreasing scar height and pain [26]. Recently, ablative fractional lasers have been reported to be effective in the treatment of burn scars [25]. The quality of burn reconstruction depends on specific factors such as; the development of materials and skin placement techniques used in the graft harvesting and securing of the skin [27]. Moreover, the surgeon’s experience, the time of treatment used, and the post-operative care play an important role in restoring the normal appearance of the skin grafted with its required function.
Conclusion
Skin burns are common in Saudi Arabia and more prevalent among females. Most skin burns occur at home and the most causative agent is hot water predominantly affecting hands. The majority of burns are treated using topical creams, and only some of them undergo skin grafting. There is a relatively higher degree of acceptance of skin burns treatment outcomes among the Saudi population. The present study may help the healthcare provider to assess the dermatology quality of life of burn-injured patients to provide much better healthcare support.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of conflict of interest
None.
References
- 1.WHO. Burn key facts 2018. Available at: https://www.who.int/news-room/fact-sheets/detail/burns.
- 2.Pitkanen J, Al-Qattan MM. Epidemiology of domestic chemical burns in Saudi Arabia. Burns. 2001;27:376–378. doi: 10.1016/s0305-4179(00)00126-1. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hoqail RA, Fadaak H, Wafa AW. Burn injuries at a university hospital in Saudi Arabia: an audit and concept of total quality management, 1997-2003. J Craniofac Surg. 2011;22:404–408. doi: 10.1097/SCS.0b013e3182077f84. [DOI] [PubMed] [Google Scholar]
- 4.Mazharinia N, Aghaei S, Shayan Z. Dermatology life quality index (DLQI) scores in burn victims after revival. J Burn Care Res. 2017;28:312–317. doi: 10.1097/BCR.0B013E318031A151. [DOI] [PubMed] [Google Scholar]
- 5.Balci DD, Inandi T, Dogramaci CA, Celik E. DLQI scores in patients with keloids and hypertrophic scars: a prospective case-control study. J Dtsch Dermatol Ges. 2009;7:688–691. doi: 10.1111/j.1610-0387.2009.07034.x. [DOI] [PubMed] [Google Scholar]
- 6.Van Loey NE, Van Son MJ. Psychopathology and psychological problems in patients with burn scars. Am J Clin Dermatol. 2003;4:245–272. doi: 10.2165/00128071-200304040-00004. [DOI] [PubMed] [Google Scholar]
- 7.Agbenorku P, Bukari AR, Effah AT, Agbenorku M, Asare NYO, Bayuo J. Burn injury in epileptics: the trend and risk factors in the middle belt of Ghana. Burns. 2018;2:122–125. [Google Scholar]
- 8.Garland K, Nahiddi N, Trull B, Malic C. Epidemiological evaluation paediatric burn injuries via an outpatient database in eastern Ontario. Burns Open. 2018;2:204–207. [Google Scholar]
- 9.Blome-Eberwein S, Lozano D, Amani H. Utility of negative pressure wound therapy with installation in a Burn Center. Burns Open. 2018;2:208–212. [Google Scholar]
- 10.Patino G, Zheng MY, Breyer BN, Cohen AJ. Skin grafting applications in urology. Rev Urol. 2019;21:8–14. [PMC free article] [PubMed] [Google Scholar]
- 11.Miyanaga T, Kishibe M, Yamashita M, Kaneko T, Kinoshita F, Shimada K. Minced skin grafting for promoting wound healing and improving donor-site appearance after split-thickness skin grafting: a prospective half-side comparative trial. Plast Reconstr Surg. 2019;144:475–483. doi: 10.1097/PRS.0000000000005868. [DOI] [PubMed] [Google Scholar]
- 12.Wang Q, Wang M, Xu Y, Ni XD, Cang ZQ, Yuan SM. Treatment of large scars in children using artificial dermis and scalp skin grafting. J Craniofac Surg. 2019;30:891–896. doi: 10.1097/SCS.0000000000005381. [DOI] [PubMed] [Google Scholar]
- 13.Abstracts from the 12th Asia Pacific Burn Congress: Singapore. 14-17 August 2019. Burns Trauma. 2019;7(Suppl 1):27. doi: 10.1186/s41038-019-0172-1. Published 2019 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anwer MO, Rauf MU, Chishti N, Anwer S. Etiology and characteristics of burn injuries in patients admitted at Burns Center, Civil Hospital Karachi. Indian J Burns. 2016;24:36–40. [Google Scholar]
- 15.Ndiaye L, Sankale AA, Ndiaye A, Foba ML, Coulibaly NF. Management of axillary burn contracture: about of 67 cases. Burns Open. 2018;2:109–113. [Google Scholar]
- 16.Kenworthy P, Phillips M, Grisbrook TL, Gibson W, Wood FM, Edgar DW. Monitoring wound healing in minor burns-A novel approach. Burns. 2018;44:70–76. doi: 10.1016/j.burns.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 17.Zou KJ, Medina A, Tredget EE. Important developments in burn care. Plast Reconstr Surg. 2017;139:120e–138e. doi: 10.1097/PRS.0000000000002908. [DOI] [PubMed] [Google Scholar]
- 18.Aziz Z, Abdul Rasool Hassan B. The effects of honey compared to silver sulfadiazine for the treatment of burns: a systematic review of randomized controlled trials. Burns. 2017;43:50–57. doi: 10.1016/j.burns.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Shahid F, Ismail M, Khan S. Assessment of quality of life in post burn survivors: a cross-sectional single-center first validation study from Pakistan. Burns Open. 2018;2:35–42. [Google Scholar]
- 20.Van Loey NE, Faber AW, Taal LA. Do burn patients need to burn specific multidisciplinary outpatient aftercare: research results. Burns. 2001;27:103–110. doi: 10.1016/s0305-4179(00)00099-1. [DOI] [PubMed] [Google Scholar]
- 21.Lawrence J, Rosenberg LE, Fauerbach JA. Comparing the body esteem of pediatric survivors of burn injury with the body esteem of an age-matched comparison group without burns. Rehabil Psychol. 2007;52:370–379. [Google Scholar]
- 22.Retrouvey H, Chan J, Shahrokhi S. Comparison of two-dimensional methods versus three-dimensional scanning systems in the assessment of total body surface area estimation in burn patients. Burns. 2018;44:195–200. doi: 10.1016/j.burns.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 23.Yoshino Y, Kubomura K, Ueda H, Tsuge T, Ogawa R. Extension of flaps associated with burn scar reconstruction: a key difference between island and skin-pedicled flaps. Burns. 2018;44:683–691. doi: 10.1016/j.burns.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Jaspers ME, Brouwer KM, van Trier AJ, Groot ML, Middelkoop E, Zuijlen PP. The effectiveness of autologous fat grafting in adherent scars: results obtained by a comprehensive scar evaluation protocol. Plast Reconstr Surg. 2017;139:212–219. doi: 10.1097/PRS.0000000000002891. [DOI] [PubMed] [Google Scholar]
- 25.Munster AM, Smith-Meek M, Sharkey P. The effect of early surgical intervention on mortality and cost-effectiveness in burn care, 1978-91. Burns. 1994;20:61–4. doi: 10.1016/0305-4179(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 26.Ault P, Plaza A, Paratz J. Scar massage for hypertrophic burns scarring-A systematic review. Burns. 2018;44:24–38. doi: 10.1016/j.burns.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Willows BM, Ilyas M, Sharma A. Laser in the management of burn scars. Burns. 2017;43:1379–1389. doi: 10.1016/j.burns.2017.07.001. [DOI] [PubMed] [Google Scholar]


