Abstract
Anatomical and functional imaging plays a decisive role for detection and staging, of prostate cancer both primarily and post-treatment. While multiparametric MRI offers anatomic imaging with excellent soft tissue contrast, hybrid imaging based on positron emission tomography in combination with computed tomography (PET/CT) contributes functional imaging capacities. Since 68Ga-PSMA-11 was expected to be more efficient than the prior Choline-based PET radiotracers, it was the aim of the study to evaluate the diagnostic performance of the 68Ga-PSMA-11 PET/CT and multiparametric MRI in patients with recurrent prostate cancer and low PSA levels. 32 out of a cohort of 128 prostate cancer patients with biochemical relapse were referred for 68Ga-PSMA-11 PET/CT, MRI and bone scintigraphy. According to the histopathologically or clinically defined reference standard all results were classified as true positive, false positive, true negative or false negative. Local recurrence was present in 11/32 patients, lymph node metastases - in 13/32 patients and, bone metastases - in 6/32 patients. Against the standard of reference, sensitivity, specificity and accuracy for local recurrence of PET/CT were 63.6 %; 73.7%; 77.8%, respectively. MRI reached 90.9%; 94.7%; 92.3%, respectively. For local lymph node metastases PET/CT - 83.3%; 80.0% and 90.6%, respectively. MRI - 41.7%; 94.4%; 72.0%, respectively. For evaluation of bone metastases in PET/CT - 83.3%; 92.0%; 71.0%, respectively. Bone scintigraphy - 50.0%; 84.0%; 77.4%, respectively. In conclusion, mpMRI offered the better diagnostic accuracy in the detection of local recurrence and while PSMA PET/CT was superior in the detection of distant and lymph node metastases.
Keywords: PSMA-PET/CT, MRI, prostate cancer, recurrence, restaging, 68Ga-PSMA
Introduction
In patients with prostate cancer (PCa), prostate-specific antigen (PSA) blood levels are the most commonly used biomarker as well for prostate cancer screening as for assessment of treatment efficacy, but this does not reflect cancer stage and tumor volume. Therefore, anatomical and functional imaging plays a decisive role for tumor detection and staging, both primarily and post-treatment.
Imaging follow-up after treatment is particularly challenging. While patients with biochemical recurrence are best treated as early as possible, accurate localization of recurrent prostate cancer at low PSA values is difficult and the performance of the current standard imaging approach is limited. 99mTc-MDP whole-body bone scan as a highly sensitive imaging method has been used for decades to evaluate PCa bone metastasis based on its availability and low cost, but because of accumulation of this radiotracer in degenerative, inflammatory and traumatic lesions, the specificity is relatively low. If PSA values are less than 7 ng/ml, bone scintigraphy has a detection rate of only 5% and CT has a low sensitivity of 11-14% for detection of local recurrence and lymph node metastases [1].
Therefore, development of imaging protocols and definition of appropriate imaging biomarkers in multimodal prostate cancer and recurrence diagnostics are subject to ongoing investigations. Beyond bone scintigraphy, new radiopharmaceuticals have substantially increased the role of radionuclide imaging in the diagnosis of recurrent prostate cancer and treatment strategy.
Positron emission tomography in combination with computed tomography (PET/CT) is a functional and anatomical non-invasive radiological hybrid imaging modality. Several radiopharmaceuticals with different diagnostic sensitivity and specificity are used in the detection and staging of prostate cancer, in the evaluation of treatment efficacy and localization of recurrence. When compared with other diagnostic modalities, e.g. MRI, CT or bone scintigraphy, the single PET/CT examination can be more accurate to find very small metastases in both - soft tissues and bones. used in prostate cancer PET/CT examinations [2].
The introduction of prostate-specific radiopharmaceuticals such as 68Ga-PSMA-11 (gallium 68 prostate-specific membrane antigen 11) has led to further improvement in PET/CT diagnosis of prostate cancer and its recurrence. 68Ga-PSMA-11 is even more efficient than the prior choline-based radiotracers (carbon 11/fluorine-18 (F-18) choline) which showed only low detection rates in patients with low PSA levels: 30% for PSA levels under 1.5 ng/mL [3,4].
PSMA (Prostate-Specific Membrane Antigen) is a glycoprotein that is hyper expressed in prostate cancer tissues while its degree of expression correlates with tumor aggressiveness, androgen resistance, metastatic disease, and disease recurrence. It is an excellent tool for radionuclide diagnostics and prostate cancer treatment for several reasons: it expresses in prostate cancer cells during all stages of the disease on the cell surface as an integral part of the membrane and does not go into blood circulation. Therefore, PSMA radiopharmaceuticals are increasingly being used in prostate cancer patients. In combination with 68Ga it accumulates even at low PSA levels in small tumor lesions, lymph node, bone or visceral metastases due to low background signal. Sensitivity of PSMA PET/CT increases with PSA levels (42, 58, 76 and 95% for PSA categories 0-0.2, 0.2-1, 1-2 and >2 ng/ml, respectively [4].
Data about correlation of PSMA Hybrid imaging with other parameters and biomarkers such as Gleason score and PSA level are so far sparse. However, in the setting of PCa recurrence, other studies have confirmed a higher sensitivity of PSMA PET/CT compared to other imaging modalities for detecting sites of recurrence and metastatic disease, even at very low serum PSA values [5].
Therefore, 68Ga-PSMA-11 and 18F-PSMA-1007 PET/CT can be considered a valuable alternative to the current imaging standards [6]. At the same time 68Ga-PSMA-11 ligands have a drawback in the assessment of small local recurrences due to excretion via the kidneys and high accumulation in the urinary bladder [7].
The most promising solution for the detection of local recurrence therefore appears to be multiparametric magnetic resonance imaging (mpMRI). It performs excellent in the detection of aggressive prostate cancer with negative (NPV) and positive predictive value (PPV) of 63-98% and 34-68%, respectively, and it was assumed that it might as well serve for the detection of local recurrence or residual disease. First studies have indeed shown, that despite treatment induced changes, mpMRI is effective even at low PSA levels below 2 ng/ml [8].
However, while both approaches appear to be efficient in the low PSA scenario, but also expensive, reasonable protocols and PSA cut-off values are need to be defined to ensure clinical value and cost-effectiveness. It was therefore the aim of this study, to determine the potential diagnostic yield and clinical value of PET/CT with the current tracers and multiparametric MRI or a combination of both at low PSA levels.
Materials and methods
Patients
Out of a cohort of 128 consecutive patients that underwent 68Ga-PSMA-11 PET/CT at our institution, 32 patients with biochemical PCa recurrence met the inclusion criteria and were enrolled. The study was approved by the Ethics Committee and informed written consent was obtained from all patients.
Three subgroups were defined, related to PSA level and previous treatment within the last 10 years: 1. group: 21 patients followed up after radical prostatectomy and current PSA level 0.2-5.0 ng/ml. 2. group: 4 patients followed up after treatment with radical pelvic radiation therapy and current PSA level 2.0-10.0 ng/ml. 3. group: 7 patients followed up after radical prostatectomy and adjuvant radiotherapy and current PSA level 0.2-10.0 ng/ml.
Only patients who matched the criteria of the Eastern Cooperative Oncology Group (ECOG) 1-2 category were included. Full access to previous clinical history (previous treatment, prostate biopsy histology results, Gleason score, at least two previous serum PSA level results) was a prerequisite. Exclusion criteria were decreased renal function (glomerular filtration rate <45 ml/min); pelvic radiation therapy within 6 months before PET/CT and MRI; chemotherapy before PET/CT and MRI and patients with history of additional oncological disease (Table 1).
Table 1.
Inclusion criteria for study participants
| Inclusion criteria |
|
|
| Treatment (RP and/or RT) for prostate cancer within the last 10 years |
| Biochemical PCa recurrence |
| PSA 0.2-10.0 ng/ml 3 subgroups: |
| 1. post RP 0.2-5.0 ng/ml |
| 2. post RT 2.0-10.0 ng/ml |
| 3. post PR and pelvic RT 0.2-10.0 ng/ml |
| Available relevant clinical history (previous treatment, prostate biopsy histology results, Gleason score, at least two previous serum PSA level results) |
| Eastern Cooperative Oncology Group (ECOG) category 1-2 |
|
|
| Exclusion criteria |
|
|
| Impaired renal function (glomerular filtration rate <45 ml/min) |
| Pelvic radiation therapy within 6 months |
| Chemotherapy before PET/CT and MRI |
| History of additional oncological disease |
Besides 68Ga-PSMA PET/CT all patients underwent PET/CT pelvic multiparametric mpMRI and bone scintigraphy within ≤3 months before or ≤1 month after PET/CT.
Imaging
PSMA PET/CT
All included patients underwent 68Ga-PSMA-11 PET/CT using a commercially available clinical system equipped with 18 cm size PET detector and integrated 64 row detector CT unit (Gemini TF64, Philips, Koninklijke Philips N.V., Best, Netherlands). The administered 68Ga-PSMA dose per patient ranged from 1.8-2.2 MBq/kgBW (mean individual dose 189 mBq) and the CT part was acquired with intravenous contrast material application (1 ml/kgBW iodixanol (Visipaque, GE Healthcare, Boston, USA). To improve image quality each patient received diuretic injection with Furosemide 20 mg intravenous immediately after receiving PSMA injection [9].
The scanning protocol was individually adjusted for patient size. Every scanning protocol contained a whole body PET/CT scan from head to middle thigh 51-81 minutes after radiotracer injection (mean time 55 min, IQR 44-61 min) with both arms elevated above the head as far as tolerated), starting with low-dose CT, following by PET. Additional pelvic PET/CT scans were acquired in 18 patients with low serum PSA level or in patients with unclear findings on PET/CT: two patients received an early PET/CT scan for the prostate bed/periprostatic region of interest (from aortic bifurcation to small pelvis included) 44 min. and 61 min. after radiotracer injection; 16 patients received a late PET/CT for region of interest (from aortic bifurcation to small pelvis included) 120-147 min. after radiotracer injection.
The typical acquisition time per bed position was 2 minutes. In 25 patients acquisition times per bed position were increased to 3 minutes for selected regions of interest (from aortic bifurcation to the small pelvis) or towards the end of the examination to compensate for radiotracer decay.
The CT scans were acquired in the portal venous phase 70 seconds after intravenous contrast injection covering the whole body from the skull base to the symphysis pubis. PET image reconstructions (attenuation correction and 3D) were made using commercial software (IntelliSpace Portal, Philips N.V., Best, Netherlands).
68Ga-PSMA-11 was synthesized on site using an automated synthesis module Modular-Lab EAZY (Eckert & Ziegler, Berlin, Germany) and reagents, materials provided by the manufacturer. Commercially available GMP-grade peptide precursor is the most commonly used PSMA-11 trifluoroacetyl salt, manufactured by ABX advanced biochemical compounds GmbH, Radeberg, Germany.
Production and quality control of Ga-PSMA-11
68Ga-PSMA-11 was synthesized using an automated synthesis module (ModularLab-EAZY, Eckert & Ziegler). Reagents and synthesis cassettes for the synthesis module were purchased from the manufacturer. The GMP-grade peptide PSMA-11 was purchased from ABX Chemicals, which is the most commonly used peptide for 68Ga-PSMA-11 productions [10].
The first step of the production of 68Ga labelled peptides is the preparation of 68Ga. For our study, we applied the most common approach using a 68Ge/68Ga generator [11]. The reagent was eluted with 0.01 M hydrochloric acid. After elution, the eluate was directly pushed to the reactor with the previously prepared peptide (peptide was solved using 0.4 ml of acetyl buffer solution and transferred to synthesis reactor). After radio-labeling, the preparation was purified using a CM cartridge. The preparation was finalized by dilution of the product with 0.9% NaCl.
Quality controls for radiochemical purity and identification of each of the 68Ga-PSMA-11 preparations were carried out using TLC [12], gamma-ray spectrometry and activity measurement were used for determination of radionuclide purity and identification. All injectable solutions were tested for sterility and BET.
The yield of synthesis 68Ga-PSMA-11 is 80 ± 8% (non decay corrected). For QC we did not specify the range of specific activity, but only activity concentration per volume. But with back calculation the specific activity of 68Ga-PSMA-11 was ranging from 10-50 MBq/nmol, dependent of number of generators and their expiry.
MpMRI
Pelvic MRI was acquired using commercial 1.5 T (Magnetom/Avanto, Siemens, Germany) (14 pats.); and 3T (Ingenia, Philips Healthcare, Best, Netherlands) (18 pats) systems. Anatomy-specific phased-array surface coils were used, all sequences with small FOV images covering the prostate position and one sequence with large FOV for whole pelvis were obtained.
Dynamic contrast-enhanced (DCE) imaging after i/v bolus administration of 0.1 mmol/kg of Gadobutrolum (Gadovist, Bayer Pharma) using a power injector (administration rate of 3-4 mL/s). A total of 10-20 contrast-enhanced acquisitions were performed. Subtraction of each contrast-enhanced acquisition from the unenhanced acquisition was automatically performed at the MRI console during the examination, and the resulting images were saved on the PACS.
In a sub-group of 18 patients, additional diffusion-weighted whole-body imaging with background body signal suppression (DWIBS) was performed with whole-body 3.0-T Ingenia (Philips Healthcare). Images were obtained from the top of the skull base to mid-thigh. Anatomy-specific phased-array surface coils were used for all body regions.
Bone scintigraphy
All patients underwent Tc99m-MDP whole body bone scintigraphy (Philips SKYLIGHT AZ gamma camera, Philips, Best, Netherlands) in anterior and posterior projections. Patients were well hydrated prior examination, radiopharmaceutical was administered by the intravenous route by a single injection 500-700 MBq depending on patient weight and protocol used in different centers. Two to two and a half hours post injection patient were scanned by dual headed gamma camera equipped with a low-energy, high-resolution parallel-hole collimator. No special image postprocessing was done.
Image analysis
PET/CT
PET/CT images were analysed by two experienced nuclear medicine physicians-radiologists (10 and 5 years of experience) using a commercial work station. Increased 68Ga-PSMA-11 uptake was considered as pathological finding taking into account normal distribution and pitfalls of 68Ga-PSMA-11 uptake, as well as clinical information data. The standard uptake maximum values (SUVmax) were measured for each pathological lesion, lymph node size were measured in CT. A lesion was considered as pathological when at least one nuclear medicine physician classified the lesion as a pathological finding.
MRI
MRI images were analysed by two experienced diagnostic radiologists (20 and 15 years of experience). Local recurrence was assumed, if pathologic findings were consistently shown on at least two different MRI sequences: on T2WI a mass or soft-tissue area of slightly high signal intensity relative to muscle; a high focal signal intensity on the DWI with corresponding low signal intensity on the ADC; early phase hyperenhancement on DCE images (matching the lesion positive on at least one other sequence).
Criteria for pathologic lymph nodes on MRI were: round shape, size greater than 8 mm in short-axis diameter and/or inhomogeneous intranodal signal intensity on T2WI and/or irregular border and/or intensive enhancement. MRI criteria for bone metastases were: focal bone marrow lesion with hypointense signal compared to muscle on the T1WI and T2WI and/or restricted diffusion on DWI and early, intensive enhancement after contrast material injection.
Bone scintigraphy
Increased focal tracer uptake suggesting for prostate cancer metastases, its localization, size, shape and intensity was reported following clinical standards.
Further treatment
Based on PET/CT, MRI and scintigraphy results, the following treatment for recurrent prostate cancer patients was applied: 7 patients underwent lymph node dissection according to 68Ga-PSMA-11 PET/CT and/or MRI findings; hormone therapy, i.e. androgen deprivation therapy, was applied for 5 patients.
Total 8 patients received radiation therapy. 5 patients received radiation therapy of prostate area, 1 patient received radical radiation therapy of pathological lymph node, 2 patient received radiation therapy for distant metastases - scapula or ribs. External beam radiation therapy (EBRT) was used in 6 cases and stereotactic body radiation therapy was used in 2 cases (in one case for prostate bed and in one case for spina scapula). Total salvage dose for prostate bed EBRT was 66-72 Gy, divided in 2 or 3 Gy in each fraction. Pathological lymph node radiation therapy included dose 1.8 Gy in each fraction, up to total dose 45 Gy. For distant metastases radiation therapy in 3 fractions was used.
Reference standard for comparison analysis
According to reference standard all results of examinations were classified as true positive, false positive, true negative or false negative.
Histopathology: 7 patients underwent LN dissection of pathological lymph nodes seen on 68Ga-PSMA-11 PET/CT and/or MRI, therefore histopathological results were available for 7 patients. One patient with suspicion of local recurrent PC underwent surgery therefore the histopathological results were obtained.
Addition radiological examination: For one patient comprehensive diagnosis of addition imaging techniques were used as reference standard.
Follow up after radiation therapy: For 4 patients clinical follow up results after radiation therapy was taken as reference standard. 4 patients received a radiation therapy of pathologic region seen on 68Ga-PSMA-11 PET/CT and/or MRI and/or bone scintigraphy and the PSA evaluation prior and after the therapy were used to classify the results. 2 patients received radiation therapy of prostate area, 1 patient received radical radiation therapy of pathological lymph node, 1 patient received radiation therapy of scapula. If the PSA level after the radiation therapy decreased significantly then it was classified as the recurrent disease was located in the radiated localization.
Opinion on multidisciplinary team meetings: For 19 patients the reference standard was defined after opinion of a multidisciplinary team meeting (nuclear medicine physicians, radiologists, urologists). The reference standard was individually decided after the multidisciplinary team meeting discussions.
Statistical analysis
Data collection and classification were done with Microsoft Excel (version 16.0, Microsoft Office, USA). The sensitivity, specificity, positive predictive and negative predictive values were calculated by SPSS Data Analysis Software (version 20, IBM Corporation, USA) with cross tables, significance of difference among methods was evaluated by one-way ANOVA test, correlation between radiological and biochemical data was calculated.
Inter-observer agreement was assessed using (Kappa test). A p-value of <0.05 was considered as the threshold for statistical significance.
Results
32 patients with biochemical PCa recurrence were prospectively enrolled into the study (mean age: 63, SD 7 (range 49-81) years), see Table 2.
Table 2.
Patient characteristic table
| Patient nr. | Age | T category | N category | M category | Gleason score | PSA (ng/ml) value at the time of admission of the study | Previous treatment (P, R, ADT, B)* |
|---|---|---|---|---|---|---|---|
| 1 | 59 | 2C | 0 | 0 | 3+3 | 1.07 | P, R |
| 2 | 60 | 3B | 1 | 0 | 3+4 | 5.9 | P |
| 3 | 61 | 2B | 0 | 0 | 2+3 | 0.851 | P |
| 4 | 49 | 2B | 0 | 0 | 3+4 | 0.763 | P, R |
| 5 | 71 | 3B | 0 | 0 | 3+3 | 0.24 | P, R |
| 6 | 61 | 2A | 0 | 0 | 3+4 | 4 | P |
| 7 | 67 | 3 | 0 | 0 | 4+4 | 2 | R, ADT |
| 8 | 55 | 2C | 0 | 0 | 3+4 | 0.475 | P |
| 9 | 62 | 2C | 0 | 0 | 3+4 | 0.329 | P |
| 10 | 64 | 2C | 0 | 0 | 3+4 | 1.076 | P |
| 11 | 54 | 2C | 0 | 0 | 3+4 | 1.11 | P |
| 12 | 81 | 2 | 0 | 0 | 5+5 | 14.862 | P |
| 13 | 74 | 3B | 0 | 0 | 3+3 | 8.447 | P, ADT |
| 14 | 56 | 2C | 0 | 0 | 4+5 | 0.493 | P |
| 15 | 61 | 2C | 0 | 0 | 3+4 | 0.283 | P |
| 16 | 64 | 3B | 0 | 0 | 3+4 | 1.79 | P, ADT |
| 17 | 58 | 3B | 0 | 0 | 4+3 | 2.82 | P |
| 18 | 68 | 2B | 0 | 0 | 3+3 | 0.256 | P |
| 19 | 71 | 3B | 0 | 0 | 4+5 | 0.591 | P, R, ADT |
| 20 | 66 | 2C | 0 | 0 | 3+3 | 0.215 | P |
| 21 | 69 | 3A | 0 | 0 | 4+4 | 3.413 | R, ADT |
| 22 | 71 | 2C | 0 | 0 | 3+4 | 0.33 | P, R |
| 23 | 70 | 2C | 0 | 0 | 3+4 | 0.49 | P |
| 24 | 62 | 2C | 0 | 0 | 3+4 | 1.58 | P |
| 25 | 62 | 2C | 0 | 0 | 4+3 | 0.217 | P, ADT |
| 26 | 71 | 2C | 0 | 0 | 3+5 | 10.1 | B, R, ADT |
| 27 | 56 | 2C | 0 | 0 | 3+3 | 1.37 | P |
| 28 | 54 | 3B | 0 | 0 | 4+3 | 4.05 | P, R, ADT |
| 29 | 61 | 3B | 0 | 0 | 3+4 | 0.908 | P, R, ADT |
| 30 | 56 | 3B | 0 | 0 | 3+4 | 0.874 | P |
| 31 | 69 | 3B | 0 | 0 | 4+4 | 5.29 | P, ADT |
| 32 | 65 | 3B | 1 | 0 | 4+4 | 1.2 | P |
P - radical prostatectomy, R - radiation therapy, ADT - androgen deprivation therapy, B - brachytherapy.
The mean PSA value at inclusion into the study was 2.27 ng/ml (median 1.07 (range 0.22-10.10) ng/ml). In the different patient groups, the mean serum levels were 1.47, 5.99 and 3.22 ng/ml for groups 1, 2 and 3, respectively. The mean PSA doubling time was 8.11 months (median -4.63 (range -5.40-26.80) months). The mean increase of PSA per month was 0.06 ng/ml/month (median -0.07 (range -9.60-2.50) ng/ml/month).
As defined by the standard of reference, local recurrence was present in 11/32 patients. The mean maximum diameter of recurrent local lesions was 1.2 cm (median 1 cm (range 0.4-2.5 cm)). 1/11 patients presented with more than one local focus. 13/32 patients presented with lymph node metastases (13/13 local, 4/13 distant; mean number of metastases 3 (range 1-10)). Bone metastases were observed in 6/32 patients (mean number of metastases 5.3 (range 1-24), mean size 0.98 cm (median 1 cm, range 0.6-1.3 cm)). 2/32 patients had metastases in other organs.
PET/CT
A pathologic 68Ga-PSMA-11 uptake was observed in 23/32 (72%) PET/CT scans, see Table 3.
Table 3.
68Ga-PSMA PET/CT accuracy in recurrent prostate cancer (PCa)
| Finding | PPV | NPV | Se % [CI] | Sp % (CI) | Acc % [CI] | P value |
|---|---|---|---|---|---|---|
| PCa Local recurrence | 58.3 | 77.8 | 63.6 [46.6-77.8] | 73.7 [66.2-81.8] | 77.8 [63.7-82.9] | 0.01 |
| Lymph nodes | 80 | 100 | 83.3 [75.3-100] | 80.0 [60.4-96.6] | 90.6 [74.9-98.0] | 0.0001 |
| Skeletal metastasis | 71.4 | 95.8 | 83.3 [74-99.9] | 92.0 [80.3-99.9] | 71.0 [63.8-99.9] | 0.001 |
PPV - positive predictive value, NPV - negative predictive value, Se - sensitivity, Sp - specificity, Acc - Accuracy, CI - confidence interval.
This included a pathologic 68Ga-PSMA-11 uptake in the prostate or prostate bed as reported in 12/32 patients (38%; in 7 cases reported by both and in 5 cases by only one of the observers, interobserver agreement was 58%). Against the standard of reference, sensitivity, specificity, accuracy, positive predictive value and negative predictive value for local recurrence of PET/CT were 63.6%; 73.7%; 77.8; 58.3% and 77.8%, respectively.
A pathologic 68Ga-PSMA-11 uptake in lymph nodes was reported in 16/32 patients (50%; in 15 cases pathologic lymph node involvement was reported by both and in 1 case it was reported buy one of the observers; interobserver agreement was 94%). 6 of metastatic lymph nodes were smaller than 1 cm in short axis (mean short axis 0.7 cm). Against the standard of reference, sensitivity, specificity, accuracy, positive predictive value and negative predictive value of PET/CT for local lymph node metastases were 83.3%; 80.0%; 90.6%; 80% and 100%, respectively.
A pathologic 68Ga-PSMA-11 uptake in skeletal lesions was reported in 7/32 patients (22%; in 5 cases pathologic skeletal lesions were reported by both and in 2 cases buy one of the observers; interobserver agreement was 71%). Sensitivity, specificity, accuracy, positive predictive value and negative predictive value for evaluation of bone metastases in PET/CT were 83.3%; 92.0%; 71.0, 71.4% and 95.8%, respectively.
MRI
Prostate cancer recurrence was documented on 18/32 MRI scans (56%). In 2/32 patients MRI of the pelvis was not conclusive (prostate could not be evaluated due to brachytherapy artefacts in one patient and loss of data in another). In 11 of the remaining 30 patients (37%), MRI revealed PCa recurrence inside the prostate or prostate bed. Lymph node metastases were observed in 7/32 patients (22%). Evaluation of distant metastases was available for only 18 patients who underwent DWIBS MRI; In this sub-group DWIBS detected 2 cases with distant metastases (2/18; 11%).
Bone scintigraphy
23% (n=7/31) of bone scintigraphy revealed bone metastases. Evaluation of bone scintigraphy was available for 31 patients (one patient was excluded because of the technical issues).
See Table 4 for MRI and bone scintigraphy accuracy.
Table 4.
mpMRI and scintigraphy accuracy in recurrent prostate cancer (PCa)
| Finding | PPV | NPV | Se % [CI] | Sp % (CI) | Acc % [CI] | P value |
|---|---|---|---|---|---|---|
| PCa Local recurrence (MRI) | 90.9 | 94.7 | 90.9 [77.8-95.2] | 94.7 [76.2-99.1] | 92.3 [73.7-99.9] | 0.0001 |
| Lymph nodes (MRI) | 83.3 | 70.8 | 41.7 [29.3-58.1] | 94.4 [56.4-96.6] | 72.0 [54.9-80.0] | 0.01 |
| Skeletal metastasis (Scintigraphy) | 42.8 | 87.5 | 50.0 [41.2-71.9] | 84.0 [76.3-90.9] | 77.4 [63.8-98.0] | 0.01 |
PPV - positive predictive value, NPV - negative predictive value, Se - sensitivity, Sp - specificity, Acc - Accuracy, CI - confidence interval.
Accuracy comparison of 68Ga-PSMA-11 PET/CT, MRI and bone scintigraphy
Local recurrence
The evaluation is based on 30 cases with completed PET/CT and MRI (after exclusion of 2/32 in which MRI was not conclusive, see above). Against the standard of reference, sensitivity, specificity, accuracy, positive predictive value and negative predictive value for local recurrence of PET/CT were 63.6%; 73.7%; 77.8%; 58.3% and 77.8%, respectively. MRI reached 90.9%; 94.7%; 92.3%, 90.9% and 94.7% respectively.
Lymph node involvement
The evaluation is based on 32 cases with completed PET/CT and MRI. Against the standard of reference, sensitivity, specificity, accuracy, positive predictive value and negative predictive value of PET/CT for local lymph node metastases were 83.3%; 80.0%; 90.6%; 80% and 100%, respectively. MRI reached 41.7%; 94.4%; 72.0%; 83.3% and 70.8, respectively.
Bone metastases
The evaluation is based on 31 cases with completed PET/CT and bone scintigraphy including a total of 32 metastases in 7 patients. Sensitivity, specificity, accuracy, positive predictive value and negative predictive value for evaluation of bone metastases in PET/CT were 83.3%; 92.0%; 71.0%, 71.4% and 95.8%, respectively. Bone scintigraphy reached 50.0%; 84.0%; 77.45%; 42.8% and 87.5%, respectively. The full MRI protocol including diffusion weighted imaging (DWIBS) was available in only 18 patients (among these 7 with a total of 32 bone metastases). In this sub-group MRI reached a sensitivity, specificity, positive predictive value and negative predictive value of 100%; 100%; 100%; 100%, respectively. One case had to be excluded from bone evaluation, because this case asked for further examination to clear the findings (it was not available at the time of study).
See examples of findings in PET/CT, mpMRI and scintigraphy in Figures 1, 2 and 3. Figure 1 shows case where lesion in prostate bed was not detected in 68Ga-PSMA PET/CT due to excretion of the tracer in urinary bladder. MRI of this 62 years old patient after radical prostatectomy four years ago reveals contrast enhanced lesion. PSA level at the time of the study was 1.58 ng/ml. Confirmative histopathology from the bladder neck region according to MRI findings showed local recurrence Gl (Gleason Score) 3+4=7. After the studies, patient received radiation therapy locally for prostate bed.
Figure 1.
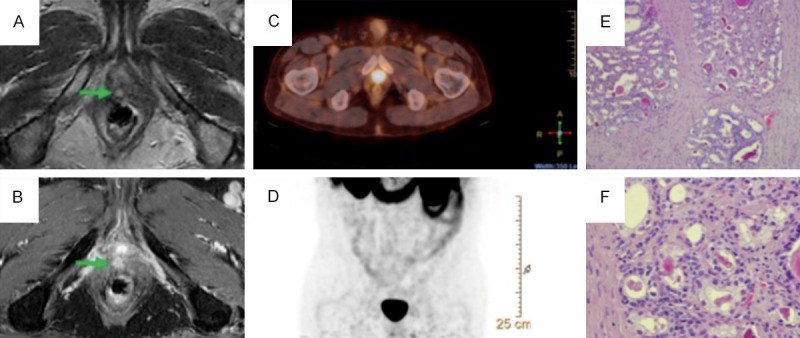
MRI of patient (62 y) after radical prostatectomy four years ago, PSA 1.58 ng/ml. (A and B) reveals contrast enhanced lesion (arrow) in prostate bed that was not detected in 68Ga-PSMA PET/CT (C, D) due to excretion of the tracer. Histopathology from the lesion: Hematoxylin eosin (HE) stain, original magnification (OM) 100× (E)- complexes of acinar adenocarcinoma, small circular glands, “infiltrative growth” pattern, with intraluminal crystalloids and OM 400× (F)- acinar structures with increased cell nuclear-cytoplasmic ratio, rare nucleoli, no pronounced nuclear polymorphism, absence of basal cell layer (Gl 3+4=7).
Figure 2.
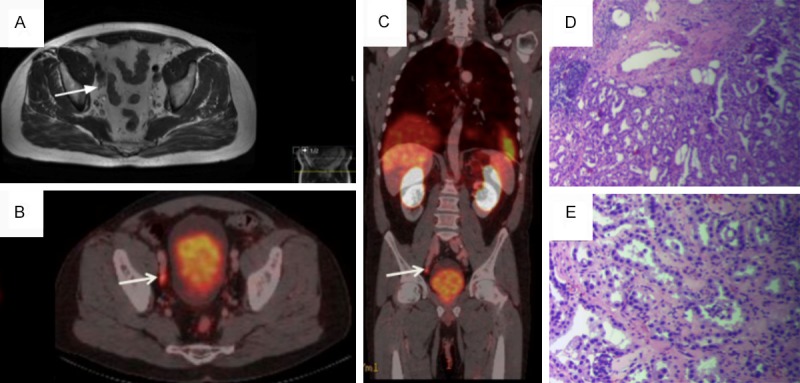
MRI T2 (A) and PET/CT (B, C) of patient (59 y) after radical prostatectomy (Gleason Score 6=3+3) three years ago, current PSA 1.07 ng/ml. (A)- in MRI unspecific pelvic lymph node is seen. (B, C)- shows 68Ga-PSMA uptake in the right iliac lymph node with SUVmax=5 (arrow). Histopathology from the lesion: Hematoxylin eosin (HE) stain, original magnification (OM) 40× (D)- lymph node with prostate carcinoma complexes and OM 200× (E)- glandular structures with distinct cellular atypia.
Figure 3.
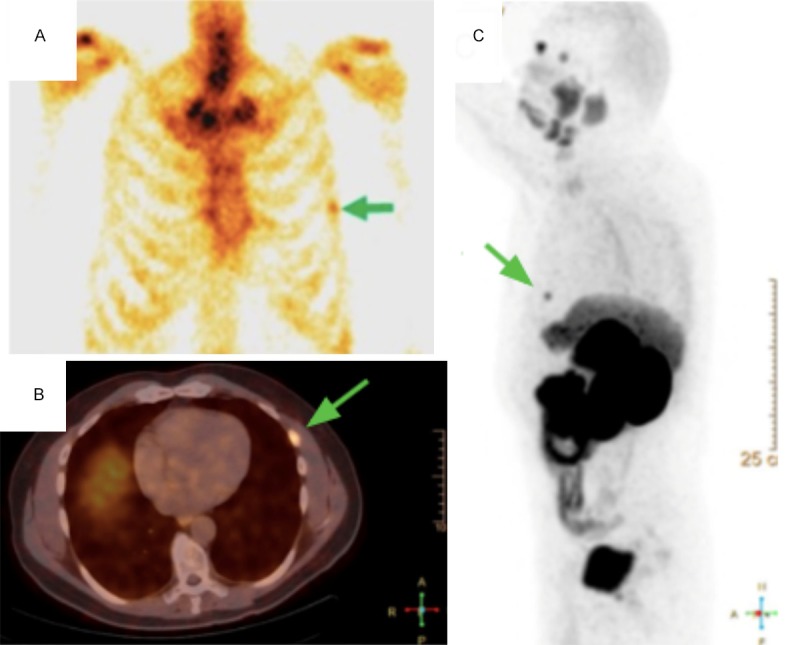
Bone scintigraphy (A) and PET/CT (B, C) of patient (56 y) after radical prostatectomy (Gleason Score 9=4+5) with PSA 0.49 ng/ml shows pathological 99mTc uptake (A) and pathological 68Ga-PSMA uptake (B, C) of the left rib (arrows).
In Figure 2 MRI and PET/CT findings of 59 years old patient after radical prostatectomy (Gleason Score 6=3+3) three years ago are compared, current PSA level -1.07 ng/ml. In MRI nonspecific right iliac lymph node is seen, but PET/CT shows 68Ga-PSMA uptake in the lymph node with SUVmax=5. After surgery the lymph node metastasis was confirmed histopathologically.
Figure 3 shows case where both bone scintigraphy and PET/CT confirmed distant metastasis - pathological 99mTc and 68Ga-PSMA uptake of the left rib in 56 years old patient after radical prostatectomy (Gleason Score 9=4+5) with PSA 0.49 ng/ml.
Discussion
Recurrence after treatment of prostate cancer is usually detected by an increase of serum PSA levels. Depending on the localization, focal ablation or resection of the recurrent lesion offers the opportunity to control oligometastatic disease saving the option of systemic therapy for a later time point [13].
Local recurrence
Similar positive effects are discussed for focal ablation of local recurrence as for salvage lymph node resection in patients with loco-regional lymph node metastases [14]. However, for both concepts, early detection and precise localization of the recurrence is a prerequisite while the traditional imaging approach with CT and bone scintigraphy lacks the necessary sensitivity and specificity in particular in patients with low PSA values [15]. Both, PSMA PET/CT and mpMRI are valuable candidates to replace the traditional diagnostic pathways. E.g. a meta-analysis by Perera et al. reports a summary per-patient sensitivity and specificity of PSMA PET/CT to detect a metastasis of 86% and 86%, respectively [16]. However, on the way towards substantial changes in the clinical pathways, appropriate studies are required to confirm the benefits of the new modalities in respect to patient benefit and cost effectiveness to justify the additional effort. Our study contributes to this comparing the accuracies of PET/CT, MRI and bone scintigraphy in early serologic recurrence of prostate cancer with mpMRI superiority in comparison to the PET/CT (sensitivity, specificity, accuracy, positive predictive value and negative predictive value for local recurrence in mpMRI -90.9%; 94.7%; 92.3%, 90.9% and 94.7% vs 63.6%; 73.7%; 77.8%; 58.3% and 77.8% in PET/CT).
Lymph nodes
Rauscher et al. [17] reported sensitivity, specificity and accuracy (compared with histology material of totally 179 resected lymph nodes) of per lesion and per patient-based analysis of Ga-PSMA PET for metastatic lymph node detection in recurrent PC - 77.9%, 97.3%, 89.9% vs. 100%, 50%, 93.8% respectively. Hijazi et al. represents a study of lymph node detection with PET/CT of recurrent and primary high-risk PC-patients compared with histology results of total of 213 removed nodes with a sensitivity, specificity, PPV and NPV 94%; 99%; 89%, 99.5% [18]. While the available results of PET/CT for detection of lymph node involvement are very promising, meanwhile MRI capability stays up to 2 times behind, in our study (sensitivity, specificity, accuracy, positive predictive value and negative predictive value -83.3%; 80.0%; 90.6%; 80% and 100% vs. 41.7%; 94.4%; 72.0; 83.3% and 70.8%). Meta-analysis represents a sensitivity of MRI to detect a lymph node metastasis by prostate cancer patients with pooled sensitivity and specificity 39% and 82%, respectively. Noteworthy is the fact, that the sensitivity and specificity between MRI and CT were not statistically significant [19]. Mauer et al. published a comparison study between 68Ga-PSMA-PET and morphological imaging (CT and MRI) for lymph node staging with sensitivity, specificity and accuracy 65.9%, 98.9%, 88.5% vs. 43.9%, 85.4%, 72.3% [20].
Bone metastasis
A wide meta-analysis represents a sensitivity per-patient based detection of bone metastases of MRI and bone scintigraphy 97% and 79%, and specificity of MRI and bone scintigraphy -95% and 82%, respectively [21]. The comparison study by Ryka et al. with 126 included patients revealed a sensitivity and specificity for PSMA PET/CT 98.7-100% and 88.2-100% versus bone scintigraphy 86.7-89.3% and 60.8-96.1% [22]. Our results showed an inferior detection rates of scintigraphy compared, but with the same tendency for PSMA PET/CT to be superior and complimentary results by body diffusion MRI (sensitivity, specificity, accuracy, positive predictive value and negative predictive value of bone scintigraphy - 50.0%; 84.0%; 77.45%; 42.8% and 87.5%, in PET/CT -83.3%; 92.0%; 71.0%, 71.4% and 95.8%, in DWIBS -100%; 100%; 100%; 100%; 100%). Although for adequate correlation we would suggest the need of comparison study between SPECT/CT and PSMA PET/CT.
Limitations of our study
One of main limitations of this study is the small number of patients. Secondly, only 8 patients underwent histopathological assessment of pathological lesions seen on 68Ga-PSMA-11 PET/CT and/or MRI. Other results were evaluated without histopathological proof; therefore, it gives a possible space for inaccuracy of the results. As another aspect we would like to mention that 68Ga-PSMA-11 PET/CT examination was made using one PET/CT scanner for all patients, but MRI were made in different medicine centres, using similar imaging protocols with insignificant variety of MRI scanning parameters according to MRI systems from different vendors. Only a sub-group of 18 patients could be investigated with whole body diffusion-weighted MRI (DWIBS). Two patients received only a decreased dose of 68Ga-PSMA-11 dose due to technical problems with the Ga-PSMA generator. In another patient, PET/CT scanning time was shortened by dysfunction of the scanner. Nevertheless, the results for Gallium-based PET/CT were very promising and deserve to be reported here. Based on these first experiences, further investigation is warranted.
As a contribution to the current discussions of a possible relationship between androgen deprivation therapy and PSMA expression in PET/CT scan [23,24], we would like not to miss reporting a single patient out of our study group who received androgen deprivation therapy before the PET/CT and MRI examinations and who presented with surprisingly high 68Ga-PSMA-11 tracer uptake. This might be interpreted as the result of PSMA-ligand uptake upregulation due to androgen deprivation therapy and further investigation in a dedicated study is warranted.
Conclusion
In conclusion, this relatively small sample study already confirms excellent diagnostic capacities of both, 68Ga-PSMA-11 PET/CT and mpMRI, in staging early recurrent prostate cancer at low PSA levels. Both methods are complementary, with mpMRI offering the better diagnostic accuracy in the detection of local recurrence and PSMA PET/CT being superior in the detection of distant and lymph node metastases. Taking into account the advantages regarding patient survival after early re-intervention as discussed in the literature and the high number of positive findings in our study with potential options for intervention, a combination of both techniques should be considered, when increasing PSA levels suggest early recurrence of prostate cancer. Although body diffusion MRI was performed in only a small sub-group of the study, but with very convincing results (in consistency with the available literature clearly superior to bone scintigraphy), it has to be taken into account, that diffusion might be the complimentary technique for the detection of bone metastases. Based on these very promising results, further studies are warranted to quantify the clinical benefit of this approach in a long term.
Acknowledgements
Nuclear Medicine Clinic colleagues: Jazeps Malnacs, Kristine Tiltina, Vitalijs Skrivelis, Andis Lacis. Pauls Stradins Clinical University Hospital, Department of Histopathology colleague: Inese Briede.
Disclosure of conflict of interest
None.
References
- 1.Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T, Zattoni F, Mottet N. EAU guidelines on prostate cancer. Part II: treatment of advanced, relapsing, and castration-resistant prostate cancer. Eur Urol. 2014;65:467–479. doi: 10.1016/j.eururo.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Rowe SP, Macura KJ, Mena E, Blackford AL, Nadal R, Antonarakis ES, Eisenberger M, Carducci M, Fan H, Dannals RF, Chen Y, Mease RC, Szabo Z, Pomper MG, Cho SY. PSMA-based (18)DCFPyL PET/CT Is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol. 2016;183:411–419. doi: 10.1007/s11307-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwenck J, Rempp H, Reischl G, Kruck S, Stenzl A, Nikolaou K, Pfannenberg C, la Fougère C. Comparison of (68)Ga-labelled PSMA-11 and (11)C-choline in the detection of prostate cancer metastases by PET/CT. Eur J Nucl Med Mol Imaging. 2017;44:92–101. doi: 10.1007/s00259-016-3490-6. [DOI] [PubMed] [Google Scholar]
- 4.Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, Bolton D, Lawrentschuk N. Sensitivity, specificity, and predictors of positive 68Ga prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 5.Futterer JJ, Surcel C, van den Bergh R, Borgmann H, Briganti A, Gandaglia G, Kretschmer A, Ost P, Sooriakumaran P, Tilki D, Valerio M, Ploussard G, De Visschere PJL, Tsaur I. Prostate cancer working party imaging modalities in synchronous oligometastatic prostate cancer. World J Urol. 2019;37:2573–2583. doi: 10.1007/s00345-018-2416-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hope TA, Afshar-Oromieh A, Eiber M, Emmett L, Fendler WP, Lawhn-Heath C, Rowe SP. Imaging prostate cancer with prostate-specific membrane antigen PET/CT and PET/MRI: current and future applications. AJR Am J Roentgenol. 2018;211:286–294. doi: 10.2214/AJR.18.19957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freitag MT, Radtke JP, Hadaschik BA, Kopp-Schneider A, Eder M, Kopka K, Haberkorn U, Roethke M, Schlemmer HP, Afshar-Oromieh A. Comparison of hybrid 68Ga-PSMA PET/MRI and 68Ga-PSMA PET/CT in the evaluation of lymph node and bone metastases of prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:70–83. doi: 10.1007/s00259-015-3206-3. [DOI] [PubMed] [Google Scholar]
- 8.Mertan FV, Greer MD, Borofsky S, Kabakus IM, Merino MJ, Wood BJ, Pinto PA, Choyke PL, Turkbey B. Multiparametric magnetic resonance imaging of recurrent prostate cancer. Top Magn Reson Imaging. 2016;25:139–147. doi: 10.1097/RMR.0000000000000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rauscher I, Maurer T, Fendler WP, Sommer WH, Schwaiger M, Eiber M. 68 Ga-PSMA ligand PET/CT in patients with prostate cancer: how we review and report. Cancer Imaging. 2016;16:14. doi: 10.1186/s40644-016-0072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nanabala R, Anees MK, Sasikumar A, Joy A, Pillai MR. Preparation of [68 Ga] PSMA-11 for PET-CT imaging using a manual synthesis module and organic matrix based 68 Ge/68 Ga generator. Nucl Med Biol. 2016;43:463–469. doi: 10.1016/j.nucmedbio.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 11.García Garzón JR, de Arcocha Torres M, Delgado-Bolton R, Ceci F, Alvarez Ruiz S, Orcajo Rincón J, Caresia Aróztegui AP, García Velloso MJ, García Vicente AM Oncology Task Force of Spanish Society of Nuclear Medicine and Molecular Imaging. Imaging 68Ga-PSMA PET/CT in prostate cancer. Rev Esp Med Nucl Imagen Mol. 2018;37:130–138. doi: 10.1016/j.remn.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 12.Vis R, Lavalaye J, van de Garde EM. GMP-compliant 68 Ga radiolabelling in a conventional small-scale radiopharmacy: a feasible approach for routine clinical use. EJNMMI Res. 2015;5:27. doi: 10.1186/s13550-015-0105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Battaglia A, De Meerleer G, Tosco L, Moris L, Van den Broeck T, Devos G, Everaerts W, Joniau S. Novel insights into the management of oligometastatic prostate cancer: a comprehensive review. Eur Urol Oncol. 2019;2:174–188. doi: 10.1016/j.euo.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 14.Fossati N, Suardi N, Gandaglia G, Bravi CA, Soligo M, Karnes RJ, Shariat S, Battaglia A, Everaerts W, Joniau S, Van Poppel H, Rajarubendra N, Gill IS, Larcher A, Mottrie A, Schmautz M, Heidenreich A, Kalz A, Osmonov D, Juenemann KP, Herlemann A, Gratzke C, Stief C, Montorsi F, Briganti A. Identifying the optimal candidate for salvage lymph node dissection for nodal recurrence of prostate cancer: results from a large, multi-institutional analysis. Eur Urol. 2019;75:176–183. doi: 10.1016/j.eururo.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alipour R, Azad A, Hofman MS. Guiding management of therapy in prostate cancer: time to switch from conventional imaging to PSMA PET? Ther Adv Med Oncol. 2019;11:175883591987682. doi: 10.1177/1758835919876828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perera M, Papa N, Christidis D, Wetherell D, Hofman MS, Murphy DG, Bolton D, Lawrentschuk N. Sensitivity, specificity, and predictors of positive 68Ga-prostate-specific membrane antigen positron emission tomography in advanced prostate cancer: a systematic review and meta-analysis. Eur Urol. 2016;70:926–937. doi: 10.1016/j.eururo.2016.06.021. [DOI] [PubMed] [Google Scholar]
- 17.Rauscher I, Maurer T, Beer AJ, Graner FP, Haller B, Weirich G, Doherty A, Gschwend JE, Schwaiger M, Eiber M. Value of 68Ga-PSMA HBED-CC PET for the assessment of lymph node metastases in prostate cancer patients with biochemical recurrence: comparison with histopathology after salvage lymphadenectomy. J Nucl Med. 2016;57:1713–1719. doi: 10.2967/jnumed.116.173492. [DOI] [PubMed] [Google Scholar]
- 18.Hijazi S, Meller B, Leitsmann C, Strauss A, Meller J, Ritter CO, Lotz J, Schildhaus HU, Trojan L, Sahlmann CO. Pelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga-PSMA-positron emission tomography/computerized tomography. Prostate. 2015;75:1934–1940. doi: 10.1002/pros.23091. [DOI] [PubMed] [Google Scholar]
- 19.Hövels AM, Heesakkers RA, Adang EM, Jager GJ, Strum S, Hoogeveen YL, Severens JL, Barentsz JO. The diagnostic accuracy of CT and MRI in the staging of pelvic lymph nodes in patients with prostate cancer: a meta-analysis. Clin Radiol. 2008;63:387–395. doi: 10.1016/j.crad.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Maurer T, Gschwend JE, Rauscher I, Souvatzoglou M, Haller B, Weirich G, Wester HJ, Heck M, Kübler H, Beer AJ, Schwaiger M, Eiber M. Diagnostic efficacy of 68 Gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J Urol. 2016;195:1436–1443. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 21.Shen G, Deng H, Hu S, Jia Z. Comparison of choline-PET/CT, MRI, SPECT, and bone scintigraphy in the diagnosis of bone metastases in patients with prostate cancer: a meta-analysis. Skeletal Radiol. 2014;43:1503–1513. doi: 10.1007/s00256-014-1903-9. [DOI] [PubMed] [Google Scholar]
- 22.Pyka T, Okamoto S, Dahlbender M, Tauber R, Retz M, Heck M, Tamaki N, Schwaiger M, Maurer T, Eiber M. Comparison of bone scintigraphy and 68 Ga-PSMA PET for skeletal staging in prostate cancer. Eur J Nucl Med Mol Imaging. 2016;43:2114–2121. doi: 10.1007/s00259-016-3435-0. [DOI] [PubMed] [Google Scholar]
- 23.Bakht MK, Oh SW, Youn H, Cheon GJ, Kwak C, Kang KW. Influence of androgen deprivation therapy on the uptake of PSMA-targeted agents: emerging opportunities and challenges. Nucl Med Mol Imaging. 2017;51:202–211. doi: 10.1007/s13139-016-0439-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.sHope TA, Truillet C, Ehman EC, Afshar-Oromieh A, Aggarwal R, Ryan CJ, Carroll PR, Small EJ, Evans MJ. 68Ga-PSMA-11 PET imaging of response to androgen receptor inhibition: first human experience. J Nucl Med. 2017;58:81–84. doi: 10.2967/jnumed.116.181800. [DOI] [PMC free article] [PubMed] [Google Scholar]


