Abstract
The goal of this study was to assess pulmonary artery calcification in healthy controls and subjects with suspicion of stable angina pectoris through the usage of quantitative 18F-sodium fluoride positron emission tomography/computed tomography (NaF-PET/CT). We hypothesized that these ‘at-risk subjects’ would demonstrate increase pulmonary artery NaF uptake compared to healthy controls. Retrospectively, 15 healthy controls were compared to 15 at-risk subjects, all of whom underwent full-body NaF-PET/CT scans. The healthy controls and at-risk patients were all randomly sampled from larger datasets. The two sampled groups were male-dominated and similar in age. The global mean standard uptake value (SUVmean), the max standard uptake value (SUVmax), and the mean target-to-background ratio (TBRmean) were acquired through mapping of regions of interest (ROI’s) around the pulmonary artery of the subjects. A two-tailed Mann-Whitney U test was used to determine the significance of difference between the two groups. For global SUVmean (0.79 compared to 0.58), global TBRmean (1.15 compared to 0.93), and global SUVmax (1.78 compared to 1.60), the NaF uptake was significantly higher in the at-risk patients compared to the controls (all P<0.05). NaF-PET/CT is a suitable imaging modality for quantification of molecular calcification in the pulmonary artery. Additionally, the connection between atherosclerosis and the risk factor of angina pectoris is further reinforced. We believe that future studies are needed to validate our proof-of-concept, and better confirm the clinical future of NaF-PET/CT as a tracer of atherosclerotic plaques.
Keywords: PET/CT, atherosclerosis, pulmonary artery, 18F-NaF, calcification
Introduction
Atherosclerosis is a chronic cardiovascular disease characterized by the buildup of lipid-derived plaque on the inner lumen of arteries. The subsequent calcification has been implicated as a major risk factor for cardiovascular disease (CVD) such as stroke and vascular dementia [1]. Indeed, this calcification can cause plaque rupture in the fibrous cap, upon which blood clots impairing blood flow can form. This connection between calcification and CVD has been described extensively for the aorta, coronary arteries, and carotid arteries [2-4]. However, only a handful of studies have examined atherosclerotic plaques within the pulmonary artery. Yet, pulmonary arterial calcification and plaque formation are a frequent finding on the autopsy of individuals with cardiopulmonary mechanisms of death, such as pulmonary embolism [5,6]. Therefore, early detection of pulmonary arterial disease may be favorable for patients suffering from cardiovascular risk factors including high body mass index, hypertension, diabetes, and angina pectoris.
Atherosclerotic structural changes may be visualized using a number of imaging modalities. Computed tomography (CT) remains the standard of care, since the scans created accurately, can depict macro-calcifications that effectively precede plaque formation [7]. In addition, ultrasonography techniques such as echocardiograms may also be used to contract two- and three-dimensional representations of affected vasculature and magnetic resonance imaging can utilize magnetic fields to analyze the structure of arterial plaques [8,9]. Positron emission tomography/computed tomography (PET/CT) may be the superior imaging technique, as it visualizes the atherosclerotic pathway on the molecular scale. 18F-fluorodeoxyglucose (FDG) is a common radiotracer that has been used extensively to detect inflammatory processes, including arteritis [10]. However, recent evidence suggest that 18F-sodium fluoride (NaF)-PET/CT may be the tool of choice to detect early intravascular calcifications, as this is one of the main defining features of the atherosclerotic process [11,12]. NaF-PET/CT has currently not yet been utilized clinically to detect atherosclerosis within the pulmonary arteries. This in mind, the objectives of the present study are two fold; 1) to prove that NaF-PET/CT may be used to detect and quantify the extent of pulmonary artery atherosclerosis, and 2) to demonstrate that patients with known cardiovascular risk factors exhibit a greater extent of atherosclerotic calcification within the pulmonary artery.
Methods
Patient population
The Cardiovascular Molecular Calcification Assessed by NaF-PET/CT (CAMONA) protocol (ClinicalTrials.gov (NCT01724749) provided both the healthy controls and at-risk subjects used in this study. The parameters of the CAMONA protocol have been previously presented in the existing literature by Blomberg et al. [13,14]. The healthy controls were defined as not having any cardiovascular risk factors, existing diseases, or drug-related issues [15]. Patients with chest pain (the defining symptom of stable angina pectoris) were recruited from those referred for a coronary CT-angiography. Only patients with a 10-year risk for fatal cardiovascular disease equal to or above 1%, as calculated by the body mass index (kgm-2) based Systematic Coronary Risk Evaluation (SCORE) tool, were eligible for inclusion and categorized as ‘at-risk subjects’. The CAMONA study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the Danish National Committee on Health Research Ethics. All participants provided written informed consent.
Fifteen healthy controls were randomly sampled out of 87 possible subjects, while fifteen at-risk subjects were randomly sampled out of 52 possible subjects. For the healthy controls, the average age was 45 years old (SD ± 8) and for the at-risk subjects, the average age was 56 years old (SD ± 11). For the healthy controls, all subjects were male while for the at-risk subjects, 9 subjects were male while 6 were female.
Imaging acquisition
NaF-PET/CT scans were acquired in accordance with the European Association of Nuclear Medicine (EANM) guidelines, which include quality control, calibration and harmonization of the scanner and SUV calculations [16,17]. The PET/CT scanners underwent regular quality and calibration control that met all EANM Research Ltd. (EARL) standards but were not EARL accredited. For both the healthy controls and at-risk subjects, whole-body NaF-PET/CT scans were created by hybrid PET/CT scanners with comparable spatial resolution (GE Discovery RX, STE, and 690/710 imaging systems (General Electrical Healthcare, Chicago, Milwaukee, WI, USA)). At the discretion of the department’s booking system the subjects were allocated to a PET/CT system. The injected dose of NaF was approximately 2.2 MBq/kg per subject and image acquisition was performed 90 minutes after injection with a emission acquisition duration per bed position of 2.5 min. In order to reduce radiation delivered to the subjects, only low-dose unenhanced CT scans (140 kV, 30-110 mA, noise index 25, 0.8 second/rotation, slice thickness 3.75) were carried out in the CAMONA study. The low-dose CT scan was used for attenuation correction and anatomical localization.
Image analysis
OsiriX MD software (version 7.04; Pixmeo SARL, Bernex, Switzerland) served as the DICOM viewer of choice. Uptake of the tracer was matched with anatomy through the fusion of PET and CT scans. Manual regions of interest (ROI’s) were drawn around the borders of the main, right, and left pulmonary artery on axial PET/CT images in order to quantify the NaF uptake [18]. We calculated the global SUVmean for both groups by summing and averaging standardized uptake values (SUVs) based on the ROI’s drawn on the axial PET/CT slices, while also accounting for the area and thickness of the slices. We calculated the global TBRmean by dividing the SUVmean by the blood pool activity of the superior vena cava (through drawing ROIs around the lumen) in both subject groups [19,20]. Lastly, we calculated the global SUVmax for both groups by averaging the maximum SUVs for each subject’s ROI and adjusting this value for the area and thickness of each axial slice.
Statistical analysis
The two-tailed Mann-Whitney U test was used to compare the differences in terms of global TBRmean, global SUVmean, and global SUVmax between the healthy controls and at-risk subjects through STATA software (Stata/IC Version 10.1, StataCorp, College Station, TX). A p-value of less than 0.05 was defined as significant.
Results
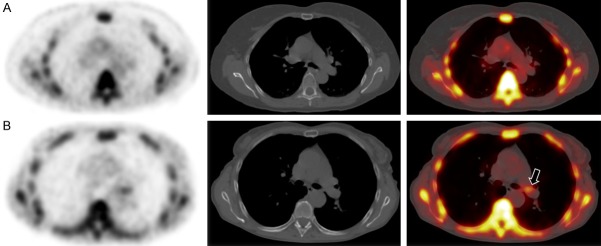
Of the 30 subjects included, 15 served as healthy controls while the remaining 15 exhibited symptoms of angina pectoris and are therefore categorized as at-risk for CVD. Examples of the pulmonary artery of subjects from control and at-risk the two different groups are in Figure 1, denoting the difference in visual intensity of certain nodes of the pulmonary artery between the two subjects. These focal intensities correspond with NaF uptake.
Figure 1.
Axial NaF PET, CT, and fused NaF PET/CT images of the pulmonary artery of a (A) 62-year-old female with no cardiovascular risk factors and a (B) 63-year-old female with symptoms of chest pain (angina pectoris). Note how patient (A) demonstrates no focal uptake of NaF in the pulmonary artery, whereas patient (B) does, as shown by the black arrow.
Table 1 shows the difference in global SUVmean, TBRmean, and SUVmax of the pulmonary artery between both of the groups. The global SUVmean (0.79 compared to 0.58), the global TBRmean (1.15 compared to 0.93), and the global SUVmax (1.78 compared to 1.60) were significantly greater in the at-risk subjects compared to the healthy controls (all P<0.05). These results are depicted graphically in Figure 2. Taken together, these data demonstrate that subjects at risk for CVD have greater uptake of NaF within the pulmonary arteries, potentially demonstrating the presence of calcifications within the pulmonary vasculature.
Table 1.
Global pulmonary artery SUVmean, TBRmean, and SUVmax in healthy controls and at-risk subjects (± SEM)
| Global SUV*mean | Global TBR#mean | Global SUV*max | |
|---|---|---|---|
| Controls | 0.58 ± 0.14 | 0.93 ± 0.12 | 1.60 ± 0.15 |
| At-Risk | 0.79 ± 0.24 | 1.15 ± 0.38 | 1.78 ± 0.10 |
| p-value | 0.00906 | 0.00104 | 0.01778 |
SUV = standardized uptake value;
TBR = target-to-background ratio.
Figure 2.
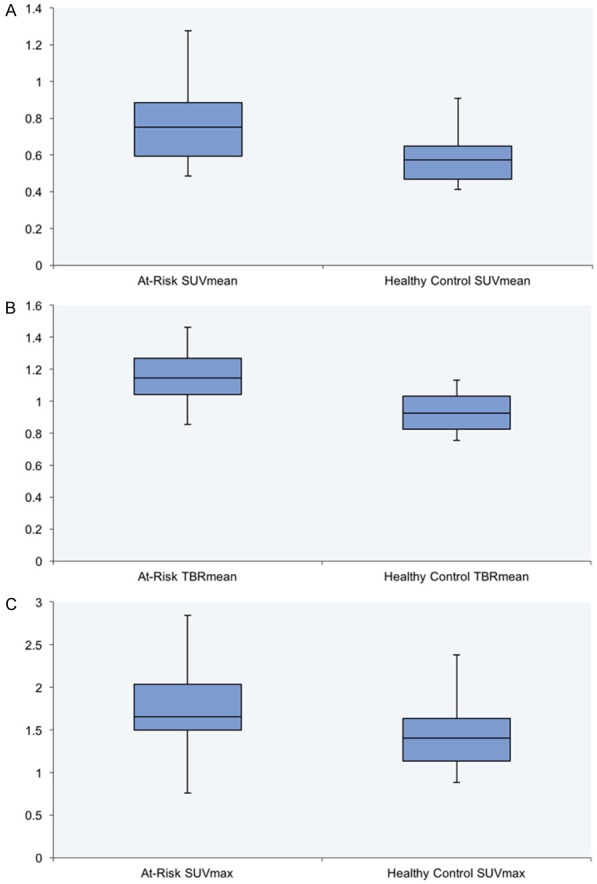
Box-and-whisker plot (± 1.5 IQR) showing significant differences in (A) global SUVmean, (B) global TBRmean, and (C) global SUVmax between at-risk subjects and healthy controls (all P<0.05).
Discussion
In the current study, NaF uptake in the pulmonary artery (measured by PET/CT) was observed to be significantly greater in the at-risk patients when compared to healthy controls (Figure 2). To our knowledge, this is the first study to investigate the role of NaF-PET/CT in assessing atherosclerotic calcification of the pulmonary artery.
Atherosclerotic plaque has traditionally been detected through clinical and structural abnormalities [21]. FDG-PET/CT has been used to identify atherosclerosis-induced inflammation, and this inflammation is an increasingly interesting target for future therapies [15,22,23]. Nevertheless, FDG-PET/CT has variable efficacy in the detection of atherosclerosis. Emamzadehfard et al. demonstrated that FDG uptake within the common carotid arteries is not significantly associated with cardiovascular risk factors [24]. Likewise, Høilund-Carlsen et al. have reviewed the existing literature and come to the conclusion that only calcification, and not arterial inflammation, are correlated consistently with 10-year Framingham risk scores- this could possibly explain why aortic and coronary arterial inflammation as quantified by FDG uptake are not necessarily indicative of cardiovascular risk [23].
There are several possible explanations for these negative findings; the most probable is the suboptimal spatial resolution of PET instruments, as the drawn ROI can either miss the most FDG avid area or include contribution from adjacent FDG avid tissue, the non-specificity of FDG for detecting inflammation in the plaques, and the scan performed at the right time point/window for the inflammatory phase that can be much shorter than the post-inflammatory phase [25,26]. Specifically, for the pulmonary artery, this line of thinking fits. The physiological uptake of FDG in the myocardium challenges the use of FDG-PET/CT to differentiate and identify inflammatory plaques in this artery. Even after a strict diet without carbohydrates, prolonged fasting and a heparin injection, the suppression of FDG uptake in the myocardium can fail [27-29].
NaF-PET/CT, meanwhile, offers an exciting novel imaging modality for the detection of atherosclerotic plaques on the molecular level by being able to detect the micro-calcification process early on in disease pathogenesis. CT has extensively been used to detect structural macro-calcification in the coronary and other major arteries over the last decade. However, structural calcification as detected on CT is of limited clinical value because it reveals the irreversible, latter stages of the disease. When macro-calcification is noted on CT, it is very likely that the disease process has been ongoing for decades and has therefore already led to permanent damage. Arani et al. demonstrated that NaF, but not FDG, uptake within the abdominal aorta is correlated with atherosclerotic risk factors and age [30]. Similarly, a previous study by Blomberg et al. demonstrated that aortic calcification detected via NaF-PET/CT is correlated with cardiovascular disease [11]. Finally, recent work by Castro et al. implicate left common carotid atherosclerotic calcifications with increased cardiovascular risk factors, including age, Framingham risk score, and various hematologic biomarkers [31-33]. These studies point toward NaF as a superior molecular biomarker in the early detection of atherosclerotic plaques. The specific advantage of NaF compared to FDG is the low background activity and high specificity with regards to atherosclerosis [34].
Pulmonary artery atherosclerosis has been associated with various cardiovascular risk factors, including cigarette smoking and lipid- or cholesterol-heavy diet [35]. Furthermore, pulmonary artery atherosclerosis is a rare comorbidity of certain diseases, such as pulmonary emphysema and right ventricular hypertrophy [5]. Interestingly, pulmonary artery atherosclerosis may still be underdiagnosed, as it is most commonly discovered only upon autopsy [5,6,36].
Typically, angina pectoris is diagnosed through complaints of chest pain after an instance of decreased vascular blood flow in the coronary arteries and aorta [37]. Pathologically, angina pectoris and atherosclerosis are inherent comorbidities, given that atherosclerotic plaques are what block arteries, leading to decreased vascular blood flow and thus angina pectoris. Zoll et al. proved this, by showing that all 848 patients with angina pectoris in their study ended up having coronary heart disease in the form of atherosclerotic artery blockages [39]. Since angina pectoris is an intrinsic indicator for the presence of plaques in the coronary arteries, we wanted to test whether or not this association would also extend to the pulmonary artery. Unsurprisingly, given the connection between the cardiovascular risk factor of angina pectoris and pulmonary artery atherosclerosis, our results showed a positive correlation between the two. Since we proved that this association did in fact extend to the pulmonary artery, future studies should be run testing the relationship between angina pectoris and generalized atherosclerosis in different arteries, through the usage of NaF-PET/CT as a measuring block.
However, our results should be taken within the confines of our study. First, we performed a retrospective analysis in a relatively small sample of predominantly male subjects. Secondly, the use of different PET/CT scanners are another limitation. The PET/CT scanners and the imaging protocol applied were standardized to international practice guidelines and the PET/CT systems were calibrated and harmonized to a phantom, which reduces the variation of quantitation parameters [40]. Furthermore, it remains challenging to cross-calibrate to overcome differences in PET/CT scanners in both hardware and software, even as the PET/CT systems were from the same vendor. The present study used only PET/CT scanners from GE Healthcare. Both the SNMMI and EANM have addressed these challenges and tries to resolve the differences in PET/CT systems and quantifications to improved inter-scan agreement studies. The PET/CT scanners used in this study were not EARL accredited but underwent regular quality and calibration control that fully met all EARL requirements and standard. To account for machine differences in our calculations, we used TBR by quantifying blood-pool activity, through looking at the lumen of the superior vena cava. This came with its own set of problems, given that blood-pool activity can be affected by many factors including differential uptake in circulating blood, blood glucose levels, scanner differences. Measurement of blood pool activity could also occasionally be challenging, as the superior vena cava was found to be small/compressed in certain subjects, in correlation to hydration levels [41]. Lastly, our study did not include histological data; in future studies, we would like to confirm our findings by the presence or absence of calcification under the microscope.
Conclusion
NaF uptake in the pulmonary artery was found to be significantly greater in patients with suspicion of angina pectoris compared to healthy controls. Future prospective studies with larger groups of subjects are needed to affirm our results, which can be of clinical use in early detection of pulmonary artery atherosclerosis.
Acknowledgements
The Jørgen and Gisela Thrane’s Philanthropic Research Foundation, Broager, Denmark, financially supported the CAMONA study.
Disclosure of conflict of interest
None.
References
- 1.Borja AJ, Hancin EC, Zhang V, Revheim ME, Alavi A. Potential of PET/CT in assessing dementias with emphasis on cerebrovascular disorders. Eur J Nucl Med Mol Imaging. 2020 doi: 10.1007/s00259-020-04697-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 2.Arad Y, Goodman KJ, Roth M, Newstein D, Guerci AD. Coronary calcification, coronary disease risk factors, c-reactive protein, and atherosclerotic cardiovascular disease events: The St. francis heart study. J Am Coll Cardiol. 2005;46:158–65. doi: 10.1016/j.jacc.2005.02.088. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, Zhang Y, Yu CM, Ji QW, Cai M, Zhao YX, Zhou YJ. Current understanding of coronary artery calcification. J Geriatr Cardiol. 2015;12:668–75. doi: 10.11909/j.issn.1671-5411.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol. 2004;15:2959–64. doi: 10.1097/01.ASN.0000145894.57533.C4. [DOI] [PubMed] [Google Scholar]
- 5.Cicconi M, Bonsignore A, Orcioni GF, Ventura F. Primary pulmonary arteries atherosclerosis: discovering an unusual cause of death in forensic practice. Rom J Leg Med. 2012;20:177–80. [Google Scholar]
- 6.Moore GW, Smith RR, Hutchins GM. Pulmonary artery atherosclerosis: correlation with systemic atherosclerosis and hypertensive pulmonary vascular disease. Arch Pathol Lab Med. 1982;106:378–80. [PubMed] [Google Scholar]
- 7.Wang Y, Osborne MT, Tung B, Li M, Li Y. Imaging cardiovascular calcification. J Am Heart Assoc Cardiovasc Cerebrovasc Dis. 2018:7. doi: 10.1161/JAHA.118.008564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Steinl DC, Kaufmann BA. Ultrasound imaging for risk assessment in atherosclerosis. Int J Mol Sci. 2015;16:9749–69. doi: 10.3390/ijms16059749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salonen JT, Salonen R. Ultrasound B-mode imaging in observational studies of atherosclerotic progression. Circulation. 1993;87(Suppl):II56–65. [PubMed] [Google Scholar]
- 10.Borja AJ, Hancin EC, Dreyfuss AD, Zhang V, Mathew T, Rojulpote C, Werner TJ, Patil S, Gonuguntla G, Lin A, Feigenberg SJ, Swisher-McClure S, Alavi A, Revheim ME. 18F-FDG-PET/CT in the quantification of photon radiation therapy-induced vasculitis. Am J Nucl Med Mol Imaging. 2020;10:66–73. [PMC free article] [PubMed] [Google Scholar]
- 11.Rojulpote C, Borja AJ, Zhang V, Aly M, Koa B, Seraj SM, Raynor WY, Kothekar E, Kaghazchi F, Werner TJ, Gerke O, Høilund-Carlsen PF, Alavi A. Role of 18F-NaF-PET in assessing aortic valve calcification with age. Am J Nucl Med Mol Imaging. 2020;10:47–56. [PMC free article] [PubMed] [Google Scholar]
- 12.Borja A, Werner T, Alavi A. Role of PET/CT in vascular dementia. J Nucl Med. 2019;60(Suppl 1):1153–1153. [Google Scholar]
- 13.Blomberg BA, Thomassen A, Takx RA, Hildebrandt MG, Simonsen JA, Buch-Olsen KM, Diederichsen AC, Mickley H, Alavi A, Høilund-Carlsen PF. Delayed (1)(8)F-fluorodeoxyglucose PET/CT imaging improves quantitation of atherosclerotic plaque inflammation: results from the CAMONA study. J Nucl Cardiol. 2014;21:588–97. doi: 10.1007/s12350-014-9884-6. [DOI] [PubMed] [Google Scholar]
- 14.Blomberg BA, Thomassen A, de Jong PA, Lam MGE, Diederichsen ACP, Olsen MH, Mickley H, Mali WPTM, Alavi A, Høilund-Carlsen PF. Coronary fluorine-18-sodium fluoride uptake is increased in healthy adults with an unfavorable cardiovascular risk profile: results from the CAMONA study. Nucl Med Commun. 2017;38:1007–14. doi: 10.1097/MNM.0000000000000734. [DOI] [PubMed] [Google Scholar]
- 15.Blomberg BA, Thomassen A, Takx RA, Vilstrup MH, Hess S, Nielsen AL, Diederichsen AC, Mickley H, Alavi A, Høilund-Carlsen PF. Delayed sodium 18F-fluoride PET/CT imaging does not improve quantification of vascular calcification metabolism: results from the CAMONA study. J Nucl Cardiol. 2014;21:293–304. doi: 10.1007/s12350-013-9829-5. [DOI] [PubMed] [Google Scholar]
- 16.Beheshti M, Mottaghy FM, Paycha F, Behrendt FFF, Van den Wyngaert T, Fogelman I, Strobel K, Celli M, Fanti S, Giammarile F, Krause B, Langsteger W. (18)F-NaF PET/CT: EANM procedure guidelines for bone imaging. Eur J Nucl Med Mol Imaging. 2015;42:1767–77. doi: 10.1007/s00259-015-3138-y. [DOI] [PubMed] [Google Scholar]
- 17.Boellaard R, Delgado-Bolton R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, Verzijlbergen FJ, Barrington SF, Pike LC, Weber WA, Stroobants S, Delbeke D, Donohoe KJ, Holbrook S, Graham MM, Testanera G, Hoekstra OS, Zijlstra J, Visser E, Hoekstra CJ, Pruim J, Willemsen A, Arends B, Kotzerke J, Bockisch A, Beyer T, Chiti A, Krause BJ. FDG PET/CT: EANM procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Demir Y, Houshmand S, Salavati A, Werner T, Alavi A. Assessment of pulmonary artery FDG uptake in pulmonary obstruction: a pilot study. J Nucl Med. 2014;55(Suppl 1):2012–2012. [Google Scholar]
- 19.Lensen KDF, van Sijl AM, Voskuyl AE, van der Laken CJ, Heymans MW, Comans EFI, Nurmohamed MT, Smulders YM, Boellaard R, Garg P. Variability in quantitative analysis of atherosclerotic plaque inflammation using 18F-FDG PET/CT. PLoS One. 2017;12:e0181847. doi: 10.1371/journal.pone.0181847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bucerius J, Hyafil F, Verberne HJ, Slart RHJA, Lindner O, Sciagra R, Agostini D, Ubleis C, Gimelli A, Hacker M. Position paper of the Cardiovascular Committee of the European Association of Nuclear Medicine (EANM) on PET imaging of atherosclerosis. Eur J Nucl Med Mol Imaging. 2016;43:780–92. doi: 10.1007/s00259-015-3259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD, Ohayon J, Pettigrew R, Sabatine MS, Tearney GJ, Waxman S, Domanski MJ, Srinivas PR, Narula J. Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging. 2012;5:941–55. doi: 10.1016/j.jcmg.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kottoor SJ, Arora RR. The utility of antiinflammatory agents in cardiovascular disease: a novel perspective on the treatment of atherosclerosis. J Cardiovasc Pharmacol Ther. 2018;23:483–93. doi: 10.1177/1074248418778548. [DOI] [PubMed] [Google Scholar]
- 23.Høilund-Carlsen PF, Moghbel MC, Gerke O, Alavi A. Evolving role of PET in detecting and characterizing atherosclerosis. PET Clin. 2019;14:197–209. doi: 10.1016/j.cpet.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Emamzadehfard S, Castro S, Werner T, Høilund-Carlsen PF, Alavi A. Does FDG PET/CT precisely detect carotid artery inflammation? J Nucl Med. 2018;59(Suppl 1):1550–1550. [Google Scholar]
- 25.Meirelles GS, Gonen M, Strauss HW. 18F-FDG uptake and calcifications in the thoracic aorta on positron emission tomography/computed tomography examinations: frequency and stability on serial scans. J Thorac Imaging. 2011;26:54–62. doi: 10.1097/RTI.0b013e3181d9c9f9. [DOI] [PubMed] [Google Scholar]
- 26.Arani LS, Gharavi MH, Zadeh MZ, Raynor WY, Seraj SM, Constantinescu CM, Gerke O, Werner TJ, Høilund-Carlsen PF, Alavi A. Association between age, uptake of 18F-fluorodeoxyglucose and of 18F-sodium fluoride, as cardiovascular risk factors in the abdominal aorta. Hell J Nucl Med. 2019;22:14–9. doi: 10.1967/s002449910954. [DOI] [PubMed] [Google Scholar]
- 27.Cussó L, Vaquero JJ, Bacharach S, Desco M. Comparison of methods to reduce myocardial 18F-FDG uptake in mice: calcium channel blockers versus high-fat diets. PLoS One. 2014;9:e107999. doi: 10.1371/journal.pone.0107999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morooka M, Moroi M, Uno K, Ito K, Wu J, Nakagawa T, Kubota K, Minamimoto R, Miyata Y, Okasaki M, Okazaki O, Yamada Y, Yamaguchi T, Hiroe M. Long fasting is effective in inhibiting physiological myocardial 18F-FDG uptake and for evaluating active lesions of cardiac sarcoidosis. EJNMMI Res. 2014;4:1. doi: 10.1186/2191-219X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang JY, Choe W. Factors affecting myocardial FDG uptake by F18-FDG PET/CT for cancer screening in healthy subjects. J Nucl Med. 2015;56(Suppl 3):293–293. [Google Scholar]
- 30.Arani L, Gharavi M, Saboury B, Al-Zaghal A, Jahangiri P, Khosravi M, Pournazari K, Werner T, Høilund-Carlsen PF, Alavi A. Assessment of the role of age and cardiovascular risk factors on 18F-Fluorodeoxyglucose (18F-FDG) and 18F-Sodium Fluoride (NaF) uptake in abdominal aortic artery. J Nucl Med. 2018;59(Suppl 1):1539–1539. [Google Scholar]
- 31.Castro S, Muser D, Acosta-Montenegro O, Emamzadehfard S, Shamchi SP, Desjardins B, Werner T, Thomassen A, Høilund-Carlsen PF, Alavi A. Common carotid artery molecular calcification assessed by 18F-NaF PET/CT is associated with increased cardiovascular disease risk: results from the CAMONA study. J Nucl Med. 2017;58(Suppl 1):34–34. [Google Scholar]
- 32.Castro S, Emamzadehfard S, Muser D, Acosta-Montenegro O, Werner T, Shamchi SP, Desjardins B, Thomassen A, Høilund-Carlsen PF, Alavi A. Aged related differences and cardiovascular molecular calcification a 18-NaF PET/CT quantification of vascular calcification in the common carotid artery study. J Nucl Med. 2017;58(Suppl 1):445–445. [Google Scholar]
- 33.Castro S, Acosta-Montenegro O, Muser D, Emamzadehfard S, Shamchi SP, Werner T, Desjardins B, Thomassen A, Høilund-Carlsen PF, Alavi A. Association between common carotid artery molecular calcification assessed by 18F-NaF PET/CT and biomarkers of vulnerable atheromatous plaques: results from the CAMONA study. J Nucl Med. 2017;58(Suppl 1):443–443. [Google Scholar]
- 34.McKenney-Drake ML, Moghbel MC, Paydary K, Alloosh M, Houshmand S, Moe S, Salavati A, Sturek JM, Territo PR, Weaver C, Werner TJ, Høilund-Carlsen PF, Sturek MAlavi A. 18 F-NaF and 18 F-FDG as molecular probes in the evaluation of atherosclerosis. Eur J Nucl Med Mol Imaging. 2018;45:2190–200. doi: 10.1007/s00259-018-4078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu BQ, Sun YP, Sievers RE, Isenberg WM, Glantz SA, Parmley WW. Passive smoking increases experimental atherosclerosis in cholesterol-fed rabbits. J Am Coll Cardiol. 1993;21:225–32. doi: 10.1016/0735-1097(93)90741-i. [DOI] [PubMed] [Google Scholar]
- 36.Malik AR. Association of increased lipid peroxide levels in the aorta in comparison to the pulmonary artery with the presence of coronary artery disease. Biomed Rep. 2016;4:479–84. doi: 10.3892/br.2016.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kannel WB, Evans JC, Piper S, Murabito JM. Angina pectoris is a stronger indicator of diffuse vascular atherosclerosis than intermittent claudication: framingham study. J Clin Epidemiol. 2008;61:951–7. doi: 10.1016/j.jclinepi.2007.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guthrie RB, Vlodaver Z, Nicoloff DM, Edwards JE. Pathology of stable and unstable angina pectoris. Circulation. 1975;51:1059–63. doi: 10.1161/01.cir.51.6.1059. [DOI] [PubMed] [Google Scholar]
- 39.Zoll PM, Wessler S, Blumgart HL. Angina pectoris. Am J Med. 1951;11:331–57. doi: 10.1016/0002-9343(51)90170-2. [DOI] [PubMed] [Google Scholar]
- 40.Dorbala S, Di Carli MF, Delbeke D, Abbara S, DePuey EG, Dilsizian V, Forrester J, Janowitz W, Kaufmann PA, Mahmarian J, Moore SC, Stabin MG, Shreve P. SNMMI/ASNC/SCCT guideline for cardiac SPECT/CT and PET/CT 1.0. J Nucl Med. 2013;54:1485–507. doi: 10.2967/jnumed.112.105155. [DOI] [PubMed] [Google Scholar]
- 41.Kapur S, Paik E, Rezaei A, Vu DN. Where there is blood, there is a way: unusual collateral vessels in superior and inferior vena cava obstruction. Radiographics. 2010;30:67–78. doi: 10.1148/rg.301095724. [DOI] [PubMed] [Google Scholar]