Abstract
Learning and memory deficits characterize the diagnosis of amnestic mild cognitive impairment (aMCI), which is widely viewed as a clinical precursor to Alzheimer’s type dementia. There is a growing interest in non-pharmacologic interventions, such as mnemonic strategies, for improving learning and memory in patients with aMCI as well as for maintaining functioning in healthy older adults. Using an ecologically relevant object-location association paradigm, we conducted a randomized, controlled, single-blind study in which healthy older adults and patients with aMCI were randomized to either mnemonic strategy training or a control group that was matched for stimulus exposure. We previously reported that mnemonic strategy training resulted in significantly greater learning and memory improvements compared to the matched exposure condition, in both aMCI patients and healthy controls. The current study examined changes in neocortical activation during encoding in a subset of participants who underwent functional magnetic resonance imaging (fMRI) scanning both before and after training. To minimize potential confounds in between-group comparisons, we employed non-linear cortex based alignment and included only correctly encoded stimuli in our analyses. When re-encoding stimuli learned during training (i.e., trained stimuli), we found a general enhancement of activation in right prefrontal and parietal regions, possibly reflecting practice-related improvement in coordinate spatial processing in all but the aMCI exposure group. Left hemisphere activation was typically only evident in the mnemonic strategy trained participants, regardless of diagnostic status, with the ventrolateral prefrontal cortex appearing especially important for strategy use. While encoding relatively novel stimuli, both mnemonic strategy groups (aMCI patients and healthy controls) demonstrated increased activation in a subset of regions showing change for the trained stimuli, indicating a mnemonic strategy-induced change in the processing of new information. These findings could not be explained by repeated exposure since there was little to no activation overlap in the respective exposure control groups. The current results reinforce the potential benefits of cognitive interventions in these growing populations and indicate that neuroplastic change in key rostral and lateral prefrontal regions mediate this behavioral change.
Keywords: cognitive training, cognitive rehabilitation, Alzheimer’s disease, aging, MCI, dementia, fMRI
The worldwide prevalence of Alzheimer’s disease (AD) and other dementias is expected to increase dramatically in the coming decades (Ferri et al., 2005), thereby threatening to overwhelm healthcare resources. This realization motivated efforts to identify those at risk of developing dementia and led to creating the diagnostic category of mild cognitive impairment (MCI) (Albert et al., 2011; Petersen, 2004; Petersen et al., 1999). Learning and memory deficits typify the cognitive profile of those with amnestic MCI (aMCI) and are generally attributed to medial temporal lobe atrophy and dysfunction (Dickerson & Sperling, 2008; Jack et al., 1997). However, the prefrontal cortex is known to play an important role during the encoding of new memories in healthy individuals (Baddeley, 2003; Spaniol et al., 2009) and the contributions of this region appear to increase during “normal” aging (Cabeza, 2002; Cramer et al., 2011; Dennis et al., 2008; Reuter-Lorenz & Lustig, 2005; Voss et al., 2010). A growing body of evidence suggests that patients with aMCI and dementia of the Alzheimer’s type (DAT) demonstrate additional dysfunction within the prefrontal cortex and associated parietal cortical regions during encoding (Hampstead et al., 2011b; Hampstead et al., 2016; Machulda et al., 2009; Mandiza et al., 2009; Schwindt & Black, 2009). Thus, clinically significant memory deficits may emerge as interactions between the prefrontal-parietal network and hippocampal memory system break down, which is consistent with findings of disrupted connectivity between these regions in patients with aMCI and DAT (Allen et al., 2007; Wang et al., 2011). As a result, interventions that reengage the frontoparietal cortices may help maximize residual hippocampal functioning, thereby improving memory.
Cognitively oriented treatments, including the use of mnemonic strategies, have long been applied to “traditional” rehabilitation populations like those with traumatic brain injury and stroke (Cicerone et al., 2005; Cicerone et al., 2011). Evidence is accumulating that such techniques can also be effective in patients with MCI (Jean, Bergeron, Thivierge, & Simard, 2010; Li et al., 2011; Simon, Yokomizu, & Bottino, 2012; Stott & Spector, 2011). The current report focuses specifically on mnemonic strategy training (MST), which includes techniques like semantic organization, semantic elaboration, and mental imagery. Healthy young adults have shown MST-related increases in activation within ventrolateral and dorsolateral prefrontal cortex and parietal regions (intraparietal sulcus, inferior parietal lobule) (Kondo et al., 2005; Leshikar, Duarte, Hertzog, 2012; Miotto et al., 2006) as well as the hippocampus (Nyberg et al., 2003). Prior work has shown that MST improves learning and memory in cognitively intact older adults (Verhaeghen, Marcoen, & Goossens, 1992) and patients with aMCI (Belleville et al., 2001; Belleville et al., 2006; Hampstead, Sathian, Moore, Nalisnick, Stringer, 2008; Kinsella et al., 2009). A few prior studies in those with aMCI have used functional magnetic resonance imaging (fMRI) and revealed that these cognitive changes are accompanied by increased activation in the above-noted lateral frontoparietal regions (e.g., inferior frontal gyrus/sulcus, middle frontal gyrus, angular gyrus, intraparietal sulcus; Hampstead et al., 2011a; Belleville et al., 2011; Balardan et al., 2016).
Although these results are encouraging, several limitations persist in this area of research. First, there are a limited number of studies in “normal” aging and across the dementia-spectrum, so additional work is needed. Second, most studies have used word lists or object/item memory paradigms that may have limited ecological relevance. In contrast, our approach has been to develop face-name (Hampstead et al., 2011a) and object-location associative memory paradigms (Hampstead et al., 2011b) that may be analogous to tasks patients find difficult in everyday life. Associative memory is especially appropriate for study, since it is dependent on medial temporal lobe structures, particularly the hippocampus (Mayes et al., 2008), and is impaired in those with aMCI. As a result, mnemonic strategy training may ultimately benefit everyday functioning by targeting dysfunctional memory systems. Third, previous studies have generally lacked control groups, especially groups containing aMCI patients, that directly control for practice effects and other non-specific factors.
The current report describes the neocortical changes in the blood oxygen level-dependent (BOLD) signal arising from a single-blind, randomized, controlled trial that compared MST to repeated exposure in cognitively intact (“healthy”) older adults (HOA) and those with aMCI. We previously reported the primary behavioral outcome results, which revealed greater object location association (OLA) memory test improvement in participants receiving MST than those receiving only exposure, regardless of whether they were HOA or patients with aMCI (Hampstead et al., 2012a; see Behavioral Results below). These behavioral benefits persisted at a 1-month follow up, thereby replicating our previous findings (Hampstead et al., 2008) and reinforcing the potential benefits of MST in patients with aMCI. Moreover, only aMCI participants (Hampstead et al., 2012b) who underwent MST showed partial restoration of activation in the left hippocampus.
Based on the literature reviewed above, we predicted that MST would enhance use of lateral frontoparietal cortices that are involved in cognitive control. Postma and colleagues (2008) posited that the right cerebral hemisphere is critical for coordinate spatial processing of OLAs (i.e., knowing the exact location of objects in space) whereas the left hemisphere mediates categorical processing (i.e., knowing the relationship between features/objects). Our MST approach (described below) teaches participants to utilize both types of processing and, as a result, should facilitate bilateral activation within the lateral prefrontal and parietal regions discussed above. Conversely, the exposure groups simply practiced the visuospatial task and, as a result, were expected to primarily engage regions in the right cerebral hemisphere (i.e., coordinate processing). We examined training-related changes in different ways. First, we identified the training-specific changes using stimuli that had been learned during the training sessions (i.e., “trained stimuli”). In this case, activation likely represents a combination of both the retrieval of the stimuli as well as the re-encoding of this information, presumably using the approach taught during training. Although the focus of the current study was on teaching content rather than the independent use of mnemonic strategies, our previous findings (Hampstead et al., 2011a) suggested that patients engage lateral prefrontal and parietal regions as they attempt to learn relatively novel stimuli. Therefore, our second set of analyses examined the changes in activation during the encoding of “untrained” (novel) stimuli, which were only seen during the pre- and post-training fMRI sessions. Many of the above referenced prior studies used novel stimuli during each time point to evaluate MST induced changes. Our approach is unique in that we can directly examine the extent to which comparable networks are engaged for both trained and untrained stimuli, which would support the possibility that participants were attempting to generalize strategy use after MST, especially if such activations are distinct from those shown by the exposure only groups. Importantly, both within- and between-group contrasts were performed after a non-linear cortex based alignment procedure that maximized inter-subject alignment and thereby minimized variability that can confound group-based neuroimaging studies arising from variations in sulcal and gyral patterns as well as neocortical atrophy.
Methods
Participants:
A total of 34 right-handed participants (16 HOA; 18 aMCI) completed the fMRI portion of the current randomized, controlled, single-blind study. The behavioral outcomes of this study were previously described in detail (Hampstead et al., 2012a) as were changes specific to the hippocampus (Hampstead et al., 2012b; which led us to focus on only neocortical changes in the current report). Clinical and demographic details about these participants have been provided earlier (Hampstead et al., 2011b; Hampstead et al., 2012a, 2012b), so only a summary is provided here.
Each aMCI patient (n=18) was diagnosed according to Petersen’s criteria (Petersen, 2004) during a consensus conference that included neuropsychologists, neurologists, geriatricians, and other key clinical staff. Specifically, the diagnosis of aMCI required a subjective report of cognitive decline (provided by the patient or an informant) and objective evidence of memory impairment within the context of generally preserved global cognitive functioning and activities of daily living. As seen in Table 1, these groups demonstrated characteristic learning (about 1SD below the mean) and memory (about 1.6 SD below the mean) as well as relative weaknesses in language (due to reduced semantic fluency); a profile consistent with that expected of those with underlying Alzheimer’s disease.
Table 1.
Demographic, baseline neuropsychological test results, and global brain volumes (in percent of intracranial volume – ICY, obtained via NeuroQuant©) for the healthy older adults (HOA) and aMCI groups that underwent mnemonic strategy training (MST) or exposure training (modified with permission from Hampstead et al., 2012b). Standard deviations are provided in parentheses. Analyses used a 2 (diagnostic group) x.2 (intervention group) multivariate analysis of variance. MMSE = mini-mental state exam; RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; GOS = Geriatric Depression Scale; FAQ = Functional Activities Questionnaire.
| HC | MCI | Main Effect of Diagnosis F(1,30)= | Main Effect of Intervention F(1,30)= | Diagnosis x Intervention F(1,30)= | |||
|---|---|---|---|---|---|---|---|
| MS (n=8) | Exposure (n=8) | MS (n=9) | Exposure (n=9) | ||||
| Age (years) | 72.1 (7.5) | 72. 1 (7.6) | 71.7 (10.2) | 70.8 (7.2) | 0.10, p=.75 | 0.03, p=.88 | 0.03, p=.88 |
| Education (years) | 15.8 (3.2) | 16.5 (2.3) | 17.4 (1.8) | 16.8 (2.4) | 1 35, p=26 | 0.00, p= 96 | 0.70, p=.41 |
| MMSE (raw score) | 28.4 (1.6) | 27.3 (2.3) | 26.8 (2.2) | 26.7 (2.5) | 2.08, p=.16 | 0.67, p= 42 | 0.45, p=.51 |
| RBANS Indices (Standard Scores) | |||||||
| Immediate Memory | 105.1 (12.1) | 106.5 (15.5) | 87.8 (11.6) | 86.3 (15.3) | 15.83, p<.001 | 0.00, p=.99 | 0.09, p=.77 |
| Visuospatial/construction | 95.3 (13.4) | 103.8 (15.0) | 99.0 (19.6) | 90.9 (6.6) | 0.72, p=.40 | 0.00, p= 97 | 2.39, p=.13 |
| Language | 104.6 (14.5) | 101.9 (16.8) | 93.8 (7.6) | 90.6 (6.6) | 7.33, p=.011 | 0.53, p=.47 | 0.00, p= 95 |
| Attention | 111.9 (11.1) | 108.9 ( 12.9) | 105.8 (13.5) | 106.0 (10.6) | 1.17, p=.29 | 0.11, p=.74 | 0.15, p=.70 |
| Delayed Memory | 104.1 (9.3) | 103.4 (9.0) | 75.9 (16.1) | 74.0 (14.9) | 41.99, p<.001 | 0.09, p=.77 | 0.02, p=.90 |
| Total Score | 105.5 (12.5) | 107.5 (16.9) | 89.9 (11.7) | 85.7 (9.0) | 18.43, p<.001 | 0.07, p= 80 | 0.51, p=.48 |
| Trails A (T-scores) | 49.0 (9.4) | 49.0 (8.5) | 42. 1 (7.6) | 49.6 (14.9) | 0.76, p=.39 | 1.05, p= 31 | 1.05, p=.31 |
| Trails B (T-scores) | 48.8 (9.7) | 52.0 (8.6) | 48.6 (6.3) | 46.9 (7.4) | 0.93, p=.34 | 0.08, p=.78 | 0.80, p=.38 |
| GDS (raw scores) | 1.4 (2.4) | 0.8 (1.5) | 1.1 (l.3) | 2.0 (2.4) | 0.54, p=.47 | 0.04, p= 85 | 1.27, p=.27 |
| FAQ (raw scores) | 0.6 (0.9) | 0.0 (0) | 3. 1 (3.7) | 4.1 (4.3) | 10.65, p=.003 | 0.03, p= 85 | 0.65, p=.43 |
| Brain volumes (% ICV) | |||||||
| Total Gray Matter | 29.6 (2.2) | 30.6 (2.0) | 29.0(1.4) | 29.2 (1.8) | 2.38, p= 13 | 0.74, p= 40 | 0.44, p=.51 |
| Lateral Ventricles | 2.06 (0.7) | 2.34 (0.8) | 2.88 (0.98) | 2.72 (1.1) | 3.61, p=.067 | 0.04, p= 85 | 0.51, p=.48 |
HOA (n=16), recruited from a longitudinal registry and the greater Atlanta area, were free of subjective complaints and objective evidence of cognitive impairment, and were also independent in activities of daily living. As seen in Table 1, the groups were highly similar in all demographic and neuropsychological abilities.
Exclusion criteria for all participants included a history of neurologic injury or disease such as dementia, stroke, epilepsy, or head injury resulting in loss of consciousness as well as psychiatric disorders (e.g., major depressive disorder, bipolar disorder, schizophrenia) and current or past alcohol or drug abuse.
All participants completed a brief neuropsychological protocol within approximately 1 month of taking part in the study in order to: ensure that (1) aMCI patients had neither reverted to normal nor progressed to dementia and (2) HOA did not demonstrate significant memory deficits. Both the Emory University Institutional Review Board and the Atlanta VAMC Research and Development Committee approved the study. Participants provided informed written consent.
OLA stimuli:
We created two lists of 9 rooms that are encountered in daily life (bathroom, bedroom, dining room, garage, kitchen, laundry room, living room, office, recreational room) using a 3-dimensional design program (www.Plan3d.com). A total of 90 objects were selected because they were highly concrete, familiar, imageable, and frequently used. Objects were split into two comparable lists of 45 OLAs (List A and List B). In each room, we pseudorandomly placed five objects within locations that spanned the width and, to the extent possible, height of the room so that any of the objects could have reasonably appeared in any of the locations within a given room (see Hampstead et al., 2012a for additional details). Following prior research (We created 2 additional rooms with one object in each room – these are referred to as the “repeated” stimuli hereafter. Subtracting these “repeated” stimuli from those in the “trained” or “untrained” lists allowed us to control for basic perceptual processes associated with the object-location pairs during scanning as described in earlier studies (e.g., see Hampstead et al., 2011; Sperling et al., 2003). We verified the location of each object was feasible in order to avoid any “oddball” type stimuli. Sample stimuli can be seen in Figure 1.
Figure 1.
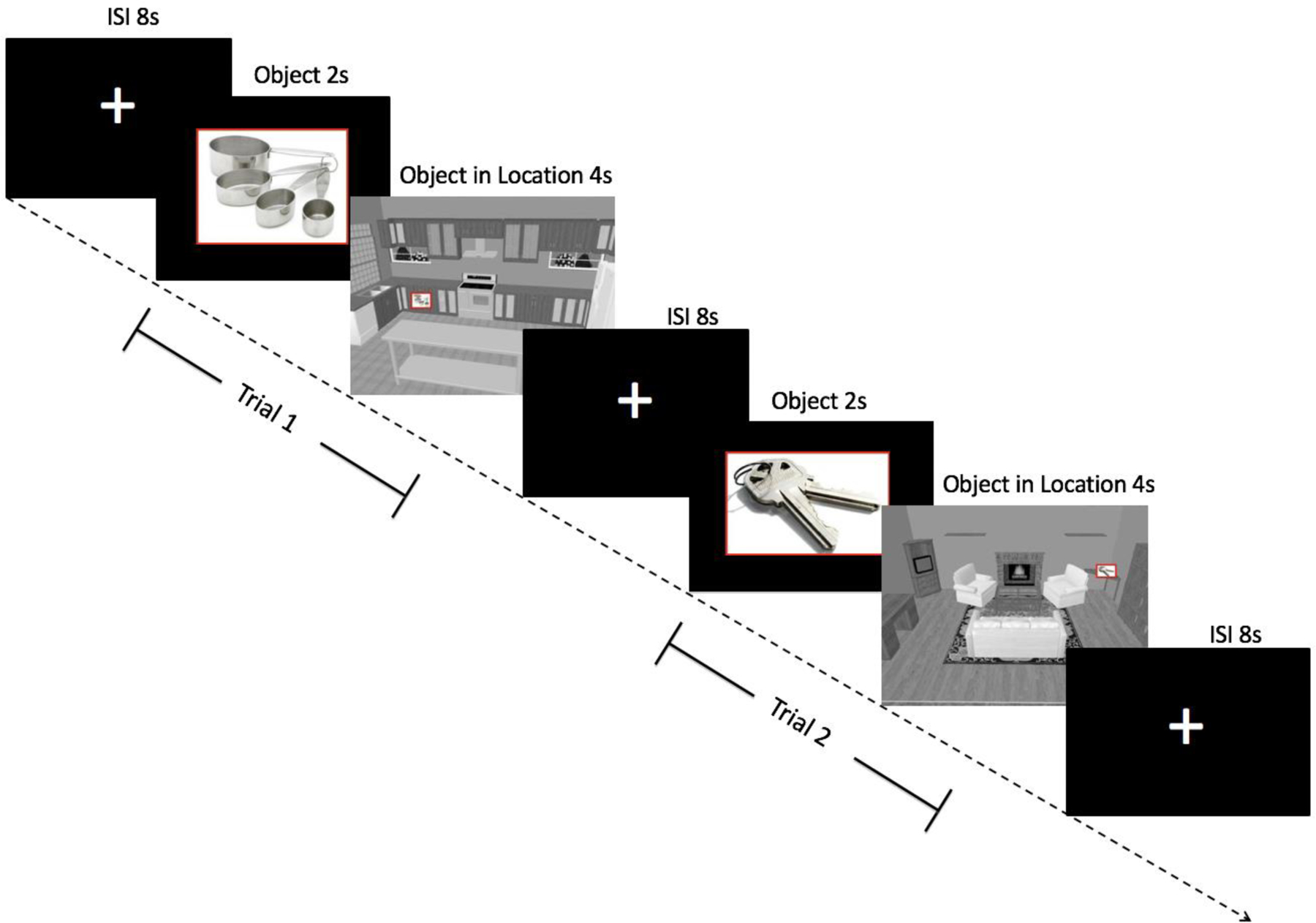
Design of the object-location association paradigm (used with permission from Hampstead et al.,2011b). Participants saw an object for 2s followed by the object within a specific location of a room for 4s. An 8s inter-stimulus interval separated each trial.
Randomization and training procedures:
Participants were randomized to either mnemonic strategy training or an exposure-matched control condition on a 1:1 basis within each diagnostic group. The general study design can be seen in Figure 2. All groups were comparable in terms of demographic variables (see Table 1 and Hampstead et al., 2012a).
Figure 2.
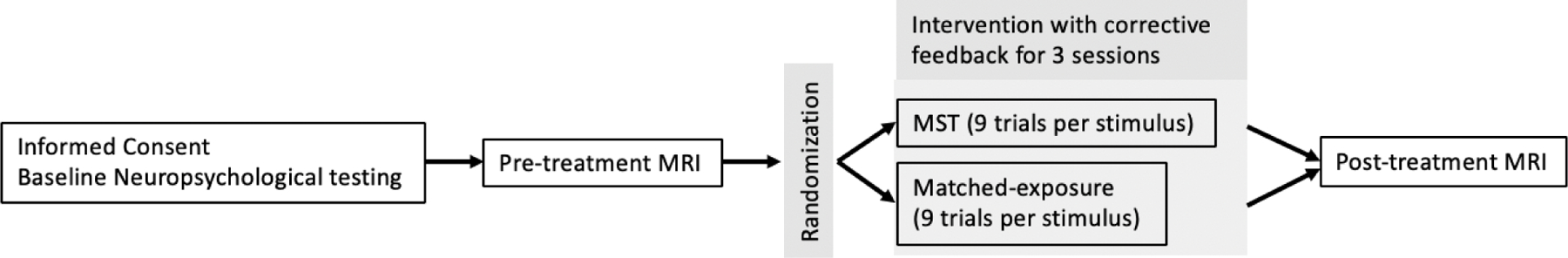
Overall study design. Randomized participants completed 3 training sessions in which they learned a total of 15 stimuli using the designated approach (i.e., MST or exposure). Each stimulus was presented a total of 9 times during the session, with corrective feedback provided following each trial as necessary.
All participants completed 5 sessions within a 2-week period of time. fMRI scanning was performed during the 1st (pre-training) and 5th (post-training) sessions. After the first fMRI session (sessions 2–4), participants were randomly assigned (1:1) to learn the 45 OLAs from either List A or B using either MST or a matched-exposure control group. This list is referred to as the “trained” list. The other list of OLAs was seen only during the fMRI scanning sessions and is referred to as the “untrained” list. These untrained stimuli provide a set of relatively novel stimuli through which it is possible to examine training-related changes (i.e., transfer/generalization effects).
During sessions 2–4, participants in both the MST and exposure groups received an initial study period for each of 15 OLAs (see Hampstead et al., 2012a for complete details). Presentation of these 15 OLAs occurred in 3 sets of 5 stimuli. During the initial presentation of each stimulus, those in the MST group practiced using the steps outlined below. To control for the time-demands of MST use, participants in the matched-exposure control group were given approximately 15 seconds to study each stimulus. All participants then received 6 “test” trials with these small sets of 5 stimuli as well as an additional 3 “test” trials with all 15 stimuli (9 total “test” trials per stimulus). During the “test” trials, participants were ultimately required to state the location of the targeted object from among 5 choices that spanned the length and height of each room. Both groups received corrective feedback as needed. The only difference in training approach was that the MST groups also used a 3-step process requiring them to 1) identify a salient feature within the room that was close to the object, 2) use a verbally-based “reason” that related the object to the specific feature, and 3) form a corresponding mental image. Thus, during the “test” trials, participants in the MST group recalled, in order, the feature, reason, and location so as to promote a specific series of steps that could be applied to other OLAs. In contrast, the matched-exposure group was simply required to recall the location of each object. A more detailed description of the training procedures can be found in our previous report (Hampstead et al., 2012a).
fMRI scanning (sessions 1 & 5):
MRI scans were performed on a Siemens Trio 3T MRI scanner (Siemens Medical Solutions, Malvern, PA), using a 12-channel head coil. For blood oxygenation level-dependent (BOLD) contrast, T2*-weighted functional images were acquired using a single-shot, gradient-recalled, echo-planar imaging (EPI) sequence with the following parameters: repetition time (TR) 2000 ms, echo time (TE) 30 ms, field of view (FOV) 220 mm, flip angle (FA) 90°, 29 axial slices of 4 mm thickness, in-plane resolution 3.4×3.4 mm, and in-plane matrix 64×64. High-resolution anatomic images were acquired using a 3D MPRAGE sequence (TR 2300 ms, TE 3.9 ms, TI 1100 ms, FA 8°) consisting of 176 sagittal slices of 1 mm thickness (FOV 256 mm, in-plane resolution 1×1 mm, in-plane matrix 256×256).
During each of 5 functional runs, participants viewed 9 trained, 9 untrained, and 9 repeated (i.e., two stimuli that were repeated in alternating fashion) stimuli in an event-related design. Each 6s trial consisted of presenting the object (2s) followed immediately by the object in its location (4s). Trials were separated by 8s inter-stimulus intervals (ISI) and the three trial types were pseudorandomly distributed within each run. Six 10s baseline periods were pseudorandomly distributed in each run to allow for signal normalization. Total run length was 7’18” and the order of the 5 runs was randomized for each participant. During these ‘encoding” runs, participants were instructed to remember the object’s location. One hour later, participants selected the object’s location from among 3 choices during a separate fMRI session (retrieval data will be reported separately). Each of the choices used during the retrieval phase was an actual target location within that room; a design intended to promote recollection over familiarity. These same procedures were repeated during session 5. Importantly, all fMRI data analyses included only trials in which trained or untrained stimuli were successfully remembered.
Imaging data preprocessing:
Image processing and analysis were performed using BrainVoyager QX v2.4 (Brain Innovation, Maastricht, The Netherlands). Functional runs were motion-corrected in real time using Siemens 3D-PACE (prospective acquisition motion correction). For each subject, the functional images were realigned to the first image of the series. Images were preprocessed using trilinear-sinc interpolation for motion correction, cubic spline interpolation for slice scan time correction, and high-pass temporal filtering to 2 cycles/run to remove slow drifts in the data. They were then co-registered with anatomic images and transformed into Talairach space (Talairach & Tournoux, 1988), which is standard in BrainVoyager.
The inter-individual variability in sulcal and gyral patterns can present a challenge when performing group-based comparisons, especially in populations with varying degrees of atrophy. To more accurately compare the patterns of neocortical activation across groups, we used the moving-target group-averaging approach of the cortex-based alignment procedure that is available within BrainVoyager (Goebel, Esposito, & Formisano, 2006). This technique consists of two general steps. First, folded cortical representations (i.e., meshes) were created from each Talairach normalized brain. The meshes from each hemisphere were automatically morphed into spheres and then iteratively aligned to the group average based on corresponding sulci and gyri. Second, the Talairach normalized, unsmoothed functional data (volume time course (vtc) files in BrainVoyager) were transformed into mesh time course (mtc) files using only the voxels contained within the outermost 4mm (i.e., the cortical mantle). For each participant, we then combined the individual mtc files from the left and right hemisphere so that subsequent surface-based analyses would be performed on both hemispheres together. For group analysis, the mtc data were spatially smoothed with an isotropic Gaussian kernel (full-width half-maximum = 5 mm). Data were normalized across runs and subjects using data where the predictor values were at or near zero (≤0.1).
All analyses were performed using random effects, general linear models (GLMs) for the aligned neocortical data. All activation maps were corrected by imposing a cluster-volume threshold for contiguous vertices passing a vertex-wise significance threshold of p < .05 (500 iterations of a permutation test were performed), which is available in BrainVoyager and based on Monte-Carlo simulations arising from the work of Forman and colleagues (1995). The specific contrasts and associated rationale used to address each aim are presented within the Results section below.
Behavioral data analysis:
As previously reported (Hampstead et al., 2012a&b), treatment efficacy was calculated using a modified change score that quantified the percentage improvement relative to that possible after accounting for pre-training (Session 1) performance: [(Session 5 % correct – Session 1 % correct)/(100 – Session 1 % correct)] x 100. This formula provides a standard metric that can be directly compared across groups and is less susceptible to ceiling effects than change scores using raw data. Scores for trained and untrained stimuli were analyzed separately, using a 2 (healthy controls vs. aMCI) x 2 (mnemonic strategy vs. exposure) analysis of variance (ANOVA) in SPSS 18.
Results
Behavioral
The behavioral results for the current participants were previously reported (Hampstead et al., 2012b; see Table 2). Briefly, the analyses revealed that MST participants demonstrated significantly greater improvement than the exposure groups for the trained stimuli. There was no interaction between intervention and diagnosis, suggesting comparable magnitude of improvement in HOA and MCI.
Table 2.
Mean (standard deviation) improvement on the object location association test for the healthy older adults (HOA) and aMCI groups who underwent mnemonic strategy training (MST) or exposure training. Bold typeface indicates a statistically significant difference.
| HOA | aMCI | Main Effect of Diagnosis F(l,30)= | Main Effect of Intervention F(l,30)= | Diagnosis x Intervention F(l,30)= | |||
|---|---|---|---|---|---|---|---|
| MST (n=8) | Exposure (n=8) | MST (n=9 | Exposure (n=9) | ||||
| Trained stimuli (SD) | 91.85 ( 11.59) | 84.92 (11.79) | 75.41 (21.57) | 54.71 (19.94) | 15.68, p<.00I | 5.5, p=.026 | 1.37, p=.25 |
| Untrained stimuli (SD) | 52.49 (34.80) | 43.33 (20.19) | 12.08 (28.55) | 18.27 (15.66) | 13.75, p=.00I | 0.03, p=.87 | 0.76, p=.39 |
For the untrained stimuli, HOA improved more than aMCI but no intervention or interaction effects were evident.
fMRI
Pretraining differences (session 1):
Within each diagnostic group (healthy controls or aMCI), there were no significant differences on either the trained > repeated or untrained > repeated contrasts.
Contrasts:
We used two interaction contrasts to examine training-induced changes in activation. Training-specific effects were measured via the (trained [post > pre] > repeated [post > pre]) contrast. Changes potentially related to transfer of the trained approach were examined via the (untrained [post > pre] > repeated [post > pre]) contrast (BrainVoyager automatically displays the opposite contrast as well (i.e., post < pre) – see Supplemental Tables). The inclusion of the matched-exposure control group in the current study increases our confidence that any differences between the intervention groups are, in fact, due to MST.
We first applied these contrasts within each of the four intervention groups separately to identify intervention-related effects. We then examined treatment-specific effects (i.e., MST vs. exposure for the trained and untrained contrasts described in the previous paragraph) within each diagnostic group separately (i.e., HOA or aMCI). This was deemed appropriate given the baseline differences between the diagnostic groups (Hampstead et al., 2011b) and the possibility that different brain regions / networks mediate changes in the HOA and aMCI groups.
Activation Changes for the Trained Stimuli:
HOA groups (Figure 3; Supplemental Tables 1-3):
Figure 3.
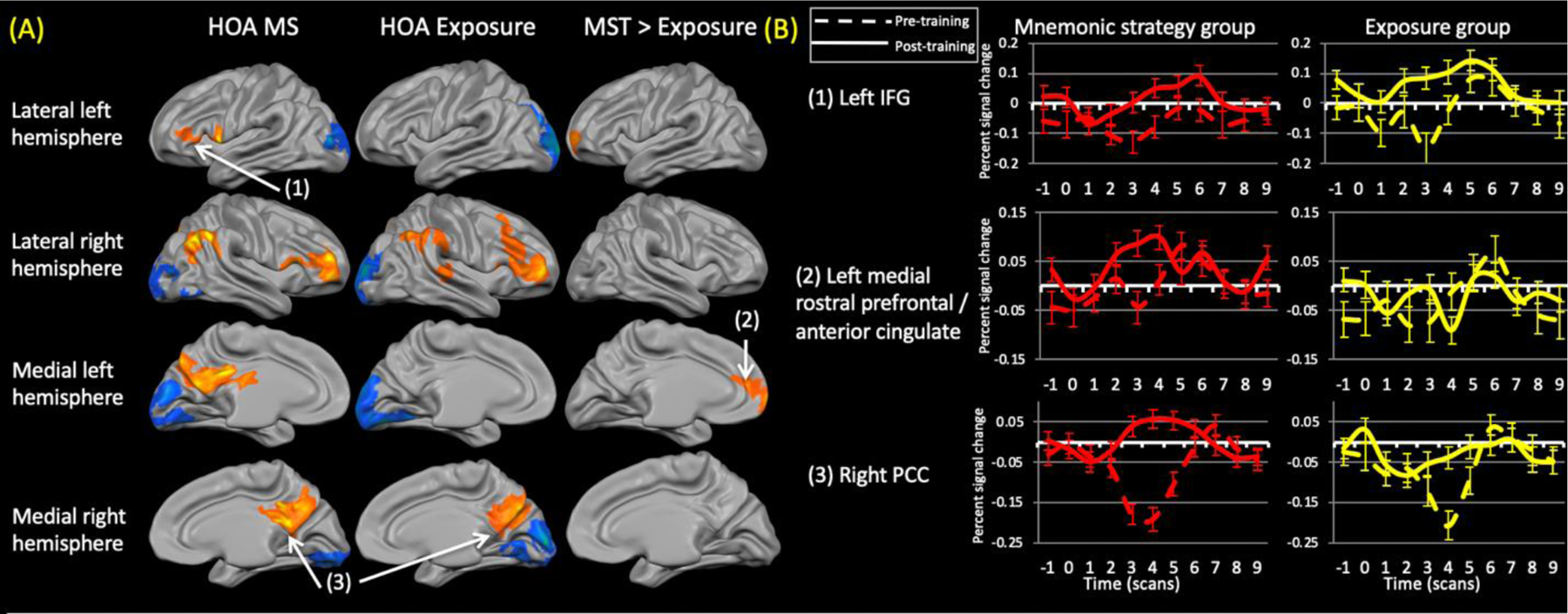
(A) Training-related changes in activation within healthy older adults (HOA) groups (left and middle column) (contrast: (trained (post > pre)> repeated (post > pre)). The right column shows the areas that were significantly different between the groups. Both groups showed reduced activation within the occipital cortex. The exposure group did not show any regions of increased activation relative to the mnemonic strategy training (MST) group. (B) Representative time courses from the numbered regions within (A). Dashed lines represent pre-training time courses while solid lines represent post-training time courses. IFG = inferior frontal gyrus; PCC = posterior cingulate cortex.
The HOA MST group demonstrated training-related increases in activation in the right rostral prefrontal cortex and right inferior parietal cortex. Bilateral increases were evident in the ventrolateral prefrontal and posteromedial parietal cortex. Training-related decreases in activation, suggestive of repetition suppression effects, occurred bilaterally within various regions of the occipital cortex.
The HOA exposure group demonstrated activation increases only in the right cerebral hemisphere, with changes in the ventrolateral and dorsolateral prefrontal cortex, inferior and posteromedial parts of parietal cortex, and in the superior temporal gyrus. This group also showed activation decreases in the occipital cortex bilaterally, with some extension into ventral temporal regions; again consistent with repetition suppression effects.
Comparing the healthy control groups revealed greater activation increases in the left rostral prefrontal cortex, extending medially into the dorsal anterior cingulate cortex, in the MST relative to the exposure group. There were no areas where activation increases were greater for exposure relative to MST.
aMCI groups (Figure 4; Supplemental Tables 4-6):
Figure 4.
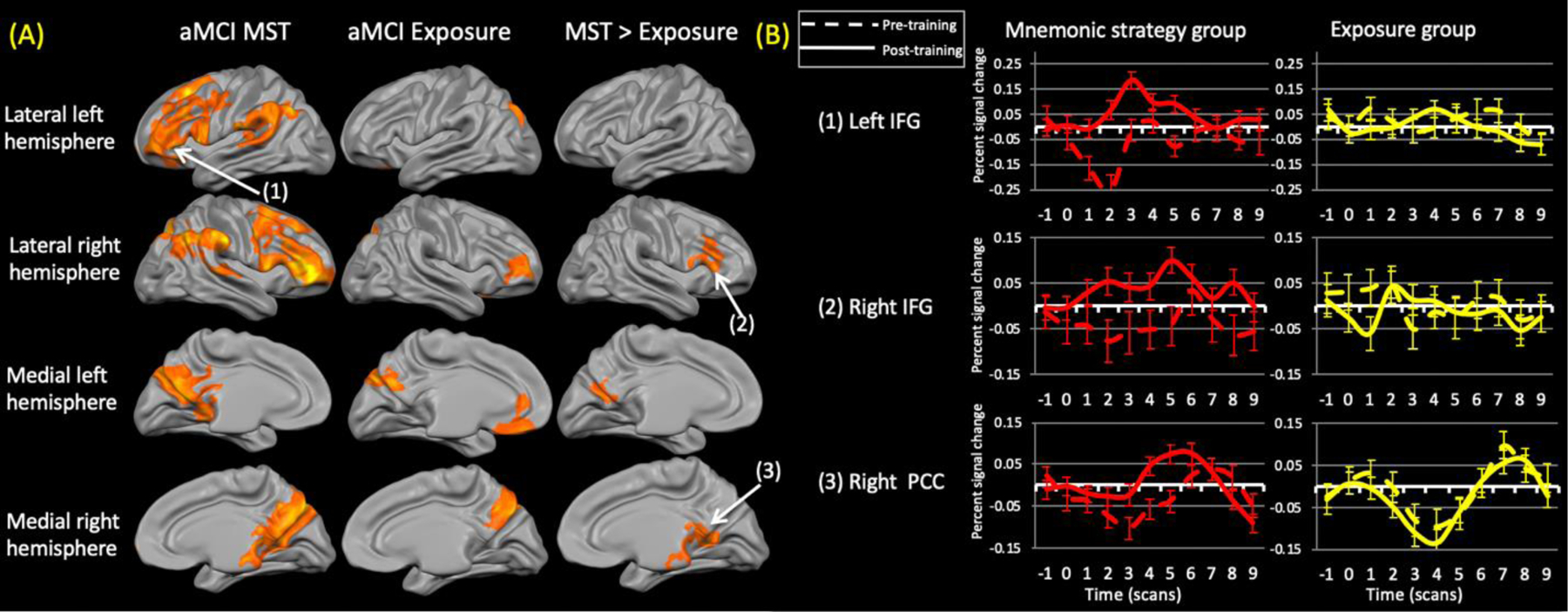
(A) Training-related changes in activation within the aMCI groups (left and middle column) (contrast: (trained (post > pre)> repeated (post > pre)). The right column shows the areas that were significantly different between the groups. Neither group demonstrated decreased activation. The exposure group did not show any regions of increased activation relative to the mnemonic strategy training (MST) group. (B) Representative time courses from the numbered regions within (A). Dashed lines represent pre-training time courses while solid lines represent post-training time courses IFG = inferior frontal gyrus; PCC = posterior cingulate cortex.
The aMCI MST group demonstrated widespread bilateral training-related increases in activation in lateral prefrontal (ventrolateral, dorsolateral, superior, and rostral prefrontal) as well as in the inferior and posteromedial parietal and superior temporal. There were no areas of reduced activation after training.
The aMCI exposure group demonstrated a more limited pattern of increased activity, restricted to the right rostral prefrontal cortex, left anteromedial frontal cortex, as well as the precuneus (bilaterally) with some extension into the dorsal occipital and (right) parietal cortices. There were no areas of reduced activation.
Comparing the aMCI groups revealed significantly greater increases after MST relative to exposure within the right ventrolateral and bilateral posteromedial parietal cortices. No areas showed greater activation increases after exposure than MST.
Activation Changes for the Untrained (novel) Stimuli:
HOA groups (Figure 5; Supplemental Table 7-8):
Figure 5.
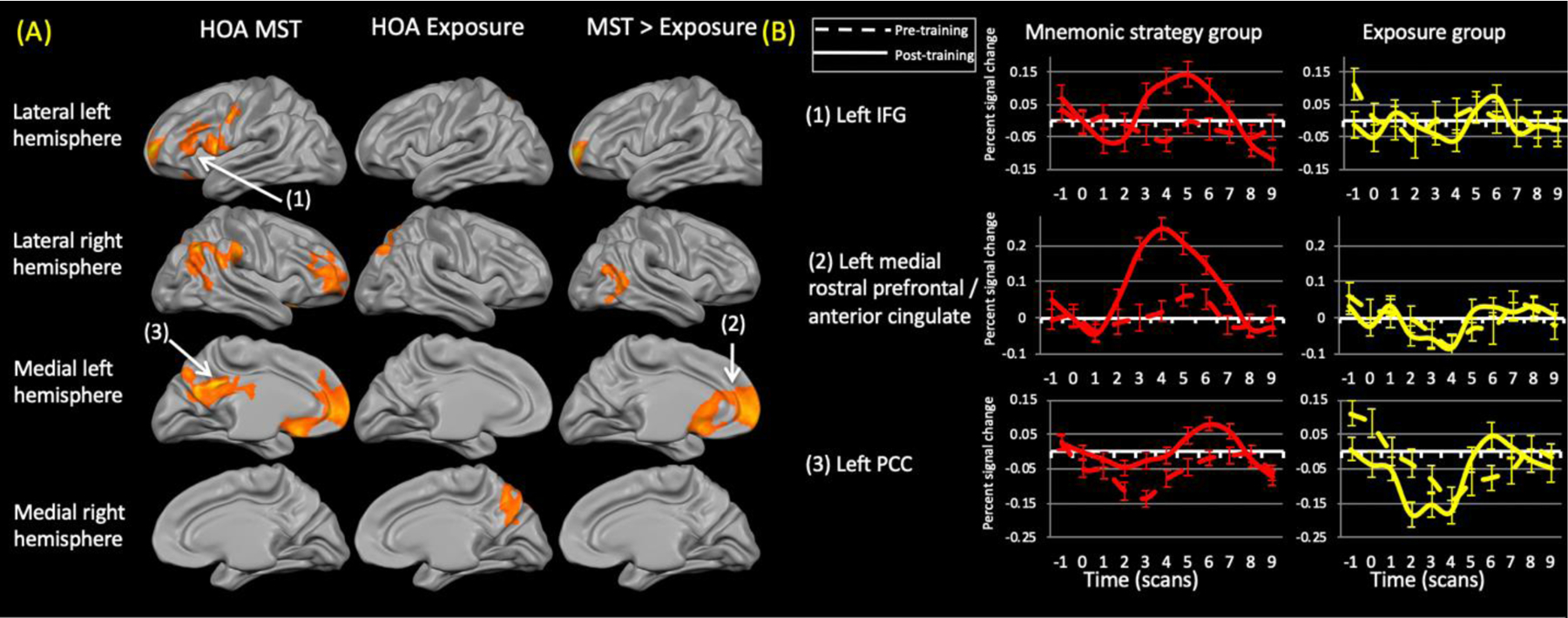
(A) Changes in activation for the untrained stimuli within healthy older adult (HOA) groups (left and middle column) (contrast: (untrained (post> pre) > repeated (post > pre)). The right column shows the areas that were significantly differnet between the groups. Neither group demonstrated reduced activation. The exposure group did not show any regions of increased activation relative to the mnemonic strategy training (MST) group. (B) Representative time courses from the numbered regions within (A). Dashed lines represent pre-training time courses while solid lines represent post-training time courses. IFG = inferior frontal gyrus; PCC= posterior cingulate cortex.
While encoding the untrained stimuli, the MST group demonstrated increased activation in several regions of prefrontal cortex (left: ventrolateral, dorsolateral, rostral, orbitofrontal; right: dorsolateral, rostral) as well as the left posteromedial parietal cortex. Increases were also evident in the right inferior parietal cortex. Many of these regions were analogous to those seen for the trained stimuli. There were no areas of reduced activation.
The only significant changes within the exposure group were increased activation in the right superior parietal lobule, precuneus, and superior occipital cortex. There were no areas of reduced activation.
Activation increases in the left anteromedial frontal cortex and right mid-to-inferior temporal cortex were significantly greater in the MST than the exposure group. These regions were virtually identical to those seen for the trained stimuli.
aMCI groups (Figure 6; Supplemental Tables 9-10):
Figure 6.
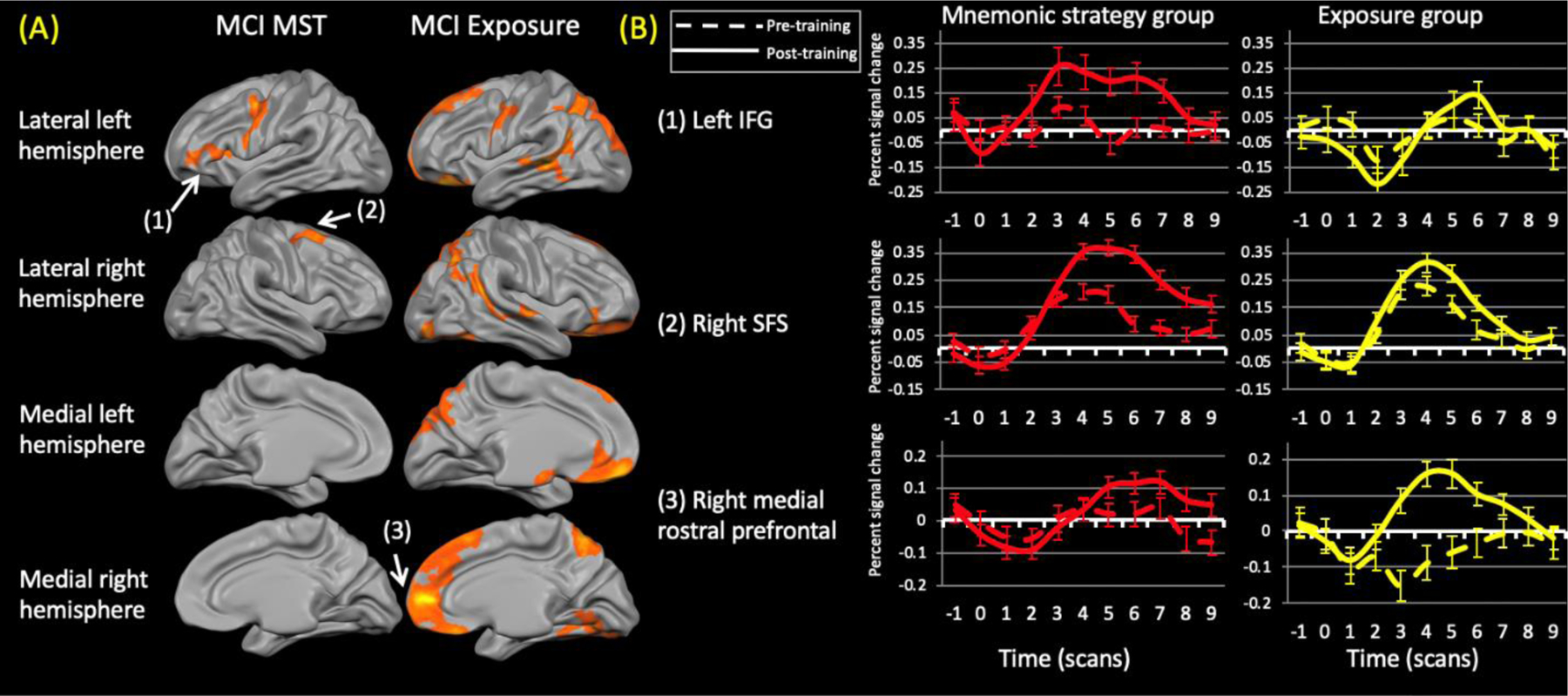
(A) Changes in activation for the untrained stimuli within the aMCI groups. Neither group demonstrated reduced activation (contrast: (untrained (post> pre) > repeated (post > pre)). No between group differences survived correction procedures. MST = mnemonic strategy Training group. (B) ) Representative time courses from the numbered regions within (A). Dashed lines represent pre-training time courses while solid lines represent post-training time courses. IFG = inferior frontal gyrus; SFS = superior frontal sulcus.
The MST group demonstrated significantly increased activation in the left ventrolateral and posterior prefrontal cortex abutting the precentral gyrus and in the right posterior superior frontal sulcus (at the location of the frontal eye field). No areas of decreased activation were evident.
The aMCI exposure group showed widespread activation increases bilaterally in prefrontal, lateral temporal, and superior parietal cortices. Increased activation was also evident in the left precentral gyrus as well as the right occipital and ventral temporal cortices.
Although these groups showed activation increases in distinct regions there were no significant differences between groups.
Discussion
This is one of only a few randomized, controlled, single-blind trials of cognitively oriented treatment(s) in older adults and aMCI patients to include fMRI and to include an ecologically relevant task.We compared MST with a tightly matched active control group that received the exact same number of trials as the MST group, thereby allowing us to conclude that the observed behavioral and fMRI effects are specific to MST. Our paradigm included both trained stimuli (i.e., stimuli learned during the training sessions) and “untrained” stimuli (i.e., relatively novel stimuli that were seen only a single time during the fMRI sessions). This design provides the opportunity to also examine potential generalization (or transfer) of the trained technique as well as the neural basis of such generalization. fMRI data were analyzed after non-linear cortex-based alignment, which minimizes confounds attributable to cortical atrophy and inter-individual differences in sulcal and gyral patterns. We also analyzed only trials in which stimuli were correctly encoded, thereby removing possible confounds associated with unsuccessful learning attempts. Thus, the current study addressed several critical methodological limitations of this line of research.
Our MST approach required participants to 1) select and attend to a salient feature, 2) use a verbally-based explanation (i.e., semantic organization/elaboration) that explicitly linked the object, the feature, and the location, and 3) develop/rehearse a mental image. The associated patterns of activation change in the MST groups are consistent with these steps and presumably account for the improved memory test performance relative to the matched (repeated) exposure control condition. Postma and colleagues (2008) posited that lateral frontoparietal regions in the right hemisphere mediate coordinate spatial processing (i.e., the knowledge of precise spatial details related to the object’s location); a process presumably engaged by our first step (i.e., selecting and attending to a salient feature). We previously reported the aMCI patients showed significantly reduced activation in these same areas relative to the HOA (Hampstead et al., 2011). Thus, it appears that MST had restorative effects in the aMCI patients as reflected by re-engagement of this “normal” network; a possibility reinforced by the absence of lateral prefrontal cortex, inferior parietal lobule, or posterior cingulate cortex change in the aMCI matched-exposure group. The significant differences in dorsolateral and ventrolateral prefrontal activation between the aMCI groups reinforces the probability that these changes are directly attributable to MST. In contrast, all four groups showed change in the rostral prefrontal cortex and precuneus, which likely reflect the mere act of repeated exposure and testing that was constant across all groups.
Our second MST step focuses on the use of verbally based “reason” that linked the object and the selected environmental feature. This process aligns with Postma and colleagues’ (2008) categorical spatial processing, which involves locating one object relative to another or to a landmark. The most consistent changes were again in the ventrolateral prefrontal cortex and replicate previous reports of MST-related increases in both aMCI patients (Hampstead et al., 2011a; Balardan et al., 2016) and healthy adults (Hales & Brewer, 2012). This region is also known to be critical for successful encoding (Bergmann et al., 2012; Kuhl, Bainbridge, and Chun, 2012; Spaniol et al., 2009), perhaps due to its concurrent role in semantic processing (Binder et al., 2009), verbal working memory (Nee et al., 2013), and other elements of cognitive control (Derrfus, Brass, & von Cramon, 2004; Sundermann & Pfleiderer, 2012).
Both MST groups also demonstrated increased activation in the left posteromedial parietal cortex (which comprises the precuneus, posterior cingulate, and retrosplenial cortex). These regions have rich reciprocal connections with one another and are also structurally and functionally connected with the hippocampal memory system (Torta & Cauda, 2011). MST has been shown to increase activation in these regions in both healthy individuals (Kondo et al., 2005; Miotto et al., 2006; Nyberg et al., 2003) and aMCI patients (Hampstead et al., 2011a). Particularly relevant to our third MST step of creating a mental image are prior findings that activation in the left anterior precuneus reflected the vividness of mental images used to encode information (Leshikar, Duarte, Hertzog, 2012). Such an effect would explain the significant differences that were evident in this area between the aMCI groups.
Despite these areas of overlap, there were several notable differences between the MST groups that raise the possibility that effects are at least partially mediated by cognitive phenotype. For example, the HOA MST demonstrated increased activation in the left rostral prefrontal cortex that was significantly greater than in the HOA exposure group. This general region is believed to mediate metacognitive control (Burgess et al., 2007; Stuss, 2011) over other cognitive processes often associated with these areas (e.g., response inhibition, verbal generation, coordinated integration of internal and external stimuli; see Cabeza et al., 2000; Torta & Cauda, 2011). In contrast, the aMCI MST group showed robust changes in the dorsolateral and superior prefrontal cortices that may reflect use of working memory (Baddeley, 2003) and relational encoding (Blumenfeld, Parks, Yonelinas, & Ranganath, 2011). Interestingly, the aMCI group engaged the left inferior parietal lobule in what appears to be a compensatory manner given the lack of baseline hypoactivation (see Hampstead et al., 2011b) or comparable response in the HOA MST group. This finding replicates our earlier study with face-name associations (Hampstead et al., 2011a) and complement those of Belleville and colleagues (2011), who demonstrated increased right inferior parietal lobule activation while aMCI patients encoding verbally based information.
Evidence of generalization (transfer)?
The behavioral portion of the current study was designed to examine whether MST improved memory for specific information, the answer to which was affirmative, as previously reported (Hampstead et al., 2012a). Although we did not explicitly promote generalization during the training process in the current study, we provided extensive practice with our 3-step approach with the hope that participants would alter their approach to learning new OLAs. Including the untrained stimuli in the fMRI paradigm provides an opportunity to study the neural correlates of generalization. However, the single, relatively brief exposure to each untrained stimulus means that participants probably did not have enough time to fully develop and/or rehearse strategies – a limitation that likely explains the lack of memory test improvement. Regardless, our design allows us to ask whether activation changes for the untrained stimuli comprise a similar set of regions as for the trained stimuli, as suggested by our previous study (Hampstead et al., 2011a), and whether the pattern of change differs by treatment group.
The HOA MST group demonstrated activation changes that were strikingly similar for the untrained and trained stimuli; a finding that argues in favor of generalization and against the possibilities they arose from mere exposure or the retrieval and re-encoding of the trained stimuli. Further, the HOA matched exposure control group showed increased activation only in the right superior and medial parietal cortex despite both groups showing substantial changes in accuracy for memory test performance for the untrained stimuli. Together, these findings reinforce the conclusion that HOA modified their approach to encoding OLAs in a manner consistent with the trained techniques and that engaged frontoparietal cortices bilaterally.
As with their healthy counterparts, the aMCI MST group showed increased activation for untrained stimuli within a subset of regions that showed change for the trained stimuli. The increases in the left ventrolateral prefrontal cortex are noteworthy given that they were found in both MST groups but neither of the exposure groups. These findings further reinforce the importance of this region in mnemonic strategy use, regardless of clinical status (i.e., healthy vs. aMCI) or stimulus familiarity (i.e., trained vs. untrained) and across distinct memory paradigms (face-name associations in Hampstead et al., 2011a; OLAs in the present study). This may have the “downstream” effect of maximizing residual hippocampal functioning in aMCI patients, which could explain our findings of increased activation in the left hippocampus in the aMCI MST group (Hampstead et al, 2012b). Interestingly, the aMCI MST group also demonstrated changes in areas consistent with the use of attentional saccades (i.e., right frontal eye fields; Grosbras, Laird, & Paus, 2005), which our prior effective connectivity data revealed to be significantly greater in aMCI than HOA. Reliance on this area could therefore reflect a phenotype-dependent effect of MST that may be compensatory in nature. and is reasonable since our training methods required participants to select specific and salient environmental features. Both aMCI groups showed increased activation in the left ventral premotor region that was previously implicated in working memory for object identity and location (Rottschy et al., 2012). Otherwise, the aMCI exposure group demonstrated a distinct pattern of increased activation within dorsal frontal and parietal as well as lateral temporal cortices that may reflect a general increase in spatial processing (e.g., Mishkin et al., 1983; Weissman & Prado, 2012). Together, these findings suggest that repeated exposure increased the use of spatial attention, which may be suboptimal by itself (i.e., absent cognitive control processes) in those with aMCI.
Limitations and improvements of the present study
Several of our study methods enhanced scientific rigor relative to many prior studies, including 1) our consensus-diagnosed cohort, 2) randomized controlled design, and 3) active control group that was precisely matched for experience/exposure. While clinical phenotypes can be problematic, 80% of our participants showed stable or declining clinical courses at 1–2 years after completion of the study, while the other 20% did not return to the clinic; no differences existed between the intervention groups (see Hampstead et al., 2012a). Biomarkers were not widely available at the time of the current study; however, their inclusion would unquestionably enhance future studies. Another limitation relates to the challenges associated with comparing different groups, especially those susceptible to cerebral atrophy, in fMRI-based research. Our within subject pre-post design minimizes concern about vascular coupling effects, while our use of task-by-session interactions allowed isolation of effects attributable to training (at the cost of power). We used cortex-based alignment (Goebel et al., 2006) to maximize the sulcal and gyral alignment between participants and analyzed the aligned functional data using surface-based statistics, which have demonstrated improved power compared to traditional methods (Anticevic et al., 2008). Despite these efforts, we fully acknowledge that our relatively small sample sizes and limited number of trials for the untrained stimuli may have affected our ability to detect within- and between-group differences. For example, the cluster-correction method required clusters to be 300–400mm2 (or larger). Although this may omit smaller regions that are also important to consider, it increases our confidence that the observed differences are, in fact, real. Despite a limited number of trials for the untrained stimuli, our results largely replicated our previous study using MST for face-name associations in those with aMCI (Hampstead et al., 2011). However, the lack of behavioral difference for the untrained stimuli within or between groups reinforces that, given a comparable number of trials, there were training specific BOLD signal effects. We also included the associated time-course data to demonstrate the specificity of the BOLD response. We acknowledge using a relatively liberal threshold for the fMRI analyses but believe it to be appropriate given the above noted methodological advances and our replication of prior findings using the same threshold. While our consideration of potential neural mechanisms was largely based on reverse inference and while this concern is mitigated by our adherence to task-based reverse inference (Hutzler, 2014), future studies will need to be specifically designed to address the neural mechanisms underlying the observed behavioral and fMRI changes. Finally, we cannot rule out the possibility that the matched-exposure groups independently developed and applied their own “strategies” when performing the task; a possibility we directly examined by including alternative control conditions for MST in subsequent studies (e.g., subtracting cues, spaced retrieval; associated manuscripts are in progress).
Conclusions
The current randomized, controlled, single-blind study demonstrated both behavioral (i.e., memory test) benefits of MST and the associated neocortical changes, relative to a matched-exposure control condition in HOA and patients with aMCI. Importantly, MST increased activation bilaterally in multiple prefrontal and parietal regions that are known to play roles in different aspects of cognitive control and spatial processing. fMRI data also suggest that participants attempted to generalize MST to new stimuli. Overall, the current findings add to the growing body of literature showing that non-pharmacologic interventions hold promise for improving cognitive functioning and that they do so by capitalizing on neuroplasticity, even in older adults and those with MCI.
Supplementary Material
Highlights.
Mnemonic strategy training increased memory and neocortical activation bilaterally
Similar neocortical changes were seen in cognitively intact and patients with MCI
Mnemonic strategies appear to (re)engage the frontoparietal cognitive control areas
Acknowledgements:
This work was supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and Rehabilitation Research and Development Service (IRX001534 and B6366W to BMH; B6662R to KS) and by the Emory Alzheimer’s Disease Research Center (NIA: 2P50AG025688) and Michigan Alzheimer’s Disease Center (NIA: 5P30AG053760–5). Support to KS from the National Institutes of Health [K24 EY017332] is also acknowledged. The contents of this manuscript do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
No author has any conflict of interest.
References
- Albert MS, DeKosky ST, Dickson D, Dubois B, Feldman HH, Fox NC, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging and Alzheimer’s Association workgroup. Alzheimer’s & Dementia 2011; 7: 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen G, Barnard H, McColl R, Hester AL, Fields JA, Weiner MF, et al. Reduced hippocampal functional connectivity in Alzheimer’s disease. Archives of Neurology 2007; 64: 1482–1487. [DOI] [PubMed] [Google Scholar]
- Anticevic A, Dierker DL, Gillespie SK, Repovs G, Csernansky JG, Van Essen DC, & Barch DM Comparing surface-based and volume-based analyses of functional neuroimaging data in patients with schizophrenia. NeuroImage 2008; 41: 835–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley A Working memory: Looking back and looking forward. Nature Reviews Neuroscience 2003; 4: 829–839. [DOI] [PubMed] [Google Scholar]
- Balardan JB, Batistuzzo MC, Martin MAG, Sato JR, Sato JR, Smid J, Porto C, Savage CR, Nitrini R, Amaro E, Miotto E (2015). Differences in prefrontal cortex activation and deactivation during strategic episodic verbal memory encoding in mild cognitive impairment. Front Aging Neurosci, 7, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleville S, Clement F, Mellah S, Gilbert B, Fontaine F, & Gauthier S Training-related brain plasticity in subjects at risk of developing Alzheimer’s disease. Brain 2011; 134: 1623–1634. [DOI] [PubMed] [Google Scholar]
- Belleville S, Gilbert B, Fontaine F, Gagnon L, Menard E, & Gauthier S Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: Evidence from a cognitive intervention program. Dementia and Geriatric Cognitive Disorders 2006; 22: 486–499. [DOI] [PubMed] [Google Scholar]
- Bergmann H, et al. , Distinct neural correlates of associative working memory and long-term memory encoding in the medial temporal lobe. NeuroImage 2012; 63: 989–997. [DOI] [PubMed] [Google Scholar]
- Binder R, Desai RH, Graves WW, Conant LL Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies Cereb. Cortex, 19 (12) (2009), pp. 2767–2796, 10.1093/cercor/bhp055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld RS, Parks CM, Yonelinas AP, Ranganath C Putting the pieces together: The role of dorsolateral prefrontal cortex in relational memory encoding. Journal of Cognitive Neuroscience 2011; 23: 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R and Nyberg L Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience 2000; 12: 1–47. [DOI] [PubMed] [Google Scholar]
- Cabeza R Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychology and Aging 2002; 17: 85–100. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Dhalberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, et al. Evidence-based cognitive rehabilitation: Update review of literature from 1998 through 2002. Archives of Physical Medicine and Rehabilitation 2005; 86: 1681–1692. [DOI] [PubMed] [Google Scholar]
- Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence-Based Cognitive Rehabilitation: Updated Review of the Literature From 2003 Through 2008. Archives of Physical Medicine and Rehabilitation 2011; 92: 519–530. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trajanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain 2011; 134: 1591–1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettell SA, & Cabeza R Effects of aging on the neural correlates of successful item and source memory encoding. Journal of Experimental Psychology: Learning, Memory, and Cognition 2008; 34: 791–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, & Sperling RA Functional abnormalities of the medial temporal lobe memory system in mild cognitive impairment and Alzheimer’s disease: Insights from functional MRI studies. Neuropsychologia 2008; 46: 1624–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrfuss J, Brass M, von Cramon Y Cognitive control in the posterior frontolateral cortex: evidence from common activations in task coordination, interference control, and working memory. NeuroImage 2004; 23: 604–612. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005; 366: 2112–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC Improved assessment of significant activation in functional magnetic resonance imaging (fMRI) - Use of a cluster size threshold. Magnetic Resonance in Medicine 1995; 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Goebel R, Esposito F, Formisano E Analysis of functional image analysis contest (FIAC) data with BrainVoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Human Brain Mapping 2006; 27: 392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras MH, Laird AR, Paus T Cortical regions involved in eye movements, shifts of attention, and gaze perception. Human Brain Mapping 2005; 25: 140–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales JB & Brewer JB The path to memory is guided by strategy: Distinct networks are engaged in associative encoding under visual and verbal strategy and influence memory performance in healthy and impaired individuals. Journal of Cognitive Neuroscience 2012; 24: 1398–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM, Sathian K, Moore AB, Nalisnick C, & Stringer AY Explicit memory training leads to improved memory for face-name pairs in patients with mild cognitive impairment: results of a pilot investigation. J Int Neuropsychol Soc 2008; 14: 883–889. [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Sathian K, Phillips PA, Amaraneni A, Delaune WR, & Stringer AY Mnemonic strategy training improves memory for object location assiciations in both healthy elderly and patients with amnestic mild cognitive impairment: A randomized, single-blind study. Neuropsychology 2012a; 26: 385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF, Giddens M, & Sathian K Mnemonic strategy training partially restores hippocampal activity in patients with mild cognitive impairment. Hippocampus 2012b; 22: 1652–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF, Deshpande G, Hu XP, Moore AB, et al. Activation and Effective Connectivity Changes Following Explicit-Memory Training for Face-Name Pairs in Patients With Mild Cognitive Impairment: A Pilot Study. Neurorehabilitation and Neural Repair 2011a; 25: 210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF, Amaraneni A, & Sathian K Where did I put that? Patients with amnestic mild cognitive impairment demonstrate widespread reductions in activity during the encoding of ecologically relevant object-location associations. Neuropsychologia 2011b; 49: 2349–2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampstead BM,Khoshnoodi M, Yan W, Deshpande G, Sathiank K (2016). Patterns of effective connectivity during memory encoding and retrieval differ between patients with mild cognitive impairment and healthy older adults. NeuroImage, 124(Part A): 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutzler F (2014). Reverse inference is not a fallacy per se: Cognitive processes can be inferred from functional imaging data. NeuroImage, 84, 1061–1069. [DOI] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu YC, Waring SC, OBrien PC, Tangalos EG, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology 1997; 49: 786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean L, Bergeron ME, Thivierge S, & Simard M Cognitive Intervention Programs for Individuals With Mild Cognitive Impairment: Systematic Review of the Literature. American Journal of Geriatric Psychiatry 2010; 18: 281–296. [DOI] [PubMed] [Google Scholar]
- Kinsella GJ, Mullaly E, Rand E, Ong B, Burton C, Price S, et al. Early intervention for mild cognitive impairment: a randomized controlled trial. Journal of Neurology Neurosurgery and Psychiatry 2009; 80: 730–736. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Suzuki M, Mugikura S, Abe N, Takahashi S, Ilijama T, Fujii T Changes in brain activation associated with use of a mnemonic strategy: a functional MRI study. NeuroImage 2005; 24: 1154–1163. [DOI] [PubMed] [Google Scholar]
- Kuhl B, Bainbridge W, and Chun M, Neural reactivation reveals mechanisms for updating memory. The Journal of Neuroscience 2012; 32: 3453–3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshikar ED, Duarte A, Hertzog C Task-selective memory effects for successfully iimplement encoding strategies. Plos One 2012; 7: e38160. doi: 10.1371/journal.pone.0038160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Li JA, Li NX, Li B, Wang PY, & Zhou T Cognitive intervention for persons with mild cognitive impairment: A meta-analysis. Ageing Research Reviews 2011; 10: 285–296. [DOI] [PubMed] [Google Scholar]
- Machulda MM, Senjem ML, Weigand SD, Smith GE, Ivnik RJ, Boeve BF, et al. Functional magnetic resonance imaging changes in amnestic and nonamnestic mild cognitive impairment during encoding and recognition tasks. Journal of the International Neuropsychological Society 2009; 15: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandzia JL, McAndrews MP, Grady CL, Graham SJ, & Black SE Neural correlates of incidental memory in mild cognitive impairment: An fMRI study. Neurobiology of Aging 2009; 30: 717–730. [DOI] [PubMed] [Google Scholar]
- Mayes A, Montaldi D, Migo E Associative memory and the medial temporal lobes. Trends in Cognitive Sciences 2007; 11: 126–135. [DOI] [PubMed] [Google Scholar]
- Miotto EC, Savage CR, Evans JJ, Wilson BA, Martins MGM, Iaki S, & Amaro E Bilateral activation of the prefrontal cortex after strategic semantic cognitive training. Human Brain Mapping 2006; 27: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA Object vision and spatial vision - 2 cortical pathways. Trends in Neurosciences 1983: 6: 414–417. [Google Scholar]
- Nee D, et al. , A meta-analysis of executive components of working memory. Cerebral Cortex 2013; 23: 264–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyberg L, Sandblom J, Jones S, Neely AS, Petersson KM, Ingvar M, & Backman L Neural correlates of training-related memory improvement in adulthood and aging. Proceedings of the National Academy of Sciences 2003; 100: 13728–13733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen RC Mild cognitive impairment as a diagnostic entity. Journal of International Medicine 2004; 256: 183–194. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, & Kokmen E Mild cognitive impairment - Clinical characterization and outcome. Archives of Neurology 1999; 56: 303–308. [DOI] [PubMed] [Google Scholar]
- Postma A, Kessels RPC, van Asselen M How the brain remembers and forgets where things are: The neurocognition of object-location memory. Neuroscience and Biobehavioral Reviews 2008; 32: 1339–1345. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz P, & Lustig C Brain aging: reorganizing discoveries about the aging mind. Current Opinion in Neurobiology 2005; 15: 245–251. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB et al. Modelling neural correlates of working memory: A coordinate-based meta-analysis. NeuroImage 2012; 60: 830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwindt GC, & Black SE Functional imaging studies of episodic memory in Alzheimer’s disease: a quantitative meta-analysis. Neuroimage 2009; 45: 181–190. [DOI] [PubMed] [Google Scholar]
- Spaniol J, Davidson PSR, Kim ASN, Han H, Moscovitch M, & Grady CL Event-related fMRI studies of episodic encoding and retrieval: Meta-analysis using activation likelihood estimation. Neuropsychologia 2009; 47: 1765–1779. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rozen BR, et al. (2003). fMRI studies of associate encoding in young and elderly controls and mild Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 74(1), 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SS, Yokomizo JE, & Bottino CMC Cognitive intervention in amnestic mild cognitive impairment: A systematic review. Neuroscience and Biobehavioral Reviews 2012; 36: 163–1178. [DOI] [PubMed] [Google Scholar]
- Stott J, & Spector A A review of the effectiveness of memory interventions in mild cognitive impairment (MCI). International Psychogeriatrics 2011; 23: 526–538. [DOI] [PubMed] [Google Scholar]
- Stuss DT Functions of the frontal lobes: Relation to executive functions. Journal of the International Neuropsychological Society 2011; 17: 759–765. [DOI] [PubMed] [Google Scholar]
- Sundermann B & Pfleiderer B Functional connectivity profile of the human inferior frontal junction: involvement in a cognitive control network. BMC Neuroscience 2012; 13: article #: 119. DOI: 10.1186/1471-2202-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, & Tournoux P Co-planar stereotaxic atlas of the human brain Thieme Medical Publishers; 2008; New York. [Google Scholar]
- Torta DM & Cauda F Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. NeuroImage 2011; 56: 2157–2172. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Marcoen A, & Goossens L Improving memory performance in the aged through mnemonic training - a meta-analytic study. Psychology and Aging 1992; 7: 242–251. [DOI] [PubMed] [Google Scholar]
- Voss MW, Prakash RS, Erickson KI, Chandramallika B, Chaddock L, Kim JS, et al. Plasticity of brain networks in a randomized intervention trial of exercise training in older adults. Frontiers in Aging Neuroscience 2012, 2: Article 32 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Liang PP, Jia XQ, Qi ZG, Yu L, Yang YH, et al. Baseline and longitudinal patterns of hippocampal connectivity in mild cognitive impairment: Evidence from resting state fMRI. Journal of the Neurological Sciences 2011; 309: 79–85. [DOI] [PubMed] [Google Scholar]
- Weissman DH, & Prado J Heightened activity in a key region of the ventral attention network is linked to reduced activity in a key region of the dorsal attention network during unexpected shifts of covert visual spatial attention. NeuroImage 2012; 61: 798–804. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


