Abstract
Attachment-related learning (that is, forming preferences for cues associated with the parent) defies the traditional rules of learning in that it seems to occur independently of apparent reinforcement1—young children prefer cues associated with their parent, regardless of valence (rewarding or aversive), despite the diversity of parenting styles2. This obligatory attraction for parental cues keeps the child nearby and safe to explore the environment; thus, it is critical for survival and sets the foundation for normal human cognitive–emotional behaviour. Here we examined the learning underlying this attraction in preschool-age children. Young children underwent an aversive conditioning procedure either in the parent’s presence or alone. We showed that despite disliking the aversive unconditioned stimulus, children exhibited a behavioural approach for conditioned stimuli that were acquired in the parent’s presence and an avoidance for stimuli acquired in the parent’s absence, an effect that was strongest among those with the lowest cortisol levels. The results suggest that learning systems during early childhood are constructed to permit modification by parental presence.
Maintaining proximity to parents has evolved as one of the most important survival strategies for human children and young animals that depend on parental care. Children learn to prefer cues and contexts associated with parents3,4, a preference that ensures that children remain close to parents and return to parents after separations. This type of learning, which is observed in young altricial animals, forms the foundation of what are collectively known as ‘attachment behaviours’1,5. Notably, this infantile preference for newly learned stimuli is acquired regardless of the apparent desirability of the stimuli, and appears even if stimuli possess aversive qualities2. Thus, parental cues seem to switch avoidance learning to attraction learning. The learning underlying attachment-related cues seems to defy traditional principles of learning in that it can occur regardless of reinforcement type1. This process occurs because the survival value of attachment-related (that is, preference) learning trumps other learning processes (for example, avoidance) during early postnatal life. Despite the fundamental importance of this behaviour, very little is known about the developmental mechanisms promoting this learning behaviour in young children.
The rodent literature has shown that early in postnatal development, the mere presence of the dam alters her pup’s learning systems to uniformly produce a preference, regardless of the pleasantness or aversive value of stimuli6. While the preference for positively valenced conditioned stimuli is intuitive, the preference for aversively valenced conditioned stimuli is less so. During a sensitive period of brain development, classical conditioning with aversive stimuli in the presence of the dam results in the pup producing a preference for new cues learned in her presence, even if they are aversive6,7. That is, even when a cue is paired with a mild tail shock, if this pairing is learned in the dam’s presence, the dam ‘buffers’ or attenuates aversive learning systems, thereby switching the pup’s learning to preference-promoting systems. The result is that the pup exhibits a behavioural approach towards the cue learned in the presence of the dam, whereas the pup would normally exhibit an avoidance for the same cue if it is learned in her absence. This seemingly counterintuitive behavioural approach following aversive conditioning is nonetheless critical for normative development; it promotes preference for parental cues (that is, attachment learning) and by doing so, promotes exploration of novel stimuli (even if potentially aversive) if the dam is there for protection. The cue has now acquired attachment qualities for the pup by virtue of being learned in the presence of the attachment figure. This pattern of behaviour focuses our attention to the fundamental element of attachment-related learning that is common across species. Human children also learn to attach to parental cues, regardless of warmth or maltreatment7,8, and this type of learning permits increased exploration of unknown and potentially dangerous stimuli when in the presence of parents.
It is not known whether parents exert such effects during childhood, and aversive conditioning paradigms provide an opportunity to assess these parameters of learning in children. While this process has been shown to be central to normal rat pup development, children are much more cognitively complex and, by many metrics9, they are more biologically mature than the postnatal rat. Thus, it is important to establish whether children exhibit parental modification of learning because childhood is extended in humans relative to other species10 and requires much more prolonged parental care. While initial learning of the attachment figure begins in infancy, it continues through early childhood11,12, and may therefore be a powerful influence over the subsequent learning that occurs in the presence of the attachment figure. This period has been identified as a ‘transitional sensitive period’ in the rodent6, during which stimuli learned in the dam’s presence produce a preference for those stimuli. Here we assessed whether a similar parental modulation of aversive conditioning occurs in preschool-age children. The characteristics of the preschool period share a developmental ecology with the rat pup during this transitional sensitive period13: namely, children begin initial momentary separations from parents; stress neurobiology and affective learning systems are still immature and changing rapidly14–16; and parental presence ‘buffers’ or modulates the neurobiology that mediates aversive learning17–19. The biology of children at this age situates them for the fundamental task of ttachment-related learning. Therefore, we predicted that parental modification of affective learning processes would be observed in the young child. We hypothesized that parental presence would switch preschool-age children’s learning of aversive stimuli from avoidance to attraction.
A total of 106 children (3–5 yr old, mean (s.d.) = 4.4 (0.1) yr, 48 male and 58 female) were tested in the laboratory procedure (Fig. 1). Ninety-eight children contributed data to the study (subjects were excluded if partial conditions were completed; see Supplementary Information). Using a computer, children were exposed to pairings of a conditional stimulus (CS+), a geometric shape (for example, a blue square), that coterminated 50% of the time with an aversive unconditional stimulus (US; a loud, high-frequency metallic scraping noise delivered through headphones; with volume calibrated to each participant’s tolerance to avoid pain) (see Methods) (Fig. 1a). A different shape (for example, a purple triangle) served as the CS−, which was never paired with the US. Pre-testing ratings obtained from the child participants indicated that the US was judged more negatively than a neutral ‘ding’ tone (F(1,83) = 30.53, P < 0.001, partial eta-squared , 90% confidence interval (CI) for effect size =0.14, 0.39). Children were instructed to press a button as quickly as possible in response to each geometric shape. Using a within-subjects design, in one condition, children underwent this conditioning procedure without the parent (‘alone’), and in the other they were seated next to their parent (‘parent’). The parent was seated slightly behind the child, so that the child could not see the parent but knew that he or she was present. There was no interaction with the parent, who was occupied with paperwork. A female experimenter always remained in the room. Conditions were counterbalanced and new stimuli were used for each condition. All statistical tests were two-sided, and an α level of 0.05 was used for statistical tests. All analyses controlled for the age and sex of the child.
Fig. 1 |. Schematic of study design.
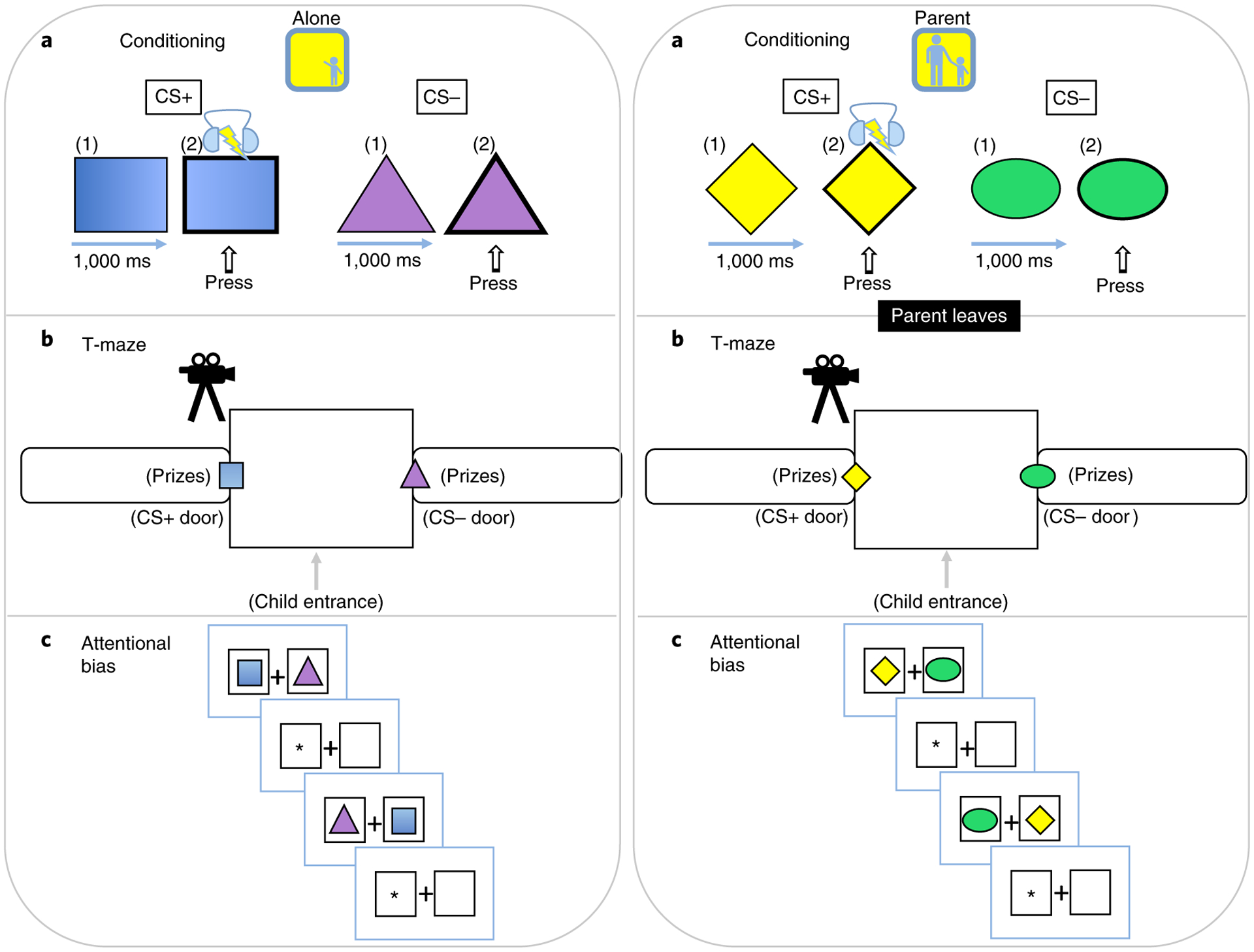
a, Conditioning phase. Children were presented with pairings of a CS+, a geometric shape that coterminated (50% reinforcement) with an aversive US through headphones. A different shape served as the CS− that was never paired with the US. Using a within-subjects design, children learned these associations alone (alone condition) or with their parent present (parent condition). b, T-maze phase. After conditioning, the parent left the room (if present for the parent condition), and children were placed in a T-maze for five trials. One path doorway displayed the CS+ and the other displayed the CS−, and on each trial children were instructed to select a doorway to retrieve a prize. Before the trial, the children were shown that the same buckets of treats (small toys) were behind both doors. c, Attentional bias phase. On a computer screen, the CS+ and CS− were simultaneously presented just before an asterisk, which appeared either behind the CS+ or the CS−. Children were instructed to indicate the location of this dot as quickly as possible using a keyboard.
During acquisition, multiple behavioural and physiological measures demonstrated success of acquiring the US–CS pairings. Children’s button-press reaction times and accuracy, heart rate and skin conductance responses (SCRs) each indicated that the children learned to discriminate the CS+ from the CS− in both the alone and parent conditions (Fig. 2; see Supplementary Information for additional results). In response to the CS+, relative to the CS−, children responded more slowly (F(1.75,125.76) = 14.94, P < 0.001, , 90% CI = 0.08, 0.26), showed higher accuracy (lower false alarm rate; (F(2,126) = 17.55, P < 0.001, , 90% CI = 0.11, 0.31)), exhibited heart rate deceleration (F(2,106) = 7.80, P < 0.001, , 90% CI = 0.04, 0.22) and exhibited greater SCRs (F(1,59) = 53.97, P < 0.001, , 90% CI = 0.32, 0.59). Thus, children acquired the US–CS pairings in both the alone and parent conditions, with no differences in acquisition between the alone and parent conditions. Further, the heart-rate deceleration together and SCR increase in response to the CS+ suggest greater attentional deployment20 and arousal21, respectively. Although we did not predict sex differences, a condition × stimulus × sex interaction (F(2,106) = 3.29, P = 0.04, , 90% CI = 0.00, 0.13) showed that boys were more likely to show increased heart rate in response to the CS+ reinforced in the parent condition.
Fig. 2 |. Behavioural and physiological evidence of CS+–CS− learning.
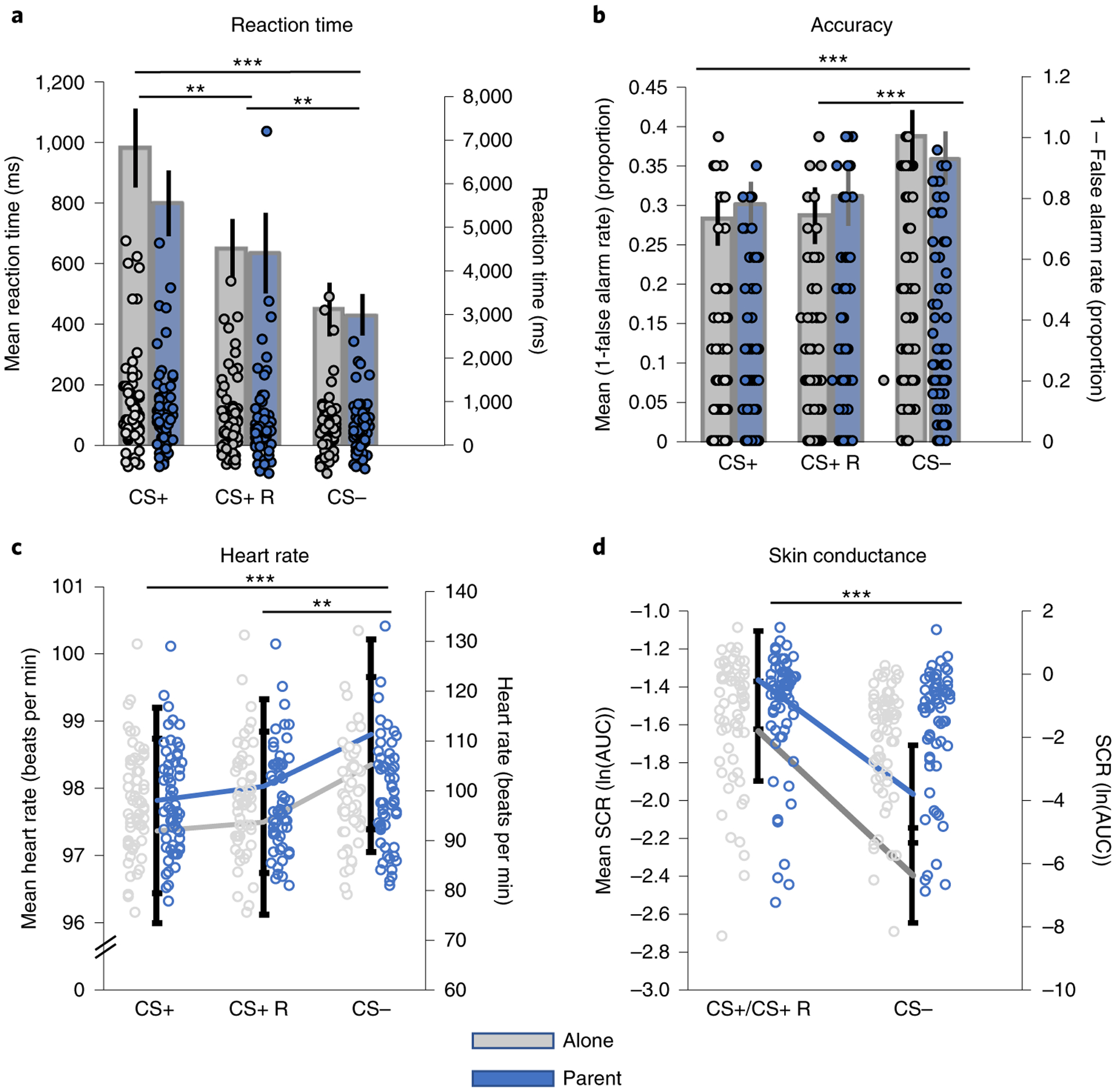
a–d, Learning was evidenced through multiple measures. Children (n = 69) exhibited slower reaction times (a; F(1.75,125.76) = 14.94, P < 0.001, , 90% CI = 0.09, 0.28) and fewer false alarms (b; F(2,126) = 17.55, , 90% CI = 0.11, 0.31) to the CS+ relative to the CS−. Heart rate (n = 59) showed deceleration to the CS+ stimuli relative to the CS− (c; F(2,106) = 7.80, P < 0.001, , 90% CI = 0.04, 0.22), consistent with attentional deployment, and sSCR (n = 65) increased the CS+ relative to the CS− (d; F(1,59) = 53.97, P < 0.001, , 90% CI = 0.32, 0.59). CS+ R, trials of the conditioned stimulus with reinforcement present. Note that for SCR, CS+/CS+ R refers to conditioned stimuli both with and without reinforcement present. Left y axes show mean ± s.e.m. (represented by height of the bars). Because of the high variability in the mean data, individual points are represented on the right y axes, where indicated. *P < 0.05, **P < 0.01 and P < 0.001. AUC, area under the curve.
Having demonstrated that children acquired the US–CS pairings, behavioural preference was tested in a T-maze (with parents having left the room). The children were placed in a T-maze for five trials, in which one path doorway displayed the CS+ and the other displayed the CS− (Fig. 1b). In each trial, the children were instructed to select a doorway from which they could retrieve a prize (they were shown that both doors held the same prizes before each trial). We reasoned that the presence of the parent during conditioning would attenuate aversive conditioning, and may even promote preference learning as observed in rodent studies6. Consistent with our hypotheses that parental presence would alter learning, when conditioned alone, children exhibited aversive learning with a bias away from the CS+ door (preferring the CS− door) (Fig. 3a). However, in the presence of the parent, children’s aversive conditioning was replaced by a modest, yet significant, CS+ preference (>50%) (F(1,85) = 11.80, P < 0.001, , 90% CI = 0.03, 0.23, post hoc one-sample t-test results: alone condition, (t(90) = −12.78, P < 0.001, Cohen’s d = 1.34, 95% CI = 1.05, 1.62); parent condition, (t(90) = 2.99, P = 0.004, Cohen’s d = 0.31, 95% CI = 0.10, 0.52). Although not predicted, a sex × age × condition interaction (F(2,49) = 3.20, P = 0.05, , 90% CI = 0.00, 0.24) showed that boys at each age were more likely to choose the CS+ in the parent condition relative to the alone condition, whereas influence of the parent condition on girls decreased with age. To rule out the possibility that the main effect of the parent condition was the result of noisy preschooler performance in general, a confirmatory analysis was conducted that removed those children who did not show aversive conditioning in the alone condition, producing a subsample of 55 preschoolers who clearly demonstrated a CS+ avoidance in the alone condition (that is, preference <50%). With this subsample, a significant preference for the CS+ in the parent condition remained (Fig. 3a, F(1,49) = 77.51, P < 0.001, , 90% CI = 0.00, 0.98) (see Supplementary Information). This effect of the parent condition was not an artefact of the parent distracting the child; the acquisition data showed that children learned equally well in both alone and parent conditions, and a distraction artefact would produce no preference for either stimulus in the T-maze—not the avoidance and preference patterns observed here. Instead, parental presence biased the learning towards preference learning. This finding suggests that physical proximity of the parent switches avoidance learning to approach learning in young children.
Fig. 3 |. Effect of parental presence on behavioural preference.
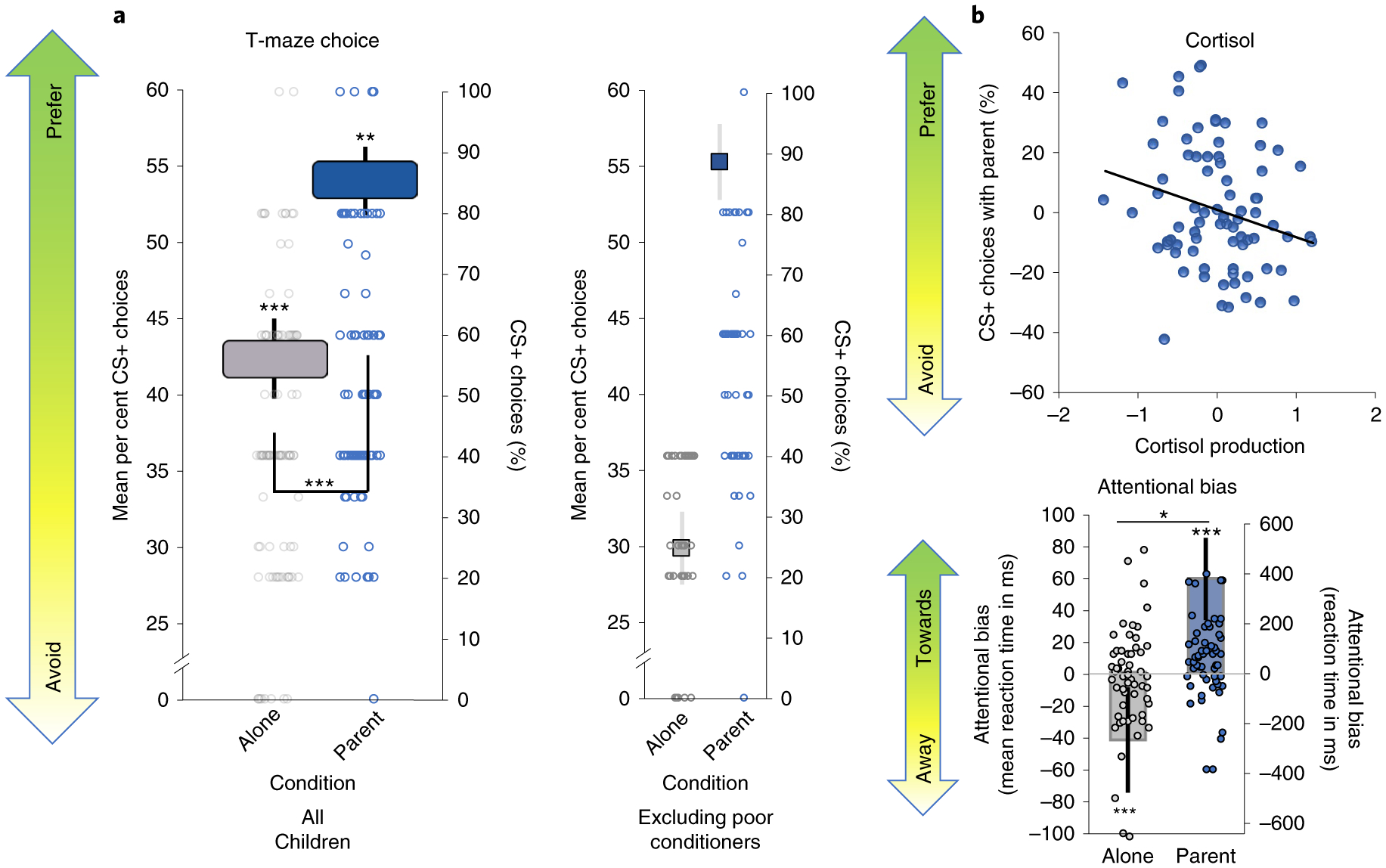
a, T-maze choice (n = 91). Children showed a behavioural avoidance of the CS+ when conditioned alone, but switched to a behavioural preference when conditioned with their parent present (F(1,85) = 11.80, P < 0.001, , 90% CI = 0.03, 0.23; post hoc one-sample t-test: alone condition, t(90) = −12.78, P < 0.001, Cohen’s d = 1.34, 95% CI = 1.05, 1.62; parent condition, t(90) = 2.99, P = 0.004, Cohen’s d = 0.31, 95% CI = 0.10, 0.52). Side bar shows that this preference remains when only considering children who demonstrated avoidance learning in the alone condition (that is, ‘good’ aversive conditioners) (F(1,49) = 77.51, P < 0.001, , 90% CI = 0.46, 0.70). b, Cortisol (n = 80). Parental effects on preference learning were more likely in children with lower cortisol levels and less likely in children with higher cortisol levels (β = −0.23, t(75) = −2.10, P = 0.04, R2 = 0.15, , Fchange(1,75) = 4.14, PFchange = 0.04). The x axis represents residuals for cortisol values controlling for age, sex, and percentage of CS+ choices (alone condition), hence the possibility of negative values. The y axis represents residuals for the percentage of CS+ choices (parent condition) controlling for age, sex and percentage of CS+ choices (alone condition), hence the possibility of negative values. c, Attentional bias (n = 56). Reaction time differences on the dot-probe task showed that children developed an attentional bias away from the CS+ when conditioned alone, whereas they developed an attentional bias towards the CS+ when conditioned with parental presence (F(1,50) = 5.57, P = 0.02, , 90% CI = 0.01, 0.24) (post hoc one-sample t-test on predicted values for alone: t(56) = −4.07, P < 0.001, Cohen’s d = 0.54, 95% CI = 0.27, 0.81); (post hoc one-sample t-test on predicted values for parent: t(56) = 6.92, P < 0.001, Cohen’s d = 0.92, 95% CI = 0.60, 1.22). Data are mean ± s.e.m (represented by height of the bars). Because of the high variability in preschooler’s mean data, individual points are plotted on the right y axes where indicated. *P < 0.05, **P < 0.01 and ***P < 0.001.
We assessed whether parental effects on learning would vary as a function of cortisol production. In the rodent, the ability of the parent to modulate learning is limited by the production of cortisol6. Cortisol is produced at low levels in the young animal, and once cortisol production exhibits its developmental increase, the parent becomes less effective in attenuating the neurobiology underlying aversive learning. Cortisol exhibits age-related changes across childhood22. We hypothesized that children’s salivary cortisol level would be associated with parental influence over conditioned behaviour. We found support for this hypothesis, in that higher levels of cortisol were associated with fewer CS+ choices in the parent condition (β = −0.23, t(75) = −2.10, P = 0.04, R2 = 0.15, , Fchange(1,75) = 4.14, PFchange = 0.04) (Fig. 3b; see Supplementary Information). While this finding is consistent with the interpretation that the lower cortisol levels in early childhood—perhaps resulting from parental buffering of cortisol—enable parental presence to modulate aversive learning, future testing with older children is needed to determine whether this type of learning is attenuated with increasing maturity as has been shown in other species2,6.
Thus far, we have found that children do not exhibit an avoidance of the CS+ learned in the parent’s presence but instead exhibit preference learning, a phenomenon that diminishes with higher cortisol levels. The final procedure investigated whether parents exert this effect by modulating children’s attentional focus. We used a dot-probe task to investigate children’s attentional biases to the conditioned stimuli (see Methods). The CS+ and CS− were simultaneously presented (for 3,000 ms) on a computer screen just before an asterisk (500 ms) appeared on the same side as either the CS+ or the CS− (Fig. 1c). Children were instructed to indicate the location of this asterisk as quickly as possible by pressing a button, and slower reaction times indexed a greater attentional bias for the stimulus that preceded the asterisk. The results showed that attentional biases differed according to the acquisition condition (F(1,50) = 5.57, P = 0.02, , 90% CI = 0.01, 0.24) (Fig. 3c). The CS+ acquired in the alone condition resulted in an attentional avoidance of the CS+ (post hoc one-sample t-test on predicted values: t(56) = −4.07, P < 0.001, Cohen’s d = 0.54, 95% CI = 0.27, 0.81). In the current design, we cannot distinguish whether the attentional bias away from the CS+ was the result of attention directed away from the CS+ or towards the CS− safety signal, but this result is consistent with previous findings of attentional avoidance of threat cues in healthy children23. By contrast, when the CS+ was learned in the parent condition, the opposite bias developed, namely a greater attention bias to the CS+ (post hoc one-sample t-test on predicted values: t(56) = 6.92, P < 0.001, Cohen’s d = 0.92, 95% CI = 0.60, 1.22). The parental effect on attention bias indicates that the presence of the parent changes emotional learning in part because the parent’s presence switches the child’s distribution of attention away from the CS+ when alone and towards the CS+ in the parent’s presence. This pattern supports the view of the parent as providing a safety signal for offspring24, a cue that enables exploration of a novel stimulus that would otherwise be avoided.
That parents influence emotional development is well established25–30; the current study provides an understanding of a fundamental learning mechanism underlying this influence. These data demonstrate that the mere physical presence of parents changes children’s early approach–avoidance learning. This finding provides a deeper explanation of how parents scaffold children’s emotional learning. Parental presence changes the nature and content of associative learning processes. In the absence of the parent, children learn to avoid an aversively conditioned stimulus. This learning is modulated by parental presence, which instead produces a behavioural preference for that same stimulus. Our findings suggest that this process is not simply a result of the parent calming the child. Indeed, during acquisition, the parent did not have an effect on acquisition measures. Instead, the parent’s presence changed learning, which was observed later during recall events (that is, T-maze and dot-probe tasks) when the parent was no longer present. These findings suggest that the nature of what was learned (for example, consolidation) was changed by the parent, as suggested by the rodent model30. Parental behaviours were not manipulated in this study, and thus the effect of parental behaviour should be examined in future work.
The learning processes examined here in young children have previously been investigated in the rat. Whether these processes are mechanistically identical and conserved across species or simply developmental analogues of each other cannot be determined from this study, but these behaviours may serve similar functions across species13. Rat pups exhibit a behavioural preference (for example, approach in a Y-maze) for a CS that was previously paired with either an aversive or rewarding stimulus (for example, tail shock or stroke, respectively) if the pairing occurred in the parent’s presence6, whereas they would avoid the aversive CS in the absence of the parent. In both the rodent model and with the children in the current study, this effect of the parent does not manifest until the choice behaviour occurs, suggesting that the parent’s presence changes the nature of what is learned (for example, consolidation processes)31. In rodent models, it has been shown that this modulation by the parent occurs because parental cues suppress the offspring’s neurobiology (that is, the amygdala) responsible for aversive learning6, thereby allowing competing learning systems to promote approach behaviours. The result of this cascade is an acquired preference for associations formed in the parent’s presence. These parental effects must occur during a sensitive period in early brain development, when immature glucocorticoid levels permit parental buffering of the amygdala. The current study cannot address whether the observed effects in children reflect sensitive-period effects. However, we also found an association between cortisol and the effect of the parent on learning. One interpretation of this association is that HPA axis maturation and corresponding increases in cortisol production (perhaps reflecting parents’ decreasing ability to buffer cortisol and/or age-related cortisol increases in the preschool period) terminate this form of learning. Preschoolers’ cortisol responses to stressors have been shown to be buffered by parents32, and age-related cortisol increases have been identified in the current age range22. Although cortisol was not collected as part of a formal stress paradigm in the current study, the present findings provide support for this interpretation, in that preschool-age children with higher cortisol levels were less responsive to parental effects during aversive conditioning. Whether or not this effect is age-limited cannot be determined from these data. Although the current study can make no claims about a sensitive period in humans, there are some results that motivate future work to address the possibility. For example, the age × sex × condition interaction in the T-maze procedure was not anticipated; although all children’s learning was modulated by the parent, boys at all ages showed a larger effect than girls, and the effect waned with increasing age in girls. If boys’ socio-emotional development tends to lag behind that of girls33, this age ×sex × condition interaction motivates future longitudinal studies to test whether there is a developmental window for the observed effect of parents (that girls exit out of before boys). This developmental window, during which parental presence or absence differentially engages learning systems, coincides with the increasing yet far from complete physical independence from the parent observed in the childhood period13.
This learning pattern fits the developmental ecology of the young child in that when children are alone, adult-like threat-learning processes are deployed13. By contrast, these same adult-like strategies are rendered unnecessary when parents are available, and are actually counterproductive for attachment-related behaviours. Thus, a behavioural preference is produced instead. This pattern of behaviour is predicted by previous findings showing that parental cues phasically modify the fronto-amygdala circuit responsible for aversive learning18. It has been argued that this seemingly paradoxical preference learning places the young child at a survival advantage by maximizing preference for parent-associated stimuli (that is, attachment behaviours), even in the context of noxious stimuli2,6. An alternative interpretation of the current findings is that the presence of the parent during acquisition subsequently facilitates exploration of the conditioned stimulus during the test phase (that is, the T-maze or dot-probe task). Indeed, a large body of literature has shown that parental presence increases exploratory behaviour in young children5. However, in somewhat of a contrast to previous work, parents’ effects on children’s exploratory behaviour did not occur during acquisition (that is, when the parent was present); there was no main effect of the parent on acquisition indices. Instead, parental effects were observed later during the test phases (that is, when the parent was no longer present). Thus, the parental effects described in this study do not seem to reflect the parent simply calming the child and thereby increasing exploration. It is possible that learning in the presence of the parent increased later exploration of the CS+, so that the child was testing whether that CS+ would lead to the prize; however, this possibility is tempered by the fact that children were routinely (before each trial) shown that each CS in the T-maze contained the same prizes. The dot-probe results suggest that the CS+ acquired in the parent’s presence heightened children’s attentional biases, suggesting that parental presence may increase the relevance or salience of the cue. In their original descriptions of attachment behaviours, Bowlby and Ainsworth posited that these two processes, maintaining proximity to parental cues and exploring novel stimuli in the context of the parent, are complementary processes1,5; thus a further alternative interpretation of the current findings is that these two processes co-occur—the presence of the attachment figure permits greater exploration of novel stimuli (even if risky or aversive) and subsequent preference formation of that cue is associated with the parent.
For the young altricial animal, approaching parent-associated cues is so critical for offspring survival that it will occur regardless of whether the parent is nurturing or abusive, as shown in several laboratory models including rats, monkeys and dogs2. Although these findings significantly inform the normative construction of human affective development, they also have large implications for emotional development following maltreatment. The current findings, namely that children develop a preference for cues associated with the parent even if aversive, provide some explanation to family courts and case workers for children’s sometimes bewildering attachments to parents. Understanding such learning systems during development might begin to answer the question of why attachments develop to parents, even in the context of abuse, and future examination of these learning processes in the context of abusive care is needed. The seemingly paradoxical approach behaviours in the parents’ presence is a startling reminder that the developing brain is not simply an immature version of the adult brain, but is instead designed to collaborate with the expected caregiving ecology.
Methods
Participants.
All protocols were approved by the University of California Institutional Review Board. Parents provided written consent for their own and their child’s participation, and children provided verbal assent for participation. A total of 106 children (3–5 yr old, mean (s.d.) = 4.4 (0.1) yr old, 48 male, 58 female) were recruited from a community sample via local birth records, Institutional Review Board-approved local newspaper ads and online classifieds. Parents indicated that 86 children were European-American or Caucasian, 19 were Asian-American, 8 were African-American or black, 6 were Native American or Alaskan Native, 3 were Native Hawaiian or Pacific Islander, and 10 indicated ‘other’ (note that participants could have endorsed more than one race) (data were missing for 6 children). Eighteen children were also identified as Hispanic or Latino(a). Out of these, 98 children provided usable and complete data for either the T-maze (n = 91) or the dot-probe (n = 62) tasks. Seventy-six per cent of parents were born in the United States. Modal education level for parents was ‘Masters degree’ although parental education ranged from ‘high school degree’ to ‘professional degree’. Modal family income was ‘>US$200,000 yr−1’, although income ranged from ‘<US$10,000 yr−1’ to ‘>US$200,000 yr−1’. The home environment was assessed through a modification of the ‘home’ short form34 that parents were asked to complete for this study; this measure assesses the quality of a child’s home environment and includes cognitive stimulation and emotional support provided by a child’s family. Overall, the average home environment score (mean (s.d.), range = 19.08 (2.51), 11–24) was comparable to values from the National Longitudinal Study of Youth35, which included a nationally representative cross-section of youth. Parents’ depression (Beck Depression Inventory36, mean (s.d.) = 4.20 (5.11) and anxiety (Spielberger trait anxiety37 mean (s.d.) = 47.48 (5.43)) scores were in the normal range. Parents reported a mean (s.d.) of 2.21 (2.10) stressful events in the past year on the Life Events Questionnaire38. Among the children, 15.6% had no siblings, 55.6% had at least one older sibling and 47.8% had at least one younger sibling. Children’s behaviour problems were in the normal range (Child Behaviour Checklist39, total problems T score mean (s.d.) = 42.6, 9.82). Parents average self-reported parenting was overall warm (mean (s.d.), range = 61.29 (14.27), 34–107 out of a possible 132) and moderately restrictive (mean (s.d.), range = 98.05 (6.15) (75–108) out of a possible 108) according to scores on the Modified Child Rearing Practices Report40.
Conditioning procedure.
Individually, 98 children, wearing headphones, sat in front of a computer in a quiet testing room. The computer screen presented repeated serial blocks of two shapes (for example, square as the CS+ and a triangle as the CS−, presented in blocked fashion). There was a total of 10 blocks of 4 shapes per block, resulting in 20 presentations of the CS+ and 20 presentations of the CS− during acquisition. Each shape had an average visual angle of 15°. The shape would be on screen for 1,000 ms, at which point the border of the shape thickened slightly (Fig. 1a). For the CS+, this thickening of the border coincided with the onset of the US (an aversive noise). An aversive noise has been shown to be an effective US in aversive conditioning41,42. Conditioning sound volume was calibrated to each child to reach a point where the noise was very loud but not painful. The CS+ was reinforced with the US 50% of the time. Between each shape presentation, there was a 1,000 ms inter-trial interval. Thus, each block lasted approximately 20 s long, and each block was separated by 4 s. The entire conditioning procedure lasted approximately 3 min. Children were instructed to press a mouse button as quickly as possible when they saw the border thicken. All stimuli would terminate when the child pressed the button. This button-press procedure was included to provide behavioural metrics of acquisition (that is, reaction times and accuracy).
The female experimenter remained in the room with the children. In one condition (alone) children underwent this conditioning procedure alone, and in the other (parent) they were seated to the left and in front of their parent. This order was randomly counterbalanced across subjects using a random number generator. Ninety-two parents were mothers and six were fathers. Parents were instructed not to interact with their child, and there was no interaction between the child and the parent, who was occupied with paperwork. Thus, children were conditioned twice, each time using different geometric shapes as the CS, with shapes stimuli counterbalanced across participants. Because of the nature of data collection, which required that the parent be in the room with the experimenter or not, data collection and analyses were not performed blind to the conditions of the experiment.
Conditioning pre-testing of US.
Before conditioning, children reported on how much they liked or disliked the US and another noise (a computerized chime). Children reported on a nine-point Likert scale that ranged from ‘really don’t like’ to ‘really like a lot’.
Conditioning physiological methods.
SCR and heart rate were collected using the BioPac system. SCR sensors were placed on the medial plantar surface of the foot and the big toe (because young children seem to tolerate this placement without excess motion artefact better than on the hand)21. Like hands, feet contain eccrine sweat glands and can produce skin responses43. SCR sensors were secured with medical tape. Heart-rate sensors were placed on the front of the body under each clavicle and on the lower left back. After about a 5 min waiting period, data were collected continuously with Acqknowledge software during the conditioning procedure.
Offline, SCR data were submitted to a low-pass filter and smoothing over 200 samples. SCRs were averaged starting from 500 ms from the start of the stimulus onset across a period of 4,500 ms after stimulus onset for the duration of the stimulus block (that is, stimuli of the same condition presented in consecutively in blocks of four) and averaged for each condition. Note that the SCR responses to CS+ reinforced and non-reinforced were presented within the same block and thus could not be resolved, and are presented in average here. Additionally, the first trial in each block was not included in computations to avoid signal bleed from the previous block. SCR area under the curve values were positively skewed and were therefore transformed using natural logarithm. The SCR data were divided into four categories: CS+ alone, CS− alone, CS+ with parent and CS− with parent. For each stimulus presentation, average heart rate and area under the curve for SCR was computed. Heart rate was averaged, starting from the start of the stimulus onset across 1,000 ms after stimulus onset, and these data were divided into six categories: CS+ alone, CS+ reinforced alone, CS− alone, CS+ with parent, CS+ reinforced with parent and CS− with parent.
T-maze procedure.
Following each conditioning procedure, children underwent the T-maze preference test. In the same room where the conditioning procedure occurred, a tent was set up. If the child had just completed the parent conditioning session, the parent left the room and waited in the adjoining room. The tent served as the human T-maze; there was a main door through which the child entered (that is, crawled through), and inside the tent, there were two additional doors (see Fig. 1b). One door had a laminated picture of the CS+ velcroed to it, and the other door had the CS− velcroed to it. Children were told, “Now we’re going to play a game with signs and treats. I want you to go into the tunnel five times. Each time you go in, I want you to look at the signs on both doors. Then, I want you to decide which door you want to reach behind to get a treat. Then you can get one treat from behind one of the doors. The treats on both sides are the same.” The children were shown that the same buckets of treats (small toys) were behind both doors. Children would enter the tent and look at both doors before choosing a door. After each trial, the experimenter would change the CS+ and CS− locations on the basis of a predetermined random order and she would mix up the buckets of treats (children could not see what experimenter was doing). Before each subsequent trial, children would be told, “Are you ready? Remember to first look at both signs on the doors. Only then decide which door you want to reach through to get your treat. The treats on both sides are the same.” Children were shown that the same treats were behind both doors before each trial. Video recordings were made of this session to ensure data usability. There were five trials. Any trial in which the child did not look at both doors before making a choice was excluded. The final score of interest was the percentage of valid trials in which the child chose the CS+ door. Both parent and alone conditions were collected for 91 out of the 98 children (7 did not complete both sessions due to fatigue or time limitations).
Cortisol procedure.
Eighty-six children provided a usable laboratory sample of salivary cortisol (one did not provide a sample, nine did not provide enough saliva for assay and two had extreme values >3 s.d. from the mean). Participants provided saliva samples approximately 30 min after arriving in the laboratory, during which time they remained with their parents (this time was occupied by explanation of the experimental procedure, signing consents and visiting the restroom). Parents were instructed to have their child avoid eating for at least 1 h before visiting the laboratory. Using a health diary, parents reported no child illness, medication use and/or unusual levels of activity. Following Salimetrics protocol for children under 6 yr old, children placed two sorbettes under their tongue for 1 min per sample. The time of collection ranged from 09:00 to 17:30, with the average time of collection at 12:35 (s.d. = 2 h 11 min). Samples were stored in a locked freezer at −20 °C until they were posted in dry ice to Technical University Dresden by C. Kirschbaum (Biological Psychology Laboratory). After thawing, samples were centrifuged at 3,000 r.p.m. for 5 min, which resulted in a clear supernatant of low viscosity. Salivary cortisol concentrations were measured using commercially available chemiluminescence immunoassays with high sensitivity (IBL International). The intraassay and interassay coefficients for cortisol were below 8% (ref.44). Evidence suggests that within the hours in which the experiment was performed, time of day matters less for cortisol levels in children than it does for wake-up and bedtime45,46. In the current sample, there was no association between time of day and cortisol levels (r(76)P = −0.13, P = 0.24, 95% CI = −0.08, 0.35, controlling for age and sex). In addition, we repeated our analysis including time of day as a covariate. Cortisol values, which were expressed in nM, were positively skewed and were therefore transformed using natural logarithm.
Attentional biases via dot-probe procedure.
We used a dot-probe task to investigate children’s attentional biases to the conditioned stimuli. Dot-probe tasks have been used as effective measures of attentional biases23 because although the children’s overt task is simple (identify the location (right or left) of an asterisk), reaction times vary with the location congruency between the asterisk and the affective stimulus, thus providing an index of attentional bias. Of the total of 98 children, 62 provided usable dot-probe task data (6 not completed due to fatigue, 19 only completed one dot-probe task and 11 had difficulty with the button pressing). The children were seated in a room separately from their parent. On a computer screen, the CS+ and CS− were simultaneously presented (500 ms) just before an asterisk, which appeared behind the CS+ or the CS− for up to 3,000 ms (Fig. 1c). Children were instructed to indicate the location of this dot as quickly as possible using the keyboard. The trial ended when children pressed the button for the asterisk. There was a 1,500 ms inter-trial interval between trials, and a total of 10 trials, with 50% congruent and 50% incongruent with the CS+. To ensure that children were on-task, an accuracy cut-off was used (accuracy >50%), excluding an additional 6 children with low accuracy for a final sample of 56 children. A bias score was calculated by subtracting the response time when the asterisk was behind the CS+ (that is, congruent) from when it was behind the CS− (that is, incongruent). Positive scores indicate a greater attentional allocation to the CS+, and negative scores indicate avoidance of the CS+ (or bias towards the CS−). Two bias scores were computed from reaction times (from correct trials only) for each child:
where RT is the reaction time. These bias scores were entered into a repeated measures analysis of variance with bias as the dependent variable and condition (alone, parent) as the independent variable, controlling for age and sex.
Reporting Summary.
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary Material
Acknowledgements
This research was supported in part by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Grant R37HD083217 (to R.M.S.) and the National Institute of Mental Health Grant R01MH091864 (to N.T.) The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript. The authors thank A. Galván for her helpful feedback on previous drafts.
Footnotes
Data availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Code availability
The code that supports the findings of this study is available from the author on reasonable request.
Competing interests
The authors declare no competing interests.
Supplementary information is available for this paper at https://doi.org/10.1038/s41562-019-0656-9.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bowlby J Attachment and Loss (Basic Books, 1969). [Google Scholar]
- 2.Rajecki DW, Lamb ME & Obmascher P Towards a general theory of infantile attachment: a comparative review of aspects of the social bond. Behav. Brain Sci 3, 417–464 (1978). [Google Scholar]
- 3.Stafleu A, Van Staveren WA, De Graaf C, Burema J & Hautvast JG Family resemblance in beliefs, attitudes and intentions towards consumption of 20 foods; a study among three generations of women. Appetite 25, 201–206 (1995). [DOI] [PubMed] [Google Scholar]
- 4.Krumhansl CL & Zupnick JA Cascading reminiscence bumps in popular music. Psychol. Sci 24, 2057–2068 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Ainsworth MD Object relations, dependency, and attachment: a theoretical review of the infant–mother relationship. Child Dev. 40, 969–1025 (1969). [PubMed] [Google Scholar]
- 6.Moriceau S & Sullivan RM Maternal presence serves as a switch between learning fear and attraction in infancy. Nat. Neurosci 9, 1004–1006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stronach EP et al. Child maltreatment, attachment security, and internal representations of mother and mother–child relationships. Child Maltreat. 16, 137–145 (2011). [DOI] [PubMed] [Google Scholar]
- 8.Ainsworth MD & Bell SM Attachment, exploration, and separation: illustrated by the behavior of one-year-olds in a strange situation. Child Dev. 41, 49–67 (1970). [PubMed] [Google Scholar]
- 9.Clancy B, Finlay BL, Darlington RB & Anand KJ Extrapolating brain development from experimental species to humans. Neurotoxicology 28, 931–937 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thompson JL & Nelson AJ Middle childhood and modern human origins. Hum. Nat 22, 249–280 (2011). [DOI] [PubMed] [Google Scholar]
- 11.Hoffman KT, Marvin RS, Cooper G & Powell B Changing toddlers’ and preschoolers’ attachment classifications: the circle of security intervention. J. Consult. Clin. Psychol 74, 1017–1026 (2006). [DOI] [PubMed] [Google Scholar]
- 12.Fisher PA & Kim HK Intervention effects on foster preschoolers’ attachment-related behaviors from a randomized trial. Prev. Sci 8, 161–170 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Callaghan BL et al. The developmental ecology of fear neurobiology across development. Annu. Rev. Clin. Psychol 15, 345–369 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bailey D et al. Marked biological variance in endocrine and biochemical markers in childhood: establishment of pediatric reference intervals using healthy community children from the CALIPER cohort. Clin. Chem 59, 1393–1405 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Gee DG et al. A developmental shift from positive to negative connectivity in human amygdala–prefrontal circuitry. J. Neurosci 33, 4584–4593 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Silvers JA et al. The transition from childhood to adolescence is marked by a general decrease in amygdala reactivity and an affect-specific ventral-todorsal shift in medial prefrontal recruitment. Dev. Cogn. Neurosci 25, 128–137 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hostinar CE, Johnson AE & Gunnar MR Parent support is less effective in buffering cortisol stress reactivity for adolescents compared to children. Dev. Sci 18, 281–297 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gee DG et al. Maternal buffering of human amygdala–prefrontal circuitry during childhood but not during adolescence. Psychol. Sci 25, 2067–2078 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Callaghan BL et al. Decreased amygdala reactivity to parent cues protects against anxiety following early adversity: an examination across 3 years. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 10.1016/j.bpsc.2019.02.001 (in the press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casey BJ & Richards JE Sustained visual attention in young infants measured with an adapted version of the visual preference paradigm. Child Dev. 59, 1514–1521 (1988). [PubMed] [Google Scholar]
- 21.Bach DR, Flandin G, Friston KJ & Dolan RJ Modelling event-related skin conductance responses. Int J. Psychophysiol 75, 349–356 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flannery JE et al. Diurnal cortisol after early institutional care-age matters. Dev. Cogn. Neurosci 25, 160–166 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vasey MW, el-Hag N & Daleiden EL Anxiety and the processing of emotionally threatening stimuli: distinctive patterns of selective attention among high- and low-test-anxious children. Child Dev. 67, 1173–1185 (1996). [PubMed] [Google Scholar]
- 24.Raineki C et al. Paradoxical neurobehavioral rescue by memories of early-life abuse: the safety signal value of odors learned during abusive attachment. Neuropsychopharmacology 40, 906–914 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenberg N, Cumberland A & Spinrad TL Parental socialization of emotion. Psychol. Inq 9, 241–273 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sroufe LA, Coffino B & Carlson EA Conceptualizing the role of early experience: lessons from the minnesota longitudinal study. Dev. Rev 30, 36–51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Campos JJ Human Emotions: Their New Importance and Their Role in Social Referencing. Annual Report 1–7 (Research & Clinical Center for Child Development, 1981). [Google Scholar]
- 28.Kernberg OF Early ego integration and object relations. Ann. N. Y. Acad. Sci 193, 233–247 (1972). [DOI] [PubMed] [Google Scholar]
- 29.Winnicott DW The theory of the parent-infant relationship. Int. J. Psychoanal 41, 585–595 (1960). [PubMed] [Google Scholar]
- 30.Baumrind D Effects of authoritative parental control on child behavior. Child Dev. 37, 887–907 (1966). [Google Scholar]
- 31.Roth TL & Sullivan RM Consolidation and expression of a shock-induced odor preference in rat pups is facilitated by opioids. Physiol. Behav 78, 135–142 (2003). [DOI] [PubMed] [Google Scholar]
- 32.Kertes DA et al. Inhibited temperament and parent emotional availability differentially predict young children’s cortisol responses to novel social and nonsocial events. Dev. Psychobiol 51, 521–532 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schore AN All our sons: the developmental neurobiology and neuroendocrinology of boys at risk. Infant Ment. Health J 38, 15–52 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Caldwell BM,. & Bradley RH Home Observation for Measurement of the Environment. (University of Arkansas at Little Rock:, 1984). [Google Scholar]
- 35.Baharudin R & Luster T Factors related to the quality of the home environment and children’s achievement. J. Fam. Issues 19, 375–403 (1998). [Google Scholar]
- 36.Beck AT, Steer RA, Ball R & Ranieri W Comparison of Beck depression inventories YIA and YII in psychiatric outpatients. J. Pers. Assess 67, 588–597 (1996). [DOI] [PubMed] [Google Scholar]
- 37.Spielberger CD (Psychological Assessment Resources, 1988).
- 38.Coddington RD The significance of life events as etiologic factors in the diseases of children. II. A study of a normal population. J. Psychosom. Res 16, 205–213 (1972). [DOI] [PubMed] [Google Scholar]
- 39.Achenbach TM (University of Vermont, Department of Psychology, 1991).
- 40.Rickel AU & Biasatti LL Modification of the block child rearing practices report. J. Clin. Psychol 38, 129–134 (1982). [Google Scholar]
- 41.Neumann DL & Waters AM The use of an unpleasant sound as an unconditional stimulus in a human aversive Pavlovian conditioning procedure. Biol. Psychol 73, 175–185 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Silvers JA et al. Previous Institutionalization is followed by broader amygdala–hippocampal–PFC network connectivity during aversive learning in human development. J. Neurosci 36, 6420–6430 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ham J & Tronick E A procedure for the measurement of infant skin conductance and its initial validation using clap induced startle. Dev. Psychobiol 50, 626–631 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kirschbaum C & Hellhammer DH Salivary cortisol. Encycl. Stress 3, 379–383 (2000). [Google Scholar]
- 45.Watamura SE, Donzella B, Kertes DA & Gunnar MR Developmental changes in baseline cortisol activity in early childhood: relations with napping and effortful control. Dev. Psychobiol 45, 125–133 (2004). [DOI] [PubMed] [Google Scholar]
- 46.Sumner MM, Bernard K & Dozier M Young children’s full-day patterns of cortisol production on child care days. Arch. Pedia. Adolesc. Med 164, 567–571 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


