Abstract
Background:
Cervical insufficiency is a risk factor for spontaneous midtrimester abortion or early preterm birth. Intra-amniotic infection has been reported in 8–52% of such patients, and intra-amniotic inflammation in 81%. Some professional organizations have recommended perioperative antibiotic treatment when emergency cervical cerclage is performed. The use of prophylactic antibiotics is predicated largely on the basis that they reduce the rate of complications during the course of vaginal surgery. However, it is possible that antibiotic administration can also eradicate intra-amniotic infection/inflammation and improve pregnancy outcome.
Objective:
To describe the outcome of antibiotic treatment of patients with cervical insufficiency with either intra-amniotic infection/inflammation.
Study design:
The study population consisted of 22 women who met the following criteria: 1) singleton pregnancy; 2) painless cervical dilatation of >1cm between 16.0–27.9 weeks of gestation; 3) intact membranes and absence of uterine contractions; 4) transabdominal amniocentesis performed for the evaluation of the microbiologic and inflammatory status of the amniotic cavity; 5) presence of intra-amniotic infection/inflammation; and 6) antibiotic treatment (regimen consisted of ceftriaxone, clarithromycin, and metronidazole). Amniotic fluid was cultured for aerobic and anaerobic bacteria and genital mycoplasmas, and polymerase chain reaction (PCR) for Ureaplasma spp. was performed. Intra-amniotic infection was defined as a positive amniotic fluid culture for microorganisms or a positive PCR for Ureaplasma spp., and intra-amniotic inflammation was suspected when there was an elevated amniotic fluid white blood cell count (≥19 cells/mm3) or a positive rapid test for metalloproteinase-8 (MMP-8) (sensitivity 10 ng/mL). For the purpose of this study, the “gold standard” for diagnosis of intra-amniotic inflammation was an elevated interleukin (IL)-6 concentration ≥2.6 ng/mL using ELISA. The results of amniotic fluid IL-6 were not available to managing clinicians. Follow-up amniocentesis was routinely offered to monitor the microbiologic and inflammatory status of amniotic cavity and fetal lung maturity. Treatment success was defined as resolution of intra-amniotic infection/inflammation or delivery ≥34 weeks.
Results:
1) Of 22 patients with cervical insufficiency and intra-amniotic infection/inflammation, three (13.6%) had microorganisms in the amniotic fluid; 2) of the 22 patients, six (27%) delivered within one week of amniocentesis, and the remaining 16 (73%) delivered more than one week after the diagnostic procedure. Among these, twelve had a repeat amniocentesis to assess the microbial and inflammatory status of the amniotic cavity; 3) in 75% (9/12), there was objective evidence of resolution of intra-amniotic inflammation or infection demonstrated by analysis of amniotic fluid at the time of repeat amniocentesis; and 4) of the four patients who did not have a follow-up amniocentesis, all delivered ≥34 weeks, two of whom delivered at term; thus, treatment success occurred in 59% (13/22) of cases.
Conclusion:
In patients with cervical insufficiency and intra-amniotic infection/inflammation, administration of antibiotics (ceftriaxone, clarithromycin, and metronidazole) was followed by resolution of the intra-amniotic inflammatory process or infection in 75% of patients, and was associated with treatment success in about 60% of cases.
Keywords: anti-microbial agents, interleukin-6, ceftriaxone, cephalosporins, cerclage, clarithromycin, metronidazole, MMP-8, amniotic fluid, prematurity, pregnancy, Ureaplasma urealyticum, chorioamnionitis, biomarker
Introduction
The term “cervical insufficiency” refers to a condition in which patients present with a dilated cervix in the midtrimester of pregnancy, protruding membranes in the absence of uterine contractions, or vaginal bleeding.1 The standard treatment is placement of an emergency cerclage.1–19 The best scientific evidence in support of this intervention derives from a randomized clinical trial that included patients with cervical insufficiency (i.e. membranes at or below a dilated external cervical os, before <27 weeks) treated with bedrest and antibiotics, and who were randomly assigned to emergency cerclage and indomethacin vs. bedrest only.2 Patients who received emergency cerclage, indomethacin, and antibiotics had a significantly lower rate of preterm delivery <34 weeks, than those allocated to bedrest and antibiotics (53.8% [7/13] vs. 100% [10/10], p=0.02).2
Intra-amniotic infection ascertained by amniocentesis has been reported in 8–52% of patients presenting with cervical insufficiency.20–27 The use of a cervical cerclage in such patients leads to uniformly poor results, since women develop clinical chorioamnionitis, rupture of the chorioamniotic membranes, or vaginal bleeding and require delivery.21 In contrast, patients with cervical insufficiency but without intra-amniotic infection who undergo cerclage placement have a better pregnancy outcome.21,22,25
Microorganisms and their products (i.e. endotoxin,28,29 peptidoglycans, or glucans30) can elicit an intra-amniotic inflammatory response,31–45 which is easily determined by measuring amniotic fluid pro-inflammatory cytokines [such as interleukin (IL)-6 or matrix metalloproteinase-8 (MMP-8)].46–93 Intra-amniotic inflammation can also be present in patients with cervical insufficiency23,94,95 and is associated with preterm delivery, as well as adverse pregnancy and neonatal outcomes.23,94 A subset of patients with intra-amniotic inflammation has no demonstrable microorganisms and, therefore, represents examples of sterile intra-amniotic inflammation, caused by danger signals or alarmins,96–102 which activate the inflammasome. Inflammasome activation can lead to preterm delivery.90,103–108
The traditional view has been that intra-amniotic infection in patients with cervical insufficiency cannot be successfully treated, and the same is the case for intra-amniotic inflammation. The objective of this report is to communicate a case series in which women with cervical insufficiency and intra-amniotic infection/inflammation were treated with antimicrobial agents, and this resulted in eradication of intra-amniotic infection/inflammation in a subset of patients.
Materials and Methods
Study design
This was a retrospective case series study of patients admitted to Seoul National University Hospital between January 2004 and April 2014 who met the following criteria: 1) singleton gestation; 2) midtrimester painless cervical dilatation (>1cm) with visible membranes through the cervical os and intact membranes confirmed by sterile speculum examination; 3) gestational age between 16–27.9 weeks; 4) absence of uterine contractility according to the patient and uterine tocodynamometer; 5) transabdominal amniocentesis to evaluate the microbiologic and inflammatory status of the amniotic cavity; 6) presence of intra-amniotic infection/inflammation; and 7) administration of antibiotics (regimen consisted of ceftriaxone, clarithromycin, and metronidazole).
Intra-amniotic infection was defined as a positive amniotic fluid culture or positive polymerase chain reaction (PCR) assay for Ureaplasma spp., while intra-amniotic inflammation was defined as an elevated amniotic fluid IL-6 concentration (≥2.6 ng/mL), as previously reported.72,92,109–119 Cervical dilatation was determined by visual examination at the time of sterile speculum examination.23,26 Previous studies have shown a good correlation between cervical dilatation observed visually and through digital examination.120 Every effort was made to avoid digital examination to decrease the risk of ascending intra-amniotic infection.
Amniocentesis was routinely offered to all patients admitted with the diagnosis of cervical insufficiency to assess the microbiologic status of the amniotic cavity and the presence of intra-amniotic inflammation. This is based on previous studies that report a 52% frequency of intra-amniotic infection in patients with cervical insufficiency21 and, in 81% of these cases, intra-amniotic inflammation is also present;23 the prognosis under these circumstances is poor.21–23,25,26
All patients provided written informed consent. The Institutional Review Board of the Seoul National University Hospital approved the collection and use of these samples and clinical information for research purposes. The Seoul National University has a Federal Wide Assurance with the Office for Human Research Protections (OHRP) of the Department of Health and Human Services (DHHS) of the United States.
Amniotic fluid analysis
Amniotic fluid was cultured for aerobic and anaerobic bacteria as well as genital mycoplasmas (Ureaplasma spp. and Mycoplasma hominis). Samples were also assayed for Ureaplasma spp. using PCR with specific primers, and performed in our clinical microbiology laboratory using methods previously described26 at the discretion of the attending physician. This test has been available in the clinical microbiology laboratory of the Hospital since 2007. A PCR assay for Ureaplasma spp. was performed using stored amniotic fluid which was obtained before 2007. An aliquot of amniotic fluid was examined in a hemocytometer chamber to determine the white blood cell (WBC) count.57 Between March 2005 and December 2010, a rapid MMP-8 bed-side test (MMP-8 PTD Check test, SK Pharma Co, Ltd, Kyunggi-do, Korea), was performed and used in patient management. Details of the MMP-8 rapid test have been previously described.109,121–123 In a subset of patients, MMP-8 concentration in amniotic fluid was measured in our laboratory using a commercially available enzyme-linked immunosorbent assay (ELISA) (Amersham Pharmcia Biotech, Inc., Bucks, UK) and the results were available to clinicians. Intra-amniotic inflammation was suspected when there was an elevated amniotic fluid white blood cell count (≥19 cells/mm3),67,121 a positive rapid test for MMP-8,108,123–125 or an MMP-8 concentration >23 ng/mL).23,81,124,125
Amniotic fluid not used for clinical diagnostic tests was centrifuged and stored in polypropylene tubes at −70°C. The stored amniotic fluid was analyzed for IL-6, which was measured using a commercially available enzyme-linked immunoassay (R&D Systems, Minneapolis, Minn) in 2017 and 2018. The amniotic fluid IL-6 concentrations were measured for research purposes, and the results were not used in clinical decision management. The sensitivity of the assay was 0.7 pg/mL. The intra-and inter-assay coefficients of variation were <10%. For the purpose of this study, the final diagnosis of intra-amniotic inflammation was made when IL-6 concentration was ≥2.6 ng/mL.
Clinical management
Intra-amniotic inflammation, isolation of microorganisms by amniotic fluid culture, or the detection of Ureaplasma nucleic acids was an indication for antibiotic administration. We used a combination of anti-microbial agents previously described by our group in the management of patients with preterm premature rupture of membranes (PROM),123,126 including ceftriaxone 1g (intravenous) every 24 hours, clarithromycin 500mg (oral) every 12 hours, and metronidazole (intravenous) 500mg every 8 hours. Metronidazole was administered for a maximum of 4 weeks. A follow-up amniocentesis was offered to monitor the microbiologic and inflammatory status of amniotic cavity and fetal lung maturity. The use and discontinuation of antibiotics, tocolytics and progesterone, interval of follow-up amniocentesis, or the placement of a cervical cerclage were left to the discretion of treating clinicians because of lack of uniformity among attending physicians. Tocolytics used were ritodrine, magnesium sulphate, or atosiban. Non-steroidal anti-inflammatory agents were not used as tocolytic agents in this study.
Treatment success
Treatment success was defined as the resolution of intra-amniotic infection/inflammation (defined as an IL-6 ≤2.6 ng/ml) or delivery at or after 34 weeks of gestation.
Diagnosis of chorioamnionitis and neonatal morbidity
Acute histologic chorioamnionitis was defined as the presence of acute inflammatory changes in the choriodecidua and amnion, respectively; acute funisitis was diagnosed by the presence of neutrophil infiltration into the umbilical vessel walls or Wharton’s jelly, using previously published criteria.127–131 Clinical chorioamnionitis was diagnosed in the presence of a maternal temperature of ≥37.8°C and ≥2 of the following criteria: (1) uterine tenderness; (2) malodorous vaginal discharge; (3) maternal leukocytosis (WBC count of >15,000 cells/mm3); (4) maternal tachycardia (>100 beats/min); and (5) fetal tachycardia (>160 beats/min).132,133
Significant neonatal morbidity was defined as the presence of any of the following conditions: respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage (grade ≥ II), proven congenital neonatal sepsis, and necrotizing enterocolitis. These conditions were diagnosed according to definitions previously described in detail.127,134
Statistical analysis
Continuous variables were compared between two groups using the Mann-Whitney U test. Proportions were compared with the Fisher’s exact test. A p-value <0.05 was considered statistically significant. Analysis was performed by SPSS, version 22 (SPSS Inc., Chicago, IL, USA).
Results
Characteristics of study population
During the study period, 44 patients with cervical insufficiency had one or more amniocenteses. Among these patients, stored amniotic fluid was available for IL-6 determination in 41 patients. Intra-amniotic infection/inflammation was identified in 68% (28/41). Among the 28 patients with intra-amniotic infection/inflammation, the antibiotic regimen (clarithromycin, ceftriaxone, and metronidazole) was administered in 22 cases. The reasons for not using this antibiotic regimen in the other 6 patients included: 1) intra-amniotic infection/inflammation was not suspected because the patients had a low amniotic fluid WBC count when the MMP-8 rapid test was not available (before March 2005 and after January 2011) (n=5); however, intra-amniotic inflammation was diagnosed by an elevated interleukin-6 concentration measured afterwards; 2) in one patient who had a fever, the managing clinician preferred to use another antibiotic regimen.
Twenty-two patients with cervical insufficiency had intra-amniotic infection and/or inflammation, and were treated with an anti-microbial agent combination, including ceftriaxone, clarithromycin, and metronidazole. Amniotic fluid culture was positive in 2 patients. Microorganisms identified by culture included Candida albicans (n=1) and Streptococcus anginosus (n=1). Intra-amniotic inflammation was identified in 20 patients with a negative amniotic fluid culture for microorganisms. In one instance, nucleic acids for Ureaplasma spp. were detected using a specific PCR assay.
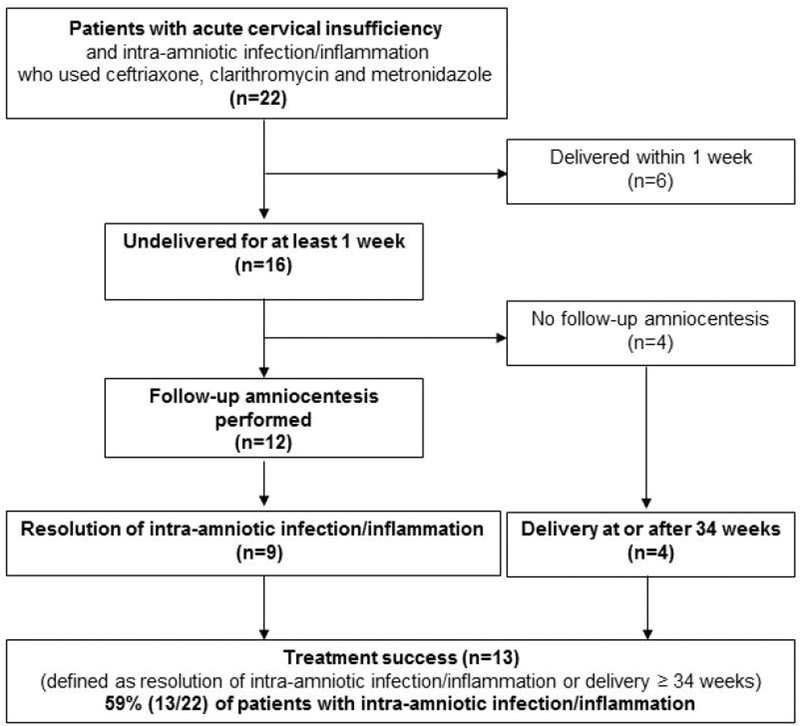
Figure 1 shows the distribution of patients. Among the 22 patients, six (27%) delivered within one week after amniocentesis, and 16 (73%) remained undelivered for at least one week after the amniocentesis. A repeat amniocentesis to evaluate the therapeutic response to anti-microbial agents was offered to patients and performed in 75% (12/16) of cases. In 75% (9/12), there was objective evidence of resolution of intra-amniotic infection or inflammation (defined as a negative culture, negative PCR, and amniotic fluid IL-6 <2.6 ng/mL). Of the four patients who did not have a follow-up amniocentesis, all delivered ≥34 weeks, two of whom delivered at term (≥37 weeks). Thus, treatment success occurred in 59% (13/22) of patients with cervical insufficiency and intra-amniotic infection/inflammation who received the antibiotic regimen (Figure 1).
Figure 1.
Flow diagram of the study population
Table 1 compares the clinical characteristics and outcomes of the patients who delivered within one week of amniocentesis and those who remained undelivered for at least one week after administration of the antibiotic regimen. Patients who delivered within one week of amniocentesis had a significantly higher median amniotic fluid IL-6 concentration and a higher rate of history of preterm delivery than those who remained undelivered for at least one week after amniocentesis (P=0.005 and P=0.025, respectively). The median amniotic fluid WBC count and the rate of clinical chorioamnionitis were higher in patients who delivered within seven days of amniocentesis than in those who were undelivered for at least one week after amniocentesis, however, the differences did not reach statistical significance (p>0.05 for each, table 1). Thus, patients with cervical insufficiency who delivered within one week of amniocentesis had a more intense intra-amniotic inflammatory response, as determined by the concentrations of a soluble component of the inflammatory response (i.e. IL-6). Vaginal progesterone was administered to two patients who remained undelivered for at least one week.
Table 1.
Clinical characteristics and outcomes of patients with cervical insufficiency who received antimicrobial agents. Patients are classified into those who delivered within 1 week of amniocentesis and those who remained undelivered for at least 1 week
| Delivery before 1 week (n=6) | Delivery after 1 week (n=16) | p-value | |
|---|---|---|---|
| Maternal age (years) | 35 (28–38) | 32 (24–39) | 0.97 |
| Nulliparity | 16.7% (1/6) | 43.8% (7/16) | 0.35 |
| History of preterm delivery | 66.7% (4/6) | 12.5% (2/16) | 0.025 |
| GA at amniocentesis (weeks) | 22.2 (19.7–25.0) | 23.2 (21.3–27.4) | 0.29 |
| Positive amniotic fluid culture | 16.7% (1/6) | 6.3% (1/16) | 0.48 |
| Positive amniotic fluid polymerase chain reaction for Ureaplasma spp. | 0% (0/4) | 7.1% (1/14) | 1.00 |
| Amniotic fluid white blood cell count (cells/mm3) | 143 (0–430) | 7 (0–2556) | 0.070 |
| Amniotic fluid white blood cell count ≥ 19 cells/mm3 | 83.3% (5/6) | 31.3% (5/16) | 0.056 |
| Amniotic fluid interleukin-6 (ng/mL) | 47.7 (5.7–49.3) | 9.4 (2.8–35.4) | 0.005 |
| Amniotic fluid interleukin-6 ≥ 2.6 ng/mL | 100% (6/6) | 100% (16/16) | 1.00 |
| Cervical dilatation > 3cm | 83.3% (5/6) | 68.8% (11/16) | 0.63 |
| Cervical cerclage for cervical insufficiency | 33.3% (2/6) | 75.0 % (12/16) | 0.14 |
| Use of tocolytics | 66.7% (4/6) | 37.5% (6/16) | 0.34 |
| Use of natural vaginal progesterone | 0% (0/6) | 12.5% (2/16) | 1.00 |
| Use of 17α-hydroxyprogesterone caproate | 0% (0/6) | 0% (0/16) | 1.00 |
| Antenatal corticosteroids administration | 33.3% (2/6) | 43.8% (7/16) | 1.00 |
| Gestational age at delivery (weeks) | 23.0 (20.0–25.7) | 37.0 (27.0–41.3) | <0.001 |
| Preterm labora | 33.3% (2/6) | 18.8% (3/16) | 0.59 |
| Preterm premature rupture of membranesa | 33.3% (2/6) | 6.3% (1/16) | 0.17 |
| Clinical chorioamnionitis | 33.3% (2/6) | 0% (0/16) | 0.065 |
| Acute histologic chorioamnionitis | 75% (3/4) | 57.1% (4/7) | 1.00 |
| Funisitis | 25% (1/4) | 42.9% (3/7) | 1.00 |
Data are median (range) or % (n/N).
Cases in which preterm delivery before 37 weeks occurred were included.
Among the 16 patients who remained undelivered for one week after amniocentesis, 12 had follow-up amniocentesis. The median interval between amniocenteses was 8 days (IQR, 7–14 days). There were no significant differences in baseline characteristics, results of amniotic fluid analysis at the time of the initial amniocentesis, and median gestational age at delivery between patients who were undelivered for at least one week and had follow-up amniocentesis, and those who had not.
Table 2 displays the clinical characteristics according to the results of amniotic fluid analysis obtained at the time of repeat amniocentesis. Resolution of intra-amniotic infection/inflammation was observed in 75% (9/12) of patients. None of the neonates born to the nine mothers with resolution of the intra-amniotic inflammatory response had significant complications. The median interval between initial amniocentesis and the follow-up amniocentesis that confirmed resolution of intra-amniotic infection/inflammation was 24 days (interquartile range, 14–30). Among the nine patients, eight delivered at or beyond 34 weeks of gestation, and six delivered at term. The median gestational age at amniocentesis and delivery of patients who delivered at or beyond 34 weeks was 23.4 weeks and 37.8 weeks, respectively. There were no significant differences in the median amniotic fluid WBC count and IL-6 concentration among the three groups of patients (p>0.1).
Table 2.
Antenatal and postnatal characteristics of patients who were treated with antimicrobial agents and underwent follow-up amniocentesis after 1 week from initial amniocentesis
| Resolution of intra-amniotic inflammation | |||
|---|---|---|---|
| Confirmed (n=9) | Not confirmed (n=3) | ||
| Delivery ≥ 34 weeks (n=8) | Delivery < 34 weeks (n=1) | Delivery < 34 weeks (n=3) | |
| Nulliparity | 50% (4/8) | 100% (1/1) | 33.3% (1/3) |
| History of preterm delivery | 12.5% (1/8) | 0% (0/1) | 0% (0/3) |
| Initial amniocentesis | |||
| Gestational age at amniocentesis | 23.4 (21.0–26.7) | 23.7 | 21.4 (21.3–27.4) |
| Positive amniotic fluid culture | 0% (0/8) | 0% (0/1) | 33.3% (1/3) |
| Positive amniotic fluid polymerase chain reaction for Ureaplasma spp. | 14.3% (1/7) | 0% (0/1) | 0% (0/3) |
| Amniotic fluid white blood cell count (cells/mm3) | 16 (0–282) | 4 | 24 (1–2556) |
| Amniotic fluid interleukin-6 (ng/mL) | 8.9 (2.8–26.2) | 3.8 | 24.8 (10.6–35.4) |
| Days from initial amniocentesis to resolutiona | 23 (6–30) | 30 | - |
| Number of amniocenteses | 3.5 (3–7) | 3 | 4 (3–5) |
| Duration of new antibiotic regimen use (days) | 27 (7–66) | 31 | 39 (9–43) |
| Cervical dilatation > 3cm (%) | 75% (6/8) | 100% (1/1) | 100% (3/3) |
| Cervical cerclage for cervical insufficiency | 50% (4/8) | 100% (1/1) | 100% (3/3) |
| Gestational age at delivery (weeks) | 37.8 (34.0–41.3)b | 29.9 | 27.4 (27.0–28.7)b |
| Days from initial amniocentesis to delivery | 101 (66–131)b | 43 | 39 (9–43)b |
| Clinical chorioamnionitis | 0% (0/8) | 0% (0/1) | 0% (0/3) |
| Acute histologic chorioamnionitis | 0% (0/3) | 100% (1/1) | 100% (3/3) |
| Funisitis | 0% (0/3) | 100% (1/1) | 66.7% (2/3) |
| Neonatal mortality | 0% (0/8) | 0% (0/1)- | 0% (0/3) |
| Significant neonatal morbidity | 0% (0/8) | 0% (0/1)- | 66.7% (2/3) |
Data are median (range) or % (n/N).
The first amniocentesis without evidence of intra-amniotic infection/inflammation
P-value < 0.05
Changes in amniotic fluid culture, intra-amniotic inflammatory markers, pregnancy and neonatal outcomes in patients with follow-up amniocenteses
Table 3 shows the characteristics and outcomes of 12 patients who received antibiotics and underwent follow-up amniocentesis. Nine patients (cases 1–9) had biochemical evidence of successful treatment of intra-amniotic inflammation. Bulging membranes (defined as the prolapse of membranes beyond the external os) were present in 10 of 12 patients (except cases 3 and 6). Three of four patients (cases 1, 2, and 4) who did not have cerclage placement had spontaneous reduction of membranes back into the uterine cavity while receiving antibiotic treatment. This was an unexpected observation made during the course of the study.
Table 3.
Characteristics and outcomes of 12 patients with administration of antimicrobial agents who underwent follow-up amniocentesis
| Gestational age
(weeks) |
Analysis of amniotic fluid
(initial amniocentesis) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | Amniocentesis | Delivery | Cervix dilatation (cm) | Bag bulging | Cerclage for cervical insufficiency | Culture | Polymerase chain reaction for Ureaplasma spp. | Interleukin-6 (ng/mL) | White blood cell count (cells/mm3) | Matrix metalloproteinase-8 rapid test | Corticosteroid for fetal lung maturity | Interval between initial amniocentesis to resolution (days)b | Acute histologic chorioamnionitis /funisitis | Outcome |
| Group A. Resolution confirmed and delivery at or beyond 34 weeks | ||||||||||||||
| 1 | 24.6 | 34 | 3 | Yes | No | Neg | Pos | 4.8 | 35 | Pos | Yes | 21 | −/− | Rupture of membranes at 25.7 weeks
Induction of labor at 34 weeks Survival without morbidity |
| 2 | 25 | 36.9 | 3 | Yes | Noa | Neg | Neg | 11.4 | 1 | N/A | No | 21 | −/− | Survival without morbidity |
| 3 | 23.9 | 38.3 | 1.5 | No | No | Neg | N/A | 18.1 | 14 | Neg | No | 14 | N/A | Survival without morbidity |
| 4 | 26.7 | 37 | 4 | Yes | No | Neg | Neg | 2.9 | 0 | Pos | Yes | 30 | N/A | Survival without morbidity |
| 5 | 22.6 | 41.3 | 3 | Yes | Yes | Neg | Neg | 10.4 | 18 | N/A | No | 27 | N/A | Survival without morbidity |
| 6 | 22.9 | 41.1 | 1.5 | No | Yes | Neg | Neg | 2.8 | 0 | N/A | No | 6 | N/A | Survival without morbidity |
| 7 | 22.7 | 37.3 | 4 | Yes | Yes | Neg | Neg | 26.2 | 282 | N/A | Yes | 30 | −/− | Survival without morbidity |
| 8 | 21.1 | 39.9 | 3 | Yes | Yes | Neg | Neg | N/A (7.4 on 22.3 week) |
144 | N/A | No | 24 | N/A | Survival without morbidity |
| Group B. Resolution confirmed but delivery before 34 weeks | ||||||||||||||
| 9 | 23.7 | 29.9 | 5.5 | Yes | Yes | Neg | Neg | 3.8 | 4 | N/A | Yes | 30 | +/+ | Spontaneous labor Survival without morbidity |
| Group C. Resolution not-confirmed and delivery before 34 weeks | ||||||||||||||
| 10 | 21.3 | 27.4 | 3 | Yes | Yes | Neg | Neg | 24.8 | 1 | Pos | Yes | - | +/+ | Spontaneous labor Respiratory distress syndrome, bronchopulmonary dysplasia, intraventricular hemorrhage |
| 11 | 21.4 | 27 | 3 | Yes | Yes | Neg | Neg | 10.6 | 24 | Pos | Yes | - | +/− | Spontaneous labor Bronchopulmonary dysplasia |
| 12 | 27.4 | 28.7 | 9 | Yes | Yes | Candida albicans | Neg | 35.4 | 2556 | N/A | Yes | - | +/+ | Persistent positive culture for candida and frequent contractions Augmentation of labor Survival without morbidity |
N/A, not assessed.
Prophylactic cerclage was performed at 14.4 weeks of gestation
The first amniocentesis without evidence of intra-amniotic infection/inflammation
Of 9 patients in whom the resolution of intra-amniotic inflammation was confirmed by IL-6 determinations retrospectively, 7 underwent additional amniocentesis after resolution. The number of amniocenteses after resolution was 1 for 6 patients and 2 for 1 patient. The procedures were performed due to the occurrence of labor or rupture of membranes (n=3), a positive result of rapid MMP-8 test at the last amniocentesis (n=1), acute elevation of maternal serum C-reactive protein and mild fever (n=1), or re-evaluation of intra-amniotic infection/inflammation at the discretion of the clinician caring for the patient (n=2).
Intra-amniotic infection was eradicated in case 1: Ureaplasma spp. had been detected by PCR. This patient had an amniotic fluid IL-6 concentration of 4.8 ng/mL, a WBC count of 35 cells/mm3, and a positive rapid test for MMP-8 at initial amniocentesis (24.6 weeks). The antibiotics were administered after the first amniocentesis. One week later (25.6 weeks), membrane rupture occurred but follow-up amniocentesis revealed improvement of inflammatory markers (IL-6 concentration of 0.19 ng/mL, WBC count of 6 cells/mm3 and a weakly positive MMP-8 rapid kit test). The PCR test for Ureaplasma spp. was still positive. The fourth amniocentesis at 27.6 weeks showed no evidence of Ureaplasma spp. by cultivation or specific PCR assay, and all parameters of intra-amniotic inflammation improved (i.e. IL-6 concentration of 0.17 ng/mL, WBC count of 6 cells/mm3and a negative MMP-8 rapid kit test). At 34 weeks of gestation, the patient underwent labor induction secondary to preterm PROM. A neonate weighing 2000 grams was delivered and did not develop any complications. There was no evidence of acute histologic chorioamnionitis or funisitis in placental pathologic examination.
Intra-amniotic infection with Candida albicans was identified in one patient (case #12). There was evidence of an intense amniotic fluid inflammatory response (i.e. IL-6 concentration of 35.4 ng/mL and WBC count of 2556 cells/mm3), and the amniotic fluid culture was eventually positive for Candida albicans. A repeat amniocentesis showed a very high WBC count (1305 cells/mm3) and the managing physician and patient elected to proceed with labor induction because of the concern of congenital candidiasis. The repeat amniotic fluid culture showed that Candida albicans was still present. After completion of corticosteroid administration for fetal lung maturation, the patient delivered at 28.7 weeks of gestation (10 days from initial amniocentesis) after the administration of oxytocin. The birthweight was 1100 grams, and Apgar scores at 1 and 5 minutes were 7 and 8, respectively. The neonate survived without any significant morbidity and placental examination showed acute histologic chorioamnionitis, and funisitis.
Resolution of intra-amniotic inflammation was noted in eight patients with a negative amniotic fluid culture and PCR (cases 2–9). Among these, seven delivered at term (cases 3–8) or near term (case 2, delivered at 36.9 weeks by elective cesarean delivery). One patient (case 9) delivered at 29.9 weeks despite the resolution of intra-amniotic inflammation. The initial amniotic fluid inflammatory markers were as follows: IL-6 concentration, 3.8 ng/mL and WBC count, 4 cells/mm3. After 4 weeks of treatment with the antibiotic regimen, the amniotic fluid IL-6 concentration decreased to 1.0 ng/mL and the amniotic fluid WBC count was 2 cells/mm3. Antibiotics were discontinued, and the patient was discharged from the hospital. Ten days later, the patient was readmitted with spontaneous preterm labor, and the amniotic fluid IL-6 concentration had increased to 2.4 ng/mL. The patient progressed to spontaneous delivery at 29.9 weeks. The neonate weighed 1610 grams and survived without any significant morbidity. Acute histologic chorioamnionitis and funisitis were diagnosed upon placental examination.
Antibiotic treatment was not successful in two patients with intra-amniotic inflammation but without intra-amniotic infection (cases 10 and 11). Of these two, microbial invasion of amniotic cavity (by Candida tropicalis after six weeks of initial amniocentesis in case 10, and by Enterococcus faecalis after eight days of initial amniocentesis in case 11) was identified in the follow-up amniocentesis. These two patients delivered at 27.4 weeks (1,220 gm) and 27 weeks (1,220gm), respectively, because of spontaneous labor. The neonates survived but had respiratory distress syndrome (case #10), bronchopulmonary dysplasia (cases 10 and 11), and intraventricular hemorrhage (case #10).
Pregnancy and neonatal outcomes in patients who did not have follow-up amniocenteses
Table 4 shows the clinical characteristics and outcomes of four patients who received antibiotics and remained undelivered for at least one week after amniocentesis, but did not have a follow-up amniocentesis. All four patients underwent an emergency cervical cerclage, and antibiotics were postoperatively administered for 4–5 days. Follow-up amniocentesis was not performed because there was no evidence of intra-amniotic infection/inflammation at initial amniocentesis (low amniotic fluid WBC count and negative culture, rapid MMP-8 test was not available at this time) in cases 14 and 16, or because of the decision made by the attending physician (cases 13 and 15). All patients in this group delivered after 34 weeks of gestation.
Table 4.
Characteristics and outcomes of 4 patients with administration of antimicrobial agents who delivered after 1 week of amniocentesis without follow-up amniocentesis
| Gestational age
(weeks) |
Analysis of amniotic
fluid |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | Amniocentesis | Delivery | Cervix dilatation (cm) | Bag bulging | Cerclage for cervical insufficiency | Culture | Polymerase chain reaction for Ureaplasma spp. | Interleukin-6 (ng/mL) | White blood cell count (cells/mm3) | Matrix metalloproteinase-8 rapid kit | Corticosteroid for fetal lung maturity | Acute histologic chorioamnionitis /funisitis | Outcome |
| 13 | 21.6 | 39 | 3 | Yes | Yes | Neg | Neg | 8.3 | 1 | Pos | No | N/A | Survival without morbidity |
| 14 | 23 | 36.1 | 2 | No | Yes | Neg | Neg | 2.8 | 2 | N/A | No | N/A | Survival without morbidity |
| 15 | 23.4 | 39.4 | 2 | Yes | Yes | Neg | N/A | 3.0 | 1 | Pos | No | N/A | Survival without morbidity |
| 16 | 24.6 | 35.7 | 2 | Yes | Yes | Neg | Neg | 16.2 | 9 | N/A | No | N/A | Survival without morbidity |
N/A, not assessed.
Table 5 summarizes the characteristics and outcomes of the six patients who delivered within one week of amniocentesis and received antibiotics. These patients had a significantly higher amniotic fluid IL-6 concentration than those who remained undelivered for at least one week after amniocentesis (P=0.005, table 1). The indication of delivery was clinical chorioamnionitis (cases 21 and 22), progression of spontaneous labor after PROM (case 19) or with intact membranes (case 20), abruptio placentae (case 17), or the patient’s refusal to maintain pregnancy (case 18). Only one neonate survived with bronchopulmonary dysplasia (case 17) and the other five neonates died shortly after birth.
Table 5.
Characteristics and outcomes of 6 patients delivered within 1 week of amniocentesis who received antibiotics
| Gestational age
(weeks) |
Analysis of amniotic
fluid |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case No. | Amniocentesis | Delivery | Cervix dilatation (cm) | Bag bulging | Cerclage for cervical insufficiency | Culture | Polymerase chain reaction for Ureaplasma spp. | Interleukin-6 (ng/mL) | White blood cell count (cells/mm3) | Matrix metallo proteinase-8 rapid kit | Corticosteroid for fetal lung maturity | Acute histologic chorioamnionitis /funisitis | Outcome |
| 17 | 24.1 | 24.9 | 2 | Yes | Yes | Neg | N/A | 47.7 | 430 | Pos | Yes | +/− | Preterm labor and delivery due to placenta
abruptio Survival with bronchopulmonary dysplasia |
| 18 | 23.6 | 24.4 | 3 | Yes | No | Neg | Neg | 5.7 | 0 | Pos | No | N/A | Patient elected termination of pregnancy at
other hospital Neonatal death |
| 19 | 20.7 | 21.6 | 4 | Yes | Yes | Neg | N/A | 26.9 | 123 | Pos | No | +/+ | Progress to spontaneous labor after rupture of
membranes Neonatal death |
| 20 | 19.7 | 20 | full dilatation | Yes | No | Neg | Neg | 48.7 | 360 | N/A | No | +/− | Spontaneous preterm labor Neonatal death |
| 21 | 19.7 | 20.3 | 4 | Yes | No | Streptococcus anginosus | Neg | 47.7 | 81 | N/A | No | N/A | Clinical chorioamnionitis Patient elected termination of pregnancy Neonatal death |
| 22 | 25 | 25.7 | 3 | Yes | No | Neg | Neg | 49.3 | 162 | Pos | Yes | −/− | Clinical chorioamnionitis after rupture of
membranes Neonatal death |
N/A, not assessed
Comment
Principal findings of the study:
1) Eradication of intra-amniotic inflammation/infection was achieved in 75% (9/12) of patients with cervical insufficiency who were treated with antimicrobial agents; 2) the overall treatment success (defined as resolution of intra-amniotic infection/inflammation or delivery ≥34 weeks) was 59% (13/22). These observations provide strong evidence that antibiotic administration can be effective in treating intra-amniotic inflammation/infection in patients with cervical insufficiency.
The recognition of cervical insufficiency as a clinical entity:
The initial recognition of cervical insufficiency is attributed to Cole, Culpepper, and Rowland in their 1658 publication “Practice of Physick”,135 and the term “cervical incompetence” was first mentioned in 1865 by Gream.136 In 1950, Lash and Lash described a condition called “incompetent internal os of the cervix” as a cause of habitual abortion.137 Currently, the term “cervical insufficiency” has replaced “cervical incompetence” to avoid the negative connotation of “incompetence”.138 The standard treatment for this treatment, cervical cerclage, was introduced in 1955 by Shirodkar139 and in 1957 by McDonald.140 The role of cerclage in obstetrics has remained controversial for more than 60 years.15,141–155
Intra-amniotic infection in cervical insufficiency
Goodlin proposed a role for intra-amniotic infection, and amniocentesis to study the microbial status of the amniotic cavity and decompression of the uterus by removing amniotic fluid prior to placement of a cerclage.20 Goodlin reported that 22.2% (2/9) of patients with cervical insufficiency had microorganisms in the amniotic cavity (one had Escherichia coli and the other had Group β streptococcus).20 He proposed treatment of intra-amniotic infection with the administration of ampicillin into the amniotic cavity at the time of amniocentesis. The patient with Group β streptococcus carried the pregnancy to term, suggesting a therapeutic value for antibiotic administration.20
Subsequent studies have shown that microorganisms are present in the amniotic cavity in 8–52% of patients with cervical insufficiency.20–27 For example, in one study including 33 patients with cervical insufficiency, 51.5% (17/33) had a positive amniotic fluid culture for microorganisms.21 Four of these patients had a cervical cerclage because the amniotic fluid Gram stain results were negative. All had complications, which consisted of rupture of membranes, clinical chorioamnionitis, or bleeding. When a cervical cerclage was not placed in patients with intra-amniotic infection, all (13/13) had a preterm delivery. On the other hand, patients without intra-amniotic infection had a favorable outcome, suggesting that the microbial status of the amniotic cavity is a major determinant of pregnancy outcome in patients with cervical insufficiency.
Intra-amniotic inflammation in cervical insufficiency
Microbial products (endotoxin or lipopolysaccharide, peptidoglycans, and glucans) and microorganisms can elicit an inflammatory response.31–45 In practice, it is easier and faster to diagnose intra-amniotic inflammation than intra-amniotic infection, because an elevated amniotic fluid WBC count or inflammatory molecules (such as IL-6 or MMP-8) can be rapidly detected in clinical practice, while the results of amniotic fluid culture or molecular microbiologic techniques will take longer than a bedside test.109,117–119,121–123,156,157 It is now recognized that some cases of intra-amniotic inflammation occur in the absence of demonstrable microorganisms detected with cultivation or molecular methods (“sterile intra-amniotic inflammation”)72,98–100,113,115,125 and this condition is more common than intra-amniotic infection in patients with a sonographic short cervix.78,113 The same has been reported in patients with cervical insufficiency with the use of cultivation techniques.23
Specifically, Lee et al.23 reported that 81% (42/52) of patients with cervical insufficiency had evidence of intra-amniotic inflammation, defined as an amniotic fluid concentration of MMP-8 >23 ng/mL. Only four patients in that study had evidence of intra-amniotic infection using cultivation methods. Therefore, most cases of intra-amniotic inflammation were not attributed to bacteria. The prevalence of intra-amniotic inflammation in other studies has ranged from 22.2% (16/72) to 64.5% (20/31) using different cutoffs of IL-6 concentrations for the definition of intra-amniotic inflammation.94,95
The prognosis of patients with cervical insufficiency and intra-amniotic inflammation is poor, with 50% delivering within seven days, and, in 84% of cases, patients have a preterm delivery at <34 weeks.23 Importantly, Lee et al. reported that there were no differences in the amniocentesis-to-delivery interval or the rate of adverse pregnancy outcome between patients with intra-amniotic inflammation and a negative amniotic fluid culture and patients with proven intra-amniotic infection.23 Collectively, these data suggest that intra-amniotic inflammation is a poor prognostic factor in cervical insufficiency.
Antimicrobial agents to treat cervical insufficiency
In this study, clinicians elected to use a combination of ceftriaxone, clarithromycin, and metronidazole based on previous studies of the pharmacokinetics of antibiotics during pregnancy.158,159 The rationale has been described in previous reports.123,126 In brief, erythromycin or azithromycin were frequently inadequate in eradicating intra-amniotic infection with Ureaplasma species, the most common microorganism identified in the amniotic fluid of patients at risk for preterm delivery, perhaps due to limited trans-placental passage, which results in inadequate antimicrobial activity in amniotic fluid (only 3% of erythromycin and 2.6% of azithromycin cross the placenta).158 Clarithromycin, on the other hand, has a much higher rate of transplacental passage, and is effective for the treatment of intra-amniotic infection with Ureaplasma species.158 Metronidazole was used because of its powerful effect against anaerobic bacteria which are frequently implicated in intra-amniotic infection.160–163 These organisms are difficult to identify using cultivation techniques.164 Ceftriaxone was included because of its enhanced coverage of aerobic bacteria and high rate of transplacental passage.159,165 In a previous study, we demonstrated successful eradication of intra-amniotic infection and/or inflammation in at least 33% of patients with preterm PROM after the administration of this antibiotic regimen.123
Can intra-amniotic infection be treated in patients with cervical insufficiency?
Goodlin20 reported the successful treatment of intra-amniotic infection in one case after the administration of a single dose of 1 gram of ampicillin into the amniotic cavity. However, since that report in 1979, there is a paucity of investigation about the treatment of intra-amniotic infection and inflammation. Antibiotics have been successful in eradicating amniotic fluid infection in animal models.166,167 Fidel et al.166 reported that antibiotic administration within 12 h of transcervical inoculations of E.coli, but not after 18 h, increased the duration of pregnancy (by reducing the rate of preterm delivery) and neonatal survival in rabbits. More recently, Grigsby et al.167 showed that maternal azithromycin can eradicate Ureaplasma spp. from the amniotic fluid and key fetal organs in a non-human primate model. Gravett et al.168 reported that the combination of immunomodulators (dexamethasone and indomethacin) and antibiotics was able to eradicate intra-amniotic infection, suppress intraamniotic and placental inflammation, and prolong gestation in a non-human primate model.
The results reported herein show that antibiotic administration can eradicate intra-amniotic infection, as demonstrated by repeat amniotic fluid analyses of one patient who had Ureaplasma spp. detected by PCR at 24.6 weeks of gestation. This patient delivered at 34 weeks and the neonate had no significant morbidity. In contrast, one patient was admitted at 27.4 weeks, received antibiotics, and the amniotic fluid culture eventually became positive for Candida albicans. A repeat amniocentesis showed a very high WBC count (1305 cells/mm3) and the managing physician and patient elected to proceed with labor induction because of the concern of congenital candidiasis. The repeat amniotic fluid culture showed that Candida albicans was still present. Such observation underscores the importance of knowing the specific microorganism involved and selecting antibiotics which are effective against the pathogen. Previous reports have shown that fluconazole may be effective in cases of intra-amniotic infection with Candida albicans.169
Antibiotics can eradicate intra-amniotic inflammation in cervical insufficiency
An observation made during the course of this case series is that antibiotic administration in patients with intra-amniotic inflammation was effective in treating intra-amniotic inflammation, even when microorganisms were not detectable. We have reported similar results in patients with preterm PROM.123,126
The mechanisms whereby antibiotics can treat intra-amniotic inflammation are not clear. One possibility is that these agents are effective against microorganisms that were not detected using cultivation techniques,99,113,114,164,170–177 or PCR-specific assays for Ureaplasma spp. Future studies using broad-range PCR assays or cell-free microbial DNA178–181 could help answer this important question.
It is also possible that macrolides (i.e. clarithromycin) suppress intra-amniotic inflammation caused by danger signals by downregulating the expression of pro-inflammatory transcription factors, such as nuclear factor κ-B (NFκ-B) and activator protein-1 (AP-1), which can induce the production of pro-inflammatory cytokines. Such observations have been made in other organ systems.182–184 It is unlikely that antibiotics were effective in reducing decidual infection because careful studies to locate microorganisms in the chorioamniotic membranes and decidua have shown that organisms first invade the amniotic cavity, and only are secondarily present in the decidua. Thus, the initial view that organisms would be present in the decidua, and from there invade the amniotic cavity, is contradicted by empirical evidence using morphologic and molecular microbiologic techniques.185
A relevant question is the relative contribution of antibiotics and cervical cerclage to the outcomes in this study. There are no data to support that cervical cerclage alone can eradicate intra-amniotic inflammation. A cervical cerclage was placed in 64% of patients, and may have had a beneficial effect in improving pregnancy outcome. However, it can be postulated that some patients with intra-amniotic infection/inflammation benefitted from the administration of the combination of antimicrobial agents, which eradicated bacteria from the amniotic cavity or downregulated the inflammatory response.
In patients in whom infection or inflammation was successfully treated, a cervical cerclage may have contributed to the prevention of preterm labor or pregnancy loss by closing the cervix and preventing further exposure of the chorioamniotic membranes to microorganisms present in the vagina. Therefore, the improved clinical outcomes observed in this study may have been due to the combination of antibiotic administration, with a direct effect on microbial invasion of the amniotic cavity and intra-amniotic inflammation, and cerclage, which prevented re-infection or recurrence of the inflammatory process. A cerclage may also have had a protective role by supporting the cervix, as cervical disorders may be a cause of preterm labor and midtrimester pregnancy loss.
Strengths and limitations
This is an observational study in which women with intra-amniotic infection/inflammation were treated with antibiotics and monitored with follow-up amniocenteses. It represents an objective demonstration that a case of intra-amniotic infection was successfully treated, and that intra-amniotic inflammation could also be down-regulated and followed by a favorable pregnancy outcome.
Although Goodlin20 reported the first case of eradication of intra-amniotic infection in cervical insufficiency, the current study reports the successful treatment of intra-amniotic inflammation in the absence of detectable microorganisms. This was possible because of the availability of rapid tests for detection of intra-amniotic inflammation, such as amniotic fluid WBC count and a rapid amniotic fluid test for MMP-8. It is unlikely that the eradication of intra-amniotic inflammation can be attributed to the administration of corticosteroids, because these agents were not administered in 69.2% (9/13) of patients in whom treatment was successful.
A limitation of this study is that it is not a randomized clinical trial, but rather a case series, and therefore, further studies to confirm these observations are desirable. Another limitation is that we combined patients with intra-amniotic infection and those with intraamniotic inflammation but without proven intra-amniotic infection. Lee et al. previously demonstrated that these two conditions have similar pregnancy outcomes.23 Additional research is required to examine whether there is a differential effect of antibiotics in patients with intra-amniotic infection vs those with “sterile” intra-amniotic inflammation. The main claim of this paper is that antimicrobial agents can be useful in eradicating intra-amniotic inflammation and, in some cases, intra-amniotic infection. While our data suggest that the frequency of complications in the neonatal period is lower, other studies will need to examine long-term follow-up.
This study included patients with cervical insufficiency diagnosed by physical examination, and therefore, does not address the entity of an asymptomatic short cervix diagnosed by ultrasound. We previously demonstrated that microbial invasion of the amniotic cavity in asymptomatic patients with a short cervix can be treated with antibiotics, as demonstrated by repeat amniocentesis in which amniotic fluid cultures became negative, and patients delivered close to term.186
The choice of antimicrobial agents used in this study, route of administration, and duration of treatment represent the choice of clinicians practicing in a University hospital that has studied intra-amniotic infection/inflammation, the microbiology of infection, and the short-and long-term consequences of these conditions. It is possible that similar success could be achieved with other antibiotic regimens which provide adequate coverage for the most frequent microorganisms found in the amniotic cavity (genital mycoplasmas) and administered in a different manner.
Medicine is evolving towards the individualized treatment of patients, and we believe that, with the application of modern molecular microbiologic techniques, it may be possible to tailor treatment for a specific patient based on the results of amniotic fluid analysis.
Conclusion
Antibiotic administration to women with cervical insufficiency and intra-amniotic infection/inflammation can eradicate these pathologic processes in a subset of patients.
AJOG at a Glance:
-
What is the research question?
Can intra-amniotic infection/inflammation in patients with cervical insufficiency be treated with antibiotics?
-
What are the key findings?
75% of patients with intra-amniotic infection/inflammation had objective evidence of resolution of the intra-amniotic inflammatory process after treatment with antibiotics.
-
What does this add to what is known?
The conventional view is that cervical insufficiency complicated by intra-amniotic infection/inflammation leads to inevitable delivery. Herein we present the first objective evidence that intra-amniotic infection/inflammation can be eradicated with antibiotic treatment, as proven by serial amniotic fluid analysis before and after treatment. This has implications for optimizing patient care.
Acknowledgments
Source of funding: This research was supported in part by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning, Republic of Korea (2017R1A2B2007958); in part by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part by Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Footnotes
Disclosure: The authors report no conflicts of interest.
Condensation: In patients presenting with cervical insufficiency and intra-amniotic infection/inflammation, antibiotic administration can eradicate the inflammatory process and be associated with improved pregnancy outcome
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American College of Obstetricians and Gynecologists. ACOG Practice Bulletin No.142: Cerclage for the management of cervical insufficiency. Obstetrics and gynecology 2014;123:372–9. [DOI] [PubMed] [Google Scholar]
- 2.Althuisius SM, Dekker GA, Hummel P, van Geijn HP, Cervical incompetence prevention randomized cerclage t. Cervical incompetence prevention randomized cerclage trial: emergency cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol 2003;189:907–10. [DOI] [PubMed] [Google Scholar]
- 3.Guzman ER, Houlihan C, Vintzileos A, Ivan J, Benito C, Kappy K. The significance of transvaginal ultrasonographic evaluation of the cervix in women treated with emergency cerclage. Am J Obstet Gynecol 1996;175:471–6. [DOI] [PubMed] [Google Scholar]
- 4.Lipitz S, Libshitz A, Oelsner G, et al. Outcome of second-trimester, emergency cervical cerclage in patients with no history of cervical incompetence. Am J Perinatol 1996;13:419–22. [DOI] [PubMed] [Google Scholar]
- 5.Latta RA, McKenna B. Emergent cervical cerclage: predictors of success or failure. J Matern Fetal Med 1996;5:22–7. [DOI] [PubMed] [Google Scholar]
- 6.Dijkstra K, Funai EF, O’Neill L, Rebarber A, Paidas MJ, Young BK. Change in cervical length after cerclage as a predictor of preterm delivery. Obstetrics and gynecology 2000;96:346–50. [DOI] [PubMed] [Google Scholar]
- 7.Novy MJ, Gupta A, Wothe DD, Gupta S, Kennedy KA, Gravett MG. Cervical cerclage in the second trimester of pregnancy: a historical cohort study. Am J Obstet Gynecol 2001;184:1447–54; discussion 54–6. [DOI] [PubMed] [Google Scholar]
- 8.Pereira L, Cotter A, Gomez R, et al. Expectant management compared with physical examination-indicated cerclage (EM-PEC) in selected women with a dilated cervix at 14(0/7)-25(6/7) weeks: results from the EM-PEC international cohort study. Am J Obstet Gynecol 2007;197:483 e1–8. [DOI] [PubMed] [Google Scholar]
- 9.Ventolini G, Genrich TJ, Roth J, Neiger R . Pregnancy outcome after placement of ‘rescue’ Shirodkar cerclage. J Perinatol 2009;29:276–9. [DOI] [PubMed] [Google Scholar]
- 10.Stupin JH, David M, Siedentopf JP, Dudenhausen JW. Emergency cerclage versus bed rest for amniotic sac prolapse before 27 gestational weeks. A retrospective, comparative study of 161 women. European journal of obstetrics, gynecology, and reproductive biology 2008;139:32–7. [DOI] [PubMed] [Google Scholar]
- 11.Abbott D, To M, Shennan A. Cervical cerclage: a review of current evidence. The Australian & New Zealand journal of obstetrics & gynaecology 2012;52:220–3. [DOI] [PubMed] [Google Scholar]
- 12.Roman A, Rochelson B, Martinelli P, et al. Cerclage in twin pregnancy with dilated cervix between 16 to 24 weeks of gestation: retrospective cohort study. Am J Obstet Gynecol 2016;215:98 e1–e11. [DOI] [PubMed] [Google Scholar]
- 13.Bernabeu A, Goya M, Martra M, et al. Physical examination-indicated cerclage in singleton and twin pregnancies: maternal-fetal outcomes. J Matern Fetal Neonatal Med 2016;29:2109–13. [DOI] [PubMed] [Google Scholar]
- 14.Ciavattini A, Delli Carpini G, Boscarato V, Febi T, Di Giuseppe J, Landi B. Effectiveness of emergency cerclage in cervical insufficiency. J Matern Fetal Neonatal Med 2016;29:2088–92. [DOI] [PubMed] [Google Scholar]
- 15.Vintzileos AM, Visser GH. Interventions for women with mid-trimester short cervix: which ones work? Ultrasound Obstet Gynecol 2017;49:295–300. [DOI] [PubMed] [Google Scholar]
- 16.Park JY, Cho SH, Jeon SJ, et al. Outcomes of physical examination-indicated cerclage in twin pregnancies with acute cervical insufficiency compared to singleton pregnancies. J Perinat Med 2018;46:845–52. [DOI] [PubMed] [Google Scholar]
- 17.Newnham JP, White SW, Meharry S, et al. Reducing preterm birth by a statewide multifaceted program: an implementation study. Am J Obstet Gynecol 2017;216:434–42. [DOI] [PubMed] [Google Scholar]
- 18.Shivani D, Quek BH, Tan PL, Shephali T. Does rescue cerclage work? J Perinat Med 2018;46:876–80. [DOI] [PubMed] [Google Scholar]
- 19.Enakpene CA, DiGiovanni L, Jones TN, Marshalla M, Mastrogiannis D, Della Torre M. Cervical cerclage for singleton pregnant patients on vaginal progesterone with progressive cervical shortening. Am J Obstet Gynecol 2018;219:397 e1– e10. [DOI] [PubMed] [Google Scholar]
- 20.Goodlin RC. Cervical incompetence, hourglass membranes, and amniocentesis. Obstetrics and gynecology 1979;54:748–50. [PubMed] [Google Scholar]
- 21.Romero R, Gonzalez R, Sepulveda W, et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol 1992;167:1086–91. [DOI] [PubMed] [Google Scholar]
- 22.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstetrics and gynecology 2000;95:652–5. [DOI] [PubMed] [Google Scholar]
- 23.Lee SE, Romero R, Park CW, Jun JK, Yoon BH. The frequency and significance of intraamniotic inflammation in patients with cervical insufficiency. Am J Obstet Gynecol 2008;198:633 e1–8. [DOI] [PubMed] [Google Scholar]
- 24.Bujold E, Morency AM, Rallu F, et al. Bacteriology of amniotic fluid in women with suspected cervical insufficiency. J Obstet Gynaecol Can 2008;30:882–7. [DOI] [PubMed] [Google Scholar]
- 25.Airoldi J, Pereira L, Cotter A, et al. Amniocentesis prior to physical exam-indicated cerclage in women with midtrimester cervical dilation: results from the expectant management compared to Physical Exam-indicated Cerclage international cohort study. Am J Perinatol 2009;26:63–8. [DOI] [PubMed] [Google Scholar]
- 26.Oh KJ, Lee SE, Jung H, Kim G, Romero R, Yoon BH. Detection of ureaplasmas by the polymerase chain reaction in the amniotic fluid of patients with cervical insufficiency. J Perinat Med 2010;38:261–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lisonkova S, Sabr Y, Joseph KS. Diagnosis of subclinical amniotic fluid infection prior to rescue cerclage using gram stain and glucose tests: an individual patient metaanalysis. J Obstet Gynaecol Can 2014;36:116–22. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Roslansky P, Oyarzun E, et al. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol 1988;158:1044–9. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol 1987;157:815–9. [DOI] [PubMed] [Google Scholar]
- 30.Pacora P, Romero R, Erez O, et al. The diagnostic performance of the beta-glucan assay in the detection of intra-amniotic infection with Candida species. J Matern Fetal Neonatal Med 2019;32:1703–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gravett MG, Hummel D, Eschenbach DA, Holmes KK. Preterm labor associated with subclinical amniotic fluid infection and with bacterial vaginosis. Obstetrics and gynecology 1986;67:229–37. [DOI] [PubMed] [Google Scholar]
- 32.Fortunato SJ, Menon RP, Swan KF, Menon R. Inflammatory cytokine (interleukins 1, 6 and 8 and tumor necrosis factor-alpha) release from cultured human fetal membranes in response to endotoxic lipopolysaccharide mirrors amniotic fluid concentrations. Am J Obstet Gynecol 1996;174:1855–61; discussion 61–2. [DOI] [PubMed] [Google Scholar]
- 33.Keelan JA, Sato T, Mitchell MD. Interleukin (IL)-6 and IL-8 production by human amnion: regulation by cytokines, growth factors, glucocorticoids, phorbol esters, and bacterial lipopolysaccharide. Biol Reprod 1997;57:1438–44. [DOI] [PubMed] [Google Scholar]
- 34.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202. [DOI] [PubMed] [Google Scholar]
- 35.Romero R, Gomez R, Chaiworapongsa T, Conoscenti G, Kim JC, Kim YM. The role of infection in preterm labour and delivery. Paediatr Perinat Epidemiol 2001;15 Suppl 2:41–56. [DOI] [PubMed] [Google Scholar]
- 36.Moss TJ, Davey MG, Harding R, Newnham JP. Effects of intra-amniotic endotoxin on lung structure and function two months after term birth in sheep. J Soc Gynecol Investig 2002;9:220–5. [PubMed] [Google Scholar]
- 37.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002;8:3–13. [DOI] [PubMed] [Google Scholar]
- 38.Grigsby PL, Hirst JJ, Scheerlinck JP, Phillips DJ, Jenkin G. Fetal responses to maternal and intra-amniotic lipopolysaccharide administration in sheep. Biol Reprod 2003;68:1695–702. [DOI] [PubMed] [Google Scholar]
- 39.Newnham JP, Kallapur SG, Kramer BW, et al. Betamethasone effects on chorioamnionitis induced by intra-amniotic endotoxin in sheep. Am J Obstet Gynecol 2003;189:1458–66. [DOI] [PubMed] [Google Scholar]
- 40.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol 2003;163:2103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moss TJ, Nitsos I, Ikegami M, Jobe AH, Newnham JP. Experimental intrauterine Ureaplasma infection in sheep. Am J Obstet Gynecol 2005;192:1179–86. [DOI] [PubMed] [Google Scholar]
- 42.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel L, Hassan S. The role of inflammation and infection in preterm birth. Semin Reprod Med 2007;25:21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novy MJ, Duffy L, Axthelm MK, et al. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci 2009;16:56–70. [DOI] [PubMed] [Google Scholar]
- 44.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science 2014;345:760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Latino MA, Botta G, Badino C, et al. Association between genital mycoplasmas, acute chorioamnionitis and fetal pneumonia in spontaneous abortions. J Perinat Med 2018;46:503–8. [DOI] [PubMed] [Google Scholar]
- 46.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993;30:167–83. [DOI] [PubMed] [Google Scholar]
- 48.Greig PC, Ernest JM, Teot L, Erikson M, Talley R. Amniotic fluid interleukin-6 levels correlate with histologic chorioamnionitis and amniotic fluid cultures in patients in premature labor with intact membranes. Am J Obstet Gynecol 1993;169:1035–44. [DOI] [PubMed] [Google Scholar]
- 49.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstetrics and gynecology 1993;81:941–8. [PubMed] [Google Scholar]
- 50.Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 1993;5:81–8. [DOI] [PubMed] [Google Scholar]
- 51.Romero R, Yoon BH, Mazor M, et al. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 1993;169:839–51. [DOI] [PubMed] [Google Scholar]
- 52.Coultrip LL, Lien JM, Gomez R, Kapernick P, Khoury A, Grossman JH. The value of amniotic fluid interleukin-6 determination in patients with preterm labor and intact membranes in the detection of microbial invasion of the amniotic cavity. Am J Obstet Gynecol 1994;171:901–11. [DOI] [PubMed] [Google Scholar]
- 53.Dudley DJ, Hunter C, Mitchell MD, Varner MW. Clinical value of amniotic fluid interleukin-6 determinations in the management of preterm labour. Br J Obstet Gynaecol 1994;101:592–7. [DOI] [PubMed] [Google Scholar]
- 54.Allbert JR, Naef RW 3rd, Perry Jr., Magann EF, Whitworth NS, Morrison JC. Amniotic fluid interleukin-6 and interleukin-8 levels predict the success of tocolysis in patients with preterm labor. J Soc Gynecol Investig 1994;1:264–8. [DOI] [PubMed] [Google Scholar]
- 55.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 1994;171:1660–7. [DOI] [PubMed] [Google Scholar]
- 56.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995;173:606–12. [DOI] [PubMed] [Google Scholar]
- 57.Yoon BH, Jun JK, Park KH, Syn HC, Gomez R, Romero R. Serum C-reactive protein, white blood cell count, and amniotic fluid white blood cell count in women with preterm premature rupture of membranes. Obstetrics and gynecology 1996;88:1034–40. [DOI] [PubMed] [Google Scholar]
- 58.Rizzo G, Capponi A, Rinaldo D, Tedeschi D, Arduini D, Romanini C. Interleukin-6 concentrations in cervical secretions identify microbial invasion of the amniotic cavity in patients with preterm labor and intact membranes. Am J Obstet Gynecol 1996;175:812–7. [DOI] [PubMed] [Google Scholar]
- 59.Garry D, Figueroa R, Aguero-Rosenfeld M, Martinez E, Visintainer P, Tejani N. A comparison of rapid amniotic fluid markers in the prediction of microbial invasion of the uterine cavity and preterm delivery. Am J Obstet Gynecol 1996;175:1336–41. [DOI] [PubMed] [Google Scholar]
- 60.Cox SM, Casey ML, MacDonald PC. Accumulation of interleukin-1beta and interleukin-6 in amniotic fluid: a sequela of labour at term and preterm. Hum Reprod Update 1997;3:517–27. [DOI] [PubMed] [Google Scholar]
- 61.Rivero-Marcotegui A, Larranaga-Azcarate C, Ceres-Ruiz R, Garcia-Merlo S. Polymorphonuclear elastase and interleukin-6 in amniotic fluid in preterm labor. Clin Chem 1997;43:857–9. [PubMed] [Google Scholar]
- 62.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998;178:546–50. [DOI] [PubMed] [Google Scholar]
- 63.Hsu CD, Meaddough E, Hong SF, Aversa K, Lu LC, Copel JA. Elevated amniotic fluid nitric oxide metabolites and interleukin-6 in intra-amniotic infection. J Soc Gynecol Investig 1998;5:21–4. [DOI] [PubMed] [Google Scholar]
- 64.Arntzen KJ, Kjollesdal AM, Halgunset J, Vatten L, Austgulen R. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med 1998;26:17–26. [DOI] [PubMed] [Google Scholar]
- 65.Greci LS, Gilson GJ, Nevils B, Izquierdo LA, Qualls CR, Curet LB. Is amniotic fluid analysis the key to preterm labor? A model using interleukin-6 for predicting rapid delivery. Am J Obstet Gynecol 1998;179:172–8. [DOI] [PubMed] [Google Scholar]
- 66.Baud O, Emilie D, Pelletier E, et al. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol 1999;106:72–7. [DOI] [PubMed] [Google Scholar]
- 67.Hsu CD, Aversa K, Meaddough E. The role of amniotic fluid interleukin-6, and cell adhesion molecules, intercellular adhesion molecule-1 and leukocyte adhesion molecule-1, in intra-amniotic infection. Am J Reprod Immunol 2000;43:251–4. [DOI] [PubMed] [Google Scholar]
- 68.Gravett MG, Hitti J, Hess DL, Eschenbach DA. Intrauterine infection and preterm delivery: evidence for activation of the fetal hypothalamic-pituitary-adrenal axis. Am J Obstet Gynecol 2000;182:1404–13. [DOI] [PubMed] [Google Scholar]
- 69.Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–81. [DOI] [PubMed] [Google Scholar]
- 70.Hitti J, Tarczy-Hornoch P, Murphy J, Hillier SL, Aura J, Eschenbach DA. Amniotic fluid infection, cytokines, and adverse outcome among infants at 34 weeks’ gestation or less. Obstetrics and gynecology 2001;98:1080–8. [DOI] [PubMed] [Google Scholar]
- 71.Maymon E, Romero R, Chaiworapongsa T, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol 2001;185:1143–8. [DOI] [PubMed] [Google Scholar]
- 72.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2001;185:1130–6. [DOI] [PubMed] [Google Scholar]
- 73.Jacobsson B, Mattsby-Baltzer I, Andersch B, et al. Microbial invasion and cytokine response in amniotic fluid in a Swedish population of women in preterm labor. Acta obstetricia et gynecologica Scandinavica 2003;82:120–8. [DOI] [PubMed] [Google Scholar]
- 74.Perni SC, Vardhana S, Korneeva I, et al. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol 2004;191:1382–6. [DOI] [PubMed] [Google Scholar]
- 75.Poggi SH, Spong CY, Ghidini A, Ossandon M. Gender differences in amniotic fluid cytokine levels. J Matern Fetal Neonatal Med 2004;15:367–71. [DOI] [PubMed] [Google Scholar]
- 76.Viscardi RM, Muhumuza CK, Rodriguez A, et al. Inflammatory markers in intrauterine and fetal blood and cerebrospinal fluid compartments are associated with adverse pulmonary and neurologic outcomes in preterm infants. Pediatr Res 2004;55:1009–17. [DOI] [PubMed] [Google Scholar]
- 77.Jacobsson B, Mattsby-Baltzer I, Hagberg H. Interleukin-6 and interleukin-8 in cervical and amniotic fluid: relationship to microbial invasion of the chorioamniotic membranes. BJOG 2005;112:719–24. [DOI] [PubMed] [Google Scholar]
- 78.Kiefer DG, Keeler SM, Rust OA, Wayock CP, Vintzileos AM, Hanna N. Is midtrimester short cervix a sign of intraamniotic inflammation? Am J Obstet Gynecol 2009;200:374 e1–5. [DOI] [PubMed] [Google Scholar]
- 79.Lee SM, Lee KA, Kim SM, Park CW, Yoon BH. The risk of intra-amniotic infection, inflammation and histologic chorioamnionitis in term pregnant women with intact membranes and labor. Placenta 2011;32:516–21. [DOI] [PubMed] [Google Scholar]
- 80.Kiefer DG, Keeler SM, Rust O, et al. Amniotic fluid inflammatory score is associated with pregnancy outcome in patients with mid trimester short cervix. Am J Obstet Gynecol 2012;206:68 e1–6. [DOI] [PubMed] [Google Scholar]
- 81.Kim SM, Romero R, Park JW, Oh KJ, Jun JK, Yoon BH. The relationship between the intensity of intra-amniotic inflammation and the presence and severity of acute histologic chorioamnionitis in preterm gestation. The Journal of Maternal-Fetal & Neonatal Medicine 2014;28:1500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oz M, Polat B, Ozgu E, Seckin KD, Tasin C, Danisman N. Interleukin-6 and Creactive protein levels in the amniotic fluid as indicators of preterm delivery in Turkish women. Clin Exp Obstet Gynecol 2015;42:801–4. [PubMed] [Google Scholar]
- 83.Kunze M, Klar M, Morfeld CA, et al. Cytokines in noninvasively obtained amniotic fluid as predictors of fetal inflammatory response syndrome. Am J Obstet Gynecol 2016;215:96 e1–8. [DOI] [PubMed] [Google Scholar]
- 84.Musilova I, Andrys C, Drahosova M, et al. Amniotic fluid prostaglandin E2 in pregnancies complicated by preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med 2016;29:2915–23. [DOI] [PubMed] [Google Scholar]
- 85.Musilova I, Andrys C, Drahosova M, et al. Amniotic fluid calreticulin in pregnancies complicated by the preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2016;29:3921–9. [DOI] [PubMed] [Google Scholar]
- 86.Lee J, Romero R, Lee KA, et al. Meconium aspiration syndrome: a role for fetal systemic inflammation. Am J Obstet Gynecol 2016;214:366 e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kesrouani A, Chalhoub E, El Rassy E, et al. Prediction of preterm delivery by second trimester inflammatory biomarkers in the amniotic fluid. Cytokine 2016;85:67–70. [DOI] [PubMed] [Google Scholar]
- 88.Kiefer DG, Peltier MR, Keeler SM, et al. Efficacy of midtrimester short cervix interventions is conditional on intraamniotic inflammation. Am J Obstet Gynecol 2016;214:276 e1– e6. [DOI] [PubMed] [Google Scholar]
- 89.Kemp MW, Molloy TJ, Usuda H, et al. Outside-in? Acute fetal systemic inflammation in very preterm chronically catheterized sheep fetuses is not driven by cells in the fetal blood. Am J Obstet Gynecol 2016;214:281 e1– e10. [DOI] [PubMed] [Google Scholar]
- 90.Oh KJ, Kim SM, Hong JS, et al. Twenty-four percent of patients with clinical chorioamnionitis in preterm gestations have no evidence of either culture-proven intraamniotic infection or intraamniotic inflammation. Am J Obstet Gynecol 2017;216:604 e1– e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Musilova I, Andrys C, Drahosova M, et al. Amniotic fluid clusterin in pregnancies complicated by the preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2017;30:2529–37. [DOI] [PubMed] [Google Scholar]
- 92.Gomez-Lopez N, Romero R, Xu Y, et al. Are amniotic fluid neutrophils in women with intraamniotic infection and/or inflammation of fetal or maternal origin? Am J Obstet Gynecol 2017;217:693 e1-e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oh KJ, Park JY, Lee J, Hong JS, Romero R, Yoon BH. The combined exposure to intra-amniotic inflammation and neonatal respiratory distress syndrome increases the risk of intraventricular hemorrhage in preterm neonates. J Perinat Med 2018;46:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jung EY, Park KH, Lee SY, Ryu A, Oh KJ. Non-invasive prediction of intra-amniotic infection and/or inflammation in patients with cervical insufficiency or an asymptomatic short cervix (</=15 mm). Arch Gynecol Obstet 2015;292:579–87. [DOI] [PubMed] [Google Scholar]
- 95.Diago Almela VJ, Martinez-Varea A, Perales-Puchalt A, Alonso-Diaz R, Perales A. Good prognosis of cerclage in cases of cervical insufficiency when intra-amniotic inflammation/infection is ruled out. J Matern Fetal Neonatal Med 2015;28:1563–8. [DOI] [PubMed] [Google Scholar]
- 96.Kim YM, Romero R, Oh SY, et al. Toll-like receptor 4: a potential link between “danger signals,” the innate immune system, and preeclampsia? Am J Obstet Gynecol 2005;193:921–7. [DOI] [PubMed] [Google Scholar]
- 97.Romero R, Chaiworapongsa T, Alpay Savasan Z, et al. Damage-associated molecular patterns (DAMPs) in preterm labor with intact membranes and preterm PROM: a study of the alarmin HMGB1. J Matern Fetal Neonatal Med 2011;24:1444–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Romero R, Miranda J, Chaiworapongsa T, et al. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Reprod Immunol 2014;72:458–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Romero R, Miranda J, Chaemsaithong P, et al. Sterile and microbial-associated intraamniotic inflammation in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med 2015;28:1394–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gomez-Lopez N, Romero R, Plazyo O, et al. Intra-Amniotic Administration of HMGB1 Induces Spontaneous Preterm Labor and Birth. Am J Reprod Immunol 2016;75:3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Plazyo O, Romero R, Unkel R, et al. HMGB1 Induces an Inflammatory Response in the Chorioamniotic Membranes That Is Partially Mediated by the Inflammasome. Biol Reprod 2016;95:130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gomez-Lopez N, Romero R, Leng Y, et al. Neutrophil extracellular traps in acute chorioamnionitis: A mechanism of host defense. Am J Reprod Immunol 2017;77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gomez-Lopez N, Romero R, Xu Y, et al. A Role for the Inflammasome in Spontaneous Preterm Labor With Acute Histologic Chorioamnionitis. Reprod Sci 2017;24:1382–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gomez-Lopez N, Romero R, Xu Y, et al. A Role for the Inflammasome in Spontaneous Labor at Term with Acute Histologic Chorioamnionitis. Reprod Sci 2017;24:934–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Panaitescu B, Romero R, Gomez-Lopez N, et al. In vivo evidence of inflammasome activation during spontaneous labor at term. J Matern Fetal Neonatal Med 2019;32:1978–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Romero R, Xu Y, Plazyo O, et al. A Role for the Inflammasome in Spontaneous Labor at Term. Am J Reprod Immunol 2018;79:e12440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gotsch F, Romero R, Chaiworapongsa T, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med 2008;21:605–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Strauss JF 3rd, Romero R, Gomez-Lopez N, et al. Spontaneous preterm birth: advances toward the discovery of genetic predisposition. Am J Obstet Gynecol 2018;218:294–314 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim KW, Romero R, Park HS, et al. A rapid matrix metalloproteinase-8 bedside test for the detection of intraamniotic inflammation in women with preterm premature rupture of membranes. Am J Obstet Gynecol 2007;197:292 e1–5. [DOI] [PubMed] [Google Scholar]
- 110.Gervasi MT, Romero R, Bracalente G, et al. Midtrimester amniotic fluid concentrations of interleukin-6 and interferon-gamma-inducible protein-10: evidence for heterogeneity of intra-amniotic inflammation and associations with spontaneous early (<32 weeks) and late (>32 weeks) preterm delivery. J Perinat Med 2012;40:329–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romero R, Kadar N, Miranda J, et al. The diagnostic performance of the Mass Restricted (MR) score in the identification of microbial invasion of the amniotic cavity or intra-amniotic inflammation is not superior to amniotic fluid interleukin-6. J Matern Fetal Neonatal Med 2014;27:757–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Combs CA, Gravett M, Garite TJ, et al. Amniotic fluid infection, inflammation, and colonization in preterm labor with intact membranes. Am J Obstet Gynecol 2014;210:125 e1–e15. [DOI] [PubMed] [Google Scholar]
- 113.Romero R, Miranda J, Chaiworapongsa T, et al. Sterile intra-amniotic inflammation in asymptomatic patients with a sonographic short cervix: prevalence and clinical significance. J Matern Fetal Neonatal Med 2014:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Romero R, Miranda J, Kusanovic JP, et al. Clinical chorioamnionitis at term I: microbiology of the amniotic cavity using cultivation and molecular techniques. J Perinat Med 2015;43:19–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Romero R, Grivel JC, Tarca AL, et al. Evidence of perturbations of the cytokine network in preterm labor. Am J Obstet Gynecol 2015;213:836 e1– e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Romero R, Chaemsaithong P, Korzeniewski SJ, et al. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J Perinat Med 2016;44:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chaemsaithong P, Romero R, Korzeniewski SJ, et al. A rapid interleukin-6 bedside test for the identification of intra-amniotic inflammation in preterm labor with intact membranes. J Matern Fetal Neonatal Med 2016;29:349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Park JY, Romero R, Lee J, Chaemsaithong P, Chaiyasit N, Yoon BH . An elevated amniotic fluid prostaglandin F2alpha concentration is associated with intra-amniotic inflammation/infection, and clinical and histologic chorioamnionitis, as well as impending preterm delivery in patients with preterm labor and intact membranes. J Matern Fetal Neonatal Med 2016;29:2563–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chaemsaithong P, Romero R, Docheva N, et al. Comparison of rapid MMP-8 and interleukin-6 point-of-care tests to identify intra-amniotic inflammation/infection and impending preterm delivery in patients with preterm labor and intact membranes(). J Matern Fetal Neonatal Med 2018;31:228–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brown CL, Ludwiczak MH, Blanco JD, Hirsch CE. Cervical dilation: accuracy of visual and digital examinations. Obstetrics and gynecology 1993;81:215–6. [PubMed] [Google Scholar]
- 121.Nien JK, Yoon BH, Espinoza J, et al. A rapid MMP-8 bedside test for the detection of intra-amniotic inflammation identifies patients at risk for imminent preterm delivery. Am J Obstet Gynecol 2006;195:1025–30. [DOI] [PubMed] [Google Scholar]
- 122.Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med 2008;36:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim SM, Romero R, Lee J, et al. About one-half of early spontaneous preterm deliveries can be identified by a rapid matrix metalloproteinase-8 (MMP-8) bedside test at the time of mid-trimester genetic amniocentesis. J Matern Fetal Neonatal Med 2016;29:2414–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Park JS, Romero R, Yoon BH, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol 2001;185:1156–61. [DOI] [PubMed] [Google Scholar]
- 125.Shim SS, Romero R, Hong JS, et al. Clinical significance of intra-amniotic inflammation in patients with preterm premature rupture of membranes. Am J Obstet Gynecol 2004;191:1339–45. [DOI] [PubMed] [Google Scholar]
- 126.Lee J, Romero R, Kim SM, et al. A new anti-microbial combination prolongs the latency period, reduces acute histologic chorioamnionitis as well as funisitis, and improves neonatal outcomes in preterm PROM. J Matern Fetal Neonatal Med 2016;29:707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yoon BH, Romero R, Kim CJ, et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–70. [DOI] [PubMed] [Google Scholar]
- 128.Redline RW. Inflammatory responses in the placenta and umbilical cord. Seminars in fetal & neonatal medicine 2006;11:296–301. [DOI] [PubMed] [Google Scholar]
- 129.Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am J Obstet Gynecol 2015;213:S29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Gomez-Lopez N, Romero R, Plazyo O, et al. Preterm labor in the absence of acute histologic chorioamnionitis is characterized by cellular senescence of the chorioamniotic membranes. Am J Obstet Gynecol 2017;217:592 e1– e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romero R, Chaemsaithong P, Docheva N, et al. Clinical chorioamnionitis at term VI: acute chorioamnionitis and funisitis according to the presence or absence of microorganisms and inflammation in the amniotic cavity. J Perinat Med 2016;44:33–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gibbs RS. Diagnosis of intra-amniotic infection. Seminars in perinatology 1977;1:71–7. [PubMed] [Google Scholar]
- 133.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. The Journal of infectious diseases 1982;145:1–8. [DOI] [PubMed] [Google Scholar]
- 134.Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol 1996;174:1433–40. [DOI] [PubMed] [Google Scholar]
- 135.Anonymous. In: Culpeper N, Cole A, Rowland W, editors. The practice of physick. London (UK): George Sawbridge; 1678. p. 502–9. [Google Scholar]
- 136.Grant A. Cervical cerclage to prolong pregnancy In: Chalmers I, Enkin M, Keirse MJNC, editors. Effective care in pregnancy and childbirth. Vol. 1 New York (NY): Oxford University Press; 1989. p. 633–46. [Google Scholar]
- 137.Lash AF, Lash SR. Habitual abortion; the incompetent internal os of the cervix. Am J Obstet Gynecol 1950;59:68–76. [DOI] [PubMed] [Google Scholar]
- 138.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol 2006;194:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shirodkar VN ea. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic 1955;52:299–300. [Google Scholar]
- 140.McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp 1957;64:346–50. [DOI] [PubMed] [Google Scholar]
- 141.Guzman ER, Forster JK, Vintzileos AM, Ananth CV, Walters C, Gipson K. Pregnancy outcomes in women treated with elective versus ultrasound-indicated cervical cerclage. Ultrasound Obstet Gynecol 1998;12:323–7. [DOI] [PubMed] [Google Scholar]
- 142.Berghella V, Ciardulli A, Rust OA, et al. Cerclage for sonographic short cervix in singleton gestations without prior spontaneous preterm birth: systematic review and metaanalysis of randomized controlled trials using individual patient-level data. Ultrasound Obstet Gynecol 2017;50:569–77. [DOI] [PubMed] [Google Scholar]
- 143.Roman A, Berghella V . Efficacy of ultrasound-indicated cerclage in twin pregnancies, REPLAY. Am J Obstet Gynecol 2016;214:132–3. [DOI] [PubMed] [Google Scholar]
- 144.Jarde A, Lutsiv O, Park CK, et al. Preterm birth prevention in twin pregnancies with progesterone, pessary, or cerclage: a systematic review and meta-analysis. BJOG 2017;124:1163–73. [DOI] [PubMed] [Google Scholar]
- 145.Adams TM, Rafael TJ, Kunzier NB, Mishra S, Calixte R, Vintzileos AM. Does cervical cerclage decrease preterm birth in twin pregnancies with a short cervix? J Matern Fetal Neonatal Med 2018;31:1092–8. [DOI] [PubMed] [Google Scholar]
- 146.Conde-Agudelo A, Romero R. Vaginal progesterone to prevent preterm birth in pregnant women with a sonographic short cervix: clinical and public health implications. Am J Obstet Gynecol 2016;214:235–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Oyelese Y. Cerclage in twin pregnancies: we should wait before making definitive recommendations. Am J Obstet Gynecol 2016;214:131–2. [DOI] [PubMed] [Google Scholar]
- 148.Sharvit M, Weiss R, Ganor Paz Y, Tzadikevitch Geffen K, Danielli Miller N, Biron-Shental T . Vaginal examination vs. cervical length -which is superior in predicting preterm birth? J Perinat Med 2017;45:977–83. [DOI] [PubMed] [Google Scholar]
- 149.Sanchez-Ramos L. Vaginal progesterone is an alternative to cervical cerclage in women with a short cervix and a history of preterm birth. Am J Obstet Gynecol 2018;219:5–9. [DOI] [PubMed] [Google Scholar]
- 150.Conde-Agudelo A, Romero R, Da Fonseca E, et al. Vaginal progesterone is as effective as cervical cerclage to prevent preterm birth in women with a singleton gestation, previous spontaneous preterm birth, and a short cervix: updated indirect comparison meta-analysis. Am J Obstet Gynecol 2018;219:10–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Romero R, Conde-Agudelo A, Da Fonseca E, et al. Vaginal progesterone for preventing preterm birth and adverse perinatal outcomes in singleton gestations with a short cervix: a meta-analysis of individual patient data. Am J Obstet Gynecol 2018;218:161–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Makrydimas G, Barmpalia Z, Sotiriadis A. Cervical cerclage for women with shortening cervix while on progesterone. Am J Obstet Gynecol 2019;220:209–10. [DOI] [PubMed] [Google Scholar]
- 153.Romero R, Conde-Agudelo A, Nicolaides KH. There is insufficient evidence to claim that cerclage is the treatment of choice for patients with a cervical length <10 mm. Am J Obstet Gynecol 2018;219:213–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Monsanto SP, Daher S, Ono E, et al. Cervical cerclage placement decreases local levels of proinflammatory cytokines in patients with cervical insufficiency. Am J Obstet Gynecol 2017;217:455 e1– e8. [DOI] [PubMed] [Google Scholar]
- 155.Oyelese Y, Powel J, Benito CW. Perhaps cerclage is the ideal treatment for the cervix <1 cm. Am J Obstet Gynecol 2018;219:213. [DOI] [PubMed] [Google Scholar]
- 156.Kacerovsky M, Musilova I, Hornychova H, et al. Bedside assessment of amniotic fluid interleukin-6 in preterm prelabor rupture of membranes. Am J Obstet Gynecol 2014;211:385 e1–9. [DOI] [PubMed] [Google Scholar]
- 157.Chaiyasit N, Romero R, Chaemsaithong P, et al. Clinical chorioamnionitis at term VIII: a rapid MMP-8 test for the identification of intra-amniotic inflammation. J Perinat Med 2017;45:539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Witt A, Sommer EM, Cichna M, et al. Placental passage of clarithromycin surpasses other macrolide antibiotics. Am J Obstet Gynecol 2003;188:816–9. [DOI] [PubMed] [Google Scholar]
- 159.Kafetzis DA, Brater DC, Fanourgakis JE, Voyatzis J, Georgakopoulos P. Ceftriaxone distribution between maternal blood and fetal blood and tissues at parturition and between blood and milk postpartum. Antimicrob Agents Chemother 1983;23:870–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gauthier DW, Meyer WJ. Comparison of gram stain, leukocyte esterase activity, and amniotic fluid glucose concentration in predicting amniotic fluid culture results in preterm premature rupture of membranes. Am J Obstet Gynecol 1992;167:1092–5. [DOI] [PubMed] [Google Scholar]
- 161.Cotton DB, Hill LM, Strassner HT, Platt LD, Ledger WJ. Use of amniocentesis in preterm gestation with ruptured membranes. Obstetrics and gynecology 1984;63:38–43. [PubMed] [Google Scholar]
- 162.Romero R, Quintero R, Oyarzun E, et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–6. [DOI] [PubMed] [Google Scholar]
- 163.Garite TJ, Freeman RK. Chorioamnionitis in the preterm gestation. Obstetrics and gynecology 1982;59:539–45. [PubMed] [Google Scholar]
- 164.DiGiulio DB, Romero R, Kusanovic JP, et al. Prevalence and diversity of microbes in the amniotic fluid, the fetal inflammatory response, and pregnancy outcome in women with preterm pre-labor rupture of membranes. Am J Reprod Immunol 2010;64:38–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lamb HM, Ormrod D, Scott LJ, Figgitt DP. Ceftriaxone: an update of its use in the management of community-acquired and nosocomial infections. Drugs 2002;62:1041–89. [DOI] [PubMed] [Google Scholar]
- 166.Fidel P, Ghezzi F, Romero R, et al. The effect of antibiotic therapy on intrauterine infection-induced preterm parturition in rabbits. J Matern Fetal Neonatal Med 2003;14:57–64. [DOI] [PubMed] [Google Scholar]
- 167.Grigsby PL, Novy MJ, Sadowsky DW, et al. Maternal azithromycin therapy for Ureaplasma intraamniotic infection delays preterm delivery and reduces fetal lung injury in a primate model. Am J Obstet Gynecol 2012;207:475 e1– e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Gravett MG, Adams KM, Sadowsky DW, et al. Immunomodulators plus antibiotics delay preterm delivery after experimental intraamniotic infection in a nonhuman primate model. Am J Obstet Gynecol 2007;197:518 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bean LM, Jackson JR, Dobak WJ, Beiswenger TR, Thorp JA. Intra-amniotic fluconazole therapy for Candida albicans intra-amniotic infection. Obstetrics and gynecology 2013;121:452–4. [DOI] [PubMed] [Google Scholar]
- 170.Jalava J, Mantymaa ML, Ekblad U, et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. BJOG 1996;103:664–9. [DOI] [PubMed] [Google Scholar]
- 171.Hitti J, Riley DE, Krohn MA, et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 1997;24:1228–32. [DOI] [PubMed] [Google Scholar]
- 172.DiGiulio DB, Romero R, Amogan HP, et al. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: a molecular and culture-based investigation. PLoS One 2008;3:e3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.DiGiulio DB, Gervasi M, Romero R, et al. Microbial invasion of the amniotic cavity in preeclampsia as assessed by cultivation and sequence-based methods. J Perinat Med 2010;38:503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.DiGiulio DB, Gervasi MT, Romero R, et al. Microbial invasion of the amniotic cavity in pregnancies with small-for-gestational-age fetuses. J Perinat Med 2010;38:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Combs CA, Garite TJ, Lapidus JA, et al. Detection of microbial invasion of the amniotic cavity by analysis of cervicovaginal proteins in women with preterm labor and intact membranes. Am J Obstet Gynecol 2015;212:482 e1– e12. [DOI] [PubMed] [Google Scholar]
- 176.Musilova I, Bestvina T, Hudeckova M, et al. Vaginal fluid IL-6 concentrations as a point-of-care test is of value in women with preterm PROM. Am J Obstet Gynecol 2016. [DOI] [PubMed] [Google Scholar]
- 177.Rowlands S, Danielewski JA, Tabrizi SN, Walker SP, Garland SM. Microbial invasion of the amniotic cavity in midtrimester pregnancies using molecular microbiology. Am J Obstet Gynecol 2017;217:71 e1– e5. [DOI] [PubMed] [Google Scholar]
- 178.Renko J, Koskela KA, Lepp PW, et al. Bacterial DNA signatures in carotid atherosclerosis represent both commensals and pathogens of skin origin. Eur J Dermatol 2013;23:53–8. [DOI] [PubMed] [Google Scholar]
- 179.Dinakaran V, Rathinavel A, Pushpanathan M, Sivakumar R, Gunasekaran P, Rajendhran J. Elevated levels of circulating DNA in cardiovascular disease patients: metagenomic profiling of microbiome in the circulation. PLoS One 2014;9:e105221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kowarsky M, Camunas-Soler J, Kertesz M, et al. Numerous uncharacterized and highly divergent microbes which colonize humans are revealed by circulating cell-free DNA. Proc Natl Acad Sci U S A 2017;114:9623–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wang Z, Tang WH, Buffa JA, et al. Prognostic value of choline and betaine depends on intestinal microbiota-generated metabolite trimethylamine-N-oxide. Eur Heart J 2014;35:904–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tamaoki J, Kadota J, Takizawa H. Clinical implications of the immunomodulatory effects of macrolides. Am J Med 2004;117 Suppl 9A:5S–11S. [DOI] [PubMed] [Google Scholar]
- 183.Shinkai M, Tamaoki J, Kobayashi H, et al. Clarithromycin delays progression of bronchial epithelial cells from G1 phase to S phase and delays cell growth via extracellular signal-regulated protein kinase suppression. Antimicrob Agents Chemother 2006;50:1738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med 2008;177:148–55. [DOI] [PubMed] [Google Scholar]
- 185.Kim MJ, Romero R, Gervasi MT, et al. Widespread microbial invasion of the chorioamniotic membranes is a consequence and not a cause of intra-amniotic infection. Lab Invest 2009;89:924–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hassan S, Romero R, Hendler I, et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med 2006;34:13–9. [DOI] [PMC free article] [PubMed] [Google Scholar]