Abstract
Background
Mast flowering (‘masting’) is characterized by mass synchronized flowering at irregular intervals in populations of perennial plants over a wide geographical area, resulting in irregular high seed production. While masting is a global phenomenon, it is particularly prevalent in the alpine flora of New Zealand. Increases in global temperature may alter the masting pattern, affecting wider communities with a potential impact on plant–pollinator interactions, seed set and food availability for seed-consuming species.
Scope
This review summarizes an ecological temperature model (ΔT) that is being used to predict the intensity of a masting season. We introduce current molecular studies on flowering and the concept of an ‘epigenetic summer memory’ as a driver of mast flowering. We propose a hypothetical model based on temperature-associated epigenetic modifications of the floral integrator genes FLOWERING LOCUS T, FLOWERING LOCUS C and SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1.
Conclusions
Genome-wide transcriptomic and targeted gene expression analyses are needed to establish the developmental and physiological processes associated with masting. Such analyses may identify changes in gene expression that can be used to predict the intensity of a forthcoming masting season, as well as to determine the extent to which climate change will influence the mass synchronized flowering of masting species, with downstream impacts on their associated communities.
Keywords: Epigenetic, floral integrator genes, ambient temperature pathway, masting, mast flowering, perennial plant, ΔT model of mast flowering
Introduction
Mast flowering, or masting, which is synchronized highly variable flowering and seed production by perennial plants, is a well-researched phenomenon at the ecological level (Kelly, 1994; Pearse et al., 2016). How this is regulated at the molecular level is not understood. In this review, we introduce masting and the predictive temperature model (Kelly et al., 2013). We briefly introduce the molecular pathways to flowering, particularly focusing on epigenetic control of flowering. We also highlight the limited knowledge of these pathways in mast flowering plants. Finally, we introduce the hypothesis of an ‘epigenetic summer memory’ as the mechanism underpinning mast flowering and present a model based on temperature-associated epigenetic modifications of FLOWERING LOCUS T (FT), encoding the flowering hormone.
MAST FLOWERING AND SEED PRODUCTION
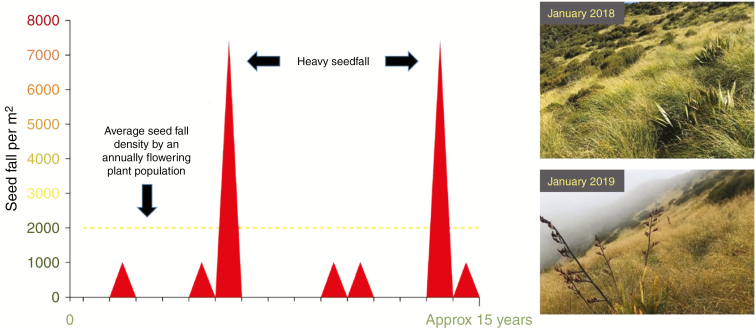
Masting is synchronized irregular seed production by a perennial plant population spread across a wide geographical area (Fig. 1). In a high seed year (mast year), a species undergoes heavy flowering and sets a large number of seeds; in other years (non-mast years), plants have moderate, low or zero flowering (Kelly, 1994; Kelly et al., 2008; Pearse et al., 2016). Masting allows a plant population to synchronize its flowering to achieve greater reproductive efficiency (Kelly and Sork, 2002). Synchronous flowering over a large area extending to hundreds or thousands of kilometres prevents the aggregation of seed consumers over a local area. Furthermore, the consumers are starved during a non-mast year and satiated with food during a mast year, leading to fluctuations in the population of the consumers over a period of time (Kelly et al., 2000). Additionally, if seed consumers firmly favour a specific fruit or seeds of a species, then less favoured species may escape seed predation by masting in synchrony with the favoured groups (Kelly and Sork, 2002).
Fig. 1.
On the left-hand side, the graph illustrates the flowering pattern of a masting plant population. A masting plant flowers heavily at irregular intervals but synchronized within the population. The plant population remains vegetative for most of its life and flowers only when the inductive signals are perceived. On the right-hand side, masting in Chionochloa pallens can be seen. These plants are present at 1070 m on Mt Hutt in Canterbury, New Zealand.
The phenomenon of masting is particularly observed in long-lived plant populations, predominantly in woody and wind-pollinated species (Herrera et al., 1998). Plants belonging to 37 different families have been shown to exhibit masting patterns, with the Pinaceae being the most studied plant family (Kelly and Sork, 2002). Much of the alpine flora of New Zealand exhibits strong masting behaviour (Kelly and Sork, 2002), providing a pulse of produce for native invertebrates and birds (Norton and Kelly, 1988).
The evolution of mast flowering in a long-lived plant species is a result of the interaction between various functional constraints at the population level and evolutionary selective forces acting at the trophic level driving the ultimate cause of masting in plants, including predator satiation and higher pollination efficiency (Tachiki and Iwasa, 2012; Pearse et al., 2016). Masting, as a reproductive strategy, is also interesting because plants delay reproduction during what would otherwise appear to be favourable conditions which results in a more density-dependent mortality (Kelly, 1994). Individually, each plant ought to suffer higher rates of intraspecific competition and seed predation or other forms of biotic attack (Kitzberger et al., 2007). Moreover, the cost of heavy flowering as a reproductive strategy is very high. The event can force a plant to exhaust its resources, and reduce future vegetative growth (Kelly and Sork, 2002; Sala et al., 2012) or produce non-viable seeds in the next season (Allen et al., 2014). However, individual plants can increase their reproductive productivity by synchronizing their reproductive timing with the timing of reproduction in other plants of the same species (Kelly, 1994). This increases the chances of survival of the offspring and decreases the cost for each surviving offspring (Kelly and Sork, 2002).
To be advantageous for a masting plant, both high individual variability (i.e. the individual flowers at irregular intervals) and high synchrony among these individuals (each individual flowering at the same time) is required (Koenig et al., 2003). Many hypotheses have been proposed to account for the masting phenomenon. These include predator satiation, wind pollination, environmental prediction, animal dispersal and weather cues (Pearse et al., 2016).
While resources are often considered an important driver of masting (Smaill et al., 2011; Miyazaki et al., 2014; Koenig et al., 2015; Monks et al., 2016; Bogdziewicz et al., 2018; Satake et al., 2019), in terms of predictive models, change in temperature has been considered the most likely cue for masting in a number of plant species (Mark, 1968; Kelly et al., 2008; Pearse et al., 2016). Temperature has the advantage of being broadly uniform over large spatial areas (Kelly et al., 2008). The generation of a high seed crop has been reported to be positively correlated with warm temperatures of the previous growing season (Tn – 1 model) in many New Zealand plant species (Schauber et al., 2002). However, the warm temperature cue has some complications when applied to explain masting behaviour. First, statistical models based on warm temperatures of a previous year have been shown not to be completely effective in terms of predicting an upcoming masting year when simulations are run over several decades. Secondly, a warm temperature season alone cannot explain why the plants do not mast during two consecutive warm years. Thirdly, it is not at all clear how the plants are able to tailor their responses to mast synchronously over a large alpine area at diverse altitudes (Pearse et al., 2016).
Since the warm temperatures of the preceding year do not explain the flowering anomaly effectively, Kelly et al. (2013) proposed the ΔT model. The ΔT model states that mast flowering is induced when plants experience a positive differential mean summer temperature, i.e. the temperature difference between the previous summer and the summer before that (Kelly et al., 2013). Mathematically, it is expressed as:
here, ΔT = the change in mean summer temperature over two previous summer seasons, Tn – 1 = the mean summer temperature in the previous year and Tn – 2 = the mean summer temperature 2 years preceding the current season.
Kelly et al. (2013) studied 15 different species belonging to five different families over 30 years and showed that the ΔT model better predicted seed fall compared with the absolute temperatures in the previous season. The Tn – 1 model displays a proximal response to a high seed crop but is unable to show the variation in the flowering output during a low seed year, while the ΔT model significantly improves the fit for masting species including the variation in the reproductive output during both low and high seed years. The ΔT model has also been shown to be a better statistical predictor of seed crops than the previous year temperature alone for Quercus lobata (Pearse et al., 2014), Picea glauca (Krebs et al., 2017), Cryptomeria japonica (Kon and Saito, 2015), Acer saccharum and Fagus grandifolia (Cleavitt and Fahey, 2017), although proving that ΔT is the underlying mechanism requires more than observational studies.
The ΔT model has several advantages over the Tn – 1 model. The model improved the best fit significantly when three decades of seed fall data were added compared with the Tn – 1 model (Kelly et al., 2013). It also solves the conundrum of how plants at distinct altitudes are able to alter their threshold local mean temperature in order to flower synchronously. Further, it explains why the plants do not flower during two consecutive warm summer years as the second warm summer has a low temperature differential (Kelly et al., 2013). The implication of ΔT is that masting plants may have a mechanism to sense and respond to two different, and temporally well separated, summer temperatures.
At the same time, an increase in the global mean temperatures may disturb the biological balance within a population, between species and at the ecosystem level where species function in a co-operative manner (Melillo et al., 1993; Cao and Woodward, 1998) by altering the timing of reproduction (Franks et al., 2007). With global climate change, the increase in variability in the weather conditions from year to year may lead to a greater variability in the ΔT values over the years, resulting in greater differences between high and low seed years (Kelly et al., 2013).
Mechanistically, the ability of a perennial plant population to undergo masting using the ΔT cue over multiple years would require the presence of a plastic memory. We suggest that this is an ‘epigenetic summer memory’ which allows the plants to ‘remember’ the differential summer temperatures over successive years. Differential epigenetic marks on the flowering time genes, in response to the ΔT cue, may then explain how synchronized flowering is driven by the epigenetic summer memory. Consequently, it is crucial to understand the molecular expression pattern of flowering pathway genes to better understand the complexity of the putative epigenetic summer memory in masting plants.
MOLECULAR CONTROL OF FLOWERING
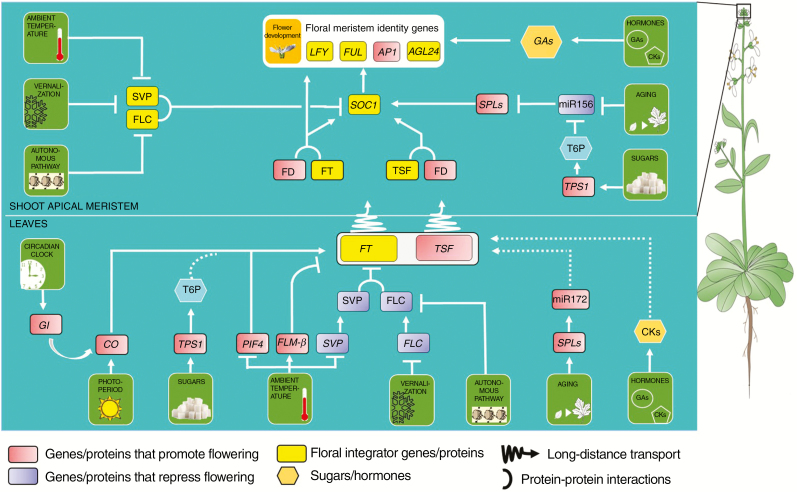
The developmental transition from a vegetative meristem to a reproductive meristem is regulated by various internal and external factors (Andres and Coupland, 2012; Romera-Branchat et al., 2014). These factors act as input signals integrated into a feedback network regulating the timing of floral induction. Studies of flowering time control in Arabidopsis thaliana (arabidopsis) have revealed seven distinct pathways regulating the reproductive transition. These comprise the autonomous, photoperiod, vernalization, hormonal, age, sugar and ambient temperature pathways (Fig. 2). Detailed reviews of each of these pathways can be found in Andres and Coupland (2012), Khan et al. (2014), Cho et al. (2017) and Susila et al. (2018).
Fig. 2.
Floral integrator pathway. FT is the mobile flowering hormone activating the transcription of the floral meristem identity genes which transform the shoot apical meristem into a floral meristem. The activation of flowering is regulated by the various pathways shown in the figure. These include photoperiod, vernalization, sugar, hormonal, ageing and thermosensory pathways. Under inductive conditions, CONSTANS (CO), encoding a zinc finger transcription factor protein, activates the transcription of FT by physically interacting with the promoter of FT. FT protein then travels via the vasculature to the shoot apical meristem to bind with FD and initiate the transcription of floral meristem identity genes. GIGANTEA (GI) is a photoperiodic activator of CO, which stabilizes the CO protein. FLC is a MADS-box transcription factor which physically interacts with SHORT VEGETATIVE PHASE (SVP) to form a floral repressor complex. SVP–FLC then physically interacts with FD, FT and SOC1 to repress their expression. Prolonged cold exposure regulates FLC expression. Endogenous gibberellin induces the expression of LEAFY, a floral meristem identity gene. MiR156 and miR172 are found to have an antagonistic role in the vegetative to reproductive transition. During early development, miR156 is highly abundant. With subsequent growth, the concentration of miR156 decreases, and that of miR172 increases. MiRNA156 targets SQUAMOSA BINDING PROTEIN LIKE (SPL) genes (promoters of flowering) and downregulates their expression, whereas miR172 negatively regulates the expression of APETALA2 (AP2) family genes (repressors of flowering). Sugars, including sucrose, glucose and maltose, can act as endogenous signals to initiate the reproductive phase. TREHALOSE PHOSPHATE SYNTHASE 1 (TPS 1) decreases the levels of miR156 and promotes flowering by increasing the transcript levels of SPL genes. Under high temperature, PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) binds more strongly to the promoter of FT to activate its transcription. At low temperature, FLOWERING LOCUS M-β (FLM-β) binds to SVP to repress flowering. Adapted from www.flor-id.org (Bouche et al., 2016).
In arabidopsis, the molecular network regulating the transition from a vegetative meristem to a reproductive meristem converges on the floral integrator genes (He, 2009) which are at the epicentre of the flowering mechanism. These include FT (FT protein is regarded as the mobile ‘florigen’ (Liu et al., 2016)), TWIN SISTER OF FT (TSF), SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1), AGAMOUS-LIKE 24 (AGL-24), FRUITFULL (FUL), FLOWERING LOCUS C (FLC), SHORT VEGETATIVE PHASE (SVP) and LEAFY (LFY) (Khan et al., 2014; Bouche et al., 2016) (Fig. 2). Once the expression of the integrator genes exceeds a threshold level (or is reduced below a certain level in the case of the repressor FLC), the transition from a vegetative meristem to an inflorescence or floral meristem is activated (Fig. 2) (Huijser and Schmid, 2011; Bouche et al., 2016). The final control of the transition is by the floral meristem identity genes which encode transcription factors that are involved in the initiation of floral development in the shoot apical meristem and include APETALA1 (AP1), LFY, AGL-24 and FUL (Fig. 2).
AMBIENT TEMPERATURE PATHWAY
As warm summer temperatures promote mast flowering, it is important to note that several genes have been reported to induce flowering in plants via the activation of the transcription of floral promoter genes in response to warm ambient temperature (Song et al., 2013; Capovilla et al., 2015a; Susila et al., 2018). FT can be silenced by the deposition of the H2A.Z histone variant in nucleosomes by the SWR1c at the transcriptional start site. However, with a rise in ambient temperature, the H2A.Z nucleosome is evicted, leaving the promoters of the floral integrator genes, including FT, more accessible to transcription factors such as PHYTOCHROME-INTERACTING FACTOR 4 (PIF4) to bind and initiate the floral transition (Kumar et al., 2012).
Additionally, FCA, encoding an RNA-binding protein, produces four distinct alternatively spliced transcripts, α, β, γ, and δ, in response to temperature change (Macknight et al., 1997). Under ambient temperature control, only γ transcripts are produced which code for a mature full-length protein with a WW protein interaction domain. The full-length protein is able to repress the activity of FLC at ambient temperatures (22–27 °C) (Quesada et al., 2003).
FLOWERING LOCUS M (FLM), a DNA-binding protein, interacts with SVP to repress flowering at low temperature. As the temperature increases, an alternative isoform of the FLM transcript is produced which is unable to bind to SVP and is thus incapable of inhibiting the expression of the floral transition genes (Capovilla et al., 2015b). MADS AFFECTING FLOWERING2 (MAF2), a MADS-box transcription factor, ensures depression of flowering until a sufficient period of cold has been experienced by the plant. Airoldi et al. (2015) showed that at lower temperatures a splice variant of MAF2, MAF2var1, represses flowering by interacting with SVP. At high temperatures, another variant of MAF2, MAF2var2, is produced which is unable to bind to the SVP to form a repressor complex and thus the floral transition is induced (Airoldi et al., 2015). If their function is conserved, these genes may have a role in the induction of flowering in masting plants by activating floral promoter genes in response to the summer temperature change.
EPIGENETIC REGULATION OF FLOWERING
Plants appear to have a plastic memory, enabling them to remember variable environmental conditions and seasonal changes. The memory is not only passed cell to cell after mitotic cell division during the growth of a plant, but can also be transgenerational (Murgia et al., 2015). Plants can erase the memory in order to re-establish sensitivity to external conditions either in the next generation or, in the case of a polycarpic plant, in the same individual over the next reproductive cycle (Bossdorf et al., 2008).
Active gene expression is often associated with histone modifications involving histone acetylation, histone 2B mono-ubiquitylation (H2Bub1), H3 lysine 36 di/trimethylation (H3K36-me/me2/me3) and histone H3 lysine 4 trimethylation (H3K4me3) (Xu et al., 2008). These epigenetic marks of active gene transcription are rendered by a heterogeneous class of enzymes collectively known as the Trithorax group (TrxG) proteins (He, 2012). Inactive or inert gene expression is mediated by epigenetic marks associated with the histones H3K27me3, H3K9me and H2A mono-ubiquitylation (H2Aub1). The polycomb group (PcG) of proteins is responsible for depositing these epigenetic marks leading to the repression of a target gene at the transcriptional start site. These epigenetic modifications can be modulated by changes in temperature in response to fluctuating environmental conditions.
The most well studied epigenetically regulated plant gene is FLC, encoding a MADS-box transcription factor which acts as a floral repressor in the Brassicaceae (Mateos et al., 2015). FLC physically interacts with SVP to form a floral repressor complex repressing the expression of FT, FD and SOC1 (Mateos et al., 2015). In arabidopsis, active expression of FLC is regulated by the ATX1 H3K4 methyltransferase and the EFS H3K36 methyltransferase to prevent precocious flowering (Xu et al., 2016). After exposure to an extended period of cold, FLC expression is downregulated through chromatin remodelling by VERNALISATION INSENSITIVE 3 (VIN3) homeodomain finger protein (Sung and Amasino, 2004), co-transcriptional RNA processing through an antisense transcript, COOLAIR, generated from the 3'-downstream region of FLC (Liu et al., 2010), and polycomb silencing through the PHD–PRC2 complex (Bastow et al., 2004). Together, histone modifications including H3K9me3, H3K27me, H3K4 and H3K36 demethylation occurred at the FLC chromatin to maintain the suppression of FLC after the winter (Bastow et al., 2004), the so-called ‘winter memory’ (Bratzel and Turck, 2015).
FT expression in arabidopsis is also controlled by epigenetic marks induced by chromatin modifiers including SWR1c, PRC2, LIKE HETEROCHROMATIN PROTEIN 1 (LHP1), REF6 H3K27 demethylase and the PKDM7B (also known as AtJMJ4 or AtJMJ14) H3K4 demethylase (Xu et al., 2016). The structure of the FT chromatin is bivalent, constituting active as well as repressive epigenetic marks (He and Amasino, 2005). The active epigenetic marks involve H3K4 trimethylation and repressive marks have H3K27 trimethylation. The balance between these epigenetic marks at the FT locus determines whether FT protein is produced (Jeong et al., 2015). The expression of FT is repressed by PcG activity depositing H3K27 trimethylation in both long and short days. REF6 demethylase removes the methylation at H3K27 to elevate the expression of FT in the vasculature under inductive conditions.
Reports suggest that PKDM7B binds to the FT chromatin and catalyses H3K4 demethylation to suppress FT expression. Loss of PKDM7B activity results in a decrease in H3K27 methylation marks and an increase in the H3K4 methylation marks, leading to FT protein production and early flowering (Jeong et al., 2009).
Expression of SOC1, another key floral integrator gene, was found to be activated by MSI1 protein via deposition of active histone marks at the H3K4 position in response to elevated temperatures, allowing arabidopsis to undergo flowering without induction of FT (Bouveret et al., 2006). As both FT and SOC1 expression can be regulated by epigenetic modifiers in response to elevated temperature, either (or both) could be considered candidates for the ‘summer memory’.
MOLECULAR REGULATION OF FLOWERING IN MASTING PLANTS
Masting is a complex phenomenon. The very nature of masting, with flowering synchronized even though the timing is highly irregular, acts as a barrier to the dissection of the relevant pathways. Flowering time is a quantitative trait associated with multiple signalling pathways (Fig. 2). There is a major gap in our molecular understanding of the masting syndrome, and relatively few researchers have attempted to demonstrate the role of flowering pathway genes in the regulation of flowering in masting plants.
For example, Kobayashi et al. (2013) identified homologous floral genes including FT, SVP, SPL and FLC in the tropical mast flowering tree Shorea beccariana. Differential expression of the flowering genes was shown in plants induced to flower by drought conditions. Most of the differentially expressed genes before the floral induction were induced by sucrose (Kobayashi et al., 2013). Recently, cold spring temperatures along with drought served as the synchronizing cue for floral induction in individual trees of S. curtisii and S. leprosula (Yeoh et al., 2017).
Miyazaki et al. (2014) identified the key floral identity genes, FcAP1, FcFT and FcLFY, from the masting plant Fagus crenata and showed a correlation between expression of these genes and the floral transition and initiation of flowering. They also demonstrated the effect of nitrogen availability on the reproductive transition by manipulating the nitrogen levels in the field. The plants treated with nitrogen showed a significant increase in the expression of FcFT along with a second round of flowering in the next year (Miyazaki et al., 2014).
More recently, Satake et al. (2019) used a non-linear time-series analysis (CCM analysis) to study causative mechanisms for the induction of flowering in F. crenata in the field. The study suggested a synergistic non-linear relationship between nitrate accumulation and activation of the floral transition by FcFT, based on the statistical analysis. The non-linear causal relationship does suggest the presence of a complex activation mechanism operational in F. crenata to induce masting (Satake et al., 2019). The approach used by Satake et al. (2019) is a powerful method enabling detection of gene regulatory networks and their causal relationship between environmental variables. A similar approach should help in the dissection of the molecular regulation of synchronized flowering and aid our understanding of masting phenology.
HYPOTHETICAL MECHANISTIC MODEL FOR MASTING PLANTS
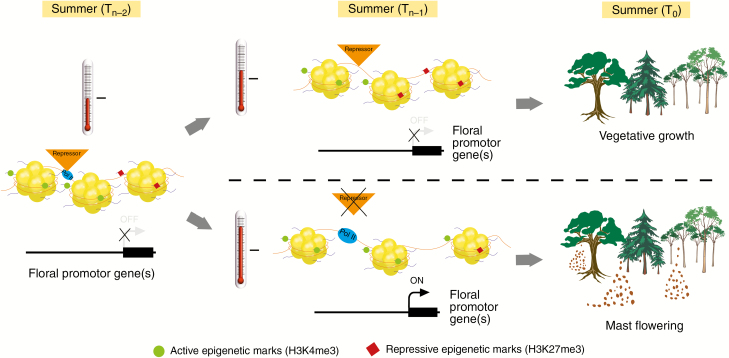
Here we propose an ‘epigenetic summer memory’ which would allow differential temperature to be used as the actual mechanism whereby masting plants respond to environmental signals to induce masting (Fig. 3). In this model, we suggest that temperature is acting as an activator leading to changes in methylation patterns of the flowering gene(s) and thus controlling developmental changes (Fig. 3). We suggest that the 2 year summer temperature requirement is necessary for the plant to fully commit to undergoing the floral transition by modulating the expression of the flowering gene(s) through changes in the histone marks at the nucleosome level.
Fig. 3.
Hypothetical mechanistic model responsible for imparting ‘summer memory’ in masting plants. The example given shows how the bivalent chromatin structure of FT, or FT-like, genes may be responsible for induction of flowering in response to the differential temperature cue. The balance between activating and repressive trimethylation marks determines whether FT is transcribed. The summer temperature of the Tn – 2 year may initiate the activation of the floral integrator genes such as FT and SOC1. However, an additional year of elevated summer temperature (Tn – 1) is required to provide sufficient activation of these genes to allow the plant to fully commit to the reproductive transition. If the Tn – 1 summer temperature is not sufficiently elevated, the balance of repressive and activating marks is in favour of no or limited flowering. In addition, if the summer temperatures of the Tn – 1 year are elevated, suppression of floral repressors may also occur, thereby releasing FT and SOC1 to be expressed.
Several floral integrator genes including FLC, SOC1 and FT have been shown to be regulated by chromatin modifiers which are active in both annual and perennial plant species (He, 2012). The activity of the chromatin modifier genes to deposit trimethylation marks at H3K27 or H3K4 nucleosomes (Jeong et al., 2015) of the floral integrator gene(s) is also modulated by temperature. These marks are associated with either the activation (H3K4me3) or the repression (H3K27me3) of the flowering pathway genes.
The FT locus has a bivalent chromatin structure which may act as a regulator to control the expression of FT and, in turn, the induction of the floral transition (Adrian et al., 2010; Verhage et al., 2014). Additionally, most perennial plants have undergone individual duplication events leading to the generation of multiple homologues of floral promoter genes (Hsu et al., 2011; Karlgren et al., 2011). For example, due to the presence of multiple homologues of FT (either orthologues or paralogues), the temperature requirement after one warm year may not be enough to efficiently activate the floral transition. The temperature of the next growing season may then additively provide sufficient signals for deposition of more trimethylation marks at H3K4/H3K36 nucleosomes, leading to the elevated expression of FT or FT-like genes and, thus, activation of the floral meristem genes.
In addition to FT, FLC is known to be epigenetically regulated. Vernalization-mediated repression of FLC is known as the ‘winter memory’ (Satake and Iwasa, 2012). FLC-like repressors could also be critical in regulating masting in response to the ∆T cue. Elevated summer temperatures in the year preceding flowering (Tn – 1) could activate the epigenetic modifiers to deposit repressive histone marks at the nucleosomes of the FLC-like repressor at H3K9/H3K27 loci. This will result in the suppression of FLC (or FLC-like repressors) which could then be maintained until the following year when the plant proceeds to flower. Consequently, this may further elevate the expression of the floral promoter genes such as FT or SOC1. Overall, we suggest it is the balance between activating epigenetic marks and repressive epigenetic marks on both promoters and repressors of flowering in response to the summer temperatures over 2 years which determines mast flowering.
This molecular network, being common across many species, where activation of floral integrator genes subsequently activates the floral meristem genes to initiate the floral transition, could provide for the strong synchrony of flowering observed during mast flowering years.
CONCLUSION
Even though various factors have been shown to be correlated with masting, few studies have probed the causative mechanism behind this mode of irregular but synchronized reproduction. Moreover, with the current rate of increase in global temperature, there is uncertainty about whether progressive warming could make masting stronger (McKone et al., 1998; Pearse et al., 2017) or weaker (Rees et al., 2002; Koenig et al., 2015), with downstream effects on higher rates of seed predation (Bogdziewicz et al., 2020). Molecular studies have the potential to be used to forecast changes in flowering behaviour and to provide an understanding of how changes in natural conditions may lead to adaptation of flowering time genes under a changing global climate. To gain a better understanding of the mechanisms underpinning masting requires a critical evaluation and analysis of the molecular flowering pathway operational in masting plants.
An approach utilizing a combination of ecological transcriptomics and ecological epigenetics may allow us to dissect the flowering mechanism in masting plants, including those plants where we lack genomic information. Identification of potential floral-promoting orthologues which may respond to inductive summer temperatures, such as FT, SOC1 and LFY, and floral repressors, similar to FLC, and their regulation under complex environmental situations, where conditions are constantly fluctuating, may then be correlated with the onset of flowering in masting plants. Epigenetic approaches such as bisulfite sequencing and methylation-sensitive amplified fragment length polymorphism (AFLP; MSAP) have shown the potential for the identification of trait-associated methylation patterns in plant species without a reference genome (Richards et al., 2017). Similar approaches, including chromatin immunoprecipitation sequencing, can now be used to characterize the ‘epigenetic summer memory’ and the activation of flowering in response to the ∆T cue. Such research will shed light on the evolution of this novel mechanism of flowering time control in masting plants.
Funding
This work was funded by the Marsden Fund Royal Society of New Zealand Grant No. UOC1401. Samarth was supported by a PhD scholarship from the Marsden Fund.
LITERATURE CITED
- Adrian J, Farrona S, Reimer JJ, Albani MC, Coupland G, Turck F. 2010. cis-Regulatory elements and chromatin state co-ordinately control temporal and spatial expression of FLOWERING LOCUS T in Arabidopsis. The Plant Cell 22: 1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airoldi CA, McKay M, Davies B. 2015. MAF2 is regulated by temperature-dependent splicing and represses flowering at low temperatures in parallel with FLM. PLoS One 10: e0126516. doi: 10.1371/journal.pone.0126516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RB, Hurst JM, Portier J, Richardson SJ. 2014. Elevation-dependent responses of tree mast seeding to climate change over 45 years. Ecology and Evolution 4: 3525–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres F, Coupland G. 2012. The genetic basis of flowering responses to seasonal cues. Nature Reviews. Genetics 13: 627–39. [DOI] [PubMed] [Google Scholar]
- Bastow R, Mylne JS, Lister C, Lippman Z, Martienssen RA, Dean C. 2004. Vernalization requires epigenetic silencing of FLC by histone methylation. Nature 427: 164–167. [DOI] [PubMed] [Google Scholar]
- Bogdziewicz M, Kelly D, Thomas PA, Lageard JGA, Hacket-Pain AJ. 2020. Climate warming disrupts mast seeding and its fitness benefits in European beech. Nature Plants. doi 10.1038/s41477-020-0592-8 [DOI] [PubMed] [Google Scholar]
- Bogdziewicz M, Steele MA, Marino S, Crone EE. 2018. Correlated seed failure as an environmental veto to synchronize reproduction of masting plants. New Phytologist 219: 98–108. [DOI] [PubMed] [Google Scholar]
- Bossdorf O, Richards CL, Pigliucci M. 2008. Epigenetics for ecologists. Ecology Letters 11: 106–115. [DOI] [PubMed] [Google Scholar]
- Bouche F, Lobet G, Tocquin P, Perilleux C. 2016. FLOR-ID: an interactive database of flowering-time gene networks in Arabidopsis thaliana. Nucleic Acids Research 44: D1167–D1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouveret R, Schonrock N, Gruissem W, Hennig L. 2006. Regulation of flowering time by arabidopsis MSI1. Development 133: 1693–1702. [DOI] [PubMed] [Google Scholar]
- Bratzel F, Turck F. 2015. Molecular memories in the regulation of seasonal flowering: from competence to cessation. Genome Biology 16: 192. doi: 10.1186/s13059-015-0770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, Woodward FI. 1998. Dynamic responses of terrestrial ecosystem carbon cycling to global climate change. Nature 393: 249–252. [Google Scholar]
- Capovilla G, Schmid M, Pose D. 2015a Control of flowering by ambient temperature. Journal of Experimental Botany 66: 59–69. [DOI] [PubMed] [Google Scholar]
- Capovilla G, Pajoro A, Immink RG, Schmid M. 2015b Role of alternative pre-mRNA splicing in temperature signaling. Current Opinion in Plant Biology 27: 97–103. [DOI] [PubMed] [Google Scholar]
- Cho LH, Yoon J, An G. 2017. The control of flowering time by environmental factors. The Plant Journal 90: 708–719. [DOI] [PubMed] [Google Scholar]
- Cleavitt NL, Fahey TJ. 2017. Seed production of sugar maple and American beech in northern hardwood forests, New Hampshire, USA. Canadian Journal of Forest Research 47: 985–990. [Google Scholar]
- Franks SJ, Sim S, Weis AE. 2007. Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proceedings of the National Academy of Sciences, USA 104: 1278–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y. 2009. Control of the transition to flowering by chromatin modifications. Molecular Plant 2: 554–564. [DOI] [PubMed] [Google Scholar]
- He Y. 2012. Chromatin regulation of flowering. Trends in Plant Science 17: 556–562. [DOI] [PubMed] [Google Scholar]
- He Y, Amasino RM. 2005. Role of chromatin modification in flowering-time control. Trends in Plant Science 10: 30–35. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Jordano P, Guitian J, Traveset A. 1998. Annual variability in seed production by woody plants and the masting concept: reassessment of principles and relationship to pollination and seed dispersal. American Naturalist 152: 576–594. [DOI] [PubMed] [Google Scholar]
- Hsu C-Y, Adams JP, Kim H, et al. 2011. FLOWERING LOCUS T duplication coordinates reproductive and vegetative growth in perennial poplar. Proceedings of the National Academy of Sciences, USA 108: 10756–10761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijser P, Schmid M. 2011. The control of developmental phase transitions in plants. Development 138: 4117–4129. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Song HR, Ko JH, et al. 2009. Repression of FLOWERING LOCUS T chromatin by functionally redundant histone H3 lysine 4 demethylases in arabidopsis. PLoS One 4: e8033. doi: 10.1371/journal.pone.0008033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Yang J, Yi J, An G. 2015. Controlling flowering time by histone methylation and acetylation in Arabidopsis and rice. Journal of Plant Biology 58: 203–210. [Google Scholar]
- Karlgren A, Gyllenstrand N, Källman T, et al. 2011. Evolution of the PEBP gene family in plants: functional diversification in seed plant evolution. Plant Physiology, 156: 1967–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly D. 1994. The evolutionary ecology of mast seeding. Trends in Ecology & Evolution 9: 465–470. [DOI] [PubMed] [Google Scholar]
- Kelly D, Sork VL. 2002. Mast seeding in perennial plants: why, how, where? Annual Review of Ecology and Systematics 33: 427–447. [Google Scholar]
- Kelly D, Harrison AL, Lee WG, Payton IJ, Wilson PR, Schauber EM. 2000. Predator satiation and extreme mast seeding in 11 species of Chionochloa (Poaceae). Oikos 90: 477–488. [Google Scholar]
- Kelly D, Turnbull MH, Pharis RP, Sarfati MS. 2008. Mast seeding, predator satiation, and temperature cues in Chionochloa (Poaceae). Population Ecology 50: 343–355. [Google Scholar]
- Kelly D, Geldenhuis A, James A, et al. 2013. Of mast and mean: differential-temperature cue makes mast seeding insensitive to climate change. Ecology Letters 16: 90–98. [DOI] [PubMed] [Google Scholar]
- Khan MR, Ai XY, Zhang JZ. 2014. Genetic regulation of flowering time in annual and perennial plants. Wiley Interdisciplinary Reviews RNA 5: 347–359. [DOI] [PubMed] [Google Scholar]
- Kitzberger T, Chaneton EJ, Caccia F. 2007. Indirect effects of prey swamping: differential seed predation during a bamboo masting event. Ecology 88: 2541–54. [DOI] [PubMed] [Google Scholar]
- Kobayashi MJ, Takeuchi Y, Kenta T, Kume T, Diway B, Shimizu KK. 2013. Mass flowering of the tropical tree Shorea beccariana was preceded by expression changes in flowering and drought-responsive genes. Molecular Ecology 22: 4767–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig WD, Kelly D, Sork VL, et al. 2003. Dissecting components of population-level variation in seed production and the evolution of masting behavior. Oikos 102: 581–591. [Google Scholar]
- Koenig WD, Knops JM, Carmen WJ, Pearse IS. 2015. What drives masting? The phenological synchrony hypothesis. Ecology 96: 184–192. [DOI] [PubMed] [Google Scholar]
- Kon H, Saito H. 2015. Test of the temperature difference model predicting masting behavior. Canadian Journal of Forest Research 45: 1835–1844. [Google Scholar]
- Krebs CJ, O’Donoghue M, Taylor S, Kenney AJ, Hofer EJ, Boutin S. 2017. Predicting white spruce cone crops in the boreal forests of southern and central Yukon. Canadian Journal of Forest Research 47: 47–52. [Google Scholar]
- Kumar SV, Lucyshyn D, Jaeger KE, et al. 2012. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484: 242–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FQ, Marquardt S, Lister C, Swiezewski S, Dean C. 2010. Targeted 3' processing of antisense transcripts triggers arabidopsis FLC chromatin silencing. Science 327: 94–97. [DOI] [PubMed] [Google Scholar]
- Liu YY, Yang KZ, Wei XX, Wang XQ. 2016. Revisiting the phosphatidylethanolamine-binding protein (PEBP) gene family reveals cryptic FLOWERING LOCUS T gene homologs in gymnosperms and sheds new light on functional evolution. New Phytologist 212: 730–744. [DOI] [PubMed] [Google Scholar]
- Macknight R, Bancroft I, Page T, et al. 1997. FCA, a gene controlling flowering time in arabidopsis, encodes a protein containing RNA-binding domains. Cell 89: 737–745. [DOI] [PubMed] [Google Scholar]
- Mark AF. 1968. Factors controlling irregular flowering in four alpine species of Chionochloa. Proceedings (New Zealand Ecological Society) 15: 55–60. [Google Scholar]
- Mateos JL, Madrigal P, Tsuda K, et al. 2015. Combinatorial activities of SHORT VEGETATIVE PHASE and FLOWERING LOCUS C define distinct modes of flowering regulation in arabidopsis. Genome Biology 16: 31. doi: 10.1186/s13059-015-0597-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKone MJ, Kelly D, Lee WG. 1998. Effect of climate change on masting species: frequency of mass flowering and escape from specialist insect seed predators. Global Change Biology 4: 591–596. [Google Scholar]
- Melillo JM, McGuire AD, Kicklighter DW, Moore B, Vorosmarty CJ, Schloss AL. 1993. Global climate change and terrestrial net primary production. Nature 363: 234–240. [Google Scholar]
- Miyazaki Y, Maruyama Y, Chiba Y, et al. 2014. Nitrogen as a key regulator of flowering in Fagus crenata: understanding the physiological mechanism of masting by gene expression analysis. Ecology Letters 17: 1299–1309. [DOI] [PubMed] [Google Scholar]
- Monks A, Monks JM, Tanentzap AJ. 2016. Resource limitation underlying multiple masting models makes mast seeding sensitive to future climate change. New Phytologist 210: 419–430. [DOI] [PubMed] [Google Scholar]
- Murgia I, Giacometti S, Balestrazzi A, Paparella S, Pagliano C, Morandini P. 2015. Analysis of the transgenerational iron deficiency stress memory in Arabidopsis thaliana plants. Frontiers in Plant Science, 6: 745. doi: 10.3389/fpls.2015.00745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton D, Kelly D. 1988. Mast seeding over 33 years by Dacrydium cupressinum Lamb. (rimu) (Podocarpaceae) in New Zealand: the importance of economies of scale. Functional Ecology 2: 399–408. [Google Scholar]
- Pearse IS, Koenig WD, Knops JMH. 2014. Cues versus proximate drivers: testing the mechanism behind masting behavior. Oikos 123: 179–184. [Google Scholar]
- Pearse IS, Koenig WD, Kelly D. 2016. Mechanisms of mast seeding: resources, weather, cues, and selection. New Phytologist 212: 546–562. [DOI] [PubMed] [Google Scholar]
- Pearse IS, LaMontagne JM, Koenig WD. 2017. Inter-annual variation in seed production has increased over time (1900–2014). Proceedings of the Royal Society B-Biological Sciences 284: 20171666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada V, Macknight R, Dean C, Simpson GG. 2003. Autoregulation of FCA pre-mRNA processing controls arabidopsis flowering time. European Molecular Biology Journal 22: 3142–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees M, Kelly D, Bjornstad O. 2002. Snow tussocks, chaos, and the evolution of mast seeding. American Naturalist 160: 44–59. [DOI] [PubMed] [Google Scholar]
- Richards CL, Alonso C, Becker C, et al. 2017. Ecological plant epigenetics: evidence from model and non-model species, and the way forward. Ecology Letters 20: 1576–1590. [DOI] [PubMed] [Google Scholar]
- Romera-Branchat M, Andres F, Coupland G. 2014. Flowering responses to seasonal cues: what’s new? Current Opinion in Plant Biology 21: 120–127. [DOI] [PubMed] [Google Scholar]
- Sala A, Hopping K, McIntire EJB, Delzon S, Crone EE. 2012. Masting in whitebark pine (Pinus albicaulis) depletes stored nutrients. New Phytologist 196: 189–199. [DOI] [PubMed] [Google Scholar]
- Satake A, Iwasa Y. 2012. A stochastic model of chromatin modification: cell population coding of winter memory in plants. Journal of Theoretical Biology 302: 6–17. [DOI] [PubMed] [Google Scholar]
- Satake A, Kawatsu K, Teshima K, Kabeya D, Han QM. 2019. Field transcriptome revealed a novel relationship between nitrate transport and flowering in Japanese beech. Scientific Reports 9: 4325. doi: 10.1038/s41598-019-39608-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber EM, Kelly D, Turchin P, et al. 2002. Masting by eighteen New Zealand plant species: the role of temperature as a synchronizing cue. Ecology 83: 1214–1225. [Google Scholar]
- Smaill SJ, Clinton PW, Allen RB, Davis MR. 2011. Climate cues and resources interact to determine seed production by a masting species. Journal of Ecology 99: 870–877. [Google Scholar]
- Song YH, Ito S, Imaizumi T. 2013. Flowering time regulation: photoperiod- and temperature-sensing in leaves. Trends in Plant Science 18: 575–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung SB, Amasino RM. 2004. Vernalization in Arabidopsis thaliana is mediated by the PHD finger protein VIN3. Nature 427: 159–164. [DOI] [PubMed] [Google Scholar]
- Susila H, Nasim Z, Ahn JH. 2018. Ambient temperature-responsive mechanisms coordinate regulation of flowering time. International Journal of Molecular Sciences 19: 3196. doi: 10.3390/ijms19103196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachiki Y, Iwasa Y. 2012. Evolutionary jumping and breakthrough in tree masting evolution. Theoretical Population Biology 81: 20–31. [DOI] [PubMed] [Google Scholar]
- Verhage L, Angenent GC, Immink RG. 2014. Research on floral timing by ambient temperature comes into blossom. Trends in Plant Science 19: 583–91. [DOI] [PubMed] [Google Scholar]
- Xu L, Zhao Z, Dong A, et al. 2008. Di- and tri- but not mono-methylation on histone H3 lysine 36 marks active transcription of genes involved in flowering time regulation and other processes in Arabidopsis thaliana. Molecular Cell Biology 28: 1348–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Hu T, Smith MR, Poethig RS. 2016. Epigenetic regulation of vegetative phase change in Arabidopsis. The Plant Cell 28: 28–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeoh SH, Satake A, Numata S, et al. 2017. Unravelling proximate cues of mass flowering in the tropical forests of South-East Asia from gene expression analyses. Molecular Ecology 26: 5074–5085. [DOI] [PubMed] [Google Scholar]