Abstract
Background and Aims
The processes that maintain variation in the prevalence of symbioses within host populations are not well understood. While the fitness benefits of symbiosis have clearly been shown to drive changes in symbiont prevalence, the rate of transmission has been less well studied. Many grasses host symbiotic fungi (Epichloë spp.), which can be transmitted vertically to seeds or horizontally via spores. These symbionts may protect plants against herbivores by producing alkaloids or by increasing tolerance to damage. Therefore, herbivory may be a key ecological factor that alters symbiont prevalence within host populations by affecting either symbiont benefits to host fitness or the symbiont transmission rate. Here, we addressed the following questions: Does symbiont presence modulate plant tolerance to herbivory? Does folivory increase symbiont vertical transmission to seeds or hyphal density in seedlings? Do plants with symbiont horizontal transmission have lower rates of vertical transmission than plants lacking horizontal transmission?
Methods
We studied the grass Poa autumnalis and its symbiotic fungi in the genus Epichloë. We measured plant fitness (survival, growth, reproduction) and symbiont transmission to seeds following simulated folivory in a 3-year common garden experiment and surveyed natural populations that varied in mode of symbiont transmission.
Key Results
Poa autumnalis hosted two Epichloë taxa, an undescribed vertically transmitted Epichloë sp. PauTG-1 and E. typhina subsp. poae with both vertical and horizontal transmission. Simulated folivory reduced plant survival, but endophyte presence increased tolerance to damage and boosted fitness. Folivory increased vertical transmission and hyphal density within seedlings, suggesting induced protection for progeny of damaged plants. Across natural populations, the prevalence of vertical transmission did not correlate with symbiont prevalence or differ with mode of transmission.
Conclusions
Herbivory not only mediated the reproductive fitness benefits of symbiosis, but also promoted symbiosis prevalence by increasing vertical transmission of the fungus to the next generation. Our results reveal a new mechanism by which herbivores could influence the prevalence of microbial symbionts in host populations.
Keywords: Defensive mutualism, symbiosis, Epichloë, induced response, resistance, tolerance, transgenerational effects
INTRODUCTION
Identifying the factors that promote the persistence of symbioses (or cause their extinction) can yield new insights into the underlying ecological and evolutionary processes that lead to integrated partnerships (Kiers and West, 2015). Through symbioses, both plants and animals have acquired new functions and metabolic capabilities (e.g. White and Torres, 2009; Douglas, 2010). Plants can acquire nitrogen through symbiosis with bacteria, arthropods gain amino acids synthesized by gut symbionts, ruminants house cellulolytic bacteria to use plant cellulose as an energy source, and plants obtain anti-herbivore resistance from microbial endophytes (Zilber-Rosenberg and Rosenberg, 2008; Rodriguez et al., 2009; Kandel et al., 2017). Despite the apparent benefits of these interactions, such symbioses often involve mutual exploitation, with cryptic costs that can vary with the ecological context (Jones et al., 2015).
Most studies on symbiosis emphasize reciprocal fitness effects as the key driver of interaction dynamics (e.g. Sachs and Simms, 2006). However, while fitness effects are clearly important to the prevalence of symbiosis, the mode and rate of symbiont transmission can also play major roles (Rudgers et al., 2010; Gundel et al., 2011; Bibian et al., 2016). Symbiont transmission can be vertical, in which microorganisms pass exclusively from parents to offspring; horizontal, in which hosts contagiously acquire symbionts from the environment (Bright and Bulgheresi, 2010); or mixed, with a combination of vertical and horizontal transmission (Brem and Leuchtmann, 2003). Unlike horizontal transmission, vertical transmission tightly couples the fitness of the host and symbiont, selecting for mutualism via partner fidelity feedback (Ewald, 1987). Despite the central role of transmission dynamics in models of host–symbiont interactions (Lipsitch et al., 1995; Genkai-Kato and Yamamura, 1999; Gundel et al., 2008), relatively few empirical studies have investigated ecological controls on the rate and mode of transmission (Douglas, 2010; Gundel et al., 2011; Sneck et al., 2019). However, observations suggest that abiotic environmental context, such as climatic variability and type of soil, could underlie variable transmission (Gundel et al., 2009; Gibert and Hazard, 2013; Sneck et al., 2017). Ecological interactions with other species could also influence transmission dynamics, but have received little study.
In plants, the prevalence of foliar endophytes within and among natural host populations is highly variable (Semmartin et al., 2015), suggesting that fitness outcomes are context-dependent (Davitt et al., 2011; Rho et al., 2018) or that variable transmission rates create frequent opportunities for symbiont loss from individual hosts (e.g. Gundel et al., 2011, 2012; Gibert and Hazard, 2013). Symbioses between plants and foliar fungal endophytes are ubiquitous (Rodriguez et al., 2009), and the best-studied of these occur between cool-season grasses (subfamily Pooideae) and systemic fungal endophytes (genus Epichloë; Ascomycota, Clavicipitaceae). As in other symbioses, empirical studies of Epichloë species have focused primarily on fitness benefits as drivers of symbiont prevalence, rather than on the transmission process (Gundel et al., 2008, 2011; Rudgers et al., 2010). Epichloë can increase plant resistance to herbivores and pathogens as well as improve plant performance under abiotic stresses (see reviews by: Clay and Schardl, 2002; Malinowski and Belesky, 2019). Alternatively, endophytes may reduce plant tolerance to herbivory by reducing regrowth after defoliation (Qin et al., 2016), particularly if plants have less carbon for regrowth because of allocation to the symbiosis. Whether the fungal endophytes that confer herbivore resistance constrain or enhance the ability of host plants to regrow after damage (tolerance to herbivory) remains unresolved because few experiments have been conducted (Partida-Martínez and Heil, 2011).
In addition to this high potential for variable fitness benefits, endophyte transmission modes can also be highly variable, ranging from exclusively vertical to mixed to exclusively horizontal (Brem and Leuchtmann, 2003; Rodriguez et al., 2009). Vertical transmission occurs with asexual hyphal growth into developing seeds (Liu et al., 2017), whereas horizontal transmission occurs when the fungal fruiting bodies (stromata) arrest development of grass inflorescences, which may reduce opportunities for vertical transmission if fewer seeds are produced (Leuchtmann et al., 2000; Schardl et al., 2004). If the ecological context alters these transmission processes, there is strong potential for changes in the prevalence of symbiosis in host populations to arise from transmission dynamics rather than context-dependency in the fitness benefits of symbiosis (Gundel et al., 2011; Cavazos et al., 2018).
The ecological factors that generate variation in vertical transmission rates or influence the relative amounts of vertical vs. horizontal transmission remain unclear. Exogenous ecological contexts, such as climate or species interactions, could affect symbiont transmission, particularly if symbionts contribute to transgenerational (or maternal) effects (Gundel et al., 2017). Maternal provisioning is one of the most common mechanisms through which progeny acquire traits associated with the environment experienced by their parents (Herman and Sultan, 2011; Zas and Sampedro, 2015). Induced mechanisms of maternal (or transgenerational) effects can increase progeny fitness via protection against abiotic or biotic stressors (Pieterse, 2012). For example, plants challenged with herbivores can produce more resistant offspring than plants that have not been exposed to herbivory (Agrawal et al., 1999; Kellenberger et al., 2018). Transgenerational effects mediated by microorganisms could occur if maternal plants provision offspring with protective symbionts through the process of vertical transmission (Gundel et al., 2017). Thus, the dynamics of grass–Epichloë symbioses, in which fungal-derived alkaloids can protect against herbivores (Clay and Schardl, 2002; White and Torres, 2009), could be altered if herbivory or other stressors alter the process of vertical transmission from maternal plants to seed. Indeed, the accumulation of fungal alkaloids in the seeds of grasses (Gundel et al., 2018) suggests potential for a transgenerational effect mediated by endophyte symbiosis. However, to our knowledge, no previous studies have manipulated herbivory to test whether it alters the amount of vertical transmission of the grass–endophyte symbiosis.
Using the native woodland plant autumn bluegrass (Poa autumnalis) and its endophytic Epichloë species as a model system, we investigated the potential for herbivory to increase symbiont transmission and compared the magnitude of that effect to the more commonly studied fitness benefits of symbiosis. We used a common garden experiment with clipping to simulate folivory in order to test the following questions: (1) Does symbiont presence increase or decrease plant tolerance to herbivory? (2) Does folivory increase symbiont vertical transmission to seeds or hyphal density in seedlings? To contextualize experimental work within natural host–symbiont dynamics, we used observations of endophyte presence across populations to test a hypothesized trade-off between vertical and horizontal transmission, and investigated whether vertical transmission rate is associated with symbiont prevalence. Specifically, we asked: (3) Do plants with symbiont horizontal transmission have lower rates of vertical transmission than plants lacking horizontal transmission?
MATERIALS AND METHODS
Study system
Autumn bluegrass (P. autumnalis), is distributed from the North American Atlantic coast to eastern Texas, which represents the western edge of its distribution (USDA/NRCS, 2012). This caespitose grass is common in mesic, hardwood forest understoreys in eastern Texas. A preliminary field survey showed a high incidence of Epichloë species (~96 % of individuals; Rudgers et al., 2009) with some individuals producing stromata – the mechanism of horizontal transmission for sexually reproductive Epichloë species. A stroma is the fruiting body of the fungus that sterilizes the reproductive tissues of the plant (the pathogenic manifestation is known as ‘choke disease’), a process that ends in the production of sexually formed ascospores that can colonize new plants (Tadych et al., 2012; Leucthmann et al., 2014). Previous field observations revealed 50 % reduced folivory when the endophyte was present than in plants from which the endophyte was experimentally removed, and laboratory assays showed an increased preference of endophyte-disinfected plants over endophyte-symbiotic plants by aphid, grasshopper and caterpillar herbivores, as well as reduced caterpillar performance in no-choice assays (Crawford et al., 2010). In the present study, we surveyed the diversity of endophyte mating types and alkaloid genes of natural populations, including one population in which individuals produced the stromata that enable horizontal transmission.
Does symbiont presence increase or decrease plant tolerance to herbivory? Does folivory increase symbiont vertical transmission to seeds or hyphal density in seedlings?
Experimental design.
In 2009, a 3-year common garden experiment was conducted at Stephen F. Austin Experimental Forest near Nacogdoches, Texas, USA (31°29′44″N, 94°45′39″W). Endophyte status (endophyte present: E+, or absent: E−) and simulated herbivory (clipped or control) were manipulated in a 2 × 2 factorial design, with 52 replicates per treatment combination (N = 208 plants). Individuals were planted into the common garden in a rectangular grid (21 × 10 m) at 1-m spacing with no watering following planting. Plant position in the grid was assigned at random.
Endophyte treatment and propagation.
We grew plants from seeds collected from 25 individual plants at the Stephen F. Austin Experimental Forest on 2 May 2007 (SFA-3 population containing Epichloë sp. PauTG-1, Table 1). We experimentally removed the endophyte by heating seeds at 60 °C in a drying oven for 7 d (further details in Supplementary Data Appendix S1). Plants were grown in the Rice University glasshouse facility. Four weeks before field planting, each plant (genet) was divided into equally sized ramets (about five tillers each) then replanted into separate 115-mL pots. One of the ramets from each genetically independent plant was assigned to the simulated herbivory treatment while the other one was the control (see below). Only ramets for which the endophyte was present as intended (E+) or effectively eliminated (E−) were used in the experiment. The endophyte status of plants was verified by the detection of endophyte hyphae in thin sections of the inner leaf sheath stained with aniline blue lactic acid and examined at ×200 magnification (Bacon & White, 1994). The common garden was planted on 16 December 2009, adjacent to the site of seed collection (SFA-3, Table 1). Ramets were removed from pots and planted into the natural matrix of vegetation using a hand trowel, with minimal disturbance.
Table 1.
Summary of data on symbiosis and germination in 18 populations of autumn bluegrass, Poa autumnalis, with latitude and longitude for each population. Populations in bold were additionally surveyed for prevalence in seedlings produced by field-collected seeds from the surveyed adult plants.
| Site | Endophyte taxa | Collection dates | Latitude | Longitude | Adult prevalence (%) | N (plants) | Stromata adults (%) | N (seeds) | Seed prevalence (%) | N (germ.) | Seed germination (%) | N (seedlings) | Seedling prevalence (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANF-1 | 1 & 2 | 2009-04-11 2009-05-08 | 31°02′52″ | −94°23′16″ | 100.0 | 30 | 0.0 | 400 | 23.2 | 73 | 70.9 | ||
| ANF-2 | 1 | 2009-05-08 | 31°05′01″ | −94°19′21″ | 97.1 | 35 | 0.0 | 438 | 47.0 | 192 | 96.3 | ||
| ANF-3 | 1 & 2 | 2009-04-11 2009-05-08 | 31°05′13″ | −94°19′15″ | 96.7 | 43 | 20.0 | 1351 | 33.9 | 480 | 96.9 | ||
| ANF-5 | 1 & 2 | 2006-04-12 | 31°05′23″ | −94°19′21″ | 100.0 | 14 | 0.0 | 21 | 90.5 | ||||
| ANF-6 | 1 | 2006-04-12 | 31°02′52″ | −94°23′14″ | 100.0 | 12 | 0.0 | 22 | 100.0 | ||||
| BNP-1 | 1 & 2 | 2006-04-16 | 30°34′24″ | −94°38′32″ | 93.3 | 15 | 0.0 | 9 | 100.0 | ||||
| BNP-2 | 1 & 2 | 2006-04-17 | 30°29′15″ | −94°50′27″ | 100.0 | 14 | 0.0 | 20 | 100.0 | ||||
| BNP-3 | 1 | 2006-04-17 | 30°23′00″ | −94°51′00″ | 100.0 | 22 | 0.0 | 20 | 100.0 | ||||
| DNF-1 | 1 | 2009-05-04 | 31°27′56″ | −95°07′44″ | 93.3 | 30 | 0.0 | 705 | 56.0 | 259 | 99.4 | ||
| DNF-2 | 1 | 2006-05-16 | 31°27′46″ | −95°07’44’’ | 100.0 | 14 | 0.0 | 19 | 78.9 | ||||
| LOC-1 | 1 & 2 | 2006-04-26 | 31°38′57″ | −94°46′37″ | 100.0 | 20 | 0.0 | 23 | 100.0 | ||||
| SFA-1 | 1 | 2009-04-30 | 31°29′44″ | −94°45′38″ | 100.0 | 30 | 0.0 | 970 | 89.0 | 593 | 98.8 | ||
| SFA-2 | 1 | 2006-04-30 | 31°30′07″ | −94°47′18″ | 90.0 | 20 | 0.0 | 71 | 94.4 | ||||
| SFA-3* | 1 | 2007-05-02 | 31°29′44″ | −94°45′39″ | 100.0 | 25 | 0.0 | n.t. | n.t. | 98 | 95.9 | ||
| SNF-1 | 1 & 2 | 2009-04-23 | 30°30′30″ | −95°05′22″ | 100.0 | 30 | 0.0 | 780 | 35.3 | 151 | 81.6 | ||
| SNF-2 | 1 & 2 | 2006-04-12 | 30°30′30″ | −95°05′22″ | 94.1 | 17 | 0.0 | 16 | 68.8 | ||||
| SNF-3 | 1 | 2006-04-12 | 30°30′48″ | −95°07′23″ | 91.7 | 12 | 0.0 | 18 | 61.1 | ||||
| TWR-1 | 2 | 2006-04-17 | 30°21′58″ | −94°51′43″ | 91.7 | 12 | 0.0 | 21 | 100.0 |
*The SFA-3 population was used in the field experiment; the other populations were used to survey natural endophyte prevalence. Collection dates are given in ISO 8601 format. Prevalence (in adults, seeds or seedlings) is the mean percentage of individuals with the endophyte. N = sample size. n.t. = not tested.
Endophyte taxa were distinguished by PCR, taxon 1 = PauTG-1 (hybrid), taxon 2 (E. typhina subsp. poae, non-hybrid), 1 & 2 = a mixture of both PauTG-1 and E. typhina subsp. poae.
Herbivory treatment.
We used a paired design to reduce error associated with genetic variation among plant individuals that were subjected to herbivory treatments. One ramet from each of 52 genetically unique endophyte symbiotic plants (E+) and from each of 52 genetically unique endophyte-free (E−) plants was randomly assigned to a simulated herbivory (clip) treatment; the other ramet of each genotype served as a control. Clipped plants had ~25 % less leaf biomass via removal of the tips of leaves with scissors. The clipping treatment replicated damage by lepidopteran larvae and orthopterans, for which we observed an average damage at the site of 8.4 % tissue removed per leaf (maximum of 50 % of leaf tissue removed) (Crawford et al., 2010). Because plants go dormant during the summer months, after flowering, clipping occurred once per month from December 2009 to May 2010 and from October 2010 to April 2011. Control plants were visited and leaves were touched, but not clipped. All clipped material was removed from the site.
Plant fitness.
From 2010 to 2012, we scored each plant for survival and counted the number of vegetative tillers and inflorescences during 11–22 May, when plants were reproductive. As seeds matured, we collected them into coin envelopes. In 2011, we also counted the number of spikelets per inflorescence for a subset of four inflorescences per individual. We estimated total seed production per plant by multiplying the number of inflorescences × mean number of spikelets per inflorescence × mean number of seeds per spikelet. Poa autumnalis produced, on average, 2.8 ± 0.4 s.d. seeds per spikelet, regardless of endophyte status.
Fungal endophyte fitness.
In 2011, we evaluated three variables associated with fitness of the symbiont: vertical transmission to the seed and to the seedlings, and hyphal density in the seedlings. Vertical transmission was evaluated by manually dissecting ten seeds per plant at ×10 magnification following soaking overnight in 5 % sodium hydroxide. We removed the endosperm, added one or two drops of aniline blue lactic acid stain following Bacon & White (1994), and squashed each seed under a cover-slip. Seeds were scored for endophyte presence at ×200 magnification. For determination of endophyte presence, we only scored seeds from E+ plants (10 seeds × 104 plants), ~1040 seeds in total.
We examined 20 seedlings per individual plant in the common garden experiment. We removed the lemma and palea then placed ten seeds into a Petri dish containing 1 % water agar, then sealed this with parafilm. After cold stratification at 4 °C for ~4 weeks, the sealed plates were placed in the glasshouse (~23 °C) during 24–30 June 2010. After seedlings had produced at least one true leaf, we stained them with rose Bengal and evaluated them for endophyte presence at ×200–400 magnification following Belanger (1996). For each maternal plant, we determined the proportion of seeds that germinated and the proportion of seedlings with an endophyte. We scored a total of 1935 seedlings for endophyte presence.
To assess the density of Epichloë hyphae in the seedlings, we measured seedling height for a subset of randomly chosen seedlings from 11 maternal plants in each clipping treatment. We then stained the seedlings as before, and determined hyphal density per linear millimetres of leaf sheath tissue. We also corrected for the possibility that plants differed in cell size by measuring plant cell size (pixels) at ×200 magnification using ImageJ analysis software (see Schneider et al., 2012). We scored a total of 22 endophyte-symbiotic seedlings for both hyphal density and plant cell size.
Common garden experiment: data analysis
Does symbiont presence increase or decrease plant tolerance to herbivory?
If endophytes increase plant tolerance to herbivory, then when plants are subjected to a fixed amount of damage, plants with the endophyte should have greater fitness than endophyte-free plants. This result would be confirmed by a statistical interaction between the endophyte treatment and simulated herbivory (clip) treatment in analyses of plant fitness metrics. We used repeated-measures general linear mixed effects models with clip treatment, endophyte treatment, clip × endophyte interaction, and the year effect as well as interactions with year. Models included the random effect of genetic pair, and the random effect of ramet to account for the non-independence of observations on the same plant in different years (restricted maximum likelihood, Proc GLIMMIX, SAS Institute v. 9.3, Cary, NC, USA). In all models, we evaluated the a priori hypothesis that fungal endophytes modulate plant tolerance to herbivory by testing the contrast between endophyte-symbiotic (E+) vs. endophyte-free (E−) plants within the clip treatment and the same contrast within the herbivory control treatment. To meet assumptions of normality of residuals and homogeneity of variances, we log-transformed inflorescence counts, tiller counts and seed counts. For survival (0/1 data), we used a log-linear mixed effects model with a binomial distribution and the same independent factors as for plant growth and reproduction. Plant survival was high in 2010 at 98 %, but declined through time (2011: 89 %; 2012: 50 %). Including 2010 survival data resulted in models that did not converge due to extremely high survival; thus we only examined survival responses to treatments for 2011 and 2012.
Does folivory increase symbiont vertical transmission to seeds or hyphal density in seedlings?
For the subset of common garden plants with endophytes, we tested the independent factors of clip treatment (fixed) and plant genetic pair (random) on five fungal response variables: the proportion of seeds with the endophyte, the proportion of seedlings with the endophyte, the proportion of seedlings that germinated, mean hyphal density/cm of plant tissue, and mean plant cell size (mm2) (restricted maximum likelihood, Proc MIXED, SAS Institute v.9.3). All proportion data were logit-transformed following Warton et al. (2011).
Field survey: do plants with symbiont horizontal transmission have lower rates of vertical transmission than plants lacking horizontal transmission?
Field collection and microscopy.
To characterize the natural prevalence and genetic diversity of endophytes in P. autumnalis, we collected leaves and seeds from ~12–30 adult plants in each of 18 populations during the reproductive phase (April–May) (Table 1). Leaves were stored at 4 °C for up to 7 d, and seeds were stored at −20 °C. We used microscopy to detect the endophyte in leaves for all populations. Then, for a subset of seven populations (bold in Table 1), we also germinated and scored seedlings for endophyte presence, following methods for the Common Garden Experiment. Sample sizes are presented in Table 1. We used a Spearman rank correlation to relate the proportion of symbiotic adult plants in each population to the proportion of symbiotic offspring either using seeds (n = 11 populations) or seedlings (n = 7 populations). We observed stromata formation in only one of the 18 surveyed populations (ANF-3, Table 1; Supplementary Data Fig. S1). For this population, we compared the efficiency of vertical transmission for seeds of stromata-bearing plants (N = 11) against that of asymptomatic plants (N = 32) using one-way ANOVA.
Endophyte genotyping.
To determine possible endophyte diversity within and between the P. autumnalis populations, we used a PCR approach to identify endophyte infection and characterize endophyte diversity. To characterize endophyte genotypes, we used PCR on seeds from endophyte-symbiotic plants with primers specific for alkaloid genes and fungal mating types (Charlton et al., 2012). In total, 19 primer sets were used to test for endophyte presence and diversity (Table S1). Each total DNA sample was extracted from up to eight individual seeds per individual plant or from up to four seeds from a bulk collection with at least four independent samples (Table S1). Each gene primer set was evaluated in a single PCR. In 2016, a subset of seed samples (21 populations and individual plants from population ANF-3) were further tested by PCR using the protocol of Charlton et al. (2014), which used the primers in five multiplex primer combinations. These primer combinations are used to provide information on endophyte diversity within the populations examined and can highlight specific classes of bioactive alkaloids that might be produced by the endophyte, such as those known to increase plant resistance to herbivores.
The populations were split into three distinct groups containing taxon 1 only, taxon 2 only, or a mixed population with taxon 1 and taxon 2 present (Table 1). Endophytes were identified to individual taxon based on phylogenetic trees of the mating-type genes. Due to the quality of the DNA isolated from the seed in our collections, we could only confirm the phylogenetic placement using sequence data from the mating-type genes mtAC and mtBC. The phylogenetic placement of each sequenced gene was performed with phylogeny.fr (Dereeper et al., 2008, 2010). Accession numbers for mating-type genes are MN311480–MN311482.
RESULTS
Does symbiont presence increase or decrease plant tolerance to herbivory?
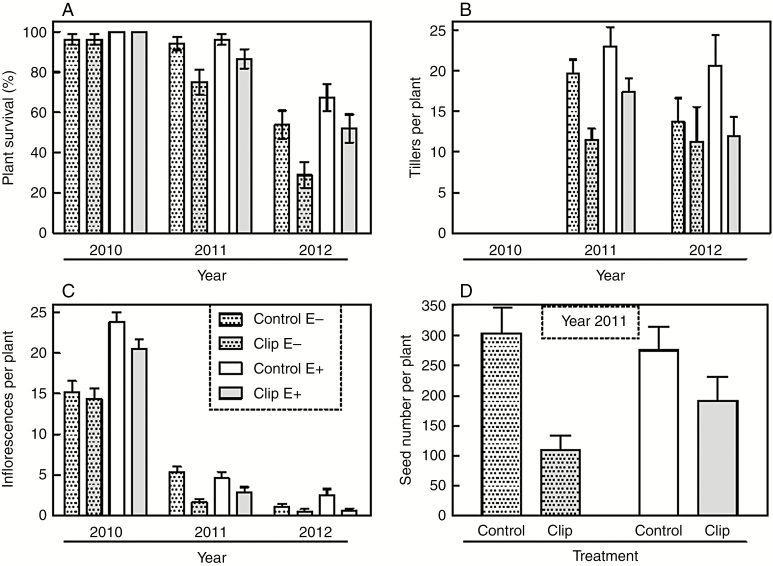
Endophyte presence increased host survival under the controlled folivory (25 % leaf tissue removed) imposed in our experiment. Over both endophyte treatments, simulated herbivory by clipping leaves with scissors reduced plant survival by 29 % (F1,78 = 12.64, P < 0.001), despite significant interannual variability in survival (Year effect: F1,101 = 57.64, P < 0.001). In the clipped treatment, plants with endophytes (E+) showed 33 % greater survival than endophyte-free (E−) plants (contrast E+ vs. E− under clipping: F1,204 = 5.27, P = 0.023), indicating improved tolerance. However, survival of E+ and E− plants was similar in the non-clipped, control treatment (F1,204 = 0.83, P = 0.363; Fig. 1A; main effect of endophyte symbiosis F1,204 = 4.33, P = 0.038). When clipped, E− plants showed 30 % lower survival than non-clipped controls, whereas E+ plants showed only a 15 % reduction in survival.
Fig. 1.
Effects of clipping to simulate folivory and the presence of Epichloë on autumn bluegrass (Poa autumnalis): (A) plant survival over the period 2010–2012, (B) number of tillers per plant recorded in 2011 and 2012, (C) number of inflorescences per plant recorded in 2010, 2011 and 2012, and (D) number of seeds per plant recorded in 2011. Symbols show means ± s.e. Sample size was n = 52 plants per treatment combination at the beginning of the experiment and declined yearly as plants died.
Endophyte-symbiotic plants did not have better tolerance to clipping than endophyte-free plants in their growth response, as indicated by a non-significant clipping × endophyte interaction for tiller production (F1,78 = 0.52, P = 0.472). Simulated herbivory caused a larger decline in plant growth compared with removal of the endophyte (Fig. 1B). Across years, E+ plants made 26 % more tillers than E− plants (F1,78 = 4.48, P = 0.037), but non-clipped plants grew 45 % larger than clipped plants (F1,78 = 7.90, P < 0.001).
The reproductive fitness effects of both simulated herbivory and endophyte presence varied among years, as evidenced by the three-way interaction among endophyte, clipping treatment and year for inflorescence production (F2,383 = 3.97, P = 0.019). First, in year 2010, E+ plants produced 40−50 % more inflorescences than E− plants, regardless of the clipping treatment. Second, in 2011, the endophyte did not increase reproduction (Fig. 1C). Total seed production per plant was recorded only in 2011 and was reduced by 88 % under clipping (F1,37 = 12.37, P < 0.001) but was not significantly increased by endophyte presence (F1,90 = 1.72, P = 0.193), mirroring the lack of an endophyte effect on inflorescence number during 2011. However, there was a trend for clipped plants to show a stronger benefit of the endophyte than controls during 2011 (contrast E+ vs. E−, clipped: F1,37 = 2.89, P = 0.097; control: F1,37 = 0.00, P = 0.953) (Fig. 1D), a closer match to the endophyte-enhanced tolerance that we observed for the plant survival response. After 3 years (by 2012), E+ plants in the control treatment made 126 % more inflorescences than E− plants (contrast, P = 0.047), but in the clipped treatment, E+ plants made a statistically equivalent number of inflorescences to E− plants (P = 0.425, Fig. 1C), demonstrating that symbiosis was beneficial only in the non-clipped treatment.
Does folivory increase symbiont vertical transmission to seeds or hyphal density in seedlings?
Simulated herbivory increased vertical transmission of the endophyte from maternal plants to seeds (F1,48 = 5.39, P = 0.025) and from maternal plants to seedlings (F1,48 = 10.13, P = 0.003). The mean difference between clipped and control plants was ~2 % for plant-to-seeds and ~5 % for plant-to-seedlings (Fig. 2A, B). In addition, endophyte hyphae were significantly denser in seedlings of maternal plants that had been clipped (F1,20 = 6.23, P = 0.021; Fig. 2C). This effect was not a consequence of plant cell size because there was no significant difference in plant cell size between seedlings from clipped vs. control maternal plants (F1,20 = 1.3, P = 0.268). Although simulated folivory increased the prevalence of vertical transmission of the endophyte, folivory had no significant effect on the proportion of seeds that germinated (F1,48 = 0.00, P = 0.982) (data not shown).
Fig. 2.
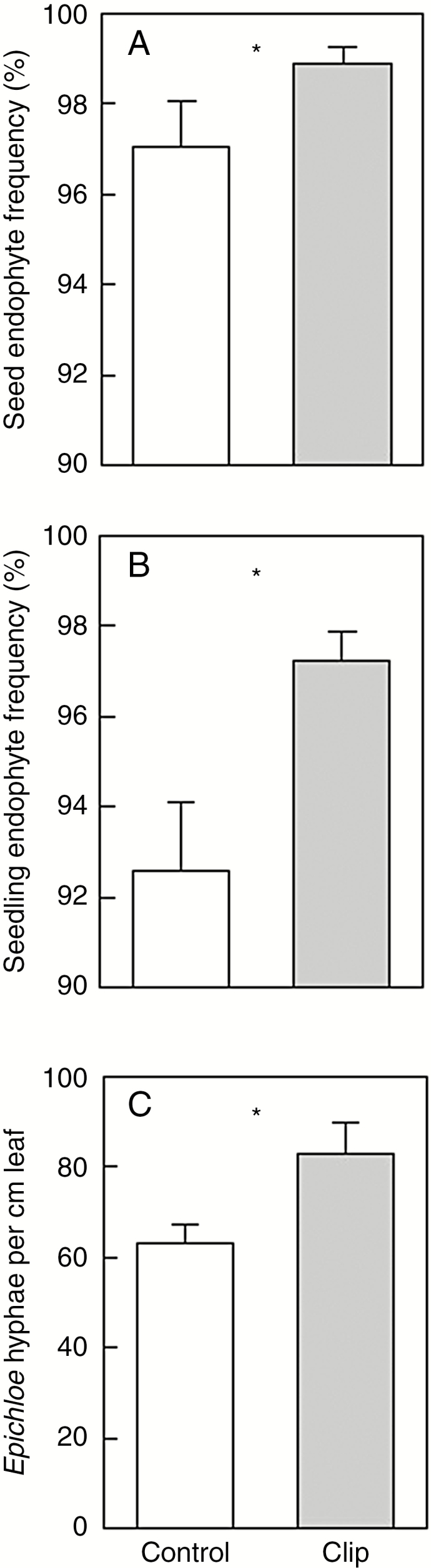
Effects of clipping to simulate folivory on autumn bluegrass (Poa autumnalis): (A) seed endophyte frequency per plant (percentage of ten seeds that had Epichloë), (B) seedling endophyte frequency per plant (percentage of 20 seedlings that had Epichloë), and (C) Epichloë hyphal density in seedlings (per centimetre of leaf tissue). Bars show means ± s.e. Sample size was 49 endophyte-bearing plant genotypes; each genotype was split into two plants, one was clipped, the other served as an unmanipulated control. Asterisks indicate significant differences (P < 0.05).
Do plants with symbiont horizontal transmission have lower rates of vertical transmission than plants lacking horizontal transmission?
Vertical transmission did not correlate positively with endophyte prevalence.
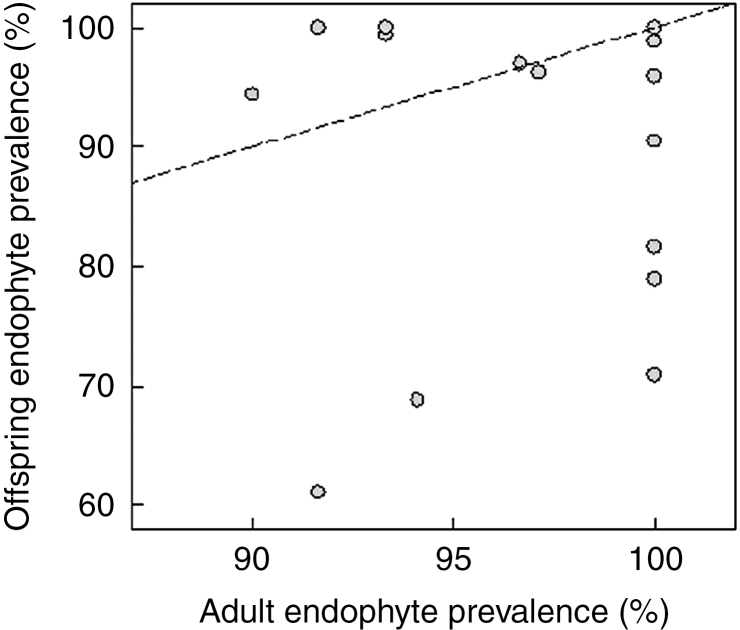
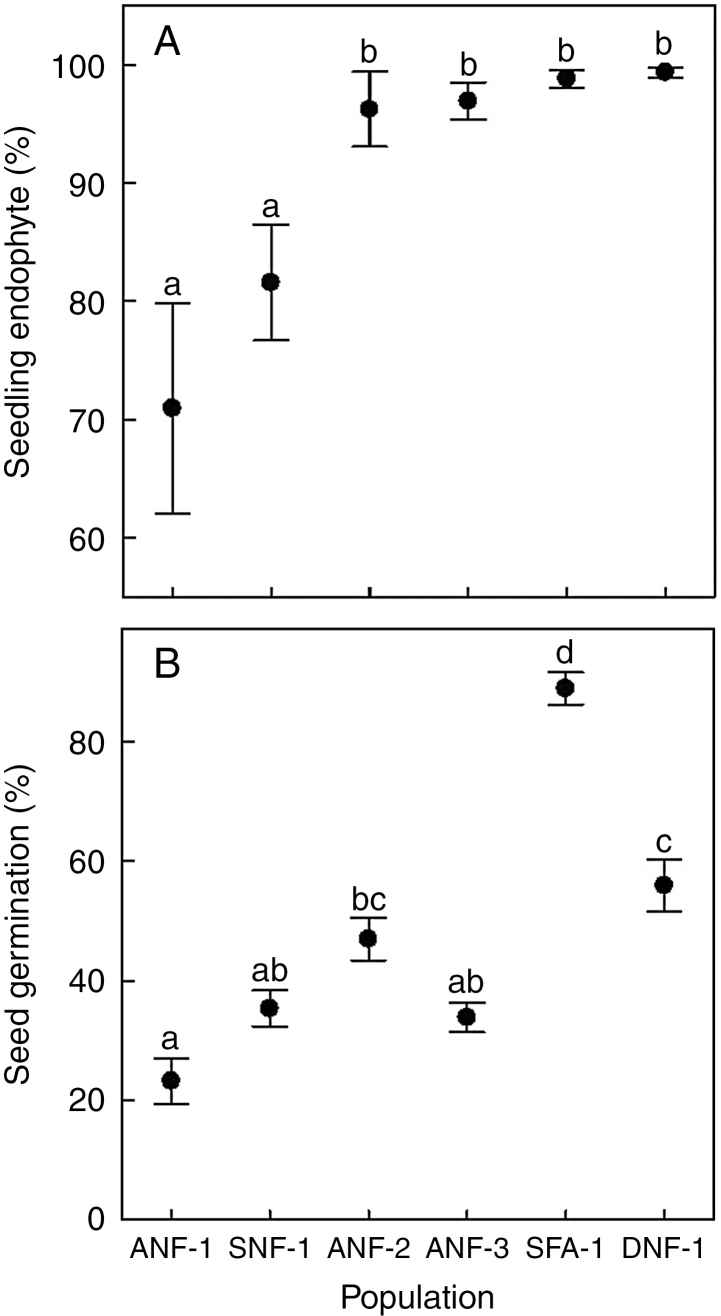
Across 18 populations, there was no significant correlation between the prevalence of symbiosis (percentage of adult plants in the population with Epichloë) and vertical transmission (percentage of seeds or seedlings produced by endophyte-bearing plants with Epichloë) (Fig. 3, Spearman’s r = 0.09, P = 0.730, N = 18). The lack of pattern did not change when we removed the one stromata-forming population (ANF-3) from the analysis. However, both variables, and particularly vertical transmission, had low variation, which constrained the ability to detect a relationship. Populations differed significantly in both the level of vertical transmission to seedlings (Fig. 4A; F5,175 = 8.47, P < 0.001) and the proportion of seeds that germinated (Fig. 4B; F5,173 = 56.36, P < 0.001). With a greater range of variation in germination than in endophyte vertical transmission, the former was positively associated with the latter across the six populations with both types of data (Fig. 4). Endophyte transmission to seedlings was highly effective (≈100 %) in five populations (Table 1), while it was ≈70–80 % in two others (Table 1).
Fig. 3.
Relationship between prevalence of Epichloë in Poa autumnalis (percentage of field-collected adult plants) and the prevalence of vertical transmission (percentage of seedlings with Epichloë) across 18 populations in Texas, USA. The dashed line shows the expectation for a 1 : 1 relationship between adult and offspring endophyte prevalence.
Fig. 4.
Variation among six populations of autumn bluegrass (Poa autumnalis) in which we assessed both (A) vertical transmission (percentage of laboratory-grown seedlings bearing Epichloë; F5,175 = 8.47, P < 0.0001, r2 = 0.19), and (B) germination (percentage of seeds that germinated in the laboratory; F5,173 = 56.36, P < 0.001, r2 = 0.62). Populations were ordered by increasing seedling endophyte prevalence. Symbols show means ± s.e. Sample sizes are given in Table 1. Different letters indicate significant differences in means (P < 0.05).
Populations varied in endophyte.
We detected two endophyte genotypes among the P. autumnalis populations surveyed that equated to two different Epichloë taxa (Supplementary Data Fig. S2). In total, ten populations contained only the interspecific hybrid endophyte E. sp. PauTG-1 (Poa autumnalis Taxonomic Group 1, taxon 1), and as yet to be named Epichloë species but previously reported endophyte (Kutil et al., 2007; Schardl et al., 2012). Four populations contained only the non-hybrid E. typhina subsp. poae endophyte (taxon 2), and eight populations contained a mixture of both endophyte taxa (Table 1; Table S2). The E. sp. PauTG-1 endophyte is considered asexual and would only transmit vertically, whereas E. typhina subsp. poae is considered a sexual endophyte and could transmit both vertically and horizontally.
Vertical transmission rates did not decline with horizontal transmission.
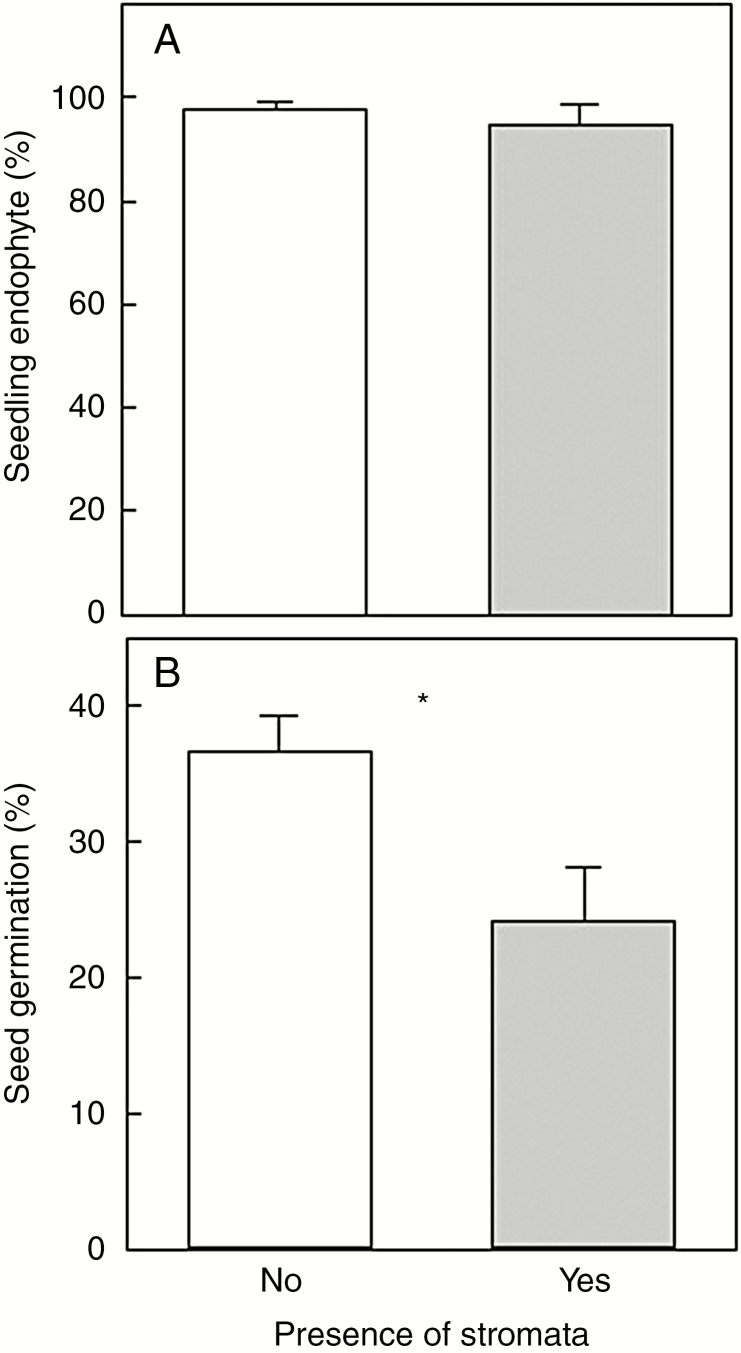
Only the ANF-3 population had plants with stromata (Table 1), and thus had potential for horizontal transmission. Evaluation of the endophytes in 20 of the 60 individual plants from ANF-3 revealed that both endophyte taxa were present. Plants with stromata all had taxon 2 (E. typhina subsp. poae), and the asymptomatic plants had either taxon 1 (asexual PauTG-1) or taxon 2 (E. typhina subsp. poae). Of the 20 ANF-3 plants tested, five contained the hybrid PauTG-1 endophyte, whereas the remaining 15 had the non-hybrid E. typhina subsp. poae. Maternal plants bearing stromata had a similar prevalence of vertical transmission to asymptomatic maternal plants (F1,41 = 0.61, P = 0.438; Fig. 5A). However, germination was ~15 % lower in seeds produced by stromata-forming plants (F1,39 = 4.83, P = 0.034; Fig. 5B) compared to plants lacking stromata, suggesting a potential fitness cost of stromata production in the ANF-3 population.
Fig. 5.
Association between presence of stromata on maternal plants of autumn bluegrass (Poa autumnalis) from population ANF-3 and two attributes of offspring (A) vertical transmission [seedling endophyte frequency (percentage of laboratory-grown seedlings with Epichloë)], and (B) germination (percentage of seeds that germinated in the laboratory). Bars show means ± s.e. Sample sizes, No: n = 9−11, Yes: n = 32. Asterisk indicate a significant difference (P < 0.05).
DISCUSSION
Here, we show that exogenous ecological interactions with simulated consumers altered the fitness benefits as well as transmission dynamics of symbioses. However, the symbiosis had variable effects on plant fitness at different life stages. Clipped plants with the endophyte survived better than endophyte-free plants, suggesting that improved tolerance to herbivory can be an additional fitness benefit of symbiosis beyond the herbivore resistance reported in our previous study (Crawford et al., 2010). However, under clipping, reproduction was equivalent between symbiotic and endophyte-free plants, and benefits of the endophyte to seed production were significant only in the absence of clipping. Altogether, several plant fitness correlates were either improved or unaffected by endophyte symbiosis in P. autumnalis under simulated folivory. For example, even when clipped, symbiotic plants made similar or greater numbers of tillers and inflorescences than non-clipped, non-symbiotic plants. Previous studies testing endophyte effects on tolerance of perennial ryegrass (Lolium perenne) or tall fescue (Schedonorus phoenix, ex. Festuca arundinacea) in response to damage yielded variable outcomes on tolerance that highlighted interactions among symbiotic status, plant species, genotype identity and timing of damage (Cheplick, 1998; Belesky and Fedders, 1996). For example, simulated grazing imposed late in the growing cycle of annual ryegrass (L. multiflorum) reduced seed production in endophyte-bearing plants (Garcia Parisi et al., 2012). The impact of a symbiont on plant recovery from herbivore attack is likely to depend also on the severity of the damage (Gundel et al., 2011), herbivore feeding guild (grazer mammals, chewing and sap-sucking arthropods) and nutritional status. It is also possible that plant and symbiont responses to actual folivory by mammals or chewing insects are stronger (or weaker) than responses to simulated clipping. Integrating the opposing effects of symbiosis during different life stages and years into a net benefit of symbiosis to population growth would require a demographic model (e.g. Rudgers et al., 2012; Bibian et al., 2016).
In addition to modulating the fitness benefits of symbiosis, simulated folivory also increased the amount of vertical transmission of the endophyte to both seeds and seedlings. Together with the increase in the density of endophyte hyphae in the seeds, these results suggest, for the first time, that symbiont vertical transmission can function as an induced response to herbivory. However, we evaluated endophyte mycelium concentration at only two stages: seeds and seedlings with one or, at most, two tillers. Therefore, although we are confident our measurements of hyphae concentration are reliable, they represent only two snapshots during host ontogeny. Fungal endophyte hyphae in adult plants are heterogeneously distributed among different parts and are variable among tillers (e.g. Philipson and Christey, 1986, Gagic et al., 2018). Further studies examining the phenology of hyphal density, in parallel with changes in fungal metabolites (e.g. Fuchs et al., 2017), could provide finer resolution on the underlying physiological mechanisms of responses to herbivory, and more precise estimates of potential effects on seedling herbivory, an aspect of plant life history that is understudied (Barton and Hanley, 2013).
Our results complement previous studies in other systems that showed herbivory-induced increases in fungal-derived alkaloids or gene expression in damaged, adult plants (e.g. Bultman and Bell, 2003; Sullivan et al., 2007; Zhang et al., 2009; Fuchs et al., 2017). Although we did not directly measure fungal-derived alkaloids in this study, we would expect higher levels of alkaloids in seeds with greater hyphal densities, based on previous studies (Spiering et al., 2005; Rasmussen et al., 2007). The E. sp. PauTG-1 endophyte identified within the SFA-3 population has been previously reported to reduce herbivory (Crawford et al., 2010) and is known to produce insect-deterring lolines (Kutil et al., 2007; Schardl et al., 2012). In accordance with previous observations that endophyte growth can be affected by exogenous factors that influence plant growth (e.g. seasonality, temperature, resources, stress; reviewed by Gundel et al., 2011), our results support the existence of transgenerational effects of consumers on symbiont dynamics.
Transgenerational effects in plants, particularly effects that are induced by herbivores, have received considerable attention in recent years, with a particular focus on understanding the underlying mechanisms and evolutionary consequences (Agrawal et al., 1999; Gundel et al., 2017). Our results suggest the new hypothesis that symbiosis with vertically transmitted microbes can be an efficient mechanism of transgenerational information transfer in response to herbivory. Given the ubiquity of both fungal and bacterial endophytes in plant seeds (Hodgson et al., 2014; Truyens et al., 2015), microbially mediated transgenerational effects may warrant additional study. Vertical transmission to seeds has been documented not only for Epichloe species in grasses, but also for other fungal endophytes including: Curvularia species that enhance heat resistance in the grass Dichanthelium lanuginosum (Redman et al., 2002); Undifilum oxytropis that produce the herbivore-deterrent toxin swainsonine in Astragalus and Oxytropis species (locoweeds) (Ralphs et al., 2011); and other Clavicipitaceae (‘Periglandula’ spp.) responsible for toxic ergot alkaloid production in the morning glory family (Steiner et al., 2006). In addition to fungi, plants can convey bacteria to offspring (reviewed by Truyens et al., 2015). However, because the mode of transmission has not been characterized for the majority of above-ground endophytes in plants, it remains unclear how widespread this possible mechanism of transgenerational effects may be.
Many Poa species have been reported with accompanying Epichloë endophytes that vary in transmission, with vertical, horizontal and mixed transmission modes (Schardl et al., 1997, 2012; Moon et al., 2004; Rudgers et al., 2009; Tadych et al., 2012; Shymanovich et al., 2017; Leuchtmann et al., 2019). Interestingly, single populations of P. autumnalis were observed to host more than one Epichloë taxon, including the non-hybrid E. typhina subsp. poae, which has been observed in other Poa species (Tadych et al., 2012; Leucthmann et al., 2014), and the interspecific hybrid PauTG-1, a new undescribed species. Some populations of P. autumnalis appear to be sympatric with both taxa, but our study was not designed to provide information on the frequency of each Epichloë taxon or compare their fitness benefits to plants. Other studies have also resolved endophyte taxonomic diversity for multiple populations of a single host, with some host species able to associate with multiple Epichloë taxa (Charlton et al., 2012, 2014; Shymanovich et al., 2017, 2019a). For example, a latitudinal transect collection of Poa alsodes populations identified two endophyte taxa and high endophyte frequency, but one Epichloë taxon was more prevalent than the other. The two Epichloë taxa of P. alsodes differed in insect defence mechanisms (Shymanovich et al., 2019b), which could also differ between the Epichloë taxa we identified in P. autumnalis.
In addition to herbivory, other environmental and genetic factors probably regulate the prevalence of endophyte symbiosis in plant populations (Rudgers et al., 2009; Gundel et al., 2011; Semmartin et al., 2015; Sneck et al., 2017, 2019) through their effects on symbiont transmission. In comparison with other studies (e.g. Afkhami and Rudgers, 2008; Gundel et al., 2009; Rudgers et al., 2009; Gibert and Hazard, 2013), our populations of P. autumnalis showed, overall, high endophyte prevalence (≥90 %), similar to that previously reported for P. alsodes (Shymanovich et al., 2019a). Although we found variation among populations in the prevalence of vertical transmission (from ~60 to 100 %, Table 1), transmission was not positively correlated with the high standing prevalence of symbiosis in populations (Fig. 3), perhaps because there was little variation in adult plant prevalence. At present, we cannot parse out how much the differences among populations were caused by plant genotype vs. endophyte genotype because we lack data on plant genotype. For example, Gibert and Hazard (2013) demonstrated that the variation in endophyte frequency among populations of Lolium perenne was largely caused by plant genotype. By contrast, in a recent study of P. alsodes, frequency of the more common E. alsodes symbiont (interspecific hybrid endophyte) was higher than that of E. schardlii var. pennsylvanica (intraspecific hybrid endophyte) and was probably due to greater compatibility with its host (Shymanovich et al., 2017, 2019a). Further investigation into the roles of plant genotype vs. fungal genotype or taxon in the P. autumnalis system, where Epichloë genotypes are diverse, could aid in determining the relative importance of genotype vs. environment in symbiont prevalence.
We expected to find a lower vertical transmission rate and greater fitness cost associated with horizontal transmission of the endophyte (stromata formation) in our mixed transmission population (ANF-3). However, there was no evident penalty in terms of reduced vertical transmission for plants associated with stromata formation, in contrast to the expectation of a trade-off between these transmission modes (e.g. Tintjer et al., 2008). We did detect a potential fitness cost via reduced seed germination from maternal plants with stromata than in those without. The predominance of vertical transmission among the populations of P. autumnalis we surveyed (Table 1) may suggest a history of selection toward mutualistic associations, for which vertical transmission creates a partner fidelity feedback (Ewald, 1987; Sachs and Simms, 2006). However, the persistence of stromata-forming variants indicates potential maintenance of some pathogenicity in this symbiosis. Our field experiment demonstrated superior net fitness of endophyte-symbiotic plants over non-symbiotic plants, consistent with previous results in this system and in other Poa species (Crawford et al., 2010; Yule et al., 2011; Shymanovich et al., 2019b). However, integration of results on both fitness effects and transmission into demographic models would further resolve the question of long-term persistence of fungal endophytes (e.g. Gundel et al., 2008; Cavazos et al., 2018).
In summary, besides the fungal alkaloid-conferred resistance to herbivores, our study provides evidence for an endophyte-mediated enhanced tolerance to herbivory. Also, our results demonstrate that simulated folivory not only mediates the relative fitness benefits of symbiosis in host plant populations, but also promotes the persistence of symbiosis by increasing endophyte vertical transmission to the next generation. The synergism between fitness and transmission mechanisms of symbiont persistence should maintain long-term symbiosis under frequent herbivory and probably explains the high prevalence of the grass–Epichloë symbiosis and low frequency of pathogenic variants in populations of P. autumnalis.
SUPPLEMENTARY DATA
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Appendix 1: Detailed methods. Figure S1: Detection of Epichloë sp. in Poa autumnalis. Figure S2: Phylogenetic tree of partial mtAC and mtBA gene sequences of representative Epichloë species. Table S1: PCR primers used in this study. Table S2: Overview of PCR-based genotyping of endophytes in natural populations of Poa autumnalis.
FUNDING
The stay of P.E.G. in the laboratory of J.A.R. was funded by a Fulbright-CONICET grant. J.A.R. and T.E.X.M. were funded by NSF DEB#1145588 and #1754433. J.A.R. was additionally funded by National Science Foundation DEB#1456955 and #1354972.
ACKNOWLEDGEMENTS
Thanks to Liz Seifert and Carolina Simao for substantial assistance in the field and laboratory, and Christopher L. Schardl (University of Kentucky) for access to PauTG-1 e55. J.A.R. conceived the study and collected population data, and J.A.R./T.E.X.M. maintained the experiment. P.S. collected data on transmission rates, N.D.C. and C.A.Y. conducted genetic analysis of endophytes, and P.E.G. and J.A.R. led the writing. All authors contributed to the manuscript. The authors declare that they have no conflicts of interest.
LITERATURE CITED
- Afkhami ME, Rudgers JA. 2008. Symbiosis lost: imperfect vertical transmission of fungal endophytes in grasses. American Naturalist 172: 405–416. [DOI] [PubMed] [Google Scholar]
- Agrawal AA, Laforsch C, Tollrian R. 1999. Transgenerational induction of defences in animals and plants. Nature 401: 60–63. [Google Scholar]
- Bacon CW, White JF Jr. 1994. Biotechnology of endophytic fungi of grasses. Boca Raton: CRC Press. [Google Scholar]
- Barton KE, Hanley ME. 2013. Seedling–herbivore interactions: insights into plant defence and regeneration patterns. Annals of Botany 112: 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belanger FC 1996. A rapid seedling screening method for determination of fungal endophyte viability. Crop Science 36: 460–462. [Google Scholar]
- Belesky DP, Fedders JM. 1996. Does endophyte influence regrowth of tall fescue? Annals of Botany 78: 499–505. [Google Scholar]
- Bibian AJ, Rudgers JA, Miller TEX. 2016. The role of host demographic storage in the ecological dynamics of heritable symbionts. American Naturalists 188: 446–459. [DOI] [PubMed] [Google Scholar]
- Brem D, Leuchtmann A. 2003. Molecular evidence for host-adapted races of the fungal endophyte Epichloë bromicola after presumed host shifts. Evolution 57: 37–51. [DOI] [PubMed] [Google Scholar]
- Bright M, Bulgheresi S. 2010. A complex journey: transmission of microbial symbionts. Nature Reviews Microbiology 8: 218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bultman TL, Bell GD. 2003. Interaction between fungal endophytes and environmental stressors influences plant resistance to insects. Oikos 103: 182–190. [Google Scholar]
- Cavazos BR, Bohner TF, Donald ML, et al. 2018. Testing the roles of vertical transmission and drought stress in the prevalence of heritable fungal endophytes in annual grass populations. New Phytologist 219: 1075–1084. [DOI] [PubMed] [Google Scholar]
- Charlton ND, Craven KD, Afkhami ME, Hall BA, Ghimire SR, Young CA. 2014. Interspecific hybridization and bioactive alkaloid variation increases diversity in endophytic Epichloë species of Bromus laevipes. FEMS Microbiology Ecology 90: 276–289. [DOI] [PubMed] [Google Scholar]
- Charlton ND, Craven KD, Mittal S, Hopkins AA, Young CA. 2012. Epichloë canadensis, a new interspecific epichloid hybrid symbiotic with Canada wildrye (Elymus canadensis). Mycologia 104: 1187–1199. [DOI] [PubMed] [Google Scholar]
- Cheplick GP 1998. Genotypic variation in the regrowth of Lolium perenne following clipping: effects of nutrients and endophytic fungi. Functional Ecology 12: 176–184. [Google Scholar]
- Clay K, Schardl C. 2002. Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. American Naturalist 160: S99–S127. [DOI] [PubMed] [Google Scholar]
- Crawford KM, Land JM, Rudgers JA. 2010. Fungal endophytes of native grasses decrease insect herbivore preference and performance. Oecologia 164: 431–444. [DOI] [PubMed] [Google Scholar]
- Davitt AJ, Chen C, Rudgers JA. 2011. Understanding context-dependency in plant–microbe symbiosis: the influence of abiotic and biotic contexts on host fitness and the rate of symbiont transmission. Environmental and Experimental Botany 71: 137–145. [Google Scholar]
- Dereeper A, Audic S, Claverie JM, Blanc G. 2010. BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evolutionary Biology 10: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A, Guignon V, Blanc G, et al. 2008. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Research 36: W465–W469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas AE 2010. The symbiotic habit. Princeton: Princeton University Press. [Google Scholar]
- Ewald P 1987. Transmission modes and evolution of the parasitism–mutualism continuum. Annals of the New York Academy of Sciences 503: 295–306. [DOI] [PubMed] [Google Scholar]
- Fuchs B, Krischke M, Mueller MJ, Krauss J. 2017. Herbivore-specific induction of defence metabolites in a grass–endophyte association. Functional Ecology 31: 318–324. [Google Scholar]
- Gagic M, Faville MJ, Zhang W, et al. 2018. Seed transmission of Epichloë endophytes in Lolium perenne is heavily influenced by host genetics. Frontiers in Plant Science 9: 1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Parisi PA, Casas C, Gundel PE, Omacini M. 2012. Consequences of grazing on the vertical transmission of a fungal Neotyphodium symbiont in an annual grass population. Austral Ecology 37: 620–628. [Google Scholar]
- Genkai-Kato M, Yamamura N. 1999. Evolution of mutualistic symbiosis without vertical transmission. Theoretical Population Biology 55: 309–323. [DOI] [PubMed] [Google Scholar]
- Gibert A, Hazard L. 2013. Genetically based vertical transmission drives the frequency of the symbiosis between grasses and systemic fungal endophytes. Journal of Ecology 101: 743–752. [Google Scholar]
- Gundel PE, Batista WB, Texeira M, Martínez-Ghersa MA, Omacini M, Ghersa CM. 2008. Neotyphodium endophyte infection frequency in annual grass populations: relative importance of mutualism and transmission efficiency. Proceedings of the Royal Society B: Biological Sciences 275: 897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundel PE, Garibaldi LA, Tognetti PM, Aragón R, Ghersa CM, Omacini M. 2009. Imperfect vertical transmission of the endophyte Neotyphodium in exotic grasses in grasslands of the Flooding Pampa. Microbial Ecology 57: 740–748. [DOI] [PubMed] [Google Scholar]
- Gundel PE, Martínez-Ghersa MA, Omacini M, et al. 2012. Mutualism effectiveness and vertical transmission of symbiotic fungal endophytes in response to host genetic background. Evolutionary Applications 5: 838–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundel PE, Rudgers JA, Ghersa CM. 2011. Incorporating the process of vertical transmission into understanding of host–symbiont dynamics. Oikos 120: 1121–1128. [Google Scholar]
- Gundel PE, Rudgers JA, Whitney KD. 2017. Vertically transmitted symbionts as mechanisms of transgenerational effects. American Journal of Botany 104: 787–792. [DOI] [PubMed] [Google Scholar]
- Gundel PE, Seal C, Biganzoli F, et al. 2018. Occurrence of alkaloids in grass seeds symbiotic with vertically-transmitted Epichloë fungal endophytes and its relationship with antioxidants. Frontiers in Ecology and Evolution 6: 211. [Google Scholar]
- Herman JJ, Sultan SE. 2011. Adaptive transgenerational plasticity in plants: case studies, mechanisms, and implications for natural populations. Frontiers in Plant Science 2: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson S, Cates C, Hodgson J, Morley NJ, Sutton BC, Gange AC. 2014. Vertical transmission of fungal endophytes is widespread in forbs. Ecology and Evolution 4: 1199–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EI, Afkhami ME, Akçay E, et al. 2015. Cheaters must prosper: reconciling theoretical and empirical perspectives on cheating in mutualism. Ecology Letters 18: 1270–1284. [DOI] [PubMed] [Google Scholar]
- Kandel SL, Joubert PM, Doty SL. 2017. Bacterial endophyte colonization and distribution within plants. Microorganisms 5: 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger RT, Desurmont GA, Schlüter PM, Schiestl FP. 2018. Trans-generational inheritance of herbivory-induced phenotypic changes in Brassica rapa. Scientific Reports 8: 3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiers ET, West SA. 2015. Evolving new organisms via symbiosis. Science 348: 392–394. [DOI] [PubMed] [Google Scholar]
- Kutil BL, Greenwald C, Liu G, Spiering MJ, Schardl CL, Wilkinson HH. 2007. Comparison of loline alkaloid gene clusters across fungal endophytes: predicting the co-regulatory sequence motifs and the evolutionary history. Fungal Genetics and Biology 44: 1002–1010. [DOI] [PubMed] [Google Scholar]
- Leuchtmann A, Bacon CW, Schardl CL, White JF Jr, Tadych M. 2014. Nomenclatural realignment of Neotyphodium species with genus Epichloë. Mycologia 106: 202–215. [DOI] [PubMed] [Google Scholar]
- Leuchtmann A, Schmidt D, Bush LP. 2000. Different levels of protective alkaloids in grasses with stroma-forming and seed-transmitted Epichloë/Neotyphodium endophytes. Journal of Chemical Ecology 26: 1025–1036. [Google Scholar]
- Leuchtmann A, Young CA, Stewart AV, Simpson WR, Hume DE, Scott B. 2019. Epichloe novae-zelandiae, a new endophyte from the endemic New Zealand grass Poa matthewsii. New Zealand Journal of Botany 57: 271–288. [Google Scholar]
- Lipsitch M, Nowak MA, Ebert D, May RM, Lipsitch M. 1995. The population dynamics of vertically and horizontally transmitted parasites. Proceedings of the Royal Society of London Series B: Biological Sciences 260: 321–327. [DOI] [PubMed] [Google Scholar]
- Liu J, Nagabhyru P, Schardl CL. 2017. Epichloë festucae endophytic growth in florets, seeds, and seedlings of perennial ryegrass (Lolium perenne). Mycologia 109: 691–700. [DOI] [PubMed] [Google Scholar]
- Malinowski DP, Belesky DP. 2019. Epichloë (formerly Neotyphodium) fungal endophytes increase adaptation of cool-season perennial grasses to environmental stresses. Acta Agrobotanica 72: 1767. [Google Scholar]
- Moon CD, Craven KD, Leuchtmann A, Clement SL, Schardl CL. 2004. Prevalence of interspecific hybrids amongst asexual fungal endophytes of grasses. Molecular Ecology 13: 1455–1467. [DOI] [PubMed] [Google Scholar]
- Partida-Martínez LP, Heil M. 2011. The microbe-free plant: fact or artifact? Frontiers in Plant Science 2: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson MN, Christey MC. 1986. The relationship of host and endophyte during flowering, seed formation, and germination of Lolium perenne. New Zealand Journal of Botany 24: 125–134. [Google Scholar]
- Pieterse CMJ 2012. Prime time for transgenerational defense. Plant Physiology 158: 545–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Gao Y, Liu H, Zhou Y, Ren A, Gao Y. 2016. Effect of endophyte infection and clipping treatment on resistance and tolerance of Achnatherum sibiricum. Frontiers in Microbiology 7: 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralphs MH, Cook D, Gardner DR, Grum DS. 2011. Transmission of the locoweed endophyte to the next generation of plants. Fungal Ecology 4: 251–255. [Google Scholar]
- Rasmussen S, Parsons AJ, Bassett S, et al. 2007. High nitrogen supply and carbohydrate content reduce fungal endophyte and alkaloid concentration in Lolium perenne. New Phytologist 173: 787–797. [DOI] [PubMed] [Google Scholar]
- Redman RS, Sheehan KB, Stout RG, Rodriguez RJ, Henson JM. 2002. Thermotolerance generated by plant/fungal symbiosis. Science 298: 1581. [DOI] [PubMed] [Google Scholar]
- Rho H, Hsieh M, Kandel SL, Cantillo J, Doty SL, Kim S-H. 2018. Do endophytes promote growth of host plants under stress? A meta-analysis on plant stress mitigation by endophytes. Microbial Ecology 75: 407–418. [DOI] [PubMed] [Google Scholar]
- Rodriguez RJ, White Jr JF, Arnold AE, Redman RS. 2009. Fungal endophytes: diversity and functional role. New Phytologist 182: 314–330. [DOI] [PubMed] [Google Scholar]
- Rudgers JA, Afkhami ME, Rua MA, Davitt AJ, Hammer S, Huguet VM. 2009. A fungus among us: broad patterns of endophyte distribution in the grasses. Ecology 90: 1531–1539. [DOI] [PubMed] [Google Scholar]
- Rudgers JA, Fischer S, Clay K. 2010. Managing plant symbiosis: fungal endophyte genotype alters plant community composition. Journal of Applied Ecology 47: 468–477. [Google Scholar]
- Rudgers JA, Miller TEX, Ziegler SM, Craven KD. 2012. There are many ways to be a mutualist: vertically transmitted symbiont reduces host survival but increases population growth. Ecology 93: 565–574. [DOI] [PubMed] [Google Scholar]
- Sachs JL, Simms EL. 2006. Pathways to mutualism breakdown. Trends in Ecology & Evolution 21: 585–592. [DOI] [PubMed] [Google Scholar]
- Schardl CL, Chung K-R, Penny D, Siegel MR. 1997. Coevolution by common descent of fungal symbionts (Epichloë spp.) and grass hosts. Molecular Biology and Evolution 14: 133–143. [Google Scholar]
- Schardl CL, Leuchtmann A, Spiering MJ. 2004. Symbioses of grasses with seedborne fungal endophytes. Annual Review of Plant Biology 55: 315–340. [DOI] [PubMed] [Google Scholar]
- Schardl CL, Young CA, Faulkner JR, Florea S, Pan J. 2012. Chemotypic diversity of Epichloae, fungal symbionts of grasses. Fungal Ecology 5: 331–344. [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. 2012. NIH Image to ImageJ: 25 years of image analysis. Nature Methods 9: 671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semmartin M, Omacini M, Gundel PE, Hernández-Agramonte IM. 2015. Broad-scale variation of fungal-endophyte incidence in temperate grasses. Journal of Ecology 103: 184–190. [Google Scholar]
- Shymanovich T, Charlton ND, Musso AM, et al. 2017. Interspecific and intraspecific hybrid Epichloë species symbiotic with the North American native grass Poa alsodes. Mycologia 109: 459–474. [DOI] [PubMed] [Google Scholar]
- Shymanovich T, Faeth SH. 2019a Environmental factors affect the distribution of two Epichloë fungal endophyte species inhabiting a common host grove bluegrass (Poa alsodes). Ecology and Evolution 9: 6624–6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shymanovich T, Musso AM, Cech NB, Faeth SH. 2019b Epichloë endophytes of Poa alsodes employ alternative mechanisms for host defense: insecticidal versus deterrence. Arthropod–Plant Interactions 13: 79–90. [Google Scholar]
- Sneck ME, Rudgers JA, Young CA, Miller TEX. 2017. Variation in the prevalence and transmission of heritable symbionts across host populations in heterogeneous environments. Microbial Ecology 74: 640–653. [DOI] [PubMed] [Google Scholar]
- Sneck ME, Rudgers JA, Young CA, Miller TEX. 2019. Does host outcrossing disrupt compatibility with heritable symbionts? Oikos 128: 892–903. [Google Scholar]
- Spiering MJ, Lane GA, Christensen MJ, Schmid J. 2005. Distribution of the fungal endophyte Neoyphodium lolii is not a major determinant of the distribution of fungal alkaloids in Lolium perenne plants. Phytochemistry 66: 195–202. [DOI] [PubMed] [Google Scholar]
- Steiner U, Ahimsa-Muller MA, Markert A, et al. 2006. Molecular characterization of a seed transmitted clavicipitaceous fungus occurring on dicotyledoneous plants (Convolvulaceae). Planta 224: 533–544. [DOI] [PubMed] [Google Scholar]
- Sullivan TJ, Rodstrom J, Vandop J, et al. 2007. Symbiont-mediated changes in Lolium arundinaceum inducible defenses: evidence from changes in gene expression and leaf composition. New Phytologist 176: 673–679. [DOI] [PubMed] [Google Scholar]
- Tadych M, Ambrose KV, Bergen MS, Belanger FC, White JF Jr. 2012. Taxonomic placement of Epichloë poae sp. nov. and horizontal dissemination to seedlings via conidia. Fungal Diversity 54: 117–131. [Google Scholar]
- Tintjer T, Leuchtmann A, Clay K. 2008. Variation in horizontal and vertical transmission of the endophyte Epichloë elymi infecting the grass Elymus hystrix. New Phytologist 179: 236–246. [DOI] [PubMed] [Google Scholar]
- Truyens S, Weyens N, Cuypers A, Vangronsveld J. 2015. Bacterial seed endophytes: genera, vertical transmission and interaction with plants. Environmental Microbiology Reports 7: 40–50. [Google Scholar]
- USDA and NRCS 2012. The PLANTS Database. Greensboro: National Plant Data Team; Available at http://plants.usda.gov (28 December 2012). [Google Scholar]
- Warton DI, Hui FKC. 2011. The arcsine is asinine: the analysis of proportions in ecology. Ecology 92: 3–10. [DOI] [PubMed] [Google Scholar]
- White JF Jr, Torres MS. 2009. Defensive mutualism in microbial symbiosis. Boca Raton: CRC Press. [Google Scholar]
- Yule KM, Woolley JB, Rudgers JA. 2011. Water availability alters the tri-trophic consequences of a plant–fungal symbiosis. Arthropod–Plant Interactions 5: 19–27. [Google Scholar]
- Zas R, Sampedro L. 2015. Heritability of seed weight in Maritime pine, a relevant trait in the transmission of environmental maternal effects. Heredity 114: 116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Nagabhyru P, Schardl CL. 2009. Regulation of a chemical defense against herbivory produced by symbiotic fungi in grass plants. Plant Physiology 150: 1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilber-Rosenberg I, Rosenberg E. 2008. Role of microorganisms in the evolution of animals and plants: the hologenome theory of evolution. FEMS Microbiology Reviews 32: 723–735. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.