Abstract
Background and Aims
Classic theory on geographical gradients in plant–herbivore interactions assumes that herbivore pressure and plant defences increase towards warmer and more stable climates found at lower latitudes. However, the generality of these expectations has been recently called into question by conflicting empirical evidence. One possible explanation for this ambiguity is that most studies have reported on patterns of either herbivory or plant defences whereas few have measured both, thus preventing a full understanding of the implications of observed patterns for plant–herbivore interactions. In addition, studies have typically not measured climatic factors affecting plant–herbivore interactions, despite their expected influence on plant and herbivore traits.
Methods
Here we tested for latitudinal variation in insect seed predation and seed traits putatively associated with insect attack across 36 Quercus robur populations distributed along a 20° latitudinal gradient. We then further investigated the associations between climatic factors, seed traits and seed predation to test for climate-based mechanisms of latitudinal variation in seed predation.
Key Results
We found strong but contrasting latitudinal clines in seed predation and seed traits, whereby seed predation increased whereas seed phenolics and phosphorus decreased towards lower latitudes. We also found a strong direct association between temperature and seed predation, with the latter increasing towards warmer climates. In addition, temperature was negatively associated with seed traits, with populations at warmer sites having lower levels of total phenolics and phosphorus. In turn, these negative associations between temperature and seed traits led to a positive indirect association between temperature and seed predation.
Conclusions
These results help unravel how plant–herbivore interactions play out along latitudinal gradients and expose the role of climate in driving these outcomes through its dual effects on plant defences and herbivores. Accordingly, this emphasizes the need to account for abiotic variation while testing concurrently for latitudinal variation in plant traits and herbivore pressure.
Keywords: Climate, Curculio spp, phenolics, phosphorus, plant–herbivore interactions, Quercus robur
Introduction
It is generally assumed that species interactions become stronger towards lower latitudes in response to higher temperatures, longer growing seasons, and higher plant and animal diversity (e.g. the classic tropical vs. temperate contrast; Dobzhansky, 1950; Janzen, 1970; Schemske et al., 2009). Empirical studies on plant–herbivore interactions testing this prediction have indeed frequently reported that plant species at lower latitudes experience higher rates of herbivory than their counterparts found at higher latitudes (Coley and Barone, 1996; Schemske et al., 2009; Pennings et al., 2009; Salazar and Marquis, 2012; Lim et al., 2015; Moreira et al., 2015), as well as correspondingly higher levels of anti-herbivore defences (Rasmann and Agrawal, 2011; Pearse and Hipp, 2012; Moreira et al., 2014; Abdala-Roberts et al., 2016a), presumably as a result of increased herbivore pressure. However, the generality of this biogeographical paradigm of plant–herbivore interactions has been scrutinized in recent years and several syntheses have pointed out that expected latitudinal patterns are less common than previously assumed (Moles et al., 2011; Anstett et al., 2016).
Recent work has identified several likely sources of variation in the sign and strength of latitudinal gradients in herbivory and plant defences, including methodological inconsistencies (e.g. herbivory measurements or resolution of analyses for chemical traits) (Anstett et al., 2016; Moles and Ollerton, 2016). Crucially, until recently most studies had reported on either patterns of herbivory or plant defences, whereas surprisingly few measured both (but see Anstett et al., 2015; Abdala-Roberts et al., 2016a; Moreira et al., 2018a). This obviously precludes a complete test of the latitudinal clines in plant–herbivore interactions and limits inferences that can be made about the evolution of plant traits in response to increased herbivore pressure (when only herbivory is measured) or the defensive role of plant traits (when only plant traits are measured). Relatedly, the plant traits measured are assumed to be associated with herbivory, but evidence supporting this assumption is weak or absent for the species studied, in part due to limited information on the natural history of plant–herbivore interactions (Anstett et al., 2016). Therefore, even when both types of responses have been measured, the latitudinal associations found may not necessarily indicate a causation (e.g. Abdala-Roberts et al., 2016a).
Another important point is that, at least until recently, studies typically have not measured abiotic factors that vary with latitude which can potentially affect plant traits and herbivory in unexpected ways (Johnson and Rasmann, 2011; Anstett et al., 2016). This is surprising considering the underlying emphasis in both classic and contemporary work on the role of climatic conditions underlying latitudinal gradients in plant–herbivore interactions (Pearse and Hipp, 2012; Moreira et al., 2015; 2018a). For example, increased temperature frequently enhances insect metabolic rates and leads to higher herbivore consumption, growth and development rates (Bale et al., 2002; O’Connor et al., 2011; Lemoine et al., 2017). In addition, climatic conditions may indirectly influence herbivory via changes in plant nutritional quality and investment in anti-herbivore defences (Huberty and Denno, 2004; Zvereva and Kozlov, 2006; Jamieson et al., 2012; Bauerfeind and Fischer, 2013). For example, warming and drought tend to increase levels of plant chemical defences (e.g. terpenoids, glucosinolates), and such changes can in turn influence the performance of herbivorous insects and their consumption of plant tissues (e.g. Llusià and Peñuelas, 1998; Gutbrodt et al., 2011). Addressing the direct and indirect effects of abiotic factors on herbivory should therefore represent a necessary component of latitudinal studies in order to gain a better understanding of how abiotic and biotic factors concurrently shape latitudinal gradients (or lack thereof) in plant–herbivore interactions.
We previously found that pedunculate oak Quercus robur (Fagaceae) populations found at lower latitudes exhibited higher levels of insect leaf herbivory and had lower concentrations of chemical defences in leaves, suggesting that latitudinal variation in herbivory was driven by an inverse gradient in chemical defences (Moreira et al., 2018a). In addition, we further found that abiotic factors influenced leaf defences and, in doing so, indirectly influenced insect leaf herbivory, suggesting a scenario whereby abiotic variation shaped plant defences, and these in turn shaped latitudinal variation in herbivory for this oak species (Moreira et al., 2018a). We here expand on this previous work and test for latitudinal variation in insect seed predation and seed traits putatively related to insect attack across Q. robur populations. If present, latitudinal clines in seed predation may be equally important or even more so than patterns of leaf herbivory (Moles and Westoby, 2003), as seed predation has direct impacts on plant fitness and seedling recruitment and seed traits may therefore be under strong selection to increase defence against seed predation. To further investigate the ecological mechanisms behind any such latitudinal gradients, we also addressed the associations between climatic factors, seed traits and seed predation to elucidate climate-determined mechanisms of latitudinal variation in seed predation. To this end, we sampled 36 oak populations distributed along a 20° latitudinal gradient extending from northern Spain to southern Finland and quantified insect seed predation and seed defensive and nutritional traits putatively associated with insect herbivory in this species (Abdala-Roberts et al., 2016b; Moreira et al., 2018a). Specifically, we addressed the following questions: (1) Are there latitudinal gradients in seed predation and seed traits? (2) Is any such latitudinal cline in seed predation associated with concurrent clines in seed traits? (3) Are climatic correlates of latitude associated with variation in seed predation and seed traits? If so, in what way are these climatic variables associated with seed predation, i.e. directly and/or indirectly via associations with seed traits? By assessing latitudinal gradients in herbivory, plant traits and abiotic (climatic) factors, the present study seeks to unveil the mechanisms behind latitudinal gradients in seed predation, a relatively understudied guild of insect herbivores within the literature of latitudinal variation in herbivory.
MATERIAL AND METHODS
Study species
We studied Quercus robur, commonly known as pedunculate oak. This is a long-lived, deciduous tree native to western Europe (Schwarz, 1964), with a distribution ranging from central Spain (39°N) to southern Fennoscandia (62°N) (Petit et al., 2002). It flowers in mid-spring, and the fruits (called acorns) ripen by mid-autumn. Acorns are 2–2.5 cm long, borne on lengthy stalks and held tightly by cupules (i.e. the cup-shaped base of the acorn).
Pre-dispersal attack of Q. robur acorns (hereafter ‘seed predation’) is mostly due to weevil larvae of Curculio spp. (Coleoptera: Curculionidae; Andersson, 1992; Crawley and Long, 1995), which are herbivores specialized on oaks. The adult female weevil bores a tiny hole through the coat of immature acorns to lay eggs (Desouhant et al., 2000). Weevil larvae then grow inside acorns feeding on the embryo cotyledons until completing development and in late autumn leave the acorn by drilling an exit hole (Bonal et al., 2012). Seed predation by moth caterpillars has also been reported for this oak species but was uncommon at the sampled sites (X. Moreira, pers. observ.).
Field sampling
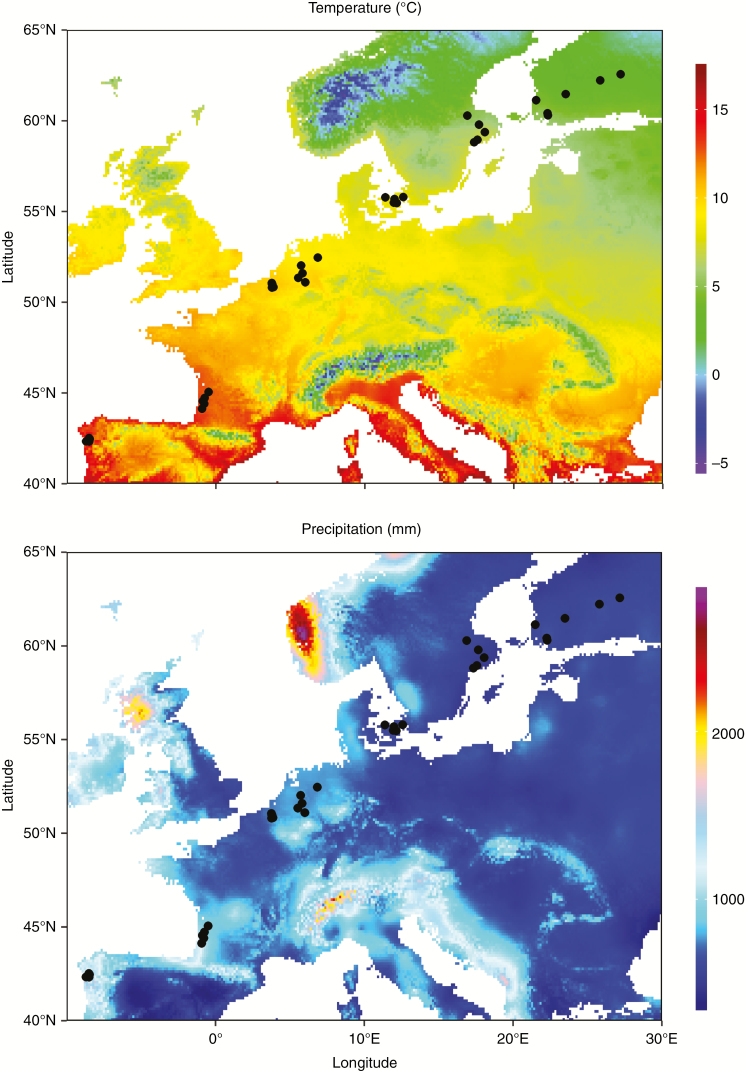
From late October to late November 2018, we sampled a total of 36 Q. robur populations distributed across Spain, France, Belgium, the Netherlands, Denmark, Sweden and Finland spanning 20° in latitude (42–62°N; Fig. 1). This transect covered almost the entire latitudinal and climatic gradient experienced by Q. robur along its distribution range (>90 % in both cases, Fig. 1). From north to south, oak trees and insect herbivores experience more than a three-fold increase in precipitation (513–1541 mm per year) and an increase of 10.5 °C in mean annual temperature (3.0–13.5°C) (Fig. 1). Sites were sampled when acorns reached maturity (from late October to late November depending on the site).
Fig. 1.
Maps of (A) mean annual temperature and (B) annual precipitation. Circles indicate the location of the 36 populations of Quercus robur sampled along a 20° latitudinal range from northern Spain to southern Finland.
At each site, the Q. robur population included at least 15 reproductive trees and pedunculate oak was usually the most abundant tree species at all sites (>50 % of mature trees). We selected five mature trees per population [mean (±s.e.) diameter at breast height = 48.20 ± 1.98 cm], and for each tree haphazardly selected two low-hanging branches (~3 m above ground level) with at least 10 ripe acorns and for each branch we haphazardly collected up to 50 acorns (mean 27.66 ± 0.92). We placed all collected acorns in paper envelopes to allow weevils to continue feeding and complete their development. We also estimated acorn density by counting all acorns and empty cupules along 1 m in length of each branch, moving from branch tip to the trunk. We sampled trees with a roughly similar reproductive output to avoid biases introduced by variation in acorn density which may influence seed predation (e.g. seed predator satiation).
Estimation of seed predation
Four weeks after collection, we estimated the proportion of infested acorns per branch and averaged values across branches to obtain a mean value per tree for statistical analyses (hereafter ‘seed predation’). By far most of insect seed predation was caused by larvae of Curculio spp. (>95 % of insect-attacked seeds). It is important to note that although we did not record acorn removal or damage by mammals, these species could influence estimates of insect attack (e.g. squirrels or rodents preferentially removing uninfested acorns; Smallwood et al., 2001). Nonetheless, we assumed that such effect was small and consistent across regions and therefore would not lead to biases. After estimating seed predation, we oven-dried all acorns for 48 h at 40 °C and randomly selected eight uninfested acorns per tree (four per branch) to be weighed to the nearest 1 mg because previous work has shown that seed mass (or size) is positively associated with weevil attack (Xia et al., 2016). In addition, we removed the acorn coat of the weighted acorns, ground the embryo cotyledons in liquid nitrogen and stored the samples for subsequent quantification of phenolic compounds and nutrients in the embryo cotyledons. We used uninfested acorns to quantify these chemical traits in order to minimize effects of local (individual acorn-level) induction. Still, we acknowledge that systemic induction at the branch level or among branches may influence results, and therefore the levels of phenolics and nutrients measured probably represented a combination of constitutive levels plus an unknown level of systemic induction (Moreira et al., 2018a).
Quantification of seed chemical defences
We chose phenolic compounds as putative defensive traits given good evidence indicating that they act as a feeding deterrent of insect herbivores in a number of oak species (e.g. Steele et al., 1993; Zhishu et al., 2007; Weckerly et al., 2011). Briefly, we extracted seed phenolic compounds using 20 mg of dry plant tissue with 0.25 mL of 70 % methanol in an ultrasonic bath for 15 min, followed by centrifugation (Moreira et al., 2014). We then transferred the extracts to chromatographic vials to perform phenolic profiling. For phenolic compound identification, we used ultra-performance liquid chromatography coupled with electrospray ionization quadrupole (Thermo Dionex Ultimate 3000 LC) time-of-flight mass spectrometry (UPLC-Q-TOF-MS/MS) (Bruker Compact). We performed chromatographic separation in a Kinetex 2.6-µm C18 82–102 Å, LC Column 100 × 4.6 mm column using a binary gradient solvent mode consisting of 0.05 % formic acid in water (solvent A) and acetonitrile (solvent B). We used the following gradient: from 5 to 30 % B (0–4 min), from 30 to 75 % B (4–15 min), from 75 to 85 % B (15–18 min), and from 85 % to 100 % B (18–20 min). The injection volume was 6 µL, the flow rate was established at 0.4 mL min−1 and column temperature was controlled at 25 °C. We operated MS analysis in a spectra acquisition range from 50 to 1200 m/z. We used negative (-) electron spin ionization (ESI) modes under the following specific conditions: gas flow 8 L min−1, nebulizer pressure 38 psi, dry gas 7 L min−1 and dry temperature 220 °C. We set capillary and end plate offset to 4500 and 500 V, respectively. We performed MS/MS analysis based on the previously determined accurate mass and retention times and fragmented by using different collision energy ramps to cover a range from 15 to 50 eV. We recorded chromatograms at 275 nm. We identified individual compounds based on the data obtained from the standard substances or published literature including retention times, λ max, ([M–H]−), and major fragment ions. We only identified phenolic compounds from two groups: ellagitannins and gallic acid derivatives (‘hydrolysable tannins’ hereafter) (n = 16) and proanthocyanidins (‘condensed tannins’ hereafter) (n = 1).
For phenolic compound quantification, we injected 6 µL of each sample (using the same column and conditions mentioned above) in a UHPLC device (Nexera LC-30AD; Shimadzu) equipped with a Nexera SIL-30AC injector and one SPD-M20A UV/VIS photodiode array detector (Moreira et al., 2018b). We quantified condensed tannins as catechin equivalents and hydrolysable tannins as gallic acid equivalents (Moreira et al., 2018b). Quantification of these phenolic compounds was done by external calibration using calibration curves at 0.25, 0.5, 1, 2 and 5 μg mL−1. We calculated total phenolics as the sum of condensed and hydrolysable tannins, and expressed phenolic compound concentrations in mg g−1 tissue on a dry weight basis.
Quantification of seed nutrients
We also measured the concentration of seed phosphorus and nitrogen, macro-nutrients that have been previously shown to correlate with insect herbivory in oak species (e.g. Abdala-Roberts et al., 2016b; Moreira et al., 2018a). Briefly, we digested ~0.1 g of dry plant tissue in a mixture of selenous sulphuric acid and hydrogen peroxide (Moreira et al., 2012). We then used a colorimetric analysis of diluted aliquots of the digestion to quantify nitrogen (indophenol blue method) and phosphorus (molybdenum blue method) concentration using a Bio-Rad 650 microplate reader (Bio-Rad Laboratories, Philadelphia, PA, USA) at 650 and 700 nm, respectively (Walinga et al., 1995). We expressed nitrogen and phosphorus concentration in mg g−1 tissue on a dry weight basis.
Geographical and climatic variables
We obtained the geographical coordinates of each Q. robur population using a Global Positioning System device (Garmin). To characterize the climatic conditions present at each site, we used a subset of the bioclimatic variables from the WorldClim database (Version 2; http://www.worldclim.org/) at a 30-second resolution. Specifically, we used BIO1 (annual mean temperature, °C), BIO4 (temperature seasonality, expressed as the standard deviation of temperature among months × 100), BIO5 (maximum temperature of the warmest month, °C), BIO6 (minimum temperature of the coldest month, °C), BIO12 (annual precipitation, mm), BIO13 (precipitation of the wettest month, mm), BIO14 (precipitation of the driest month, mm) and BIO15 (precipitation seasonality, expressed as the standard deviation of precipitation across months) as climatic variables. The procedures used to calculate these climatic variables are fully described in Fick and Hijmans (2017).
Statistical analyses
Latitudinal variation in seed predation and seed traits.
We ran generalized linear mixed models (GLMMs) testing the effect of latitude (fixed factor) on seed predation, mass, total phenolics, nitrogen and phosphorus using data at the tree level. In each model, we also accounted for population as a random factor (implemented in R via the lme4 package; R Core Team, 2018). In addition, because environmental conditions are frequently suboptimal at the margins of the species’ distribution range (e.g. Spain and Finland), the relationship between seed predation and traits with latitude could be non-linear. Accordingly, we also included the quadratic term for latitude as a fixed factor in all models. Having said this, this quadratic term was not significant for seed traits and was removed from these models (results not shown). In addition, we also initially included branch-level acorn density and tree diameter as covariates, but these predictors were not significant in any case and were also removed from the statistical models. For seed predation, we used a GLMM with a binomial distribution and logit-link function. Because the model was overdispersed, we added tree ID nested within the population as a random factor. For seed traits, we used a GLMM with a Gaussian distribution and identity-link function. We standardized latitude to zero mean and unit variance before running the models to ensure model convergence.
Correlates of latitudinal variation in seed predation and traits.
Rather than testing for associations between each climatic variable and seed predation which would inflate Type I error due to multiple tests, we summarized climatic variables using a principal component (PC) analysis (PROC FACTOR, rotation = varimax in SAS 9.4) (Moreira et al., 2015). Two axes explained 89 % of the variance in the eight climatic variables across oak populations. PC1 (hereafter ‘temperature’) was positively related to mean annual temperature and minimum temperature of the coldest month, and negatively related to temperature seasonality. PC2 (hereafter ‘precipitation’) was positively related to precipitation of the wettest month and precipitation of the driest month. We used the standardized z-scores of these PCs for structural equation modelling (SEM).
We ran a piece-wise SEMusing data at the population level to investigate direct associations among climatic factors, seed traits and seed predation, as well as the indirect relationships between climate and seed predation. We also included the associations between latitude and climatic variables to assess the direction of latitudinal clines in climatic conditions. Direct and indirect associations between latitude and seed traits or seed predation were not included to simplify the model and because we assumed that latitude acted as a proxy for climatic variables, i.e. it is these latter variables which underlie latitudinal variation in seed traits and predation. Conventional SEM simultaneously estimates the relationships between all variables, while for piece-wise SEM the association network is broken down into different independent linear regression models and then combined (Lefcheck, 2016). This approach allows us to easily incorporate specific assumptions in each of the regression models that were included in the SEM (Lefcheck, 2016). Specifically, we first ran a local analysis testing for direct relationships between seed traits and seed predation, as well as indirect associations between climate and seed predation (via seed traits), and subsequently ran another local analysis testing for direct associations between climatic variables, seed traits and seed predation. Direct associations between climatic variables and seed predation were obtained from the latter analysis, whereas direct associations between seed traits and seed predation were obtained from the former analysis. Rather than including all seed traits in this analysis, we previously selected the biologically most relevant traits by running a separate multiple regression (using PROC REG in SAS 9.4) that included seed mass, phenolics, phosphorus and nitrogen as predictors of seed predation. Results from this regression indicated that seed phenolics and phosphorus were significantly (P < 0.05) associated with seed predation (Supplementary Data Table S1). We therefore only included these two traits in the SEM to reduce parameter load and simplify the model. We estimated direct associations as standardized partial regression coefficients, whereas indirect associations were calculated by multiplying the specified coefficients for direct relationships between both the predictor and the response. We assessed the significance of direct and indirect coefficients with t-tests. The goodness of fit of the general model was evaluated with a ‘test of direct separation’ based on Fisher’s C-test (Lefcheck, 2016). SEM analysis was performed in R version 3.6.0 (R Core Team, 2018) using the piecewiseSEM package (Lefcheck, 2016). We used the psem function to obtain SEM fit parameters and the partialResid function to extract the partial effects of significant predictors on seed traits or predation accounting for all other covariates locally in the model (Lefcheck, 2016).
RESULTS
Latitudinal variation in seed predation and traits
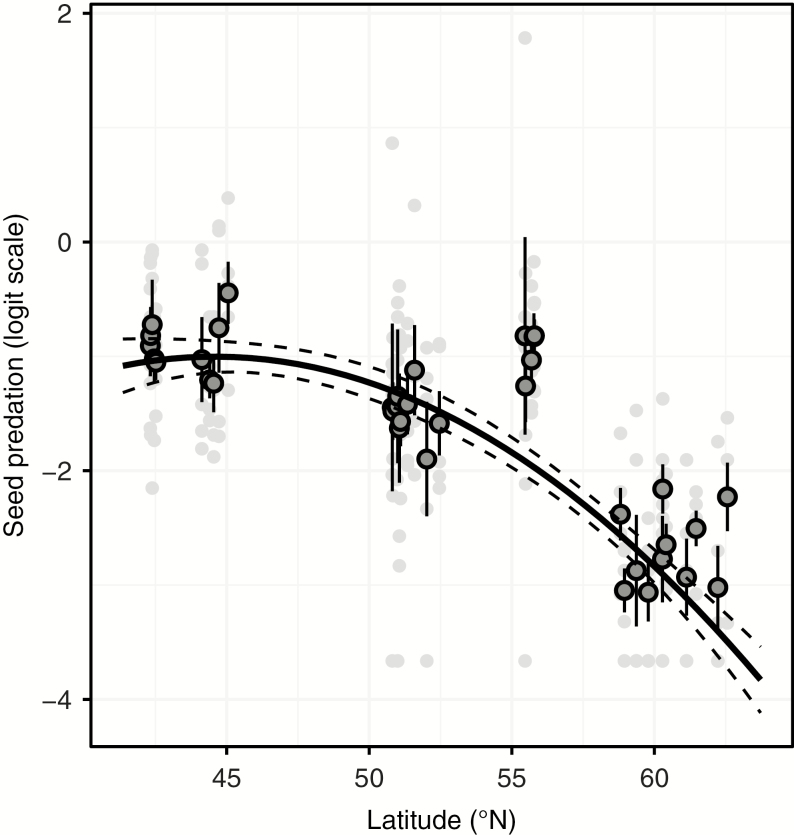
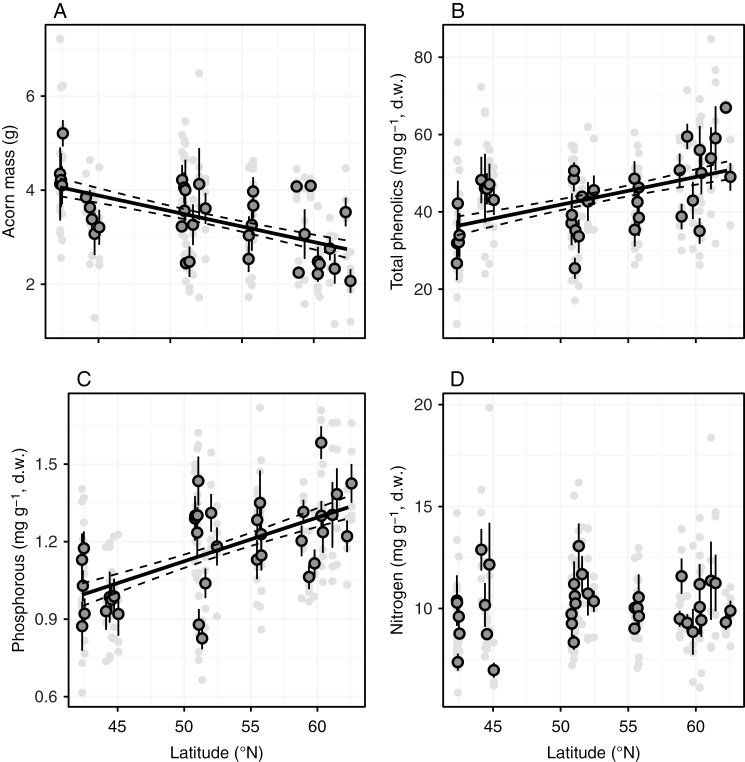
We found a significant negative association between both linear and quadratic terms of latitude and the proportion of infested seeds (Table 1), indicating a non-linear increase in seed predation towards lower latitudes (Fig. 2). In addition, we found a significant negative association between latitude and seed mass (Table 1), whereby oak populations found at lower latitudes had heavier seeds (Fig. 3A). In contrast, we found significant positive associations between latitude and seed total phenolics and phosphorus concentration (Table 1), with oak populations found at higher latitudes having higher mean values of these seed traits (Fig. 3B, C). There was no significant association between latitude and seed nitrogen concentration (Table 1; Fig. 3D).
Table 1.
Results from generalized linear mixed models testing for the effect of latitude (fixed factor) on seed predation (mean proportion of attacked seeds) and seed traits, namely: mass, total phenolics, nitrogen and phosphorus using data at the tree level
| Response | Predictor | χ2 | d.f. | P-value | Estimate ± s.e. | R m 2 (Rc2) |
|---|---|---|---|---|---|---|
| Seed predation | Latitude | 80.24 | 1 | <0.001 | −0.82 (0.09) | 0.15 (0.29) |
| Latitude2 | 10.12 | 1 | <0.001 | −0.34 (0.11) | ||
| Seed mass | Latitude | 17.80 | 1 | <0.001 | −0.44 (0.10) | 0.17 (0.39) |
| Total phenolics | Latitude | 13.04 | 1 | <0.001 | 4.80 (1.33) | 0.13 (0.35) |
| Phosphorous | Latitude | 22.06 | 1 | <0.001 | 0.11 (0.02) | 0.22 (0.49) |
| Nitrogen | Latitude | 0.41 | 1 | 0.522 | 0.14 (0.22) | 0.00 (0.18) |
In each model, we also accounted for population as a random factor. For the seed predation model, we also included the quadratic term for latitude because it was significant (see Statistical analyses section). Chi-square values (χ 2), degrees of freedom and associated significance levels (P) are shown. Significant (P < 0.05) effects are in bold. We also report the slope estimates and their standard errors (s.e.) as well as marginal and conditional R2 values (Rm2 and Rc2, respectively) which represent the proportion of variance explained by fixed (Rm2) and fixed plus random (Rc2) factors.
Fig. 2.
Latitudinal variation in insect seed predation (measured as the proportion of infested seeds) in Quercus robur individuals sampled from 36 populations distributed from northern Spain to southern Finland. Light grey circles represent individual trees (n = 180) and dark grey circles represent tree populations (n = 36; error bars are standard errors). The black solid curve depicts the predicted (significant) non-linear relationship between seed predation and latitude, and the dashed lines represent its 95 % confidence interval. Statistics are presented in Table 1.
Fig. 3.
Latitudinal variation in (A) seed mass and concentration of seed, (B) total phenolics, (C) phosphorus and (D) nitrogen of Quercus robur individuals from 36 populations distributed from northern Spain to southern Finland. Light grey circles represent individual trees (n = 180) and dark grey circles represent tree populations (n = 36; error bars are standard errors). Black solid lines represent significant (P < 0.05) relationships between seed traits and latitude, with the dashed lines representing its 95 % confidence interval. Statistics are presented in Table 1.
Correlates of latitudinal variation in seed predation and traits
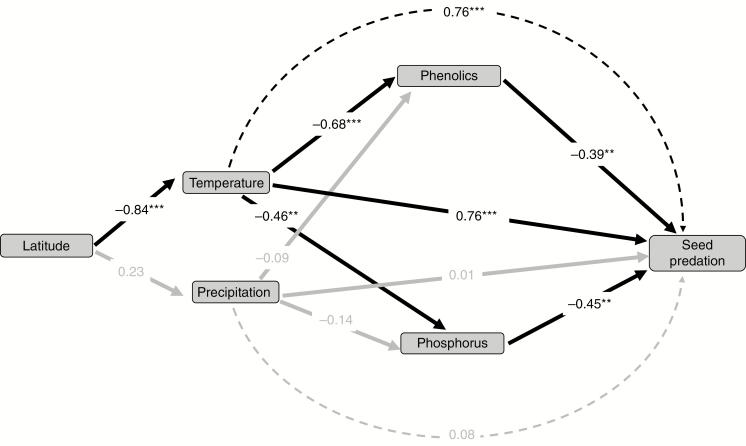
Results from the piece-wise SEM indicated a significant negative association between latitude and temperature but not precipitation (Fig. 4). There were significant direct associations between temperature and seed predation and traits (Fig. 4). Specifically, temperature was positively associated with the proportion of attacked seeds and negatively associated with seed total phenolics and phosphorus (Fig. 4). In contrast, there were no significant relationships between precipitation and seed traits or seed predation (Fig. 4). The results also indicated significant associations between seed traits and seed predation, whereby both total phenolics and phosphorus were negatively associated with the proportion of attacked seeds (Fig. 4). Finally, there was a significant positive indirect association between temperature (via seed traits) and seed predation (Fig. 4).
Fig. 4.
Diagram showing results from a piece-wise structural equation model testing for associations among latitude, climatic factors (temperature and precipitation), seed defensive and nutritional traits (total phenolics, phosphorus), and insect seed predation (measured as the proportion of infested seeds) on Quercus robur individuals sampled from 36 populations distributed from northern Spain to southern Finland. Values used were population means (n = 36). Climatic variables represent z-score values from a principal component analysis summarizing a suite of variables associated with precipitation or temperature. Values next to each arrow are path coefficients (i.e. standardized regression coefficients). Continuous arrows indicate direct associations whereas broken arrows indicate indirect associations, in this case between temperature and precipitation and seed predation (mediated by seed total phenolics and phosphorus). The model also accounted for co-variation between temperature and precipitation as well as between seed traits, but these estimates are not shown for ease of visualization. Significant (*P < 0.05, **P < 0.01, ***P < 0.001) and non-significant (P > 0.05) path coefficients are in black and grey, respectively. Explained variance: phosphorus = 0.24; phenolics = 0.47; seed predation = 0.40. Fisher’s C = 24.80, P < 0.001, corrected Akaike’s information criterion (AICc) = 48.80.
Discussion
We found strong but contrasting latitudinal clines in insect seed predation and seed traits for Q. robur, whereby populations found at lower latitudes had higher levels of seed predation and lower concentrations of seed total phenolics and phosphorus. Our findings also indicated a significant negative association between seed predation and seed total phenolics and phosphorus, as well as a strong direct association between temperature, but not precipitation, and seed predation, whereby the proportion of attacked seeds increased towards warmer climates. In addition, temperature was negatively associated with seed chemical defences and nutrients, with oak populations found at warmer sites exhibiting lower levels of total phenolics and phosphorus. Accordingly, these negative associations between temperature and seed traits in turn led to a positive indirect association between temperature and seed predation. Together, these findings highlight links between abiotic and biotic factors concurrently shaping latitudinal variation in plant defences and herbivory, and therefore move us closer to understanding the underlying drivers of latitudinal variation in plant–herbivore interactions.
A high degree of variation in the strength and magnitude of latitudinal clines in leaf herbivory has been found in recent studies (Anstett et al., 2016), including patterns reported for temperate trees such as oaks (Carmona et al., 2020). Accordingly, the few studies to date on latitudinal variation in seed predation and frugivory have shown no association between predation and latitude for a broad range of species from around the globe (Moles and Westoby, 2003), including a more recent study reporting an increase (rather than decrease) in seed predation towards higher latitudes for a subtropical herb (Moreira et al., 2015). Counter to these findings, our results were consistent with classic predictions in that insect seed predation on Q. robur increased towards lower latitudes. This finding is also consistent with our previous work showing that insect leaf herbivory negatively correlates with latitude in Q. robur (Moreira et al., 2018a). In combination, these studies provide robust evidence that pressure by insect leaf-chewers and seed predators on Q. robur progressively increases towards the lower latitudinal end of this species’ distribution.
On the other hand, the observed decrease in seed total phenolics towards lower latitudes runs against classic theory predicting higher plant defences at lower latitudes (Rasmann and Agrawal, 2011; Pearse and Hipp, 2012; Moreira et al., 2014). Still, this finding agrees with recent work by Chen and Moles (2018) providing the most complete study to date on latitudinal variation in fruit and seed defences, which reported that putative plant physical defences against seed predators in most cases increase with latitude across a number of plant species. In addition, our previous work with Q. robur showed that leaf chemical defences also increased with latitude (Moreira et al., 2018a), and so both studies combined indicate parallel latitudinal clines for putative chemical defences in seeds and leaves of this oak species. We also found a negative relationship between insect attack and total phenolic compounds in seeds, which matches previous findings linking leaf herbivory and phenolics (see Moreira et al., 2018a), and suggests that the decrease in seed defences towards lower latitudes underlies the increase in seed predation with decreasing latitude. Phosphorus was unexpectedly negatively associated with seed predation and correlated positively with latitude, suggesting that decreasing concentrations of this nutrient towards lower latitudes could have increased beetle preference or predation in southern populations. Interestingly, we also found greater seed size at lower latitudes, which agrees with a recent study by Bogdziewicz et al. (2019a) who reported that seed size in holm oaks (Q. ilex) increased towards lower latitudes. The authors found that greater seed size increased seed tolerance to seed predation as larger acorns were more likely to survive weevil infestation. Thus, while seed size was not correlated with seed predation in Q. robur, latitudinal variation in acorn tolerance to seed predation may have gone undetected and should be investigated. While the above findings suggest important features describing the nature of latitudinal variation in seed predation for this oak species, we caution that the patterns reported are correlational. Further work is needed to be able to assess the adaptive value of the measured chemical traits and the causality of associations between seed predation and, for example, phenolics (or other chemical and physical traits). This could include within-population correlations between seed traits and predation, bioassays of seed tolerance to predator infestation and insect seed choice, as well as artificial seed predation experiments testing for fitness implications of induced seed resistance.
Climatic factors, particularly temperature-related variables, were directly associated with insect seed predation. We found that seed predation increased towards warmer climates at lower latitudes, which agrees with previous studies reporting greater leaf herbivory with increasing temperatures towards lower latitudes (Zhang et al., 2016), presumably due to direct physiological or behavioural (e.g. Bale et al., 2002; O’Connor et al., 2011; Lemoine et al., 2017) effects of temperature on insects. Interestingly, our previous latitudinal study with Q. robur indicated that precipitation, rather than temperature, was associated with insect leaf herbivory (Moreira et al., 2018a), suggesting different mechanisms of abiotic control over latitudinal gradients for leaf-chewers than seed predators. We speculate that the relative importance of direct associations between temperature or precipitation and herbivory may differ based on whether insect herbivores are concealed vs. external feeders (Pincebourde and Casas, 2019). For example, external feeders may be more strongly affected by direct effects of precipitation (e.g. interference with feeding, pathogen infection; Abdala-Roberts et al., 2015) than concealed feeders. Although this would explain stronger effects of precipitation on external leaf-chewers than on seed predators, it does not explain why temperature appears to more strongly affect seed predators. Regardless of the precise mechanism, our studies with Q. robur in combination emphasize the importance of measuring clinal responses by multiple herbivore taxa or guilds and abiotic correlates underlying such patterns (Moreira et al., 2015; Anstett et al., 2015). In doing so, we can gain a better understanding of community-level variation in herbivory and underlying abiotic drivers of variation across insect taxa or functional groups.
Our results also indicated that temperature was negatively associated with Q. robur seed phenolics and phosphorus, consistent with our previous work reporting on latitudinal gradients in leaf traits for this oak species (Moreira et al., 2018a). Moreover, we found an indirect positive association between temperature and seed predation mediated by seed traits, which contrasts with our previous work with Q. robur for which we found an indirect association between precipitation and insect leaf herbivory (Moreira et al., 2018a). This result follows from the observed direct effects of temperature on seed predation and precipitation on leaf herbivory, again suggesting different mechanisms of climatic control (in this case indirect via plant traits) on these herbivore guilds. In speculating further about the potential mechanisms involved in this indirect effect, it is possible that temperature and precipitation have different effects on the secondary chemistry of vegetative vs. reproductive tissues (e.g. Abdala-Roberts et al., 2016a), which lead to corresponding differences in abiotically mediated clines for each guild. For example, thermal clines could have stronger effects on seed than on leaf secondary chemistry with this in turn leading to stronger indirect effects of temperature on seed predation.
While our findings reveal interesting patterns and several of the suggested mechanisms deserve attention, there are also some relevant limitations in our study that should be considered. First, seed predation by weevils may not necessarily select (at least not strongly) on seed secondary chemistry, and instead may have stronger selective effects on other types of traits of relevance to weevil attack. For example, oaks, as well as other taxa of long-lived plants, may evolve mechanisms to satiate seed predators via synchronous seed production (i.e. masting) or seed size (seed-level satiation) rather than through the expression of chemical traits in individual seeds (Bonal et al., 2007). Thus, the proposed link between weevil predation and seed chemical traits may be less important than assumed, particularly in masting species which are predicted to strongly rely on population-level mechanisms such as reproductive synchrony to ameliorate the impact of seed predation. Second, and related to the previous limitation, seed predation rates by weevils can vary inter-annually by several orders of magnitude, at least partly due to temporal variation in seed crop size (Moreira et al., 2017), and seed output does not necessarily take place synchronously over broad geographical scales (Bogdziewicz et al., 2019b), i.e. those scales needed to test for latitudinal gradients. Our study only captures variation within one growing season and therefore ignores temporal patterns in acorn output and weevil satiation within populations, as well as temporal (intra- or inter-annual) differences in reproductive synchrony across populations influencing seed predation. These sources of temporal variation in seed predation within and among populations may vary latitudinally and deserve attention in future multi-annual studies with masting species. Finally, our experimental approach might underestimate seed predation rates, mainly because trees may abort and drop seeds after weevil female oviposition such that sampled branches would have an over-representation of uninfested seeds. The use of seed traps would potentially overcome this limitation.
In summary, our results point at several interesting lines of research to assess the mechanisms underlying clinal variation in seed–predator interactions. First, conducting simultaneous measurements of herbivory by multiple insect guilds and investigating the influence of abiotic factors on each can yield insight into the abiotic mechanisms governing latitudinal clines in plant–insect herbivore interactions. Correlational data on relevant abiotic predictors can be complemented with experimental studies in which precipitation and/or temperature are manipulated to understand the modus operandi of abiotic controls (direct and indirect) on insect performance and behaviour. Second, conducting measurements of multiple types of defences (physical and chemical) using replicated correlational analyses within and among populations, combined with experimental studies that manipulate seed predation while controlling for other confounding factors, are needed to pinpoint relevant seed traits that have presumably been selected by and at the same time influence seed predation along environmental clines. Third, extending studies on individual plant species and their associated insect communities to several co-occurring plant species within local communities is a necessary step to establish general patterns at the community level. Expanding beyond single-species plant–insect interactions will allow us to relate plant community-level features (e.g. composition) to seed predation patterns, while overcoming the limitations from past comparisons among studies (e.g. meta-analyses) that vary in methodology or sampling design features.
Supplementary data
Supplementary data are available online at https://academic.oup.com/aob and consist of the following. Table S1: Multiple regression testing for the associations between seed traits and seed predation in Quercus robur trees belonging to 36 populations.
ACKNOWLEDGEMENTS
We thank María Lores and Víctor M. Rodríguez for their help with chemical analyses and Carla Vázquez-González for her advice on statistical analyses. Comments and suggestions by Raúl Bonal and an anonymous reviewer helped to improve the manuscript.
Funding
This research was financially supported by a Spanish National Research Grant (AGL2015-70748-R), a Regional Government of Galicia Grant (IN607D 2016/001) and the Ramón y Cajal Research Programme (RYC-2013–13230) to X.M.
LITERATURE CITED
- Abdala-Roberts L, Mooney KA, Quijano-Medina T, Campos-Navarrete MJ, González-Moreno A, Parra-Tabla V. 2015. Comparison of tree genotypic diversity and species diversity effects on different guilds of insect herbivores. Oikos 124: 1527–1535. [Google Scholar]
- Abdala-Roberts L, Moreira X, Rasmann S, Parra-Tabla V, Mooney KA. 2016a Test of biotic and abiotic correlates of latitudinal variation in defenses in the perennial herb Ruellia nudiflora. Journal of Ecology 104: 580–590. [Google Scholar]
- Abdala-Roberts L, Rasmann S, Berny-Mier y Terán JC, Covelo F, Glauser G, Moreira X. 2016b Biotic and abiotic factors associated with altitudinal variation in plant traits and herbivory in a dominant oak species. American Journal of Botany 103: 2070–2078. [DOI] [PubMed] [Google Scholar]
- Andersson C. 1992. The effect of weevil and fungal attacks on the germination of Quercus robur acorns. Forest Ecology and Management 50: 247–251. [Google Scholar]
- Anstett DN, Ahern JR, Glinos J, Nawar N, Salminen J-P, Johnson MTJ. 2015. Can genetically based clines in plant defence explain greater herbivory at higher latitudes? Ecology Letters 18: 1376–1386. [DOI] [PubMed] [Google Scholar]
- Anstett DN, Nunes KA, Baskett C, Kotanen PM. 2016. Sources of controversy surrounding latitudinal patterns in herbivory and defense. Trends in Ecology & Evolution, 31: 789–802. [DOI] [PubMed] [Google Scholar]
- Bale JS, Masters GJ, Hodkinson ID, et al. 2002. Herbivory in global climate change research: direct effects of rising temperature on insect herbivores. Global Change Biology 8: 1–16. [Google Scholar]
- Bauerfeind SS, Fischer K. 2013. Increased temperature reduces herbivore host-plant quality. Global Change Biology 19: 3272–3282. [DOI] [PubMed] [Google Scholar]
- Bogdziewicz M, Espelta JM, Bonal R. 2019a Tolerance to seed predation mediated by seed size increases at lower latitudes in a Mediterranean oak. Annals of Botany 123: 707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdziewicz M, Szymkowiak J, Fernández-Martínez M, Peñuelas J, Espelta JM. 2019b The effects of local climate on the correlation between weather and seed production differ in two species with contrasting masting habit. Agricultural and Forest Meteorology 268: 109–115. [Google Scholar]
- Bonal R, Hernandez M, Ortego J, Muñoz A, Espelta JM. 2012. Positive cascade effects of forest fragmentation on acorn weevils mediated by seed size enlargement. Insect Conservation and Diversity 5: 381–388. [Google Scholar]
- Bonal R, Muñoz A, Díaz M. 2007. Satiation of predispersal seed predators: the importance of considering both plant and seed levels. Evolutionary Ecology 21: 367–380. [Google Scholar]
- Carmona D, Moreira X, Abdala-Roberts L. 2020. Latitudinal and elevational gradients in plant defences and herbivory in temperate trees: recent findings, underlying factors, and an evolutionary perspective using genomic tools. In: Núñez-Farfán J, Valverde PL, eds. Evolutionary ecology of plant–herbivore interactions. Berlin: Springer; (in press). [Google Scholar]
- Coley PD, Barone JA. 1996. Herbivory and plant defenses in tropical forests. Annual Review of Ecology and Systematics 27: 305–335. [Google Scholar]
- Crawley MJ, Long CR. 1995. Alternate bearing, predator satiation and seedling recruitment in Quercus robur L. Journal of Ecology 83: 683–696. [Google Scholar]
- Chen SC, Moles AT. 2018. Factors shaping large‐scale gradients in seed physical defence: seeds are not better defended toward the tropics. Ecography 27: 417–428. [Google Scholar]
- Desouhant E, Debouzie D, Ploye H, Menu F. 2000. Clutch size manipulations in the chestnut weevil, Curculio elephas: fitness of oviposition strategies. Oecologia 122: 493–499. [DOI] [PubMed] [Google Scholar]
- Dobzhansky T. 1950. Evolution in the tropics. American Scientist 38: 209–221. [Google Scholar]
- Fick SE, Hijmans RJ. 2017. Worldclim 2: new 1-km spatial resolution climate surfaces for global land areas. International Journal of Climatology 37: 4302–4315. [Google Scholar]
- Gutbrodt B, Mody K, Dorn S. 2011. Drought changes plant chemistry and causes contrasting responses in lepidopteran herbivores. Oikos 120: 1732–1740. [Google Scholar]
- Huberty AF, Denno RF. 2004. Plant water stress and its consequences for herbivorous insects: a new synthesis. Ecology 85: 1383–1398. [Google Scholar]
- Jamieson MA, Trowbridge AM, Raffa KF, Lindroth RL. 2012. Consequences of climate warming and altered precipitation patterns for plant–insect and multitrophic interactions. Plant Physiology 160: 1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen DH. 1970. Herbivores and the number of tree species in tropical forests. The American Naturalist 104: 501–528. [Google Scholar]
- Johnson MTJ, Rasmann S. 2011. The latitudinal herbivory defence hypothesis takes a detour on the map. New Phytologist 191: 589–592. [DOI] [PubMed] [Google Scholar]
- Lefcheck JS. 2016. piecewiseSEM: piecewise structural equation modelling in R for ecology, evolution, and systematics. Methods in Ecology and Evolution 7: 573–579. [Google Scholar]
- Lemoine NP, Doublet D, Salminen J-P, Burkepile DE, Parker JD. 2017. Responses of plant phenology, growth, defense, and reproduction to interactive effects of warming and insect herbivory. Ecology 8: 1817–1828. [DOI] [PubMed] [Google Scholar]
- Lim JY, Fine PVA, Mittelbach GG. 2015. Assessing the latitudinal gradient in herbivory. Global Ecology and Biogeography 24: 1106–1112. [Google Scholar]
- Llusià J, Peñuelas J. 1998. Changes in terpene content and emission in potted Mediterranean woody plants under severe drought. Canadian Journal of Botany 76: 1366–1373. [Google Scholar]
- Moles AT, Bonser SP, Poore AGB, Wallis IR, Foley WJ. 2011. Assessing the evidence for latitudinal gradients in plant defence and herbivory. Functional Ecology 25: 380–388. [Google Scholar]
- Moles AT, Ollerton J. 2016. Is the notion that species interactions are stronger and more specialized in the tropics a zombie idea? Biotropica 48: 141–145. [Google Scholar]
- Moles AT, Westoby M. 2003. Latitude, seed predation and seed mass. Journal of Biogeography 30: 105–128. [Google Scholar]
- Moreira X, Castagneyrol B, Abdala-Roberts L, et al. 2018. a Latitudinal variation in plant chemical defenses drives latitudinal patterns of leaf herbivory. Ecography 41: 1124–1134. [Google Scholar]
- Moreira X, Abdala-Roberts L, Galmán A, et al. 2018. b Assessing the influence of biogeographical region and phylogenetic history on chemical defences and herbivory in Quercus species. Phytochemistry 153: 64–73. [DOI] [PubMed] [Google Scholar]
- Moreira X, Abdala-Roberts L, Parra-Tabla V, Mooney KA. 2015. Latitudinal variation in herbivory: influences of climatic drivers, herbivore identity, and natural enemies. Oikos 124: 1444–1452. [Google Scholar]
- Moreira X, Mooney KA, Rasmann S, et al. 2014. Trade-offs between constitutive and induced defences drive geographical and climatic clines in pine chemical defences. Ecology Letters 17: 537–546. [DOI] [PubMed] [Google Scholar]
- Moreira X, Pérez-Ramos IM, Abdala-Roberts L, Mooney KA. 2017. Functional responses of contrasting seed predator guilds to masting in two Mediterranean oak species. Oikos 126: 1042–1050. [Google Scholar]
- Moreira X, Zas R, Sampedro L. 2012. Genetic variation and phenotypic plasticity of nutrient re-allocation and increased fine root production as putative tolerance mechanisms inducible by methyl-jasmonate in pine trees. Journal of Ecology 100: 810–820. [Google Scholar]
- O’Connor MI, Gilbert B, Brown CJ. 2011. Theoretical predictions for how temperature affects the dynamics of interacting herbivores and plants. The American Naturalist 178: 626–638. [DOI] [PubMed] [Google Scholar]
- Pearse IS, Hipp AL. 2012. Global patterns of leaf defenses in oak species. Evolution 66: 2272–2286. [DOI] [PubMed] [Google Scholar]
- Pennings SC, Ho C-K, Salgado CS, et al. 2009. Latitudinal variation in herbivore pressure in Atlantic coast salt marshes. Ecology 90: 183–195. [DOI] [PubMed] [Google Scholar]
- Petit RJ, Brewer S, Bordács S, et al. 2002. Range wide distribution of chloroplast DNA diversity and pollen deposits in European white oaks: inferences about colonisation routes and management of oak genetic resources. Forest Ecology and Management 156: 49–74. [Google Scholar]
- Pincebourde S, Casas J. 2019. Narrow safety margin in the phyllosphere during thermal extremes. Proceedings of the National Academy of Sciences of the United States of America 116: 5588–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team 2018. R: A language and environment for statistical computing. Vienna:R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- Rasmann S, Agrawal AA. 2011. Latitudinal patterns in plant defense: evolution of cardenolides, their toxicity, and induction following herbivory. Ecology Letters 14: 476–483 [DOI] [PubMed] [Google Scholar]
- Salazar D, Marquis RJ. 2012. Herbivore pressure increases toward the equator. Proceedings of the National Academy of Sciences of the United States of America 109: 12616–12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemske DW, Mittelbach GG, Cornell HV, Sobel JM, Roy K. 2009. Is there a latitudinal gradient in the importance of biotic interactions? Annual Review of Ecology, Evolution, and Systematics, 40: 245–269. [Google Scholar]
- Schwarz O. 1964. Quercus L. In: Tutin TG, Heywood VH, Burges NA, Valentine DH, Walters SM, Webb DA, eds. Flora Europaea, vol. 1: Lycopodiaceae to Platanaceae. Cambridge: Cambridge University Press, 61–64. [Google Scholar]
- Smallwood PD, Steele MA, Faeth SH. 2001. The ultimate basis of the caching preferences of rodents, and the oak-dispersal syndrome: tannins, insects, and seed germination. Integrative and Comparative Biology 41: 840–851. [Google Scholar]
- Steele ML, Knowles TW, Bridle K, Simms E. 1993. Tannins and partial consumption of acorns: implications for dispersal of oaks by seed predators. The American Midland Naturalist 130: 229–238. [Google Scholar]
- Walinga I, Van Der Lee J, Houba VJG. 1995. Plant analysis manual. Dordrecht: Kluwer Academic Publisher. [Google Scholar]
- Weckerly FW, Sugg DW, Semlitsch RD. 2011. Germination success of acorns (Quercus): insect predation and tannins. Canadian Journal of Forest Research 19: 811–815. [Google Scholar]
- Xia K, Harrower WL, Turkington R, Tan H-Y, Zhou Z-K. 2016. Pre-dispersal strategies by Quercus schottkyana to mitigate the effects of weevil infestation of acorns. Scientific Reports 6: 37520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Zhang Y, Ma K. 2016. Latitudinal variation in herbivory: hemispheric asymmetries and the role of climatic drivers. Journal of Ecology 104: 1089–1095. [Google Scholar]
- Zhishu X, Harris MK, Zhang Z. 2007. Acorn defenses to herbivory from insects: implications for the joint evolution of resistance, tolerance and escape. Forest Ecology and Management 238: 302–308. [Google Scholar]
- Zvereva EL, Kozlov MV. 2006. Consequences of simultaneous elevation of carbon dioxide and temperature for plant–herbivore interactions: a metaanalysis. Global Change Biology, 12: 27–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.