Abstract
A 60-year-old male patient presented with a serum α-fetoprotein (AFP) level of 2940.5 ng/mL accompanied by a significant increase in serum globulin. Hepatitis B virus (HBV) DNA was 2.85 × 103 (normal value <1.0 × 103). B-mode ultrasound and magnetic resonance imaging showed characteristic manifestations and he was clinically diagnosed with hepatocellular carcinoma in January 2015. He received radiofrequency ablation and tenofovir disoproxil anti-HBV therapy and his serum AFP and globulin levels were significantly reduced. In March 2018, he presented at our Hematology Department with fatigue and a pale complexion. At that time, his serum AFP level was normal, with hemoglobin 61 g/L and globulin 64.7 g/L. He was diagnosed with multiple myeloma (MM) by bone marrow examination, and immunofixation electrophoresis. The patient received PCD chemotherapy (bortezomib 2.0 g/dL on days 1, 4, 8, and 11 plus cyclophosphamide 0.3 g/dL on days 1–4 plus dexamethasone 20 mg/dL on days 1–2, 4–5, 8–9, and 11–12). The patient finally died of MM complicated by disseminated intravascular coagulation.
Keywords: Globulin, hepatocellular carcinoma, multiple myeloma, hepatitis B virus, α-fetoprotein, disseminated intravascular coagulation
Introduction
Hepatocellular carcinoma (HCC) is the third-leading cause of cancer-related deaths worldwide and is highly prevalent in Eastern Asia, with an incidence rate of 31.9/100,000.1,2 Multiple myeloma (MM) is a clonal plasma cell malignancy that accounts for 1% of all cancers and approximately 10% of all hematologic malignancies.3,4 HCC and most MM cases are characterized by increased globulin levels. Globulin levels decrease during active treatment for HCC, but may then increase progressively, usually indicating clinical recurrence of HCC. Here we report the rare case of a patient who was clinically diagnosed with HCC whose globulin level decreased following active treatment but then increased, and he was finally diagnosed with MM.
Case report
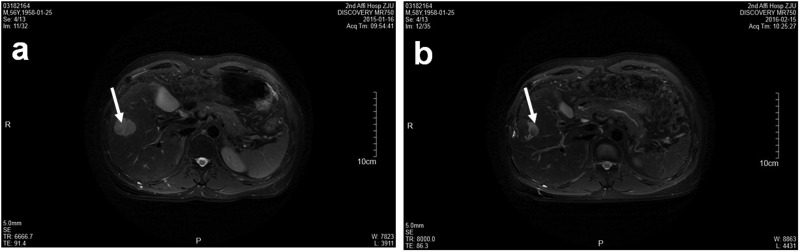
A 60-year-old man visited a local hospital for a physical examination in January 2015. His serum α-fetoprotein (AFP) was 2940.5 ng/mL (normal value <20 ng/mL). His routine biochemistry results were as follows: creatinine (Cr) 51 mol/L (reference value 40–106 mol/L), globulin 51.2 g/L (reference value 15–30 g/L), and albumin 37.1 g/L (reference value 35–52 g/L). Routine blood test results were normal. Hepatitis B virus (HBV) DNA was 2.85 × 103 (normal value < 1.0 × 103). B-mode ultrasound suggested a space-occupying right liver lesion of about 2.6 × 2.8 cm, with a clear boundary and uneven internal echo. Liver magnetic resonance imaging (MRI) suggested that the T1 signal was slightly low and the T2 signal was high in the V segment of the right lobe. After enhancement, the arterial phase was obviously enhanced and contrast medium in the portal phase was withdrawn, showing fast in and fast out (Figure 1a). The patient was therefore clinically diagnosed with HCC and treated with radiofrequency ablation and tenofovir disoproxil anti-HBV therapy. His serum AFP level fell to 140.8 ng/mL and his globulin level to 45.1 g/L after therapy.
Figure 1.
Liver magnetic resonance images. (a) Space-occupying lesion in the V segment of the right liver lobe (arrow). (b) Nodule in the lower inner margin considered as local relapse (arrow).
Liver MRI conducted in February 2016 showed mostly coagulative necrosis of the tumor in segment V, but a 21-mm nodule in the lower inner margin was suspected to indicate local relapse (Figure 1b) and a 17-mm nodule in segment VI was believed to be a new lesion. The patient received further radiofrequency ablation.
Positron emission tomography-computed tomography (PET-CT) in June 2017 revealed a right hepatic focal mass with no evidence of increased 2-fluoro-2-deoxy-D-glucose (FDG) metabolism, widespread bone destruction, and osteogenic changes. Some lesions with abnormal FDG metabolism and multiple bone metastases were assessed. The patient was then treated with sorafenib.
The patient was admitted to our Hematology Department in March 2018 with a > 1-month history of fatigue and a pale complexion. Physical examination showed stable vital signs, an Eastern Cooperative Oncology Group (ECOG) score of 4, a numeric rating scale (NRS) score of 2 to 4, a clear mind, listless and anemic appearance, multiple ecchymoses in the skin and mucosa throughout the body, no yellowing of the skin or mucosa, no enlargement of superficial lymph nodes, coarse breathing sounds and scattered wet rales in both lungs. His abdomen was soft and his liver and spleen were unaffected. There was no tenderness or rebound pain in the abdomen. Bowel sounds were active 5 to 7 times per minute, and there was edema in both lower limbs. Auxiliary examination was conducted Routine blood tests showed a white blood cell count of 5.7 × 109/L, hemoglobin level of 61 g/L, platelet count of 120 × 109/L, and reticulocyte count of 3.6%. Routine biochemistry showed Cr 56.9 mol/L, total protein 79.5 g/L, albumin 14.8 g/L, and globulin 64.7 g/L. His serum AFP level was 0.3 ng/L and HBV DNA was < 30 IU/mL. No mutations were detected. HBV surface antigen, E antigen, and core antibody were positive. Chest CT showed infectious lesions in both lungs with bilateral pleural effusion and pericardial effusion, and his sternum, cervical, and thoracic vertebrae showed multiple rib fractures. Abdominal CT revealed two oval, uniform-density nodules in his right liver; the larger nodule was about 3.34 × 4.11 cm, but no increase in the lesion was obvious on the enhanced scan.
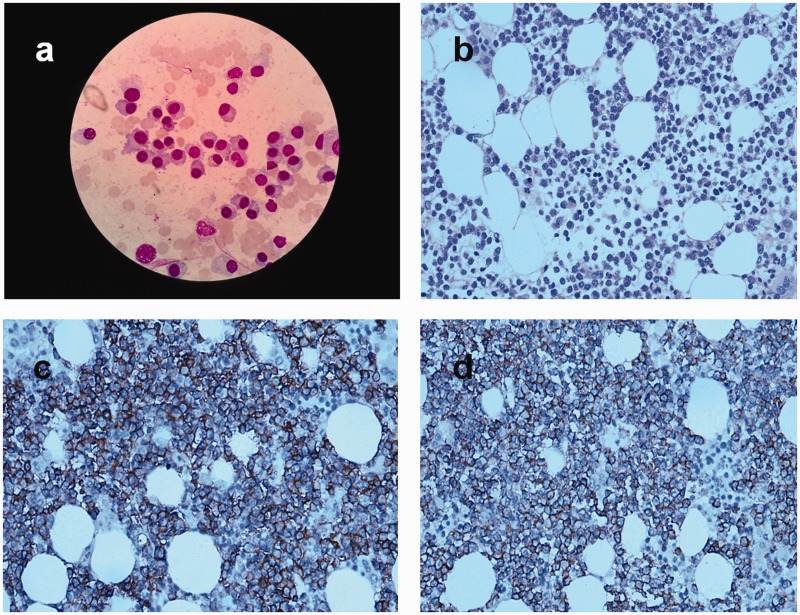
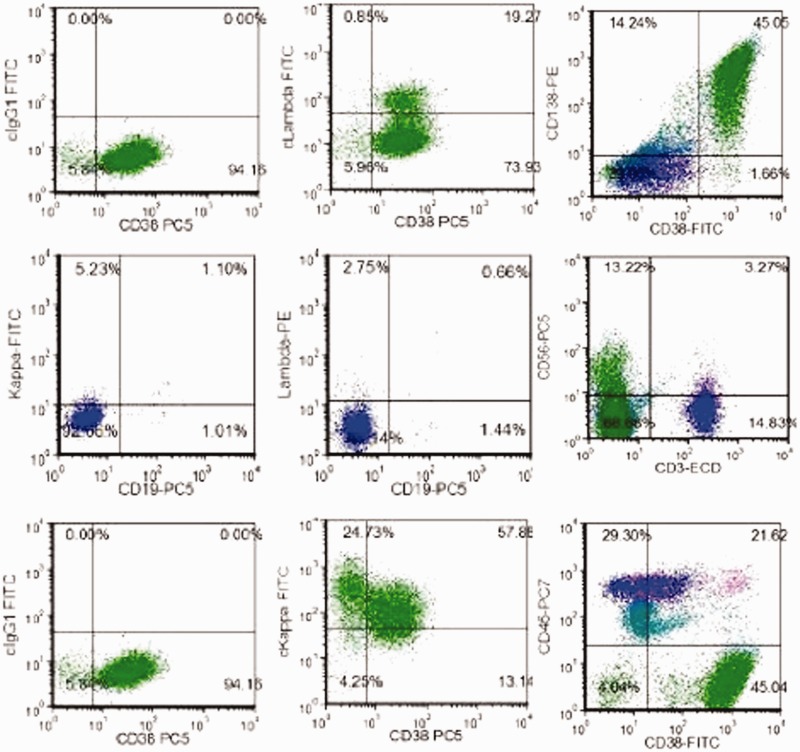
He had anemia, increased globulin level, and multiple rib fractures. We therefore considered the possibility of plasma cell disease and the patient underwent additional examinations. The results were as follows: immunoglobulin (Ig) A < 0.0667 g/L, IgG 53.9 g/L, IgM 0.16 g/L, peripheral blood β2-microglobulin 5.20 mg/L, and peripheral blood immunofixation electrophoresis suggested abnormal monoclonal bands in IgG and κ. A bone marrow smear (Figure 2a) showed decreased proliferation of granulocytes and erythroid cells. Mature red blood cells were arranged like strings of beads, and the proportion of plasma cells to nucleated cells was increased to 87.0%. Naive plasma cells were occasionally observed, with dense, blue-gray, cytoplasm with nuclear offset and thick, dense, dark purplish-red chromatin. Bone marrow biopsy showed clusters of plasma cells in the marrow puncture tissues (Figure 2b). Immunohistochemistry results showed CD3 (5%+), CD20 (3%+), CD117 (−), CD79α (2%+), CD33 (−), CD34 (−), CD43 (15%+), CD14 (5%+), CD61 (2%+), CD235α (10%+), LCA (8%+), MPO (20%+), HLA-DR (2%+), κ (+), λ (−), CD19 (−), CD56 (+), CD10 (+), CD38 (+) (Figure 2c), CD138 (+) (Figure 2d), CAM5.2 (−), and CK (−). Bone marrow flow cytometry (Figure 3) showed CD38 (++), CD138 (++), and small amounts of CD19 (+), CD56 (+), and CD20 (−). Igκ light chain restriction expression was observed in about 45.0% of the plasma cells, suggesting the presence of monoclonal plasma cells. The bone marrow karyotype was normal. The patient was diagnosed with MM (IgG - κ DS IIIA). He received PCD chemotherapy (bortezomib 2.0 g/day on days 1, 4, 8, and 11 plus cyclophosphamide 0.3 g/day on days 1 to 4 plus dexamethasone 20 mg/day on day 1 and 2, 4 and 5, 8 and 9, and 11 and 12) on 9 April 2018. The patients subsequently presented with bloody stools, decreased urine volume, and chest wall skin ecchymosis. His platelet count declined progressively to 11 × 109/L, prothrombin time and activated partial thromboplastin time were significantly prolonged, and his fibrinogen was significantly decreased. The patient died on 23 April 2018 of MM complicated by disseminated intravascular coagulation.
Figure 2.
(a) Bone marrow smear suggested occasional naive plasma cells with dense, blue-gray cytoplasm, nuclear offset, and thick, dense, dark purplish-red chromatin Wright-Giemsa stain, ×100 oil immersion). (b) Bone marrow biopsy revealed clusters of plasma cells in the marrow puncture tissues (hematoxylin–eosin, ×40). (c, d) Immunohistochemical expression of CD38 (+) and CD138 (+), respectively (×40).
Figure 3.
Bone marrow flow cytometry. CD38 (++), CD138 (++), small amounts of CD19 (+), CD56 (+), and CD20 (−), and Igκ light chain restriction expression in about 45.0% of plasma cells, suggesting monoclonal plasma cells. FITC, fluorescein isothiocyanate.
The study was approved by the Ethics Committee of Hangzhou Red Cross Hospital. The patient provided written informed consent for the procedures and his relatives provided consent for publication of this report.
Discussion
HCC ranks sixth in terms of cancer incidence and third in terms of cancer mortality worldwide.1 Its early diagnosis is therefore crucial to enable curative treatment. The diagnosis of HCC currently depends mainly on clinical symptoms, imaging evidence, laboratory indicators, and liver biopsy. The main clinical symptoms are pain, weight loss, and Budd–Chiari syndrome, while imaging evidence mainly includes ultrasonography, CT, MRI, and PET/CT. The typical vascular profile of HCC on dynamic imaging is characterized by early arterial phase enhancement followed by loss of enhancement in the portal venous phase and delayed phase compared with the surrounding liver.5
Laboratory indicators of HCC mainly include AFP, AFP-L3, GP73, and α-l-fucosidase, with serum AFP level being the most frequently used diagnostic marker for HCC. Numerous serologic markers may also be used, and the diagnostic accuracy for HCC may be improved when these markers are combined with serum AFP level.6 Liver biopsy should only be considered when the diagnostic imaging results are doubtful, for example, in patients with cirrhosis and hypovascular nodules.7
AFP and HBV DNA were significantly increased in the current patient, and both ultrasonography images and MRI showed characteristic manifestations of HCC. Some studies have reported a clear relationship between HBV infection and HCC.8 Moreover, a diagnosis of HCC can be established in high-risk patients with nodules ≥1 cm in diameter if one or two of the above imaging techniques show typical features of HCC.9 The clinical diagnosis of HCC in the current patients was therefore clear.
High globulin levels occur in patients with liver diseases, chronic inflammatory diseases, hematological disorders, infections, and malignancies.8 This parameter therefore facilitates the diagnosis of some disorders, particularly liver diseases.10–12 Moreover, globulin is also elevated in most patients with MM.13 The present patient had no anemia during the diagnosis of HCC; however, his serum AFP level was significantly increased and he had characteristic imaging features, and the diagnosis of HCC was therefore clear at that time. Unfortunately, no specimens were taken for pathological examination during the interventional treatment. However, anemia, increased globulin level, and multiple rib fractures subsequently occurred in our hospital, and the patient was diagnosed with MM after a detailed evaluation.
MM is a heterogeneous malignancy characterized by the accumulation of malignant plasma cells within the bone marrow. Common complications of MM include infections, anemia, renal failure, hypercalcemia, and osteolytic bony lesions. The median age of MM patients at diagnosis is 59 to 69 years.14 The global incidence of MM has increased in recent decades, partly because of population aging.15 MM is generally easily distinguished from HCC because these two tumors have their own unique clinical manifestations. However, liver plasma cell tumors may be difficult to distinguish from HCC, and raised AFP levels are not unique to HCC.16 A liver biopsy is required to confirm the diagnosis. We previously reported a patients with IgD-λ MM with significant AFP elevation and liver occupation, in whom a liver biopsy was conducted to establish a definite diagnosis of plasma cell tumor.17
This case highlights that, in the case of primary tumors with poor therapeutic response or worsening symptoms, clinicians should not simply consider recurrence or metastasis of the primary tumor, but should carefully evaluate the patient’s condition and perform puncture biopsy whenever possible, to identify new lesions and establish an accurate diagnosis.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iDs
Ye-Fei Shu https://orcid.org/0000-0002-1625-0421
Xiao-Feng Xu https://orcid.org/0000-0003-1590-681X
References
- 1.Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010; 127: 2893–2917. [DOI] [PubMed] [Google Scholar]
- 2.Asia–Pacific Working Party on Prevention of Hepatocellular Carcinoma . Prevention of hepatocellular carcinoma in the Asia-Pacific region: consensus statements. J Gastroenterol Hepatol 2010; 25: 657–663. [DOI] [PubMed] [Google Scholar]
- 3.Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014; 15: e538–e548. [DOI] [PubMed] [Google Scholar]
- 4.Rajkumar SV. Multiple myeloma: 2014 Update on diagnosis, risk-stratification, and management. Am J Hematol 2014; 89: 998–1009. [DOI] [PubMed] [Google Scholar]
- 5.Marrero JA, Hussain HK, Nghiem HV, et al. Improving the prediction of hepatocellular carcinoma in cirrhotic patients with an arterially-enhancing liver mass. Liver Transpl 2005; 11: 281–289. [DOI] [PubMed] [Google Scholar]
- 6.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bialecki ES, Ezenekwe AM, Brunt EM, et al. Comparison of liver biopsy and noninvasive methods for diagnosis of hepatocellular carcinoma. Clin Gastroenterol Hepatol 2006; 4: 361–368. [DOI] [PubMed] [Google Scholar]
- 8.Dispenzieri A, Gertz MA, Therneau TM, et al. Retrospective cohort study of 148 patients with polyclonal gammopathy. Mayo Clin Proc 2001; 76: 476–487. [DOI] [PubMed] [Google Scholar]
- 9.Korean Liver Cancer Study Group (KLCSG), National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol 2015; 16: 465–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamamoto K, Terada R, Okamoto R, et al. A scoring system for primary biliary cirrhosis and its application for variant forms of autoimmune liver disease. J Gastroenterol 2003; 38: 52–59. [DOI] [PubMed] [Google Scholar]
- 11.Mao MJ, Wei XL, Sheng H, et al. Clinical significance of preoperative albumin and globulin ratio in patients with gastric cancer undergoing treatment. Biomed Res Int 2017; 2017: 3083267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J, Chen S, Geng Q, et al. Prognostic value of pretreatment albumin-globulin ratio in predicting long-term mortality in gastric cancer patients who underwent D2 resection. Onco Targets Ther 2017; 10: 2155–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification and management. Am J Hematol 2016; 91: 719–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Wang L. Multiple myeloma: what do we do about immunodeficiency? J Cancer 2019; 10: 1675–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cowan AJ, Allen C, Barac A, et al. Global burden of multiple myeloma: a systematic analysis for the global burden of disease study 2016. JAMA Oncol 2018; 4: 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li MS, Li H, Li CY, et al. Cytoplasmic alpha-fetoprotein functions as a co-repressor in RA-RAR signaling to promote the growth of human hepatoma Bel 7402 cells. Cancer Lett 2009; 285: 190–199. [DOI] [PubMed] [Google Scholar]
- 17.Yang W, Zhang XJ, Wang HC, et al. IgD-λ multiple myeloma accompanying with elevated AFP level: a case report and literature review. Int J Clin Exp Med 2018; 11: 5176–5180. [Google Scholar]