Abstract
The Ageratum conyzoides L. (A. conyzoides) is commonly used as a traditional medicine, and its antitumor effects have also been studied. However, the functional roles of flavonoids in A. conyzoides in antitumor activities have not been clarified. The present study is aimed at investigating the biological effects of flavonoids in A. conyzoides on human cervical adenocarcinoma. Firstly, we detected that flavonoids in A. conyzoides significantly inhibited the proliferation, invasion, migration, and clonality of human cervical adenocarcinoma HeLa cells in vitro. Furthermore, we found that flavonoids in A. conyzoides induced significant S phase arrest and apoptosis and obviously decreased the intracellular reactive oxygen species (ROS) level in HeLa cells. Finally, we found that flavonoids in A. conyzoides significantly inhibited the HeLa xenograft tumor growth and epithelial-mesenchymal transition (EMT) in vivo. In conclusion, our results demonstrated the obvious antitumor effects of flavonoids in A. conyzoides on HeLa cells, suggesting that flavonoids in A. conyzoides could be provided as a novel therapeutic compound for human cervical adenocarcinoma.
1. Introduction
Cervical cancer is the fourth most common cancer in women worldwide [1, 2] and accounts for approximately 275,000 deaths and 528,000 diagnosed cases per year [3]. The main cause for cervical cancer is infection with the human papilloma virus (HPV), and the most common subtype of HPV in cervical cancer is HPV 16, followed by HPV 18, HPV 45, and HPV 31 [4, 5]. Current therapies for cervical cancer including surgery, chemotherapy, or radiotherapy are expensive, nonspecific, and low effective [6]. In addition, several unsatisfactory problems in tumor chemotherapy are the intrinsic or acquired drug resistance of tumor cells as well as high toxic side effects of chemotherapeutic drugs [7]. Hence, it is creditable to exploit more novel therapeutic compounds for cervical cancer treatment.
Nowadays, natural products are considered attractive candidates for new tumor therapies ascribed to their properties of chemical diversity, structural complexity, affordability, few toxic effects, and inherent biological activities [8]. Flavonoids are a large class of natural compounds that are widely found in the plant kingdom [9]. Flavonoids possess a diverse range of bioactivities, such as antioxidation, antihyperlipidemia, antifatigue, antiaging, and atherosclerosis-prevention activities [10]. Meanwhile, the antitumor effects of flavonoids have also been revealed by plentiful of studies [11–13].
A. conyzoides is an annual herb which belongs to the Asteraceae family and is generally used as a traditional medicine [14]. It has been reported that A. conyzoides exhibited considerable cytotoxic activity on human non-small-cell lung cancer and mouse leukemia cells [15]. To date, more than 21 flavonoids have been extracted from essential oil of A. conyzoides [16], and their functional roles in antitumor activities have not been clarified. In the current study, we demonstrated the significant inhibitory effects of flavonoids in A. conyzoides on human cervical adenocarcinoma HeLa cells in vivo and in vitro.
2. Materials and Methods
2.1. Cell Culture
The human cervical adenocarcinoma HeLa cells were purchased from the School of Life Sciences, Xiamen University. Cells were cultured in Dulbecco's modified Eagle medium (DMEM, HyClone, Logan, USA), supplemented with 10% fetal bovine serum (FBS, Gibco Life Technologies, NY, USA), and maintained at 37°C in a 95% O2 and 5% CO2 incubator.
2.2. MTT Cell Viability Assay
HeLa cells were seeded into a 96-well culture plate at a density of 500 cells/well and allowed to grow in DMEM supplemented with 10% FBS for 24 h before treatments. Thereafter, cells in three reduplicative wells were incubated with increasing concentrations (50, 100, 200, 300, 400, and 500 μg/mL) of flavonoids in A. conyzoides (purchased from the herbal garden of Zhangzhou Health Vocational College, Fujian, China) for 16 h. Cells incubated with sterile ddH2O were considered the control. For the MTT assay, medium in each well was carefully replaced by 150 μL fresh DMEM+10% FBS with diluted MTT (0.2 mg/mL, Amresco, USA) and incubated for 4 h at 37°C. Afterwards, the incubation medium was removed and formazan crystals were dissolved in 150 μL solution of DMSO in each well. The OD value of each well was quantified by recording the light absorbance at 630 nm using a microplate reader (Bio-Rad, Hercules, USA). The calculation equation of cell survival rate is as follows: cell survival rate (%) = ODflavonoids/ODcontrol × 100%.
2.3. Wound Healing Assay
HeLa cells were seeded into the 6-well culture plate and cultured in DMEM without serum until 95% confluence. A wound was then scratched into the cell monolayer using a sterile 10 μL pipette tip. After carefully removing the floating cells with sterile PBS, the remaining adherent cells were cultured at 37°C for 48 h in FBS-free DMEM dissolved with various concentrations of flavonoids in A. conyzoides (50, 200, and 400 μg/mL) or sterile ddH2O (control). The wound areas were photographed at the 0 h and 48 h time points under an inverted optical microscope-camera system (CK40, Olympus, Tokyo, Japan). The experiments were conducted in triplicate.
2.4. Transwell Assay
The 24-well transwell chambers with 8 μm pores (Millipore, Billerica, USA) were used for transwell assays. HeLa cells were seeded into the upper chambers at a density of 1 × 105 and incubated with FBS-free DMEM dissolved with various concentrations of flavonoids in A. conyzoides (50, 200, and 400 μg/mL) or sterile ddH2O (control). The lower chambers contained the DMEM supplemented with 10% FBS. After incubation for 48 h, cells on the internal surface of the upper chambers were washed with PBS, fixed with 4% paraformaldehyde for 20 min, stained with 0.1% crystal violet (Beyotime, Shanghai, China) for 20 min, and then rinsed with PBS for three times. Finally, three random views for each chamber were captured using an inverted optical microscope-camera system (Olympus), and the number of migration cells in each view was counted. Three independent experiments were performed.
2.5. Clonogenic Assay
HeLa cells were plated into culture dishes at a density of 1000 cells/dish and cultured in DMEM supplemented with 10% FBS overnight. Subsequently, various doses of flavonoids in A. conyzoides (50, 200, and 400 μg/mL) or sterile ddH2O (control) were added into the culture dishes, respectively. The culture medium was replaced every 7 days. After culturing for 14 days, the colonies were fixed with 4% formaldehyde for 20 min, stained with 0.1% crystal violet for 20 min, and then washed with PBS thrice. Finally, the number of colonies was counted. The experiments were performed in triplicate.
2.6. Flow Cytometric Analysis
HeLa cells were cultured in full medium dissolved with various doses of flavonoids in A. conyzoides (50, 100, 200, 300, and 400 μg/mL) or sterile ddH2O (control) for 24 h. For cell cycle analysis, HeLa cells were then collected and fixed with 70% ethanol overnight at 4°C, washed with PBS, and incubated with propidium iodide (PI, Sangon Biotech Co., Shanghai, China) for 10 min. For apoptosis analysis, HeLa cells were harvested and incubated with Annexin-V-fluorescein isothiocyanate (FITC)/PI (Sangon Biotech Co.) at room temperature in the dark for 15 min. Analyses were performed with a flow cytometer (Beckman Coulter, Miami, USA). Each experiment was repeated three times.
2.7. Detection of Intracellular Reactive Oxygen Species (ROS)
HeLa cells were seeded into 6-well plates and treated with various doses of flavonoids in A. conyzoides (50, 100, 200, 300, and 400 μg/mL) or sterile ddH2O (control) for 24 h. Cells were then harvested, washed with PBS, and centrifuged at 1000 rpm for 5 min. After abandoning the supernatant, cells were resuspended and incubated with 2′,7′-dichlorofluorescin diacetate solution (10 μM, Sigma, St Louis, USA) at 37°C for 30 min. Subsequently, cells were washed with PBS and acquired by a flow cytometer (Beckman Coulter) to detect intracellular ROS. The experiments were conducted in triplicate.
2.8. Acridine Orange/Ethidium Bromide (AO/EB) Staining
HeLa cells were plated onto the sterile glass coverslips and cultured in DMEM supplemented with 10% FBS. After culturing for 24 h, cells were treated with various doses of flavonoids in A. conyzoides (50, 200, and 400 μg/mL) or sterile ddH2O (control) for 24 h. Cells were then washed with PBS and stained with 100 μg/mL AO and 100 μg/mL EB (Amresco) in the dark. Finally, cells were viewed and photographed using a fluorescent microscope (Olympus). Cells with bright green fluorescence and orange-red fluorescence in the pyknotic nuclei were considered the early and late apoptotic cells, respectively. The experiments were performed in triplicate.
2.9. Xenograft Tumor Assay
All animal experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Fujian Agriculture and Forestry University. Four-week-old female nude mice were purchased from Vital River Laboratory Animal Technology Co. (Beijing, China) and randomly divided into the control and experimental groups (4 mice per group). HeLa cells (1 × 106) were subcutaneously injected into the back of nude mice. After injection for one week, nude mice were daily received intraperitoneal injections of flavonoids in A. conyzoides (400 mg/kg body weight) or sterile ddH2O (control). All nude mice were sacrificed by overdose of urethane at the 4th week, and the tumor weights were measured.
2.10. Immunohistochemical (IHC) Staining
The xenograft tumors were fixed with 4% formaldehyde, embedded in paraffin, and cut into 4 μm sections. After incubating with EDTA antigen retrieval solution (Beyotime) at 95°C for 15 min, the slides were incubated with endogenous peroxidase blocking solution (Beyotime) at room temperature for 10 min and then were incubated at 4°C overnight with the primary antibodies: anti-E-cadherin, anti-N-cadherin, and anti-vimentin (ab76055, ab76011, and ab92547; Abcam, Cambridge, UK). Thereafter, the slides were incubated with the corresponding secondary antibody (Beyotime) for 30 min at 37°C and orderly stained with diaminobenzidine (Beyotime) and hematoxylin. Finally, the slides were visualized and photographed under an inverted optical microscope-camera system (Olympus).
2.11. Statistical Analysis
Data are presented as the mean ± SD. Statistical analysis was performed with SPSS version 19.0 (SPSS Inc., Chicago, USA). Significant difference was analyzed using one-way analysis of variance (ANOVA), and p < 0.05 was considered statistically significant.
3. Results
3.1. Flavonoids in A. conyzoides Inhibited the Proliferation, Invasion, Migration, and Clonality of HeLa Cells In Vitro
Using the MTT assay, we found that flavonoids significantly suppressed the cell survival rate of HeLa cells in a concentration-dependent manner, and the calculated IC50 value was 334.171 μg/mL (Figure 1(a)). Next, we detected that the medium (200 μg/mL) and high (400 μg/mL) concentrations of flavonoids obviously reduced the invasion ability of HeLa cells compared to the control (0 μg/mL) and low concentration (50 μg/mL) in the wound healing assay (Figure 1(b)). Furthermore, upon the administration of increasing doses of flavonoids (50, 200, and 400 μg/mL), we found that the number of migration cells and clones was significantly decreased in the transwell and clonogenic assays of HeLa cells, and flavonoids at the concentration of 400 μg/mL possessed the most prominent inhibitory effects (Figures 1(c)–1(f)). Taken together, we suggested that flavonoids in A. conyzoides significantly inhibited the proliferation, invasion, migration, and clonality of HeLa cells.
Figure 1.
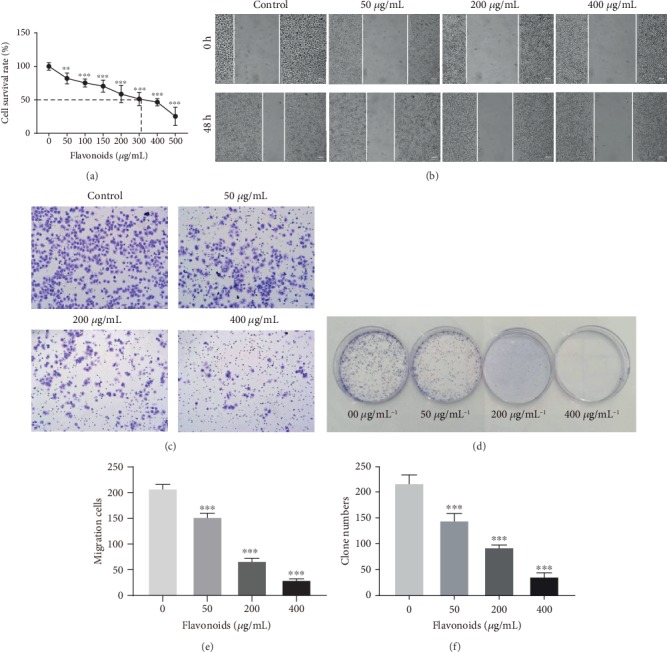
Flavonoids in A. conyzoides inhibited the proliferation, invasion, migration, and clonality of HeLa cells in vitro. (a) Effects of gradient concentrations of flavonoids (50-500 μg/mL) on the cell survival rate of HeLa cells were tested using the MTT assay (∗∗P < 0.01 and ∗∗∗P < 0.001, compared to control). After treatments with increasing doses of flavonoids (50, 200, and 400 μg/mL) for 48 h, the invasion ability of HeLa cells was tested by the wound healing assay (b), and the migration ability (c, e) and clonality (d, f) of HeLa cells were investigated using the transwell and clonogenic assays, respectively (∗∗∗P < 0.001, compared to control).
3.2. Flavonoids in A. conyzoides Induced Significant S Phase Arrest and Apoptosis in HeLa Cells
After being treated with various doses of flavonoids (0, 50, 100, 200, 300, and 400 μg/mL), the cell cycle of HeLa cells was tested using a flow cytometer (Figure 2(a)). We found that flavonoids at low concentrations (50 and 100 μg/mL) had no significant impacts on the cell cycle of HeLa cells. While we detected that flavonoids at concentrations of 200, 300, and 400 μg/mL mildly reduced the proportion of cells in G1 phase and significantly increased and decreased the proportion of cells in S phase and G2/M phase, respectively (Figure 2(b)). These data indicated that flavonoids in A. conyzoides could induce significant S phase arrest in HeLa cells.
Figure 2.
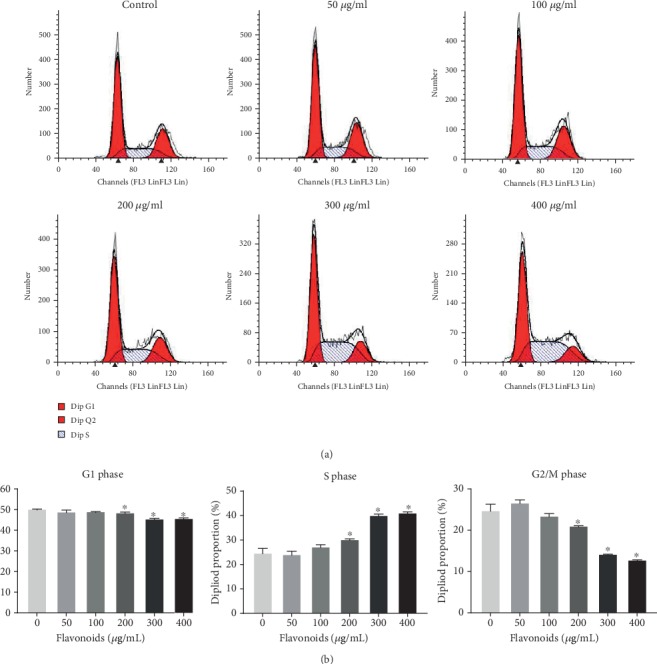
Flavonoids in A. conyzoides induced significant S phase arrest in HeLa cells. (a) Cell cycle analyses in HeLa cells upon the administration of increasing doses of flavonoids (50, 100, 200, 300, and 400 μg/mL) using a flow cytometer. (b) Statistical analyses of the proportion of HeLa cells during G1, S, and G2/M phases, respectively (∗P < 0.05, compared to control).
We further tested that whether flavonoids in A. conyzoides could induce apoptosis in HeLa cells. Under the light microscope, we found that HeLa cells without flavonoid treatment were spindle-shaped with a plump body, and there were a small number of round cells scattered in a high-power field (HPF). The low concentration (50 μg/mL) of flavonoids showed no obvious influences on the morphology of HeLa cells. The medium concentration (200 μg/mL) of flavonoids obviously increased the amount of round cells in a HPF and changed the morphology of some HeLa cells to irregular polygons. After treatment with flavonoids at a concentration of 400 μg/mL, almost all HeLa cells became round cells, and the cell density was dramatically decreased in a HPF (Figure 3(a)). Furthermore, we found that HeLa cells without or with low concentration (50 μg/mL) of flavonoid treatment emitted light green fluorescence in the homogeneous nuclei after AO/EB staining. After treatment with flavonoids at a concentration of 200 μg/mL, the amount of HeLa cells in a HPF that displayed bright green or orange-red fluorescence in the pyknotic nuclei was obviously increased. After treatment with flavonoids at a concentration of 400 μg/mL, the large majority of HeLa cells in a HPF emitted orange-red in the pyknotic nuclei (Figure 3(b)). These results indicated that the number of apoptotic HeLa cells was significantly increased by flavonoids. Finally, we confirmed that the proportion of apoptotic HeLa cells was significantly elevated by increasing doses of flavonoids (50, 200, and 400 μg/mL) using the flow cytometric analysis. Meanwhile, we found that flavonoids (200, 400 μg/mL) mainly increased the proportion of late apoptotic HeLa cells compared to the control (Figure 3(c)).
Figure 3.
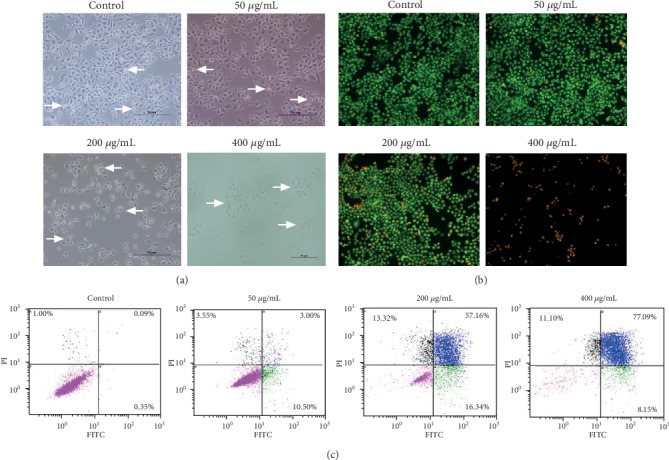
Flavonoids in A. conyzoides induced significant apoptosis in HeLa cells. (a) Influences of increasing doses of flavonoids (50, 200, and 400 μg/mL) on the common morphology and density of HeLa cells were observed under the light microscope. White arrows point to the round cells (apoptosis cells). (b) Apoptosis in HeLa cells after treatments with increasing doses of flavonoids (50, 200, and 400 μg/mL) was detected by AO/EB staining. Cells with light green fluorescence in the homogeneous nuclei were considered the survival cells. Cells with bright green fluorescence and orange-red fluorescence in the pyknotic nuclei were considered the early and late apoptotic cells, respectively. (c) Apoptotic analyses in HeLa cells after treatments with increasing doses of flavonoids (50, 200, and 400 μg/mL) using a flow cytometry (black spots: necrotic cells, blue spots: late apoptotic cells, green spots: early apoptotic cells, and pink spots: survival cells).
3.3. Flavonoids in A. conyzoides Obviously Reduced the Intracellular ROS Level in HeLa Cells
We further detected the influences of flavonoids in A. conyzoides on the intracellular ROS level in HeLa cells using a flow cytometer. We found that all the ROS peaks in HeLa cells after treatments with various doses of flavonoids (50, 100, 200, 300, and 400 μg/mL) were shifted to the left compared to that of the control group (Figure 4), indicating that the intracellular ROS level in HeLa cells was reduced by flavonoids in A. conyzoides.
Figure 4.
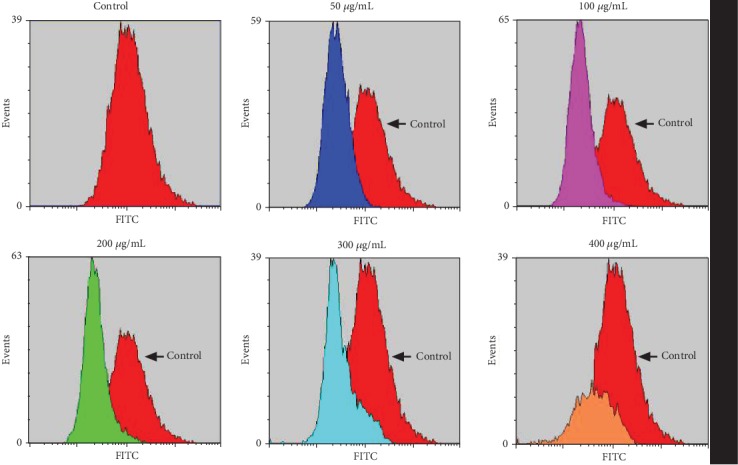
Flavonoids in A. conyzoides obviously reduced the intracellular ROS level in HeLa cells. Intracellular ROS levels in HeLa cells upon the administration of increasing doses of flavonoids (50, 100, 200, 300, and 400 μg/mL) were detected using a flow cytometer. Black arrows point the ROS peak of the control group.
3.4. Flavonoids in A. conyzoides Significantly Suppressed the HeLa Xenograft Tumor Growth and EMT In Vivo
In xenograft tumor assay, we found that no death occurred in all the experimental nude mice with daily injection of flavonoids in A. conyzoides (400 mg/kg) for three weeks. We detected that flavonoids (400 mg/kg) significantly suppressed the growth of HeLa cells compared to the control (Figure 5(a)). The weight of xenograft tumors treated by 400 mg/kg flavonoids was significantly higher than that of the control group (Figure 5(b)). Moreover, using the IHC staining, we found that flavonoids (400 mg/kg) prominently enhanced the E-cadherin expression and reduced the N-cadherin and vimentin expressions in the xenograft tumors (Figure 5(c)), indicating that flavonoids in A. conyzoides could inhibit the EMT in vivo.
Figure 5.
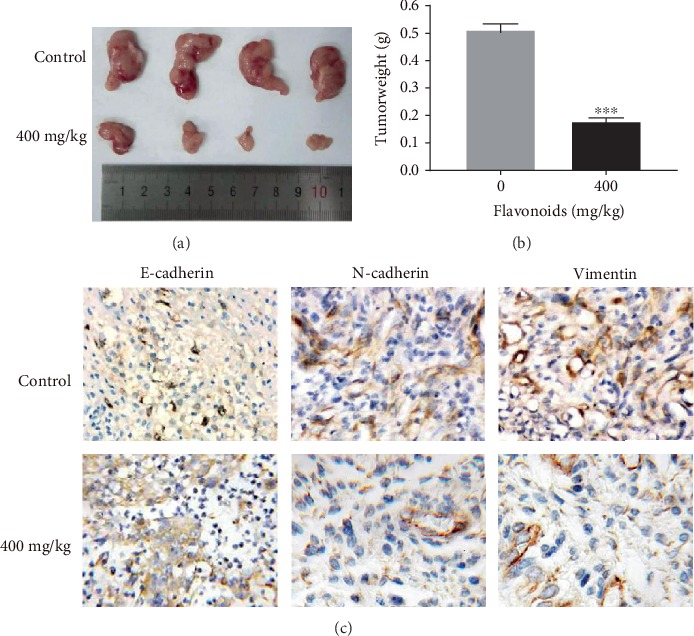
Flavonoids in A. conyzoides significantly suppressed the HeLa xenograft tumor growth and EMT in vivo. (a, b) Effects of flavonoids (400 mg/kg) on the volume and weight of HeLa xenograft tumor (∗∗∗P < 0.001, compared to control (sterile ddH2O)). (c) Expressions of E-cadherin (epithelial marker), N-cadherin, and vimentin (mesenchymal markers) in the xenograft tumors were detected using IHC staining.
4. Discussion
A previous study has reported that essential oils of A. conyzoides could significantly inhibit the proliferation of prostate cancer cell lines (LNCaP and PC-3) and glioblastoma cell lines (SF-763 and SF-767) [17]. Another research has also proven that several flavonoids isolated from the Asteraceae family of plants showed considerable suppressive effects on the growth of rat glial tumor cells (C6) [18]. Consistently, our current study demonstrated that flavonoids in A. conyzoides significantly inhibited the growth of human cervical adenocarcinoma HeLa cells in vitro and in vivo, and the calculated IC50 value was 334.171 μg/mL. It has been reported that chronic treatment with the ethanolic leaf extract of A. conyzoides at 200, 400, and 600 mg/kg body weight did not significantly change the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) expression levels in serum and liver of all experimental animals [19]. In our study, we also found that daily injection of flavonoids in A. conyzoides at 400 mg/kg body weight for three weeks did not cause death in all the experimental nude mice. Taken together, we suggest that flavonoids in A. conyzoides could be served as a novel and safe therapeutic compound for human cervical adenocarcinoma.
To our knowledge, the cell cycle is divided into four phases: G1, G2, S, and M; and each phase is found to possess its own biological function [20]. Numerous studies have clarified that flavonoids could prominently affect the cell cycle in various cancer cells. For instance, it has been reported that various kinds of flavonoids could induce G0/G1 phase or G2/M phase arrest in breast cancer cells [21]. Zhang et al. have proven that flavonoids extracted from Chinese bayberry leaves could significantly induce G1 cell cycle arrest in ovarian cancer cells [22]. Nagappan et al. have reported that flavonoids isolated from Citrus platymamma induced G2/M cell cycle arrest in A549 human lung cancer cells [23]. In our present study, we found that flavonoids in A. conyzoides induced significant S phase arrest in HeLa cells. These findings indicate that various kinds of flavonoids could exert their antiproliferation effects on diverse cancer cells via inducing different cell cycle arrests.
A number of studies have revealed that flavonoids could induce apoptosis in various cancer cells [24–26]. In the present study, we also detected obvious apoptosis in HeLa cells upon the administration of flavonoids in A. conyzoides. We think that the round cells increased by flavonoids in A. conyzoides in a HPF under the light microscope were apoptotic cells. To confirm this, we performed AO/EB staining, in which we found that the medium concentration of flavonoids in A. conyzoides slightly increased the number of early apoptotic HeLa cells (bright green fluorescence), and the medium and high concentrations of flavonoids in A. conyzoides both remarkably increased the amount of late apoptotic HeLa cells (orange-red fluorescence). Finally, using the flow cytometric analysis, we further confirmed that the medium and high concentrations of flavonoids significantly increased the percentage of late apoptotic HeLa cells compared to the control. These results indicate that flavonoids in A. conyzoides mainly induce late apoptosis in HeLa cells.
Moreover, several studies have reported that flavonoids could induce apoptosis in the bladder and hepatocellular cancer cells via enhancing the intracellular ROS level [27, 28]. On the contrary, we tested that the intracellular ROS levels in HeLa cells were obviously reduced after treatment with increasing doses of flavonoids in A. conyzoides in our study. This is consistent with a previous report that dihydromyricetin, a kind of flavonoids extracted from Ampelopsis grossedentata, could reduce ROS accumulation in human hepatoma HepG2 cells in a concentration-dependent manner [29]. Fan et al. have also reported that luteoloside, a flavone subclass of flavonoids, could significantly decrease the intracellular ROS level in human hepatoma Huh-7 and SMMC-7721 cells [30]. Based on the above findings, we suggest that flavonoids in A. conyzoides could induce apoptosis in HeLa cells through a ROS-independent mechanism.
EMT is a biological process during which epithelial cells lose their epithelial-like phenotypes and acquire the mesenchymal-like phenotypes, which include enhanced migratory potential and invasiveness [31]. It is typically characterized by the coordinated gain and loss of the mesenchymal (e.g., N-cadherin and vimentin) and epithelial (e.g., E-cadherin) markers, respectively [32]. Fan et al. have showed that two dietary flavonoids luteolin and quercetin could inhibit the EMT in squamous carcinoma cells [33]. Chen et al. have also reported that isoliquiritigenin, one of the flavonoids in licorice, significantly inhibited ovarian cancer metastasis through the suppression of EMT [34]. Consistently, in the current study, we found that flavonoids in A. conyzoides obviously increased the E-cadherin expression and decreased the N-cadherin and vimentin expressions in the xenograft tumors, which indicated the inhibition of EMT. Hence, we suggest that flavonoids in A. conyzoides could significantly inhibit the invasion and migration of HeLa cells via suppressing the EMT. Ascribed to the cells during which EMT could gain the resistance to apoptosis [31], we speculate that the EMT inhibition may be also involved in the apoptosis process of HeLa cells induced by flavonoids in A. conyzoides.
A limitation of the present study is that we only preliminarily investigated the antitumor effects of flavonoids in A. conyzoides on the human cervical adenocarcinoma HeLa cells, while cervical squamous cell carcinoma is the most common histological type of cervical cancer worldwide [35]. Therefore, the impacts of flavonoids in A. conyzoides on other human cervical squamous cell carcinoma cell lines should be also clarified to further confirm their antitumor effects on the human cervical squamous cell carcinoma. Furthermore, the possible mechanism underlying the flavonoids in A. conyzoides inducing apoptosis in HeLa cells should also be explored in the further researches.
5. Conclusions
In conclusion, the present study demonstrated that flavonoids in A. conyzoides significantly inhibited the growth of HeLa cells in vitro and in vivo via inducing S cell cycle arrest and apoptosis. Moreover, we detected that flavonoids in A. conyzoides obviously decreased the intracellular ROS level in HeLa cells. Finally, we clarified that flavonoids in A. conyzoides significantly inhibited the invasion and migration of HeLa cells probably through suppressing the EMT. Our findings indicate that flavonoids in A. conyzoides could be provided as a novel therapeutic compound for human cervical adenocarcinoma.
Acknowledgments
This study was funded by Fujian Science and Technology Special Project (2017NZ0003-1-6).
Abbreviations
- A. conyzoides:
Ageratum conyzoides L.
- AO/EB:
Acridine orange/ethidium bromide
- ALT:
Alanine aminotransferase
- AST:
Aspartate aminotransferase
- DMEM:
Dulbecco's modified Eagle medium
- EMT:
Epithelial-mesenchymal transition
- FBS:
Fetal bovine serum
- IHC:
Immunohistochemical
- PI:
Propidium iodide
- ROS:
Reactive oxygen species.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
Authors' Contributions
Z.L. and Y.H. conceived and designed the experiments; Y.L., J.S., and M.J. performed the experiments and analyzed the data; Z.L. and Y.H. wrote and revised the paper.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R. L., Torre L. A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a Cancer Journal for Clinicians. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Koh W. J., Greer B. E., Abu-Rustum N. R., et al. Uterine sarcoma, version 1.2016: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network. 2015;13(11):1321–1331. doi: 10.6004/jnccn.2015.0162. [DOI] [PubMed] [Google Scholar]
- 3.Ghasemi F., Shafiee M., Banikazemi Z., et al. Curcumin inhibits NF-kB and Wnt/β-catenin pathways in cervical cancer cells. Pathology, Research and Practice. 2019;215(10, article 152556) doi: 10.1016/j.prp.2019.152556. [DOI] [PubMed] [Google Scholar]
- 4.Sadri Nahand J., Moghoofei M., Salmaninejad A., et al. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: a review. International Journal of Cancer. 2019;146(2):305–320. doi: 10.1002/ijc.32688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nahand J. S., Taghizadeh-Boroujeni S., Karimzadeh M., et al. MicroRNAs: new prognostic, diagnostic, and therapeutic biomarkers in cervical cancer. Journal of Cellular Physiology. 2019;234(10):17064–17099. doi: 10.1002/jcp.28457. [DOI] [PubMed] [Google Scholar]
- 6.Shafabakhsh R., Reiter R. J., Mirzaei H., Teymoordash S. N., Asemi Z. Melatonin: a new inhibitor agent for cervical cancer treatment. Journal of Cellular Physiology. 2019;234(12):21670–21682. doi: 10.1002/jcp.28865. [DOI] [PubMed] [Google Scholar]
- 7.Efferth T., Kahl S., Paulus K., et al. Phytochemistry and pharmacogenomics of natural products derived from traditional Chinese medicine and Chinese materia medica with activity against tumor cells. Molecular Cancer Therapeutics. 2008;7(1):152–161. doi: 10.1158/1535-7163.MCT-07-0073. [DOI] [PubMed] [Google Scholar]
- 8.George V. C., Dellaire G., Rupasinghe H. P. V. Plant flavonoids in cancer chemoprevention: role in genome stability. The Journal of Nutritional Biochemistry. 2017;45:1–14. doi: 10.1016/j.jnutbio.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Cushnie T. P., Lamb A. J. Antimicrobial activity of flavonoids. International Journal of Antimicrobial Agents. 2005;26(5):343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui H., Lu T., Wang M., et al. Flavonoids from Morus alba L. leaves: optimization of extraction by response surface methodology and comprehensive evaluation of their antioxidant, antimicrobial, and inhibition of α-Amylase activities through analytical hierarchy process. Molecules. 2019;24(13):p. 2398. doi: 10.3390/molecules24132398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y. C., Shen S. C., Chow J. M., Ko C. H., Tseng S. W. Flavone inhibition of tumor growth via apoptosis in vitro and in vivo. International Journal of Oncology. 2004;25(3):661–670. [PubMed] [Google Scholar]
- 12.Shankar E., Goel A., Gupta K., Gupta S. Plant flavone apigenin: an emerging anticancer agent. Current Pharmacology Reports. 2017;3(6):423–446. doi: 10.1007/s40495-017-0113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh H., Mishra A., Mishra A. K. The chemistry and pharmacology of Cleome genus: a review. Biomedicine & Pharmacotherapy. 2018;101:37–48. doi: 10.1016/j.biopha.2018.02.053. [DOI] [PubMed] [Google Scholar]
- 14.Diallo A., Eklu-Gadegbeku K., Amegbor K., et al. In vivo and in vitro toxicological evaluation of the hydroalcoholic leaf extract of Ageratum conyzoides L. (Asteraceae) Journal of Ethnopharmacology. 2014;155(2):1214–1218. doi: 10.1016/j.jep.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Adebayo A. H., Tan N. H., Akindahunsi A. A., Zeng G. Z., Zhang Y. M. Anticancer and antiradical scavenging activity of Ageratum conyzoides L. (Asteraceae) Pharmacognosy Magazine. 2010;6(21):62–66. doi: 10.4103/0973-1296.59968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yadav N., Ganie S. A., Singh B., Chhillar A. K., Yadav S. S. Phytochemical constituents and ethnopharmacological properties of Ageratum conyzoides L. Phytotherapy Research. 2019;33(9):2163–2178. doi: 10.1002/ptr.6405. [DOI] [PubMed] [Google Scholar]
- 17.Bayala B., Bassole I. H. N., Gnoula C., et al. Chemical composition, antioxidant, anti-inflammatory and anti-proliferative activities of essential oils of plants from Burkina Faso. PLoS One. 2014;9(3, article e92122) doi: 10.1371/journal.pone.0092122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sánchez I., Calderón J., Ruiz B., Tellez J., Calzada L., Taboada J. In vitro cytotoxicity of flavonoids against MK2 and C6 tumour cells. Phytotherapy Research. 2001;15(4):290–293. doi: 10.1002/ptr.954. [DOI] [PubMed] [Google Scholar]
- 19.Antai A. B., Eyong E. U., Eteng M. U., Itam E. H., Eko M. E., Ita S. O. Serum protein and enzyme levels in rats following administration of ethanolic leaf extract of Ageratum conyzoides (goat weed) Nigerian Journal of Physiological Sciences. 2009;24(2):117–120. doi: 10.4314/njps.v24i2.52900. [DOI] [PubMed] [Google Scholar]
- 20.Venuto S., Merla G. E3 ubiquitin ligase TRIM proteins, cell cycle and mitosis. Cells. 2019;8(5):p. 510. doi: 10.3390/cells8050510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabała-Dzik A., Rzepecka-Stojko A., Kubina R., et al. Flavonoids, bioactive components of propolis, exhibit cytotoxic activity and induce cell cycle arrest and apoptosis in human breast cancer cells MDA-MB-231 and MCF-7 - a comparative study. Cellular and Molecular Biology. 2018;64(8) doi: 10.14715/cmb/2018.64.8.1. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y., Chen S., Wei C., Rankin G. O., Ye X., Chen Y. C. Flavonoids from Chinese bayberry leaves induced apoptosis and G1 cell cycle arrest via Erk pathway in ovarian cancer cells. European Journal of Medicinal Chemistry. 2018;147:218–226. doi: 10.1016/j.ejmech.2018.01.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagappan A., Lee H. J., Saralamma V. V., et al. Flavonoids isolated from Citrus platymamma induced G2/M cell cycle arrest and apoptosis in A549 human lung cancer cells. Oncology Letters. 2016;12(2):1394–1402. doi: 10.3892/ol.2016.4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maués L. A. L., Alves G. M., Couto N. M. G., et al. Flavonoids from the Amazon plant Brosimum acutifolium induce C6 glioma cell line apoptosis by disrupting mitochondrial membrane potential and reducing AKT phosphorylation. Biomedicine & Pharmacotherapy. 2019;113, article 108728 doi: 10.1016/j.biopha.2019.108728. [DOI] [PubMed] [Google Scholar]
- 25.Yan W., Yang J., Tang H., et al. Flavonoids from the stems of Millettia pachyloba drake mediate cytotoxic activity through apoptosis and autophagy in cancer cells. Journal of Advanced Research. 2019;20:117–127. doi: 10.1016/j.jare.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dai T. Y., Wang B., Lin S. Y., Jiang J. P., Wu L. Q., Qian W. B. Pure total flavonoids from Citrus paradisi Macfad induce leukemia cell apoptosis in vitro. Chinese Journal of Integrative Medicine. 2017;23(5):370–375. doi: 10.1007/s11655-016-2593-z. [DOI] [PubMed] [Google Scholar]
- 27.Hong S. H., Cha H. J., Hwang-Bo H., et al. Anti-proliferative and pro-apoptotic effects of licochalcone A through ROS-mediated cell cycle arrest and apoptosis in human bladder cancer cells. International Journal of Molecular Sciences. 2019;20(15, article 3820) doi: 10.3390/ijms20153820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ji X., Wei X., Qian J., et al. 2′,4′-Dihydroxy-6′-methoxy-3′,5′-dimethylchalcone induced apoptosis and G1 cell cycle arrest through PI3K/AKT pathway in BEL-7402/5-FU cells. Food and Chemical Toxicology. 2019;131, article 110533 doi: 10.1016/j.fct.2019.05.041. [DOI] [PubMed] [Google Scholar]
- 29.Liu B., Tan X., Liang J., et al. A reduction in reactive oxygen species contributes to dihydromyricetin- induced apoptosis in human hepatocellular carcinoma cells. Scientific Reports. 2015;4(1, article 7041) doi: 10.1038/srep07041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fan S. H., Wang Y. Y., Lu J., et al. Luteoloside suppresses proliferation and metastasis of hepatocellular carcinoma cells by inhibition of NLRP3 inflammasome. PLoS One. 2014;9(2, article e89961) doi: 10.1371/journal.pone.0089961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou D., Kannappan V., Chen X., et al. RBP2 induces stem-like cancer cells by promoting EMT and is a prognostic marker for renal cell carcinoma. Experimental & Molecular Medicine. 2016;48(6, article e238) doi: 10.1038/emm.2016.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y., Wang B. E., Leong K. G., et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Research. 2012;72(2):527–536. doi: 10.1158/0008-5472.CAN-11-3004. [DOI] [PubMed] [Google Scholar]
- 33.Fan J. J., Hsu W. H., Lee K. H., et al. Dietary flavonoids luteolin and quercetin inhibit migration and invasion of squamous carcinoma through reduction of Src/Stat3/S100A7 signaling. Antioxidants. 2019;8(11):p. 557. doi: 10.3390/antiox8110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen C., Huang S., Chen C. L., Su S. B., Fang D. D. Isoliquiritigenin inhibits ovarian cancer metastasis by reversing epithelial-to-mesenchymal transition. Molecules. 2019;24(20, article 3725) doi: 10.3390/molecules24203725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahasrabudhe N. M., van der Horst J. C., Spaans V., et al. MGL ligand expression is correlated to lower survival and distant metastasis in cervical squamous cell and adenosquamous carcinoma. Frontiers in Oncology. 2019;9:p. 29. doi: 10.3389/fonc.2019.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.


