Abstract
Background:
Achilles tendinopathy is a debilitating overuse injury characterized by pain, altered Achilles tendon structure, and impaired functional performance. Evaluating tendon structure as part of the physical examination may help establish a well-defined prognosis. However, the usefulness of measuring tendon structure for developing a prognosis has been questioned since structural abnormalities can exist without symptoms.
Purpose:
To determine whether initial measures of tendon morphology and mechanical properties were associated with patient-reported symptoms and calf muscle endurance at baseline, 6-month follow-up, and 1-year follow-up by prospectively following a cohort of individuals with Achilles tendinopathy.
Study Design:
Cohort study; Level of evidence, 2.
Methods:
A total of 59 participants with midportion or insertional Achilles tendinopathy completed an initial assessment and follow-up assessments at 6 months and 1 year. At the initial assessment, patient-reported symptoms, calf muscle endurance, and Achilles tendon thickening were evaluated, and Achilles tendon mechanical properties were estimated. At the 6-month and 1-year follow-up assessments, patient-reported symptoms and calf muscle endurance were reevaluated.
Results:
Greater Achilles tendon thickening at the initial assessment was consistently associated with worse patient-reported symptoms and calf muscle endurance at each assessment. Changes in symptoms over the year were moderated by the initial shear modulus of the tendon, with a lower shear modulus associated with less improvement in symptoms. Lower viscosity at the initial assessment was also associated with worse calf muscle endurance at each assessment.
Conclusion:
Measures of tendon morphology and mechanical properties appear to be associated with patient-reported symptoms and calf muscle function for patients with Achilles tendinopathy.
Keywords: tendinitis, tendinosis, ultrasound, elastography, function
Achilles tendinopathy is a debilitating overuse injury characterized by pain, impaired functional performance, and altered Achilles tendon morphology.24,40 A clinical diagnosis of Achilles tendinopathy is primarily based on patient history and physical examination. Yet, ultrasonography and magnetic resonance imaging are commonly used to confirm the diagnosis and measure morphologic features of the tendon (eg, thickness, cross-sectional area). The clinical usefulness of these morphologic measurements has been questioned because structural abnormalities can exist without symptoms.12,13,32 However, abnormal Achilles tendon structure increases the likelihood of developing symptoms.16,19,20,26 To better understand whether tendon structure is important for patient recovery, there is a need to evaluate the associations of tendon morphologic features with patient outcomes.
Although traditional measures of tendon morphology reflect the size and/or shape of the tendon, they do not necessarily represent the tendon’s ability to absorb and transfer loads during functional activities. Tendon mechanical properties reflect the structural integrity of the tendon and have become of interest to supplement measures of tendon morphology. In the context of Achilles tendinopathy, research has focused on developing techniques for estimating mechanical properties, comparing properties of healthy and tendinopathic tendons,3,6 and determining whether mechanical properties can assist with diagnosis.4,14,28 We have developed a valid and reliable technique known as continuous shear wave elastography (cSWE) that was originally described by Cortes et al10 and then further expanded upon by Corrigan et al.9 With this technique, shear waves are applied to the tendon through use of an external device and measured via high-frame-rate ultrasonography. The mechanical properties estimated with cSWE are shear modulus (resistance to a shearing force) and viscosity (rate-dependent resistance to a shearing force), which have intraclass correlation coefficients of 0.70 and 0.86, respectively, and are strongly correlated with Young’s modulus.9 Furthermore, cSWE has been used in several clinical research studies to estimate mechanical properties of healthy,39 tendinopathic,8,10 and ruptured Achilles tendons.42–44 Yet, it remains unclear whether these properties are associated with patient symptoms and function. Thus, a better understanding of Achilles tendon injury and recovery may be gained by exploring associations between tendon mechanical properties and patient outcomes.
It is currently recommended that patients with Achilles tendinopathy be treated with exercise therapy for at least 3 months before other treatments are considered.2,21 This recommendation is made regardless of symptom severity, abnormalities in tendon morphologic features, functional status, or patient characteristics (eg, age, body mass index, activity level). Although diagnostic imaging may help guide treatment decisions, findings are rarely used to adjust treatment expectations or determine treatment dosage. One of the speculated benefits of measuring tendon morphologic and mechanical properties is the ability to better predict patient outcomes, which would result in more realistic treatment expectations. It is currently unknown whether Achilles tendon morphologic and mechanical properties have this predictive value. Therefore, as a first step, this study aimed to determine whether initial measures of tendon morphologic features and estimated mechanical properties were associated with patient-reported symptoms and calf muscle endurance at baseline, 6-month follow-up, and 1-year follow-up by prospectively following a cohort of individuals with either midportion or insertional Achilles tendinopathy. We hypothesized that greater tendon thickening, lower shear modulus, and lower viscosity at the initial assessment would relate to consistently worse patient-reported symptoms and calf muscle endurance over the year. Additionally, we hypothesized that changes in patient-reported symptoms and calf muscle endurance would be moderated by measures of tendon thickening, shear modulus, and viscosity.
Methods
Study Design
This study is part of an ongoing observational cohort study that prospectively follows patients with any type of Achilles tendon injury (eg, tendinopathy, rupture) or associated injury (eg, retrocalcaneal bursitis) for 1 year. Participants complete an initial assessment, a 6-month follow-up assessment, and a 1-year follow-up assessment. Participants in this analysis were recruited from November 2014 to January 2018 through local physicians, podiatrists, and physical therapists as well as through newspaper advertisements, flyers, and social media. At the initial assessment, patient-reported symptoms, calf muscle endurance, and tendon morphologic features were evaluated and tendon mechanical properties were estimated. At the follow-up assessments, patient-reported symptoms and calf muscle endurance were reevaluated. Prior to data collection, participants were informed of the study procedures and provided written consent. The study protocol was approved by the institutional review board at the University of Delaware.
Participants
For the current study, only participants who had a clinical diagnosis of midportion or insertional Achilles tendinopathy, as defined by van Dijk et al,40 were included. The diagnosis was confirmed with a clinical examination performed by a licensed physical therapist. Participants with a history of surgery to the Achilles tendon, a comorbidity that affects pain perception (eg, multiple sclerosis, fibromyalgia), or minimal symptoms at their initial visit (ie, Victorian Institute of Sports Assessment–Achilles [VISA-A] score >90) were excluded. Additionally, participants were excluded if they did not either complete follow-up assessment or undergo the structural evaluation at the initial assessment. Demographics, anthropometrics, injury characteristics, treatment history, and physical activity levels were recorded at the initial assessment. The Physical Activity Scale, as originally described by Grimby,17 is scored from 1 to 6, with higher values indicating more physical activity.
Patient-Reported Symptoms
The VISA-A questionnaire was used to quantify symptom severity at each assessment. The VISA-A is a questionnaire developed for patients with Achilles tendinopathy that is valid, reliable, and commonly used in clinical practice.25,30 The questionnaire is scored from 0 to 100, with lower scores representing worse symptoms. The first 7 questions are each worth 10 points, and the eighth question is worth 30 points. Ideally, participants will complete all 8 questions. However, questions 6 and 8 are occasionally skipped by participants. These questions assess pain during hopping (question 6) and participation in sports (question 8). The reasons for skipping these questions were not recorded. If the sixth question was skipped, the mean score of questions 1 through 5 was used to fill in the missing response. If the eighth question was skipped, the VISA-A was not scored.
Calf Muscle Endurance
Participants performed the heel-rise endurance test at each assessment to quantify the functional status of the calf musculature. For this test, participants performed as many single-leg heel-rises as possible on a 10° incline box while keeping their knee straight and following a metronome (30 repetitions per minute). The heel-rise endurance test is valid and reliable5 and has been used to detect functional deficits in patients with Achilles tendinopathy.35 Testing was terminated when the participant was unable to perform more repetitions or maintain the testing parameters. A linear encoder (MuscleLab; Ergotest Innovations) was attached to the heel of the participant throughout testing to determine the total mechanical work (work = Σ displacement × mass) performed by the calf musculature. A zero was recorded when a participant could not complete a single-leg heel-rise.
Tendon Morphologic Assessment
Morphologic features of the Achilles tendon were evaluated during the initial assessment with ultrasonography. Participants were prone with their feet hanging naturally over the edge of a treatment table. We obtained 3 extended field-of-view images using a LOGIQ e ultrasound system (GE Healthcare) equipped with a wide-band linear array probe (5.0-13.0 MHz), as previously described.31,36 Anterior-posterior tendon thickness was measured at the thickest part of the tendon and at a standardized reference location. Achilles tendon thickening was then calculated by subtracting the thickness at the reference location from the thickness at the thickest part (Figure 1). The reference location reported in the literature is 2 cm proximal to the osteotendinous junction for patients with midportion Achilles tendinopathy.7 The osteotendinous junction was defined by the calcaneal notch, or the most proximal aspect of the osteotendinous junction. Therefore, this reference location was used for participants with midportion Achilles tendinopathy. The same reference location could not be used for participants with insertional Achilles tendinopathy because the tendon shape at this location was affected. Thus, the reference location for participants with insertional tendinopathy was immediately distal to the myotendinous junction of the soleus. This reference location was also used for participants who had equal symptoms in the midportion and insertion. Although the reference location differed between participants with midportion and insertional tendinopathy, each location reflected a region of the tendon that was relatively unaffected for each subgroup.
Figure 1.

Representative ultrasound images for measuring Achilles tendon thickening. Thickening was calculated as the thickness at the thickest location minus the thickness at the reference location. (A) A healthy Achilles tendon. Tendon thickening would be close to zero regardless of the reference location. (B) Midportion Achilles tendinopathy with a reference location 2 cm proximal to the osteotendinous junction, indicated by a star at the calcaneal notch. (C) Insertional Achilles tendinopathy with the reference location immediately distal to the soleus myotendinous junction.
Tendon Mechanical Properties
Achilles tendon mechanical properties were estimated with cSWE during the initial assessment. cSWE is a valid and reliable tool9,10 and has been used to estimate mechanical properties of healthy, tendinopathic, and ruptured Achilles tendons.8,38,42–44 cSWE provides separate elastic and viscosity measurements of the tendon. To undergo cSWE, participants were prone with their feet secured to a platform in 10° of ankle dorsiflexion. This positioning increased the likelihood that the tendon was within the linear region of the stress-stiffness curve. Shear waves were generated with an external actuator (Mini-Shaker Type 4810; Bruel & Kjaer) and applied to the Achilles tendon at 11 known frequencies (range, 322-643 Hz). While the waves propagated along the length of the tendon, a SonixMDP Q+ ultrasound system (Ultrasonix) with an L14-5/38 probe and a 128-channel data acquisition device sampled raw radiofrequency data at 6438 frames per second. Care was taken to ensure that the ultrasound probe was held parallel with the tendon fibers in the thickest part of the free tendon between the osteotendinous and myotendinous junctions. Viscoelastic modeling was used to estimate shear modulus and viscosity of the Achilles tendon, which was thoroughly described by Cortes et al.10 Three consecutive trials were performed, and the mean was used for analysis.
Statistical Analysis
After we determined eligibility of participants, we used independent t tests and chi-square tests (2 × 2 and 2 × 3 for sex and injury location, respectively) to compare participant characteristics between those included in the primary analysis and those excluded because of no follow-up data or baseline structural evaluation. Associations between patient-report symptoms (VISA-A scores) with tendon morphologic and mechanical properties were analyzed with a General Linear Mixed Model. The analysis was then repeated with calf muscle endurance (heel-rise work) as the dependent variable. All assumptions of the mixed models, including the absence of outliers at each time point, were evaluated through use of residual plots and Shapiro-Wilks tests for normality. If a single observation for an individual was identified as an outlier, it was removed from the analysis at only that time point. A first-order autoregressive correlation structure of the residuals was selected by comparing model fit through use of the Akaike and Bayesian information criteria. Fixed effects in the model included tendon thickening, shear modulus, and viscosity (initial assessment) and time (initial, 6 months, and 1 year). This analysis allowed us to determine whether greater tendon thickening, lower shear modulus, and lower viscosity at the initial assessment related to consistently worse patient-reported symptoms and calf muscle endurance over the year. Additionally, the interactions of time with tendon thickening, shear modulus, and viscosity were included, which enabled us to determine whether changes in patient-reported symptoms and calf muscle endurance were moderated by measures of tendon thickening, shear modulus, and viscosity. We assessed significant interactions post hoc by performing a simple slope analysis, following suggestions from Aiken and West.1 Thus, if VISA-A scores or heel-rise work over time was significantly moderated by a structural variable, the interaction was visualized by plotting the simple slope when the structural variable was set to the mean and 1 SD above and below the mean. For all analyses, the significance level was set to .05.
In addition to the primary models, which included patients with midportion and/or insertional Achilles tendinopathy, a stratified analysis was performed to evaluate each subgroup independently. Independent t tests and chi-square tests were used to evaluate differences in patient characteristics and initial measures of tendon structure between the 2 subgroups.
Results
Participants
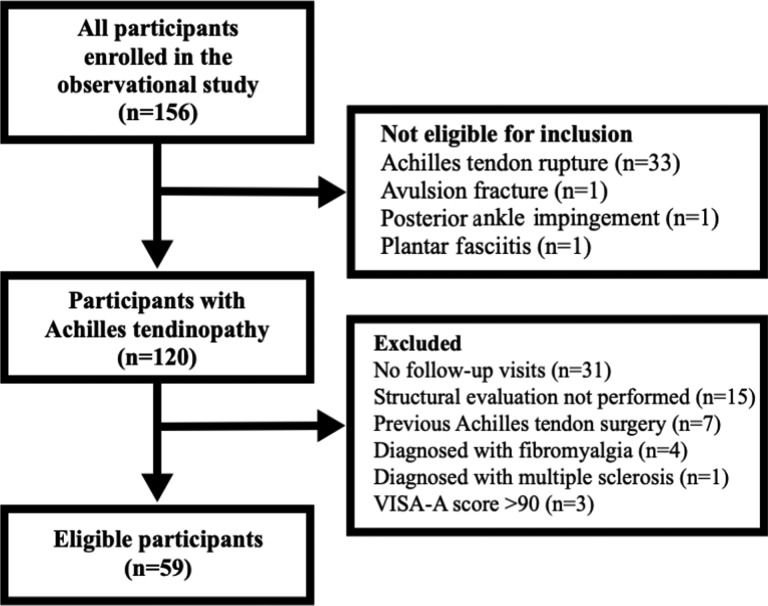
The inclusion and exclusion criteria resulted in 59 eligible participants; the flow of participants is depicted in Figure 2. Demographics, anthropometrics, injury characteristics, physical activity levels, and initial measures of morphologic and mechanical properties are reported in Table 1 for all eligible participants and subgroups of participants with midportion and insertional Achilles tendinopathy. Age, height, weight, duration of symptoms, Physical Activity Scale score, and proportions for sex and injury location were not significantly different (P > .05) between eligible participants (n = 59) and those excluded because of no follow-up data or initial structural evaluation (n = 46). Furthermore, no significant differences were found between the midportion and insertional cohorts for all characteristics, including VISA-A scores, heel-rise endurance, and tendon structural variables (Table 1).
Figure 2.
Participant flowchart. VISA-A, Victorian Institute of Sports Assessment–Achilles.
Table 1.
Initial Characteristics of Patients With Achilles Tendinopathya
| Entire Tendinopathy Cohort | Midportion Subgroup | Insertional Subgroup | P | |
|---|---|---|---|---|
| Age, y | 52 ± 15 (19-79) | 54 ± 14 (27-77) | 51 ± 16 (19-79) | .57 |
| Height, cm | 174 ± 11 (140-195) | 177 ± 11 (157-193) | 172 ± 12 (140-195) | .20 |
| Body mass, kg | 84 ± 21 (56-164)b | 81 ± 14 (58-116) | 88 ± 27 (56-164)b | .24 |
| Duration of symptoms, mo | 25.4 ± 57.5 (0.4-396.3)c | 25.3 ± 75.8 (0.4-396.3)b | 24.1 ± 30.4 (2-127.9)d | .95 |
| Physical Activity Scale score | 4.1 ± 1.4 (1-6)b | 4.2 ± 1.5 (1-6)b | 4.0 ± 1.4 (1-6) | .66 |
| VISA-A score | 55 ± 21 (18-88)b | 59 ± 18 (25-88)b | 50 ± 22 (18-88) | .13 |
| Heel-rise work, J | 1248 ± 952 (0-3697)c | 1294 ± 832 (0-2728)b | 1232 ± 1096 (0-3697)d | .82 |
| Tendon thickening, mm | 2.88 ± 1.9 (0.1-7.5) | 3.17 ± 1.9 (0.1-6.4) | 2.43 ± 1.6 (0.2-7.5) | .13 |
| Shear modulus, kPa | 100.7 ± 17.5 (63.2-139.4) | 102.4 ± 17.8 (70.8-139.4) | 102.6 ± 16.2 (80.3-133.3) | .97 |
| Viscosity, Pa*s | 50.8 ± 12.9 (20-80) | 49.3 ± 12.7 (20-76) | 50.7 ± 13.5 (25-80) | .72 |
| Sex, males/females, n | 33/26 | 19/9 | 12/13 | .14 |
| Location of injury, midportion/insertion/both, n | 28/25/6 | NA | NA | NA |
aUnless otherwise noted, results are reported as mean ± SD (range). The entire cohort includes midportion cohort, insertional cohort, and individuals with equal midportion and insertional symptoms. P values are from independent t tests and chi-square tests for differences between midportion and insertional cohorts. NA, not applicable; VISA-A, Victorian Institute of Sports Assessment–Achilles.
bOne participant did not complete.
cFour participants did not report.
dThree participants did not report.
Patient-Reported Symptoms
Entire Tendinopathy Cohort
When analyzing the associations between VISA-A scores over the year and tendon morphologic and mechanical properties at the initial assessment, we identified 2 observations as outliers and removed them from the final model. This resulted in data from 58 participants at the initial assessment, 49 participants at 6-month assessment, and 46 participants at 1-year assessment. The 2 observations were removed to satisfy the assumption of normality for mixed model. During sensitivity analyses, it was determined that the results were unchanged when the analysis was performed with all observations.
Adjusted mean VISA-A scores were 54 (95% CI, 50-60) at the initial assessment, 72 (95% CI, 67-77) at the 6-month assessment, and 78 (95% CI, 72-83) at the 1-year assessment, with a significant fixed effect of time (P = .016). The tendon’s initial shear modulus significantly moderated the relationship between VISA-A scores and time (P = .009). To illustrate the effect of low, moderate, and high shear modulus at the initial assessment on VISA-A scores over time, the findings from the simple slope post hoc analysis are plotted in Figure 3. The fixed effect of tendon thickening on VISA-A scores was also significant (P = .001). A 1-mm increase in tendon thickening was associated with a 4-point decrease in VISA-A scores across all time points. VISA-A scores were not associated with the tendon’s initial viscosity (fixed effect, P = .221; interaction with VISA-A by time, P = .270).
Figure 3.
Post hoc simple slope analysis illustrating the effect of the initial shear modulus on patient-reported symptoms (Victorian Institute of Sports Assessment–Achilles [VISA-A] scores) over time. For this analysis, the initial shear modulus was set to 3 levels, which corresponded with the mean (moderate) and 1 SD above and below the mean (high and low, respectively).
VISA-A Scores Stratified by Location of Achilles Tendinopathy
For individuals with midportion Achilles tendinopathy (n = 28 with 1 observation removed), adjusted mean VISA-A scores were 58 (95% CI, 51-66) at the initial assessment, 71 (95% CI, 64-79) at the 6-month assessment, and 82 (95% CI, 74-90) at the 1-year assessment. VISA-A scores were not associated with time (P = .214), tendon thickening (fixed effect, P = .052; interaction with VISA-A by time, P = .666), shear modulus (fixed effect, P = .489; interaction with VISA-A by time, P = .126), or viscosity (fixed effect, P = .670; interaction with VISA-A by time, P = .584).
For individuals with insertional Achilles tendinopathy (n = 25 with 1 observation removed), adjusted mean VISA-A scores were 51 (95% CI, 42-59) at the initial assessment, 72 (95% CI, 63-80) at the 6-month assessment, and 72 (95% CI, 63-82) at the 1-year assessment. Time and tendon thickening were significantly associated with VISA-A scores (P = .032 and P = .028, respectively). A 1-mm increase in tendon thickening was associated with a 6-point decrease in VISA-A score across all time points. Shear modulus (fixed effect, P = .192; interaction with VISA-A by time, P = .261) and viscosity (fixed effect, P = .182; interaction with VISA-A by time, P = .274) were not associated with VISA-A scores.
For the 6 individuals with equal midportion and insertional symptoms, a mixed model could not be performed. As an alternative, unadjusted VISA-A scores are shown in Figure 4 for these individuals, along with the adjusted means from the midportion and insertional subgroups.
Figure 4.
Victorian Institute of Sports Assessment–Achilles (VISA-A) scores over time. Unadjusted VISA-A scores for individuals with equal midportion and insertional symptoms are plotted with the mean adjusted VISA-A scores for the midportion and insertional Achilles tendinopathy subgroups as references. Error bars represent 95% CIs.
Calf Muscle Endurance
Entire Tendinopathy Cohort
When analyzing the associations between heel-rise work over the year and tendon morphologic and mechanical properties at the initial assessment, we identified 3 observations (the same participant at each time point) as outliers and removed them from the final model. Additionally, another participant who did not complete the heel-rise endurance test was removed. Removal of these 2 participants resulted in data from 54 participants at the initial assessment, 47 participants at the 6-month assessment, and 44 participants at the 1-year assessment. The outliers were removed to satisfy the assumption of normality for mixed model, and results were unchanged when a sensitivity analysis that included all observations was performed. Therefore, the following results are from the model that excluded outliers.
The adjusted means for heel-rise work were 1180 J (95% CI, 984-1376 J) at the initial assessment, 1601 J (95% CI, 1399-1803 J) at the 6-month assessment, and 1598 J (95% CI, 1387-1809 J) at the 1-year assessment, with no significant effect of time (P = .282). Tendon thickening and viscosity were significantly associated with heel-rise work (P < .001 and P = .001, respectively). A 1-mm increase in tendon thickening was associated with a 179-J decrease in heel-rise work across all time points. A 1-Pa*s decrease in viscosity was associated with a 20-J decrease in heel-rise work across all time points. The initial viscosity did not significantly moderate the relationship of heel-rise work by time (P = .053). Finally, heel-rise work was not associated with the tendon’s shear modulus (P = .132), and the heel-rise work–time relationship was not significantly moderated by tendon thickening or shear modulus (P > .05).
Heel-Rise Work Stratified by Location of Achilles Tendinopathy
For individuals with midportion Achilles tendinopathy (n = 28 with no observations removed), adjusted means for heel-rise work were 1257 J (95% CI, 1002-1512 J) at the initial assessment, 1566 J (95% CI, 1299-1834 J) at the 6-month assessment, and 1642 J (95% CI, 1356-1929 J) at the 1-year assessment. Tendon thickening and viscosity were significantly associated with heel-rise work (P = .005 and P = .021, respectively). A 1-mm increase in tendon thickening was associated with a 94-J decrease in heel-rise work across all time points. A 1-Pa*s decrease in viscosity was associated with a 26-J decrease in heel-rise work across all time points. Heel-rise work, however, did not significantly change over time (P = .616) and was not associated with shear modulus (fixed effect, P = .202; interaction with heel-rise work by time, P = .640).
For individuals with insertional Achilles tendinopathy (n = 24 with 3 observations removed), adjusted means for heel-rise work were 1138 J (95% CI, 713-1563 J) at the initial assessment, 1706 J (95% CI, 1280-2133 J) at the 6-month assessment, and 1718 J (95% CI, 1284-2152 J) at the 1-year assessment. Heel-rise work did not significantly change over time (P = .608) and was not associated with tendon thickening (fixed effect, P = .228; interaction with heel-rise work by time, P = .115), shear modulus (fixed effect, P = .968; interaction with heel-rise work by time, P = .933), or viscosity (fixed effect, P = .071; interaction with heel-rise work by time, P = .061).
Similar to VISA-A scores, a mixed model was not performed for heel-rise work for the 6 individuals with equal midportion and insertional symptoms. Instead, each individual’s unadjusted heel-rise work was plotted over time with the larger subgroups as references (Figure 5).
Figure 5.
Heel-rise work over time. Unadjusted heel-rise work for individuals with equal midportion and insertional symptoms is plotted with mean adjusted heel-rise work for the midportion and insertional Achilles tendinopathy subgroups as references. Error bars represent 95% CIs.
Discussion
The purpose of this study was to determine whether initial measures of Achilles tendon morphology and mechanical properties were associated with patient-reported symptoms and calf muscle function at baseline, 6-month follow-up, and 1-year follow-up for patients with Achilles tendinopathy. We hypothesized that greater tendon thickening, lower shear modulus, and lower viscosity at the initial assessment would consistently relate to lower VISA-A scores and less heel-rise work at each time point over the year. Notably, we found that greater tendon thickening at the initial assessment was associated with worse patient-reported symptoms and calf muscle endurance at baseline, 6 months, and 1 year. Additionally, a lower initial viscosity was consistently associated with worse calf muscle endurance. We also hypothesized that changes in patient-reported symptoms and calf muscle function over a year would be moderated by tendon thickening, shear modulus, and viscosity. Interestingly, we found that shear modulus significantly moderated VISA-A scores over time, with a lower shear modulus at the initial assessment being associated with less improvement in symptoms by 1-year follow-up. Collectively, these findings partially support our hypotheses and suggest that measures of tendon morphology and mechanical properties may play an important role in the recovery of symptoms and function for patients with Achilles tendinopathy.
Morphologic characteristics of the tendon are commonly evaluated in clinical and research settings with ultrasonography and magnetic resonance imaging. In addition to confirming a diagnosis of Achilles tendinopathy, diagnostic imaging has been used to monitor the tendon’s response to treatment15,27,33 and predict the development of symptoms.16,19,20,26 Markedly, a systematic review by McAuliffe et al26 showed that the risk of developing symptoms of Achilles tendinopathy was 7 times greater in people who had tendon structural abnormalities. Two of the most commonly measured morphologic features are tendon thickness and cross-sectional area. Although useful for between-limb and between-group comparisons, stand-alone measures of tendon thickness and cross-sectional area do not reflect the degree of structural abnormality within a tendon. Achilles tendon thickening is a novel measure of tendon morphology that reflects how uniform the tendon is, with perfect uniformity being characteristic of a healthy tendon. In the current study, we showed that a greater degree of tendon thickening was associated with worse symptoms and calf muscle function over the course of a year. This finding suggests that patient outcomes may depend on the tendon’s structural status. When we also consider that tendon thickening is related to current symptoms and physical activity levels,7 it seems that measures of tendon thickening may provide useful insights if collected in future clinical research.
In patients with Achilles tendinopathy, reduced mechanical properties accompany changes in tendon morphology.3,6 This reduction in mechanical properties negatively affects the tendon’s ability to efficiently store and release elastic energy during locomotor and explosive (eg, jumping) activities.11,22,23,41 Consequently, mechanical properties have been proposed as biomarkers for tendon health and recovery. In the current study, we found that changes in patient-reported symptoms over a year depended on the initial estimated shear modulus of the tendon (see Figure 3). Overall, it appears that symptoms were similar at the initial assessment regardless of the shear modulus. However, participants with lower shear modulus at the initial assessment had the greatest improvements in symptoms after 6 months but slightly regressed by 1 year. In contrast, participants with higher shear modulus at the initial assessment were the least improved by 6 months but had the best outcome by 1 year. Taken together, these findings appear to indicate that a lower initial shear modulus is associated with a worse long-term prognosis for patients with Achilles tendinopathy. Further research is needed to determine whether tendon mechanical properties change with treatment or whether these measures can help with making clinical decisions, such as treatment dosages and return to sport.
Another mechanical property that was estimated in this study is viscosity, which is a tendon’s rate-dependent resistance to a shearing force when measured with cSWE. Limited research has investigated tendon viscosity with elastography because of the technical inability to separate dampening behaviors from elastic behaviors in vivo. With the development of cSWE, we are able to reliably estimate tendon viscosity.9,10 This parameter may have large clinical implications because it describes the tendon’s ability to resist strain at different loading rates. In the current study, we found that lower tendon viscosity initially was associated with lower heel-rise endurance across all time points. This suggests that tendon viscosity may be an important determinant of calf muscle function. However, further research is needed to better understand how this mechanical behavior relates to function.
Currently, there is no evidence showing that alterations in tendon morphologic and mechanical properties differ between individuals with midportion and insertional Achilles tendinopathy. Therefore, the original cohort of interest for this study was all individuals with Achilles tendinopathy. To explore the potential influence of tendinopathy location on the associations, we reanalyzed the data stratifying by location of lesion. For brevity, our discussion is limited to structural variables that were significant in the entire cohort. In the stratified analysis, we found that tendon thickening was significantly associated with VISA-A scores at each time point for those with insertional Achilles tendinopathy and was nearly significant (P = .052) for those with midportion Achilles tendinopathy. The nonsignificant finding in the case of the midportion tendinopathy subgroup may be a result of type II error. In addition to tendon thickening, shear modulus significantly moderated the association of VISA-A with time in the entire cohort, but this interaction effect was not significant for either subgroup independently. Again, this may suggest type II error in the stratified analysis. For heel-rise endurance, we noted a significant association with tendon thickening and viscosity in the entire cohort. However, in the stratified analysis, it was revealed that these associations were significant only for the midportion Achilles tendinopathy subgroup. This finding indicates that the significant association of heel-rise work with tendon thickening and viscosity found in the entire cohort may have been greatly influenced by the midportion tendinopathy subgroup and the association may not exist in patients with insertional Achilles tendinopathy. Yet, because the statistical model may be underpowered in the stratified analysis, cautious interpretation is required. Last, for the 6 individuals with equal midportion and insertional symptoms, patient-reported symptoms and calf muscle endurance did not appear to be consistently better or worse than those found in the stratified cohorts. More research is needed to confirm the results of the stratified analysis and directly compare individuals with midportion and insertional Achilles tendinopathy.
Regardless of where the tendon is affected (ie, midportion or insertional), rehabilitation aims to alleviate symptoms, improve function, and allow for a safe return to recreational activities. In many cases, patients and clinicians primarily focus on symptoms to assess recovery. Although symptoms are an important part of the clinical picture, a treatment approach that mainly focuses on symptoms to guide clinical decisions may partially explain high reinjury rates and poor long-term outcomes.18,29,34 This may be because symptom resolution does not ensure full structural or functional recovery.15,37 In the current study, we showed that measures of tendon structure are associated with the recovery of patient symptoms and function. Therefore, a comprehensive approach to rehabilitation that takes symptoms, structure, and function into consideration may ultimately improve long-term outcomes and provide more realistic expectations for patients with Achilles tendinopathy. Before this comprehensive approach to tendon health can be fully implemented clinically, research is needed to evaluate changes in patient symptoms, tendon structure, and function throughout treatment and to determine whether certain measures are correlated with failure of nonoperative treatment.
This study has several limitations. One limitation is that treatment was not controlled or systematically documented. Conversely, based on information provided at the initial assessment, it was determined that all participants except 4 had either seen a medical professional or received treatment. To confirm that the untreated cases did not significantly influence the primary results, we repeated the analysis after excluding these cases. In this secondary analysis, all significant associations were maintained, which suggests that inclusion of the untreated participants did not affect the results. Another limitation of this study was including participants regardless of how long they had experienced symptoms. It can be speculated that the associations of tendon morphology and mechanical properties with symptoms and function may be influenced by a patient’s duration of symptoms. Therefore, an additional analysis was performed that stratified the entire cohort by symptoms longer and shorter than 6 months. Results from this analysis showed that VISA-A scores are associated with tendon thickening for individuals with symptoms for 6 months or less but not for individuals with symptoms for longer than 6 months. Furthermore, heel-rise work was associated with tendon viscosity for both subgroups and tendon thickening for individuals with symptoms for 6 months or less. Collectively, these findings suggest that tendon thickening may assist with defining a prognosis for patients with shorter symptoms, although viscosity may be used regardless of symptom duration. Yet, similar to the other stratified analyses performed in this study, this analysis may be underpowered and needs to be confirmed with a larger sample. The final limitation of this study was the large number of individuals who either did not complete the follow-up assessments or did not have a structural evaluation. Although the specific reasons for dropping out were not recorded, we speculate that the high attrition rate may be due to the lack of treatment or compensation. As for the 15 cases in which structural evaluation was not performed, the reasons included equipment malfunction, equipment failure, and poor positioning of the ultrasound probe. Despite the reduced sample size, it was determined that characteristics for the eligible participants did not differ from the participants who were excluded.
Conclusion
Initial measures of Achilles tendon thickening are associated with patient-reported symptoms and calf muscle function over 1 year in patients with Achilles tendinopathy. Additionally, the tendon’s initial shear modulus is associated with symptoms, although viscosity is associated with calf muscle function. Clinically, this suggests that patients and clinicians may need to adjust their expectations for rehabilitation based on the amount of structural degradation at the beginning of treatment. Future research is needed to determine whether measures of Achilles tendon structure can inform decisions regarding treatment and return to sport.
Footnotes
Final revision submitted January 3, 2020; accepted January 22, 2020.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
One or more of the authors has declared the following potential conflict of interest or source of funding: Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health (award numbers R01-AR072034-01A1 and R21-AR067390). AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
Ethical approval for this study was obtained from the University of Delaware (study No. 670923).
References
- 1. Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage; 1991. [Google Scholar]
- 2. Alfredson H, Cook J. A treatment algorithm for managing Achilles tendinopathy: new treatment options. Br J Sports Med. 2007;41(4):211–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arya S, Kulig K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J Appl Physiol. 2010;108(3):670–675. [DOI] [PubMed] [Google Scholar]
- 4. Aubry S, Nueffer J-P, Tanter M, Becce F, Vidal C, Michel F. Viscoelasticity in Achilles tendonopathy: quantitative assessment by using real-time shear-wave elastography. Radiology. 2015;274(3):821–829. [DOI] [PubMed] [Google Scholar]
- 5. Byrne C, Keene DJ, Lamb SE, Willett K. Intrarater reliability and agreement of linear encoder derived heel-rise endurance test outcome measures in healthy adults. J Electromyogr Kinesiol. 2017;36:34–39. [DOI] [PubMed] [Google Scholar]
- 6. Chang Y-J, Kulig K. The neuromechanical adaptations to Achilles tendinosis. J Physiol. 2015;15:3373–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Corrigan P, Cortes DH, Pontiggia L, Silbernagel KG. The degree of tendinosis is related to symptom severity and physical activity levels in patients with midportion Achilles tendinopathy. Int J Sports Phys Ther. 2018;13(2):196–207. [PMC free article] [PubMed] [Google Scholar]
- 8. Corrigan P, Cortes DH, Silbernagel KG. Immediate effect of photobiomodulation therapy on Achilles tendon morphology and mechanical properties: an exploratory study. Transl Sport Med. 2019;2(4):164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corrigan P, Zellers JA, Balascio P, Silbernagel KG, Cortes DH. Quantification of mechanical properties in healthy Achilles tendon using continuous shear wave elastography: a reliability and validation study. Ultrasound Med Biol. 2019;45(7):1574–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cortes DH, Suydam SM, Silbernagel KG, Buchanan TS, Elliott DM. Continuous shear wave elastography: a new method to measure viscoelastic properties of tendons in vivo. Ultrasound Med Biol. 2015;41(6):1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Debenham JR, Travers MJ, Gibson W, Campbell A, Allison GT. Achilles tendinopathy alters stretch shortening cycle behaviour during a sub-maximal hopping task. J Sci Med Sport. 2016;19(1):69–73. [DOI] [PubMed] [Google Scholar]
- 12. de Jonge S, Tol JL, Weir A, Waarsing JH, Verhaar JAN, de Vos R-J. The tendon structure returns to asymptomatic values in nonoperatively treated Achilles tendinopathy but is not associated with symptoms. Am J Sports Med. 2015;43(12):2950–2958. [DOI] [PubMed] [Google Scholar]
- 13. de Vos RJ, Heijboer MP, Weinans H, Verhaar JAN, van Schie HTM. Tendon structure’s lack of relation to clinical outcome after eccentric exercises in chronic midportion Achilles tendinopathy. J Sport Rehabil. 2012;21(1):34–43. [DOI] [PubMed] [Google Scholar]
- 14. Dirrichs T, Quack V, Gatz M, Tingart M, Kuhl CK, Schrading S. Shear wave elastography (SWE) for the evaluation of patients with tendinopathies. Acad Radiol. 2016;23(10):1204–1213. [DOI] [PubMed] [Google Scholar]
- 15. Drew BT, Smith TO, Littlewood C, Sturrock B. Do structural changes (eg, collagen/matrix) explain the response to therapeutic exercises in tendinopathy: a systematic review. Br J Sports Med. 2014;48(12):966–972. [DOI] [PubMed] [Google Scholar]
- 16. Fredberg U, Bolvig L. Significance of ultrasonographically detected asymptomatic tendinosis in the patellar and Achilles tendons of elite soccer players: a longitudinal study. Am J Sports Med. 2002;30(4):488–491. [DOI] [PubMed] [Google Scholar]
- 17. Grimby G. Physical activity and muscle training in the elderly. Acta Med Scand Suppl. 1986;711:233–237. [DOI] [PubMed] [Google Scholar]
- 18. Hägglund M, Waldén M, Ekstrand J. Lower reinjury rate with a coach-controlled rehabilitation program in amateur male soccer: a randomized controlled trial. Am J Sports Med. 2007;35(9):1433–1442. [DOI] [PubMed] [Google Scholar]
- 19. Hirschmüller A, Frey V, Konstantinidis L, et al. Prognostic value of Achilles tendon Doppler sonography in asymptomatic runners. Med Sci Sports Exerc. 2012;44(2):199–205. [DOI] [PubMed] [Google Scholar]
- 20. Jhingan S, Perry M, O’Driscoll G, et al. Thicker Achilles tendons are a risk factor to develop Achilles tendinopathy in elite professional soccer players. Muscles Ligaments Tendons J. 2011;1(2):51–56. [PMC free article] [PubMed] [Google Scholar]
- 21. Kader D, Saxena A, Movin T, Maffulli N. Achilles tendinopathy: some aspects of basic science and clinical management. Br J Sports Med. 2002;36(4):239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kubo K, Kanehisa H, Fukunaga T. Effects of viscoelastic properties of tendon structures on stretch-shortening cycle exercise in vivo. J Sports Sci. 2005;23(8):851–860. [DOI] [PubMed] [Google Scholar]
- 23. Kubo K, Miyazaki D, Shimoju S, Tsunoda N. Relationship between elastic properties of tendon structures and performance in long distance runners. Eur J Appl Physiol. 2015:1725–1733. [DOI] [PubMed] [Google Scholar]
- 24. Maffulli N, Khan KM, Puddu G. Overuse tendon conditions: time to change a confusing terminology. Arthroscopy. 1998;14(8):840–843. [DOI] [PubMed] [Google Scholar]
- 25. Martin RL, Chimenti R, Cuddeford T, et al. Achilles pain, stiffness, and muscle power deficits: midportion Achilles tendinopathy revision 2018. J Orthop Sport Phys Ther. 2018;48(5):A1–A38. [DOI] [PubMed] [Google Scholar]
- 26. McAuliffe S, McCreesh K, Culloty F, Purtill H, O’Sullivan K. Can ultrasound imaging predict the development of Achilles and patellar tendinopathy? A systematic review and meta-analysis. Br J Sports Med. 2016;50(24):1516–1523. [DOI] [PubMed] [Google Scholar]
- 27. Ohberg L, Lorentzon R, Alfredson H. Eccentric training in patients with chronic Achilles tendinosis: normalised tendon structure and decreased thickness at follow up. Br J Sports Med. 2004;38(1):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ooi CC, Schneider ME, Malliaras P, Chadwick M, Connell DA. Diagnostic performance of axial-strain sonoelastography in confirming clinically diagnosed Achilles tendinopathy: comparison with B-mode ultrasound and color Doppler imaging. Ultrasound Med Biol. 2015;41(1):15–25. [DOI] [PubMed] [Google Scholar]
- 29. Paavola M, Kannus P, Paakkala T, Pasanen M, Järvinen M. Long-term prognosis of patients with Achilles tendinopathy: an observational 8-year follow-up study. Am J Sports Med. 2000;28(5):634–642. [DOI] [PubMed] [Google Scholar]
- 30. Robinson JM, Cook JL, Purdam C, et al. The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35(5):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ryan ED, Rosenberg JG, Scharville MJ, Sobolewski EJ, Thompson BJ, King GE. Test-retest reliability and the minimal detectable change for Achilles tendon length: a panoramic ultrasound assessment. Ultrasound Med Biol. 2013;39(12):2488–2491. [DOI] [PubMed] [Google Scholar]
- 32. Ryan M, Bisset L, Newsham-West R. Should we care about tendon structure? The disconnect between structure and symptoms in tendinopathy. J Orthop Sport Phys Ther. 2015;45(11):823–825. [DOI] [PubMed] [Google Scholar]
- 33. Shalabi A, Kristoffersen-Wilberg M, Svensson L, Aspelin P, Movin T. Eccentric training of the gastrocnemius-soleus complex in chronic Achilles tendinopathy results in decreased tendon volume and intratendinous signal as evaluated by MRI. Am J Sports Med. 2004;32(5):1286–1296. [DOI] [PubMed] [Google Scholar]
- 34. Silbernagel KG, Brorsson A, Lundberg M. The majority of patients with Achilles tendinopathy recover fully when treated with exercise alone: a 5-year follow-up. Am J Sports Med. 2011;39(3):607–613. [DOI] [PubMed] [Google Scholar]
- 35. Silbernagel KG, Gustavsson A, Thomeé R, Karlsson J. Evaluation of lower leg function in patients with Achilles tendinopathy. Knee Surg Sports Traumatol Arthrosc. 2006;14:1207–1217. [DOI] [PubMed] [Google Scholar]
- 36. Silbernagel KG, Shelley K, Powell S, Varrecchia S. Extended field of view ultrasound imaging to evaluate Achilles tendon length and thickness: a reliability and validity study. Muscles Ligaments Tendons J. 2016;6(1):104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Silbernagel KG, Thomeé R, Eriksson BI, Karlsson J. Full symptomatic recovery does not ensure full recovery of muscle-tendon function in patients with Achilles tendinopathy. Br J Sports Med. 2007;41(4):276–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Suydam SM, Soulas EM, Elliott DM, et al. Viscoelastic properties of healthy Achilles tendon are independent of isometric plantar flexion strength and cross-sectional area. J Orthop Res. 2015;33:926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Suydam SM, Soulas EM, Elliott DM, Grävare Silbernagel K, Buchanan TS, Cortes DH. Viscoelastic properties of healthy Achilles tendon are independent of isometric plantar flexion strength and cross-sectional area. J Orthop Res. 2015;33(6):926–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. van Dijk CN, van Sterkenburg MN, Wiegerinck JI, Karlsson J, Maffulli N. Terminology for Achilles tendon related disorders. Knee Surg Sports Traumatol Arthrosc. 2011;19(5):835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang HK, Lin KH, Su SC, Shih TTF, Huang YC. Effects of tendon viscoelasticity in Achilles tendinosis on explosive performance and clinical severity in athletes. Scand J Med Sci Sports. 2012;22(6):147–155. [DOI] [PubMed] [Google Scholar]
- 42. Zellers JA, Cortes DH, Corrigan P, Pontiggia L, Silbernagel KG. Side-to-side differences in Achilles tendon geometry and mechanical properties following Achilles tendon rupture. Muscles Ligaments Tendons J. 2017;7(3):541–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zellers JA, Cortes DH, Pohlig RT, Silbernagel KG. Tendon morphology and mechanical properties assessed by ultrasound show change early in recovery and potential prognostic ability for 6-month outcomes. Knee Surg Sports Traumatol Arthrosc. 2019;27(9):2831–2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zellers JA, Cortes DH, Silbernagel KG. From acute Achilles tendon rupture to return to play—a case report evaluating recovery of tendon structure, mechanical properties, clinical and functional outcomes. Int J Sports Phys Ther. 2016;11(7):1150–1159. [PMC free article] [PubMed] [Google Scholar]