Abstract
Background:
Platelet-rich plasma (PRP) has wide applications in orthopaedic care. Its beneficial effects are attributed to the growth factor profile from the platelet secretome. In theory, these effects would be diminished by medications that inhibit platelet activation and/or the subsequent release of growth factors.
Purpose:
To determine whether commonly used antiplatelets, nonsteroidal anti-inflammatory drugs (NSAIDs), or anticoagulant medications affect platelet growth factor release in PRP.
Study Design:
Systematic review; Level of evidence, 2.
Method:
A systematic review of the literature related to antiplatelet, anti-inflammatory, and anticoagulant drugs was performed following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines. We used the Downs and Black objective quality scoring system. The literature search consisted of PubMed and Cochrane Library databases. Search terms consisted of 1 item selected from “platelet-rich plasma,” “platelet-derived growth factor,” and “platelet-rich plasma AND growth factor” combined with 1 item from “antiplatelet,” “aspirin,” “anticoagulant,” and “NSAID.” Only studies published within the past 25 years were included.
Results:
A total of 15 studies met the inclusion criteria: 7 studies detected no significant decrease in growth factors or mitogenesis, whereas 6 detected a decrease with antiplatelet agents, 1 detected mixed results with an antiplatelet agent, and 1 had mixed results with an antiplatelet agent/vasodilator. In terms of PRP activation, all 3 studies assessing collagen, the 2 studies analyzing adenosine diphosphate alone, and the 1 study investigating arachidonic acid found a decrease in growth factor concentration.
Conclusion:
Antiplatelet medications may decrease the growth factor release profile in a cyclooxygenase 1– and cyclooxygenase 2–dependent manner. Eight of 15 studies found a decrease in growth factors or mitogenesis. However, more studies are needed to comprehensively understand antiplatelet effects on the PRP secretome.
Keywords: platelet-rich plasma, aspirin, growth factor, nonsteroidal anti-inflammatory, cyclooxygenase inhibitor, platelet
Originally used in oromaxillofacial surgery, platelet-rich plasma (PRP) is currently used to treat many different conditions, ranging from sports injuries to androgenic alopecia.17,21 This biologic therapy is thought to promote healing by delivering the concentrated growth factors to damaged tissues20 and augment the natural healing process with mitogenesis or cellular proliferation through mitosis, chemotaxis, and other cellular processes.20 In order to release these restorative molecules, platelets must be activated. This complex process begins with binding of myriad agonists to platelet G-protein-coupled receptors (GPCRs) or immunoreceptor tyrosine-based activation motif complexes.35 These lead to signaling cascades and platelet responses, including regulating surface receptors and releasing growth factors into the environment through degranulation.
There are various medications that can inhibit the aforementioned processes. For example, nonsteroidal anti-inflammatory drugs (NSAIDs) are thought to inhibit growth factor release by competitively and irreversibly inhibiting cyclooxygenase (COX) 1 and 2. More specifically, this is thought to occur through the inability of arachidonic acid (AA) to allow downstream thromboxane-A2 (TxA2) binding to TxA2 receptor to allow for platelet activation. Consequently, many physicians recommend cessation of antiplatelet and anti-inflammatory drugs before initiating PRP therapy.
A variety of activating agents can stimulate platelets from thrombin (TBN) to epinephrine.35 The exact mechanism of platelet activation is complex and beyond the scope of this article. Therefore, this study will focus more on the effect of antiplatelet medications on PRP.9,25 To date, there have been no systematic reviews on the effects of antiplatelet medications and anti-inflammatory drugs on PRP yield and growth factor profile. Considering the inhibitory effect of these medications on platelet function, it is reasonable to hypothesize a reduction in the growth factor expression that is dependent on the activating agent. The purpose of this review was to determine whether commonly used antiplatelets, NSAIDs, or anticoagulant medications affect platelet growth factor release in PRP. It was anticipated that these findings might lead to recommendations in administering clinical PRP in the setting of NSAID, antiplatelet, or anticoagulant medication use.
Methods
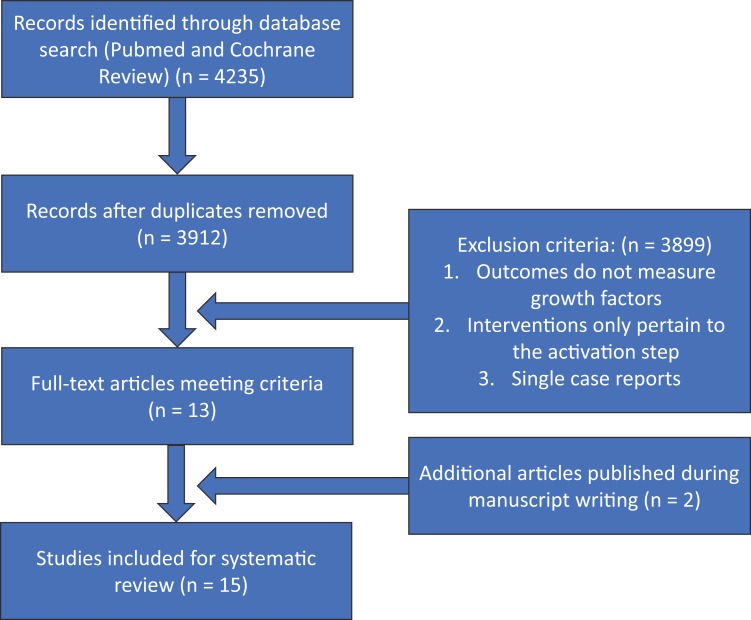
The literature search was in accordance with PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines and consisted of PubMed and Cochrane Library database inquiries for the effects of antiplatelet, anticoagulant, and anti-inflammatory drugs on PRP growth factor release. The initial search strategy used combinations of 2 keywords, 1 item selected from “platelet-rich plasma,” “platelet-derived growth factor,” and “platelet-rich plasma AND growth factor” combined with 1 item from “antiplatelet,” “aspirin,” “anticoagulant,” and “NSAID.” This method yielded 12 different combinations of inquiries per database (Table 1). The selection process, including inclusion/exclusion criteria, is illustrated in Figure 1. The initial search had a return of 4235 results, of which 323 were duplicates. Only studies published within the past 25 years were included. Both human and animal studies were included as well as in vivo or in vitro studies. We excluded articles in which the outcomes did not measure the growth factor levels and where the interventions only focused on PRP activations and not on the different medications or metabolites. Review articles and case reports were excluded. Two additional articles that fit the inclusion criteria were included at the later stages of the manuscript writing.
TABLE 1.
PubMed and Cochrane Database Search Resultsa
| Search Terms | Platelet-Rich Plasma | Platelet-Derived Growth Factor | Platelet-Rich Plasma and Growth Factor | |
|---|---|---|---|---|
| PubMed database | Antiplatelet | 390 | 70 | 9 |
| Aspirin | 306 | 39 | 22 | |
| Anticoagulant | 360 | 2447 | 55 | |
| NSAID | 130 | 210 | 8 | |
| Cochrane database | Antiplatelet | 34 | 11 | 0 |
| Aspirin | 53 | 12 | 1 | |
| Anticoagulant | 29 | 14 | 6 | |
| NSAID | 17 | 5 | 7 |
aNSAID, nonsteroidal anti-inflammatory drug.
Figure 1.
Workflow of PubMed and Cochrane Review database query following PRISMA (Preferred Reporting Items for Systematic Meta-Analyses) guidelines.
A total of 15 studies met the aforementioned inclusion criteria. Of the 15 studies included, 12 (80%) analyzed PRP,∥ 1 (6.7%) examined plasma,11 and 2 (13.33%) examined platelets directly.26,36 Of the studies that analyzed PRP, 2 used calcium chloride (CaCl2),1,2 5 used TBN,12,18,23,29,34 3 used collagen,14,16,30 2 used adenosine diphosphate (ADP),14,32 1 used AA,12 and 1 assessed both TBN and calcium gluconate as activating agents.27
All studies were evaluated using a modified 27-item Downs and Black checklist.7 We elected to use a simple 0 or 1 score for the power analysis, with papers receiving 1 point if they included power calculations showing sufficient sample size.
Data concerning changes in growth factors and/or mitogenesis, platelet activation, statistical significance, model type, activating factor, and type of medication were extracted by 1 author (C.F.) and recorded in a table. Results and statistical analysis were retrieved directly from each study. Next, factors that required methodological quality analysis for the Downs and Black checklist were extracted.
Results
Quality Assessment
The manuscript scores ranged from 13 to 25 of 28 points using our modified Downs and Black checklist (Table 2). Downs and Black scores were given the corresponding quality levels as previously reported by Hooper et al10: 26 to 28 (excellent), 20 to 25 (good), 15 to 19 (fair), and <15 (poor). Five of 15 studies were in the poor category, 6 of 15 were in the fair category, and 4 of 15 were in the good category. As expected, the clinical trials scored the highest. Most papers scored well (7 of 10 or higher) in the reporting and external validity segments; however, most papers scored low in the internal validity ratings, as only 1 was randomized or blinded. Moreover, none had calculated predetermined sample sizes to achieve adequate power or had recruitment methods that represented the target population. Owing to these limitations, most studies had a level of evidence of 2 (Table 3).3
TABLE 2.
Downs and Black7 Quality Assessment of Individual Studies
| Study | Downs and Black Score | Study | Downs and Black Score |
|---|---|---|---|
| Anitua et al2 | 13 | Takehara et al26 | 14 |
| Anitua et al1 | 13 | Tian et al27 | 20 |
| Jagroop et al11 | 19 | Vissinger et al30 | 15 |
| Jayaram et al12 | 16 | Vissinger et al29 | 15 |
| Kariyazono et al14 | 14 | Wilson et al32 | 25 |
| Lanas et al16 | 17 | Yazawa et al34 | 14 |
| Ludwig et al18 | 18 | Zhao et al36 | 22 |
| Smith et al23 | 20 |
TABLE 3.
Level of Evidence of Individual Studies
| Study | Level of Evidence | Study | Level of Evidence |
|---|---|---|---|
| Anitua et al2 | 2 | Takehara et al26 | 2 |
| Anitua et al1 | 2 | Tian et al27 | 3 |
| Jagroop et al11 | 1 | Vissinger et al30 | 1 |
| Jayaram et al12 | 2 | Vissinger et al29 | 1 |
| Kariyazono et al14 | 2 | Wilson et al32 | 1 |
| Lanas et al16 | 2 | Yazawa et al34 | 1 |
| Ludwig et al18 | 2 | Zhao et al36 | 1 |
| Smith et al23 | 2 |
Effects of Antiplatelet Medications With CaCl2-Dependent Activation on Growth Factor Profile
Only 2 studies1,2 utilized CaCl2 as an activating agent, both of which were in vivo studies in humans (Table 4). Both papers were written by Anitua et al1,2 and had a similar study design comparing the growth factor production and clotting times of participants on medications with unmedicated controls. Both papers found no significant influences on growth factor expressions for the groups assessed.
TABLE 4.
Summary of Individual Studiesa
| Author | Activating Agent | Model | N | Intervention | Growth Factors Assessed | Decrease in Factors? |
|---|---|---|---|---|---|---|
| Anitua et al2 | CaCl2 | Human, in vivo | 12 | ASA, acenocoumarol, glucosamine | PDGF-AB, VEGF, IGF-1, mitogenesis | No |
| Anitua et al1 | CaCl2 | Human, in vivo | 20 | ASA, acenocoumarol, glucosamine, glucosamine + chondroitin | HGF, PDGF-AB, TGF-β1, VEGF | No |
| Yazawa et al34 | TBN | Human, in vitro | 5 | PGE1, PGE1 + ASA + apyrase | PDGF-AB, TGF-β1 | No |
| Smith et al23 | TBN | Human, in vivo | 18 | ASA, ASA + clopidogrel | PDGF-BB, TGF-β1 | No |
| Vissinger et al29 | TBN | Human, in vivo | 12 | Dipyridamole | PDGF | No |
| Ludwig et al18 | TBN | Dog, in vivo | 10 | Carprofen | TGF-β1, PDGF-BB | No |
| Jayaram et al12 | TBN, AA, none | Human, in vivo | 12 | ASA | VEGF, PDGF-AB, TGF-β1 | With TBN, ASA slightly attenuated the release of PDGF-AB alone. With AA, ASA significantly inhibited the release of all 3 growth factors. |
| Kariyazono et al14 | Collagen, ADP, AA | Human, in vitro | 8 | ASA, cilostazol, ramatroban | TGF-β1 | Yes with ASA, cilostazol, ramatroban |
| Lanas et al16 | Collagen | Human, in vivo and fibroblast in vitro | 5 | ASA | Mitogenesis | Yes |
| Vissinger et al30 | Collagen | Human, in vivo | 12 | ASA | PDGF | Yes |
| Wilson et al32 | ADP | Human, in vivo and rat, in vitro | 50 | ASA + placebo, ASA + clopidogrel | PDGF | Yes with clopidogrel + ASA but not ASA alone |
| Tian et al27 | TBN and calcium gluconate | Human, in vivo | 44 | Either ASA or clopidogrel | EGF, FGF-2, IGF-1, VEGF-A, GDF-11, PDGF-AB/BB, PDGF-AA | Yes, FGF-2, PDGF-AA, and GDF-11 in the CVD and antiplatelet group compared with healthy controls. Growth factors were also diminished with age and diagnosis of DM. |
| Takehara et al26 | None | Human, in vivo and fibroblast in vitro | 3 | Dipyridamole, ASA, trapidil, ticlopidine | Mitogenesis, PDGF | Yes with dipyridamole |
| Zhao et al36 | None | Human, in vivo | 22 | ASA, clopidogrel, dipyridamole, ASA + clopidogrel, ASA + dipyridamole, clopidogrel + dipyridamole, ASA + clopidogrel + dipyridamole | PDGF | Yes with ASA in healthy volunteers but not in patients with stroke |
| Jagroop et al11 | None | Human, in vivo | 20 | Clopidogrel or ASA then clopidogrel + ASA | PDGF-AB | No |
aAA, arachidonic acid; ADP, adenosine diphosphate; ASA, acetylsalicylic acid; CVD, cardiovascular disease; DM, diabetes mellitus; EGF, epidermal growth factor; FGF-2, fibroblast growth factor 2; GDF-11, growth differentiation factor 11; HGF, hepatocyte growth factor; IGF-1, insulin-like growth factor 1; PDGF, platelet-derived growth factor; PGE1, prostaglandin E1; TBN, thrombin; TGF-β1, transforming growth factor beta1; VEGF, vascular endothelial growth factor.
In their 2014 publication, Anitua and colleagues2 measured platelet-derived growth factor (PDFG) AB, vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1), clotting time, and fibroblast proliferation in elderly (>60 years old) controls (no medications) against patients using chronic 100 mg/d aspirin (acetylsalicylic acid [ASA]); varying doses of acenocoumarol; a vitamin K antagonist; or 1500 mg/d glucosamine sulfate, a cartilage precursor often taken as an over-the-counter supplement.2 No significant differences were detected among clot formation time, amount of growth factor content, or cell proliferation rate of human gingival fibroblasts for any of the groups.2
Anitua and colleagues1 in 2015 conducted a similar study design analyzing different growth factors: hepatocyte growth factor (HGF), PDGF-AB, transforming growth factor beta (TGF-β1), and VEGF. Patients either had consumed 100 mg/d ASA, acenocoumarol, 1500 mg/d glucosamine sulfate, or 1500 mg/d glucosamine sulfate with 400 mg/d chondroitin (another constituent of cartilage taken as an over-the-counter supplement) or had consumed no medications for at least 1 year.1 There was a significant increase in time to achieve clot formation in the acenocoumarol group (a coumarin derivative sometimes used as an anticoagulant) compared with controls (P < .05) but no significant change in platelet activation, tendon fibroblast proliferation, or growth factor content (PDGF-AB, TGF-β1, VEGF, and HGF) of platelet-rich in growth factors when compared with the control group. Of note, cell treatment in the acenocoumarol group led to a significant decrease in fibroblast protein secretion of VEGF as well as the extracellular matrix proteins, hyaluronic acid, and fibronectin compared with the control group, which the authors attributed to matrix metalloproteinases (P < .05).1
Effects of Antiplatelet Medications With TBN-Dependent Activation on Growth Factor Profile
We identified 5 studies12,18,23,29,34 that assessed growth factors in platelets activated with TBN, 1 of which found a decrease in 1 of several growth factors (Table 4). Yazawa et al34 measured the effects of in vitro addition of prostaglandin E1 (PGE1) (a vasodilator and antiplatelet endogenous molecule) only, PGE1 with ASA and apyrase (an antiplatelet enzyme), or nothing (control group) on PRP blood samples (administered before the second centrifugation) drawn from 5 healthy human participants. After preparation, the authors measured PDGF-AB and TGF-β1 concentrations in whole blood and PRP with or without antiplatelet substances in 3 ways: direct measurement, Nonidet-P40-treated measurement, and TBN-treated measurement. Focusing on PRP that was activated with TBN, the mean PDGF was measured at 30,554 pg/mL in the control group, 120,188 pg/mL in the PGE1 only group, and 122,379 in the PGE1 + ASA + apyrase group. They found that PDGFs were concentrated to a mean of >400% in the samples with antiplatelet substances as compared with the samples without antiplatelet substances.34
Smith et al23 assessed the effect of 80 mg/d ASA, 80 mg/d ASA + 75 mg/d clopidogrel (an antiplatelet medication), and a control group (no known antiplatelet therapy regimen) on PRP produced in patients undergoing cardiac surgery. They found an insignificant decrease in the amount of TGF-β1 (P = .26) with ASA + clopidogrel compared with controls and no significant difference in platelet degranulation or PDGF-BB or TGF-β1 in any group.
Vissinger et al29 investigated the effects of dipyridamole, a molecule with vasodilator and antiplatelet properties, in healthy volunteers before and after administering 100 mg dipyridamole 3 times a day for 3 days. They found no significant difference in platelet count or platelet content of PDGF, serum PDGF concentration, or PRP PDGF concentration before and after receiving the medication.
Ludwig et al18 analyzed the growth factor levels in 10 dogs at baseline and after a 7-day or 11-day course of 4.4 mg/kg carprofen (an NSAID with more COX-2 inhibition). The PRP for all groups was divided into 4 aliquots: 2 nonactivated and 2 activated with human gamma thrombin (HGT). There were no statistically significant (P < .05) effects of the COX-2 inhibitor on the percentage of platelets positive for CD62P (a granule protein that appears on the platelet's outer surface upon fusion of the granule with the plasma membrane during platelet activation and release of α granule contents) or on concentrations of TGF-β1 or PDGF-BB. It was noted that all HGT-activated aliquots had a significantly higher platelet expression of CD62P and canine activated platelet-1 (CAP1) and released significantly higher concentrations of TGF-β1 and PDGF-BB than did platelets that were not activated.18
Jayaram et al12 analyzed leukocyte-rich PRP (LR-PRP) in 12 healthy human male participants before and after 14 days of 81 mg/d ASA. The PRP samples were collected and aliquoted into 3 groups: nonactivated, AA activated, and TBN activated, and immediately after activation TGF-β1, VEGF, and PDGF-AB were measured with enzyme-linked immunosorbent assays. Subsequently, the 12 participants took 81 mg aspirin daily for 14 days, followed by having a repeat collection of whole blood and PRP with real-time analysis. The authors found that TBN-activated PRP aliquots had a significant increase in PDGF-AB and VEGF release but not TGF-β1 release compared with nonactivated controls. Aspirin intake significantly reduced the amount of PDGF-AB release but not the VEGF release in the LR-PRP samples activated by TBN.
Effects of Antiplatelet Medications With Collagen-Dependent Activation on Growth Factor Profile
We found 3 papers14,16,30 that investigated the effects of anti-inflammatory drugs and antiplatelets on PRP using collagen for activation (Table 4). All 3 studies found a decrease in the growth factor production or mitogenesis associated with ASA use.
Kariyazono et al14 incubated PRP from healthy volunteers with 100 µmol ASA, 10 µmol cilostazol (a phosphodiesterase-3 inhibitor), or 1 µmol ramatroban (a thromboxane receptor antagonist) before stimulating the samples using ADP, collagen, and AA. Aliquots were then mixed with ASA, cilostazol, or ramatroban in vitro, and changes in TGF-β1 and platelet markers were measured. The authors found that all 3 medications significantly decreased TGF-β1 when stimulated by any of the 3 activating agents compared with vehicle control. When the samples were stimulated by collagen, TGF-β levels were decreased by roughly 80% using ASA, 30% using cilostazol, and 60% using ramatroban compared with vehicle (P < .05).
Lanas et al16 measured fibroblast mitogenesis in cells obtained from 5 patients before and after ingestion of various doses of ASA (160 mg, 320 mg, 640 mg, and 960 mg). The authors found that the mitogenic activity in collagen-stimulated PRP as measured by 3H-thymidine incorporation was reduced on average by 71.6% ± 2.5% (P = .006) using ASA and was dose dependent. Furthermore, the serum derived from collagen-stimulated PRP after ASA use was less potent in promoting fibroblast growth than the serum before ASA use (P = .01).
Last, Vissinger et al30 studied 12 healthy male volunteers and measured the values of platelet factors in human serum and in PRP stimulated with submaximal levels of collagen before and 12 hours after 1 dose of 300 mg ASA. With regard to the PRP, while there was no difference in platelet count in the PRP before and after ASA use, there was a significant decrease in concentrations of PDGF (P < .01) after ASA use.
Effects of Antiplatelet Medications With ADP-Dependent Activation on Growth Factor Profile
There were 2 studies14,32 that used ADP as an activating agent (Table 4). Both detected a negative effect on growth factors.
Wilson et al32 administered patients already using chronic 75 mg/d ASA with either a placebo or a 300-mg clopidogrel loading dose at least 12 hours before undergoing percutaneous transluminal angioplasty (PTA). Venous blood samples were taken at baseline, 1 hour pre-PTA, 1 hour post-PTA, 24 hours post-PTA, and 30 days post-PTA. In the aspirin and placebo groups, no significant differences in PDGF levels were seen pre-PTA or at any measured post-PTA time points compared with baseline. There was a significant decrease in PDGF (P = .004) in the clopidogrel group pre-PTA but no significant changes post-PTA at any of the measured time points.
In the previously described manuscript, Kariyazono et al14 found a decrease in TGF-β1 among other factors when collagen-activated PRP was incubated with ASA, cilostazol, and ramatroban. They had similar results using ADP to activate platelets. TGF-β1 was decreased by roughly 30% for each of the 3 medication groups (P < .01).
Effects of Antiplatelet Medications With AA-Dependent Activation on Growth Factor Profile
Two studies12,14 investigated the effect of ASA on AA-activated PRP (Table 4). Both studies, which have been previously mentioned, noted decreases in growth factor levels after aspirin administration.
Jayaram et al12 detected a significant decrease in all 3 growth factors tested. The authors found that TBN-activated PRP aliquots had a significant increase in PDGF-AB and VEGF release compared with nonactivated controls, but aspirin intake only partially reduced the amount of PDGF-AB release in the LR-PRP samples activated by TBN. VEGF, TGF-β1, and PDGF-AB releases were increased in the AA-activated groups compared with nonactivated controls without ASA, and all 3 growth factor releases were significantly diminished after 14 days of ASA use. Similarly, Kariyazono et al14 found that ASA, cilostazol, and ramatroban all greatly decreased TGF-β1 in PRP activated by AA use. The results are in line with the effect on PRP activated by collagen.
Effects of Antiplatelet Medications With Combined Activating Agents on Growth Factor Profile
Tian et al,27 using a combination of activating agents (Table 4), analyzed several growth factors and aging biomarkers in 4 groups: healthy and <45-year-old individuals, healthy and >45-year-old individuals, >45-year-old individuals with diabetes, and >45-year-old individuals with cardiovascular disease already taking both ASA and clopidogrel for at least 3 months. The authors then used both TBN and calcium gluconate together as activating agents for PRP and assessed different growth factor levels. They found that growth factors generally decreased with age, the presence of diabetes, and the use of antiplatelet medications. Specifically, fibroblast growth factor 2 (FGF-2), PDGF-AA, and growth differentiation factor (GDF-11) but not epidermal growth factor, VEGF-A, IGF-1, or PDGF-AB/BB were lower in the antiplatelet group than the healthy group of the same age (P < .05). There were no significant differences in growth factor levels between the diabetes and cardiovascular disease groups.
Effects of Antiplatelet Medications Without Activation on Growth Factor Profile
Four studies11,12,26,36 assessed the effects of antiplatelet medications on growth factor profile without the use of activating agents and had varying results (Table 4). Two studies detected a decrease in PDGF, whereas the other 2 studies found no effect on growth factors.
Takehara et al26 measured the mitogenic activity of skin fibroblasts, illustrating the biological activity of PDGF, incubated in the serum of 3 healthy volunteers who took 225 mg/d dipyridamole, 3g/d ASA, 300 mg/d trapidil, or 300 mg/d ticlopidine. Serum was taken before drug administration, 2 hours after the first drug administration, and 2 hours after the third day of medication administration. The authors found that dipyridamole decreased mitogenesis in fibroblasts by 65% after 1 dose and by 78% after 3 days in 1 patient. Aspirin, trapidil, or ticlopidine did not alter the mitogenic activities in the serum.
Zhao et al36 gave healthy volunteers and patients with previous ischemic stroke alternating combinations of 75 mg/d ASA, 75 mg/d clopidogrel, and 200 mg twice a day dipyridamole in a randomized multiway trial for 2 weeks. ASA significantly decreased PDGF in healthy volunteers but not in the patients with stroke. There were no significant PDGF-level changes in either the volunteer or patient groups with clopidogrel only, dipyridamole only, or any 2 or 3 drug combinations of the 3 medications.
Jagroop et al11 administered ASA and clopidogrel to patients with intermittent claudication. Patients initially received 75 mg/d of either drug as monotherapy for 8 days then received them in combination for 8 more days. Blood samples were drawn on days 8 and 16. Although the investigators utilized PRP, they only used it to study aggregation. PDGF-AB was measured in the serum, and no significant change was detected in either group.
Jayaram et al12 included a PRP aliquot without activation. These samples yielded significantly lower levels of growth factors than the activated aliquots, with low amounts of VEGF or PDGF-AB release and undetectable levels of TGF-β1 release. Nonetheless, ASA had no significant effect on any of the growth factor release amounts in nonactivated PRP.
Discussion
In this review, we analyzed the effects of common medications on the growth factor profile in PRP and further delineated the effects of activating agents. The Downs and Black objective quality scoring system was used to evaluate the methodological quality because it has been validated for both randomized and nonrandomized studies.
Overall, 812,14,16,26,27,30,32,36 of 15 studies found a decrease in growth factors or mitogenesis. Two studies1,2 using CaCl2 alone and 418,23,29,34 of 5 studies investigating TBN alone for PRP activation found no effect on growth factor production or mitogenesis with antiplatelet or anti-inflammatory medications. The fifth study, conducted by Jayaram et al,12 found a partial inhibition. Meanwhile, all 3 studies14,16,30 using collagen, 2 studies14,32 using ADP alone, and the 1 study14 utilizing AA for PRP activation found a decrease in growth factor concentration with antiplatelet or anti-inflammatory medications. Of the studies that did not use activating factors, 226,36 had mixed results, and 211,12 found no significant decrease on growth factor levels in PRP using antiplatelet or anti-inflammatory medications.
Aspirin is thought to exert its antiplatelet effect through irreversibly inhibiting COX-1 and COX-2 and attenuating the generation of downstream proaggregation factors.31 This inhibits AA conversion to prostaglandin (PG) G2 and PGH2, further preventing the synthesis of other prostaglandins and TxA2 that activate platelets. Under normal conditions, TxA2 would bind its receptor, leading to a protein kinase C (PKC)-mediated signal cascade through Rho and/or phospholipase C (PLC). This results in shape change, platelet activation, and GPIIAIIIB translocation for aggregation.24 Aspirin and other COX inhibitors are capable of inhibiting this positive feedback system, although they differ in reversibility and COX isomer selectivity. The other medications found to negatively affect growth factor production include cilostazol, clopidogrel with aspirin, dipyridamole, and ramatroban. Although each of these substances was only validated by a single study, it is possible that these compounds may alter PRP efficacy.
A variety of activating agents are being used to stimulate platelets in the preparation of PRP to elicit maximum growth factor release. CaCl2 is thought to exert its effect by directly activating platelets.28 Toyoda et al28 were unable to detect a Ca2+-sensing receptor and postulated that calcium may enter the cell through a leak pathway and directly activate cellular machinery, resulting in TxA2 synthesis and granule secretion. Cavallo et al5 found that it induced a more progressive release of growth factors than TBN or collagen. This may explain the resilience of CaCl2-activated PRP to ASA in the 2 studies.1,2
TBN primarily induces platelet activation through protease-activated receptor (PAR) 1.4 It is thought to cleave the receptor’s N-terminal exodomain, yielding a tethered ligand, which then binds the receptor. G-proteins are then activated, resulting in downstream PLC and PKC activation. Other complexes are involved, such as the activators PAR4 and glycoprotein (GP) Iba and the possible inhibitor GPV, which may check GPIba until it is cleaved. The activation of PLC and PKC by the G-proteins may offer a mechanism of platelet activation that is not dependent on TxA2 from COX-1 and COX-2, explaining the relatively robust growth factor release when ASA is introduced.
Collagen type I was found to be a weaker platelet activator than CaCl2 or TBN, with lower levels of growth factors and no clot formation.5 It is believed to activate platelets through a 2-step mechanism.15 Collagen may initially bind GPIa/IIa to hold the platelet in place and GPVI to activate the platelet. This is based on the finding that platelets with GPIa/IIa but not GPVI only elicit a partial response to TBN, activating tyrosine kinase cellular sarcoma (c-src), but not protein-tyrosine kinase p72syk (P72syk), phospholipase Cγ2 (PLCγ2), or focal adhesion kinase (P125fak). PLC then cleaves phosphatidylinositol-4,5-bisphophate to diacylglycerol and inositol 1,4,5-trisphosphate, which go on to activate PKC and release Ca2+.22 Cho et al6 hypothesized that there are 2 different pathways, one with high levels of collagen causing aggregation in platelets via integrin αIIbβ3 through a mechanism independent of PLC or TxA2 and another that responds to lower levels and requires secreted products, such as thromboaxane.
ADP mediates platelet activation through binding the GPCRs P2Y1 and P2Y12, both of which are required for complete activation.33 P2Y1 is coupled to a Gq protein that activates PLC, resulting in downstream effects, such as inducing platelet shape change and intracellular Ca2+ release and platelet aggregation.13 P2Y12 is coupled to a Gi protein that inhibits adenylate cyclase and subsequently impairs cAMP production, which normally impairs aggregation.8 This may be related to the decrease in lectin-like oxidized LDL receptor-1 (LOX-1) associated with ASA.19 Considering that LOX-1 is important for ADP-mediated platelet integrin activation, possibly mediated by PKC, it is conceivable that ASA may inhibit ADP-induced platelet activation through impairing LOX-1.
Last, AA is an upstream molecule that provides prostaglandins after getting cleaved by COX enzymes. Given that ASA directly inhibits COX-1 and COX-2, it offers a reasonable explanation for the significant attenuation of growth factor release in the 1 study using AA.12
It appears that the type of activating agent used in PRP is crucial and may allow PRP’s therapeutic activity to be maintained despite anti-inflammatory drug and/or antiplatelet use. An evidence-based support for activating agents that maintain PRP’s therapeutic activity will not only provide patients the opportunity to avoid choosing between maintaining their therapeutic regimen and utilizing PRP, but also allow providers and patients to have a more educated discussion on the risk-benefit analysis of holding antiplatelet and anti-inflammatory drugs before initiating PRP therapy.
Limitations
This study has several limitations. First, criteria were defined using terms that are commonly reported in PRP activation; however, PRP terminology is variable, which could have resulted in the exclusion of important studies. Moreover, many of the studies were level 2, marked by nonrandomized design and small sample sizes. Results from these may not be representative of the clinically relevant population. The studies in this review primarily assessed human PRP, however their characteristics of growth factor release were limited by ex vivo activation and non–freshly isolated PRP.
Conclusion
As the use of PRP and orthobiologics becomes more prevalent in clinical practice, taking into account the effects of using antiplatelets and anti-inflammatory drugs on PRP becomes more crucial. In this systematic review, we focused on the effect of using anti-inflammatory and antiplatelet medications with and without activating agents on the production of platelet growth factors. While the results were not definitive, it appeared that the use of CaCl2 or TBN alone as activation agents for PRP was, in general, not significantly affected by antiplatelets, and the use of collagen, ADP, and AA as activation agents showed a reduction in growth factors. There are limited high-quality data on the subject at this time, and future clinical research is warranted.
Final revision submitted December 27, 2019; accepted January 10, 2020.
The authors declared that they have no conflicts of interest in the authorship and publication of this contribution. AOSSM checks author disclosures against the Open Payments Database (OPD). AOSSM has not conducted an independent investigation on the OPD and disclaims any liability or responsibility relating thereto.
References
- 1. Anitua E, Troya M, Zalduendo M, Orive G. Effects of anti-aggregant, anti-inflammatory and anti-coagulant drug consumption on the preparation and therapeutic potential of plasma rich in growth factors (PRGF). Growth Factors. 2015;33(1):57–64. [DOI] [PubMed] [Google Scholar]
- 2. Anitua E, Troya M, Zalduendo MM, Orive G. The effect of different drugs on the preparation and biological outcomes of plasma rich in growth factors. Ann Anat. 2014;196;423–429. [DOI] [PubMed] [Google Scholar]
- 3. Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128(1):305–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Candia E. Mechanisms of platelet activation by thrombin: a short history. Thromb Res. 2012;129(3):250–256. [DOI] [PubMed] [Google Scholar]
- 5. Cavallo C, Roffi A, Grigolo B, et al. Platelet-rich plasma: the choice of activation method affects the release of bioactive molecules. Biomed Res Int. 2016;2016:6591717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cho MJ, Liu J, Pestina TI, et al. The roles of αIIbβ3-mediated outside-in signal transduction, thromboxane A2, and adenosine diphosphate in collagen-induced platelet aggregation. Blood. 2003;101(7):2646–2651. [DOI] [PubMed] [Google Scholar]
- 7. Downs S, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. 1998;52:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Foster CJ, Prosser DM, Agans JM, et al. Molecular identification and characterization of the platelet ADP receptor targeted by thienopyridine antithrombotic drugs. J Clin Invest. 2001;107(12):1591–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Golebiewska E, Poole A. Secrets of platelet exocytosis — what do we really know about platelet secretion mechanisms? Br J Haematol. 2014;165(2):204–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hooper P, Jutai JW, Strong G, Russell-Minda E. Age-related macular degeneration and low-vision rehabilitation: a systematic review. Can J Ophthalmol. 2008;43(2):180–187. [DOI] [PubMed] [Google Scholar]
- 11. Jagroop IA, Matsagas MI, Geroulakos G, Mikhailidis DP. The effect of clopidogrel, aspirin and both antiplatelet drugs on platelet function in patients with peripheral arterial disease. Platelets. 2004;15(2):117–125. [DOI] [PubMed] [Google Scholar]
- 12. Jayaram P, Yeh P, Patel S, et al. Effects of aspirin on growth factor release from freshly isolated leukocyte-rich platelet-rich plasma in healthy men: a prospective fixed-sequence controlled laboratory study. Am J Sports Med. 2019;47(5):1223–1229. [DOI] [PubMed] [Google Scholar]
- 13. Jin J, Kunapuli SP. Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc Natl Acad Sci USA. 1998;95:8070–8074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kariyazono H, Nakamura K, Arima J, et al. Evaluation of anti-platelet aggregatory effects of aspirin, cilostazol and ramatroban on platelet-rich plasma and whole blood. Blood Coagul Fibrinolysis. 2004;15:157–167. [DOI] [PubMed] [Google Scholar]
- 15. Kehrel B, Wierwille S, Clemetson KJ, et al. Glycoprotein VI is a major collagen receptor for platelet activation: it recognizes the platelet-activating quaternary structure of collagen, whereas CD36, glycoprotein IIb/IIIa, and von Willebrand factor do not. Blood. 1998;91(2):491–499. [PubMed] [Google Scholar]
- 16. Lanas A, Haggerty P, Hirschowitz B. Ingestion of aspirin prevents platelet-induced human fibroblast growth. Scand J Gastroenterol. 1994;29(1):17–22. [DOI] [PubMed] [Google Scholar]
- 17. Leo MS, Kumar AS, Kirit R, Konathan R, Sivamani RK. Systematic review of the use of platelet-rich plasma in aesthetic dermatology. J Cosmet Dermatol. 2015;14(4):315–323. [DOI] [PubMed] [Google Scholar]
- 18. Ludwig HC, Birdwhistell KE, Brainard BM, Franklin SP. Use of a cyclooxygenase-2 inhibitor does not inhibit platelet activation or growth factor release from platelet-rich plasma. Am J Sports Med. 2017;45(14):3351–3357. [DOI] [PubMed] [Google Scholar]
- 19. Marwali MR, Hu CP, Mohandas B, et al. Modulation of ADP-induced platelet activation by aspirin and pravastatin: role of lectin-like oxidized low-density lipoprotein receptor-1, nitric oxide, oxidative stress, and inside-out integrin signaling. J Pharmacol Exp Ther. 2007;322(3):1324–1332. [DOI] [PubMed] [Google Scholar]
- 20. Middleton KK, Barro V, Muller B, Terada S, Fu FH. Evaluation of the effects of platelet-rich plasma (PRP) therapy involved in the healing of sports-related soft tissue injuries. Iowa Orthop J. 2012;32:150–163. [PMC free article] [PubMed] [Google Scholar]
- 21. Mlynarek R, Kuhn A, Bedi A. Platelet-rich plasma (PRP) in orthopedic sports medicine. Am J Orthop. 2016;45(5):290–326. [PubMed] [Google Scholar]
- 22. Roberts D, McNicol A, Bose R. Mechanism of collagen activation in human platelets. J Biol Chem. 2004;279(19):19421–19430. [DOI] [PubMed] [Google Scholar]
- 23. Smith CW, Binford RS, Holt DW, Webb DP. Quality assessment of platelet rich plasma during anti-platelet therapy. Perfusion. 2007;22:41–50. [DOI] [PubMed] [Google Scholar]
- 24. Smyth E. Thromboxane and the thromboxane receptor in cardiovascular disease. Clin Lipidol. 2010;5(2):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stegner D, Nieswandt B. Platelet receptor signaling in thrombus formation. J Mol Med. 2011;89:109–121. [DOI] [PubMed] [Google Scholar]
- 26. Takehara K, Igarashi A, Ishibashi Y. Dipyridamole specifically decreases platelet-derived growth factor release from platelets. Thromb Res Suppl. 1990;12:73–79. [DOI] [PubMed] [Google Scholar]
- 27. Tian J, Lei X, Xuan L, Tang JB, Cheng B. The effects of aging, diabetes mellitus, and antiplatelet drugs on growth factors and antiaging proteins in platelet-rich plasma. Platelets. 2018;25:1–7. [DOI] [PubMed] [Google Scholar]
- 28. Toyoda T, Isobe K, Tsujino T, et al. Direct activation of platelets by addition of CaCl2 leads coagulation of platelet-rich plasma. Int J Implant Dent. 2018;4(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vissinger H, Husted SE, Kristensen SD, Nielsen HK. Dipyridamole and platelet release of platelet-derived growth factor. Platelets. 1994;5(2):105–108. [DOI] [PubMed] [Google Scholar]
- 30. Vissinger H, Husted SE, Kristensen SD, Nielsen HK. Platelet-derived growth factor release and antiplatelet treatment with low-dose acetylsalicylic acid. Angiology. 1993;44(8):633–638. [DOI] [PubMed] [Google Scholar]
- 31. Warner TD, Nylander S, Whatling C. Anti-platelet therapy: cyclo-oxygenase inhibition and the use of aspirin with particular regard to dual anti-platelet therapy. Br J Clin Pharmacol. 2011;72(4):619–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wilson AM, Brittenden J, Bachoo P, Ford I, Nixon GF. Randomized controlled trial of aspirin and clopidogrel versus aspirin and placebo on markers of smooth muscle proliferation before and after peripheral angioplasty. J Vasc Surg. 2009;50:861–869. [DOI] [PubMed] [Google Scholar]
- 33. Woulfe D, Yang J, Brass L. ADP and platelets: the end of the beginning. J Clin Invest. 2001;107(12):1503–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yazawa M, Ogata H, Nakajima T, Watanabe N. Influence of antiplatelet substances on platelet-rich plasma. J Oral Maxillofac Surg. 2004;62:714–718. [DOI] [PubMed] [Google Scholar]
- 35. Yun SH, Sim EH, Goh RY, Park JI, Han JY. Platelet activation: the mechanisms and potential biomarkers. Biomed Res Int. 2016;2016:9060143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao L, Gray L, Leonardi-Bee J, Weaver CS, Heptinstall S, Bath PM. Effect of aspirin, clopidogrel and dipyridamole on soluble markers of vascular function in normal volunteers and patients with prior ischaemic stroke. Platelets. 2006;17(2):100–104. [DOI] [PubMed] [Google Scholar]