Abstract
The renin–angiotensin–aldosterone system is implicated in the pathophysiology of pulmonary arterial hypertension. We undertook this study to determine the effects of spironolactone, a mineralocorticoid receptor blocker, on collagen metabolism in pulmonary arterial hypertension patients. After obtaining institutional review board approval and informed consent, 42 pulmonary arterial hypertension patients were prospectively enrolled and 35 patients completed the 16-week randomized double-blinded crossover clinical trial. Subjects received 50 mg spironolactone or placebo and at the end of week 8, treatment arm was switched. Circulating levels of collagen biomarkers, brain natriuretic peptide, and aldosterone levels were measured, and six-minute walk distance, liver function tests, and echocardiogram data were collected at weeks 0, 8, and 16. Mean age was 45 ± 15 years and 87% were females. At baseline, brain natriuretic peptide and aldosterone levels were 74 ± 95 pg/ml and 7 ± 8 pg/ml, respectively. There was no change in the levels of amino-terminal propeptide of procollagen type III (PIIINP), MMP-9, TIMP-1, and MMP-9/TIMP-1 ratio at weeks 8 and 16 compared to baseline values in placebo arm and treatment arm. The baseline six-min walk distance was 436 ± 115 meters at baseline and no change in walk distance was noted at weeks 8 and 16 (P = 0.372). None of the patients developed hyperkalemia or liver function test abnormalities at weeks 8 and 16 requiring discontinuation of study drug. Our study showed no change in collagen metabolite levels in pulmonary arterial hypertension patients treated with spironolactone. Spironolactone was safe and well tolerated by pulmonary arterial hypertension patients with no increased hyperkalemia or liver function test abnormalities.
Keywords: pulmonary arterial hypertension, echocardiogram, renin angiotensin aldosterone system, spironolactone
Introduction
Pulmonary arterial hypertension (PAH) is characterized by pulmonary vascular remodeling, vascular fibrosis, and collagen deposition in pulmonary vasculature.1 The renin–angiotensin–aldosterone system (RAAS) is an important neurohormonal pathway that augments collagen synthesis in the myocardium and systemic vasculature.2–4 Mineralocorticoid receptor (MR) blocker, spironolactone, has anti-fibrotic properties and shown to be beneficial in patients with systolic left heart failure.3,5,6. Spironolactone is frequently used as a diuretic in PAH.7 Data support RAAS contribution in the pathogenesis of PAH; however, the effects of MR blocker in PAH remains unexplored.8–10 We undertook this pilot study to determine the effect of spironolactone on indices of collagen metabolism and secondarily exercise capacity in a cohort of PAH patients.
Methods
Study design
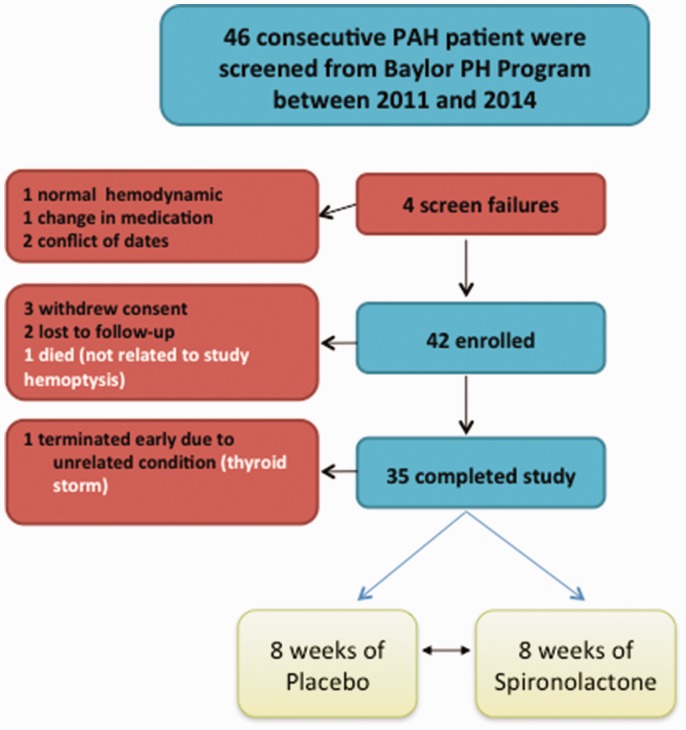
This randomized, single center placebo-controlled, crossover clinical trial was approved by the institutional review boards of Baylor College of Medicine and Houston Methodist Hospital and registered at www.clinicaltrials.gov (NCT01468571). Study monitoring was conducted by a Data and Safety Monitoring Board until completion of the study. The primary endpoint was change in collagen biomarker levels in the spironolactone-treated as compared to placebo. At week 8, patients were blindly switched such that if they were on placebo in the first eight weeks, then they received spironolactone in the second eight weeks till the completion of the study at week 16 (Fig. 1).
Fig. 1.
Enrolled patients.
PAH: pulmonary arterial hypertension.
Study participants
PAH was defined as mean pulmonary artery pressure ≥25 mm Hg and pulmonary capillary wedge pressure ≤15 mm Hg. Inclusion criteria included (1) age of 18 years or older, (2) body weight >40 kg, (3) PAH Diagnostic Group I either: (a) idiopathic, (b) hereditary, (c) related to anorexigen, (d) HIV infection, (e) collagen vascular disease, (f) congenital systemic to portal shunts, (4) stable subjects with no change in PAH specific therapy (no additions, deletions, or dose adjustments) within four weeks of study enrollment, and (5) no change in dose of background therapy (digoxin, diuretic) within two weeks excluding anticoagulation. Exclusion criteria included (1) unable to give informed consent, (2) hemodynamically unstable subjects, (3) pregnant or breast feeding subjects, (4) subjects with significant renal insufficiency (serum creatinine >2.5 mg per deciliter or required hemodialysis), (5) subjects with significant liver dysfunction (AST or ALT more than three times upper limit of normal), (6) subjects currently on aldosterone receptor blocker (spironolactone or eplerenone) or angiotensin-converting enzyme (ACE) inhibitor, (7) subjects with pulmonary hypertension (PH) due to left heart disease (Group 2–5 PH were excluded), and (8) subjects unable or unwilling to comply with study procedures.
Study procedures
Assessments
Before initiation of study medication (spironolactone or placebo), patients underwent baseline clinical and World Health Organization functional class (WHO FC) assessments, six-minute walk test (6MWT), echocardiography, and blood testing. In addition to baseline, clinical assessment was also performed at weeks 8 and 16, and telephone assessment was undertaken at weeks 4 and 12. Echocardiography was repeated at weeks 8 and 16.
Therapy
Study procedures are listed in Table 1. Patients were randomized to receive 50 mg spironolactone or placebo, and at week 8, this assignment was switched. Subjects were randomized 1:1 in blocks of four to treatment with spironolactone or placebo. Blinding of the study medication and assigning a randomization number was done by research pharmacy. Blocked randomization ensured that an equal number of subjects were assigned to the two treatment arms at mid-point and at study completion. Vital signs were checked before study drug administration and then at 30 min after study drug administration. Adverse events and concomitant medication information was collected. Study drug was dispensed for the next eight weeks and the patient was instructed to take each tablet every morning. At the follow up visit, drug accountability was performed and each tablet was accounted for. If all tablets were not taken, then the remaining study drug was taken from the patient and the number recorded and importance of compliance was reinforced.
Table 1.
Time events table.
| Procedure | Baseline | Week 4 | Week 8 | Week 12 | Week 16 |
|---|---|---|---|---|---|
| Informed consent | × | ||||
| Telephone contact, check for adverse events | × | × | × | × | × |
| Study clinic visit | × | × | × | ||
| Serum electrolytes | × | × | × | × | × |
| Echocardiogram | × | × | × | ||
| Six-min walk test, Borg dyspnea score | × | × | × | ||
| WHO functional class assessments | × | × | × | ||
| Serum biomarkers | × | × | × |
WHO: World Health Organization.
Biochemical measurements of indices of collagen metabolism
Biomarker levels were drawn three times (baseline, week 8, and week 16) during the study period for each subject. Blood drawn from a peripheral vein was transferred immediately into a glass tube and allowed to clot. Serum was separated from the blood cells by centrifugation at 3000 rpm for 10 min; supernatant was collected and immediately stored at –80℃ until simultaneous analysis. Blood was also collected in EDTA collection tubes, centrifuged, and plasma was stored at –20℃ for analysis. Enzyme-linked immunosorbent assays (ELISAs) were used to determine serum carboxy-terminal telopeptide of collagen type I levels (CITP EIA) (Orion Diagnostica, Finland, purchased through Immunodiagnostic System, Fountain Hills, Arizona). PIIINP levels were measured using double antibody radioimmunoassays according to manufacturer’s specifications (Orion Diagnostica, Finland, purchased through Immunodiagnostic Systems Inc., Fountain Hills, Arizona). The sensitivity (lower detection limit) of both the CITP and the PIIINP assays were approximately 0.3 ng/ml. Coefficients of variation (CV) derived from duplicate measurements were <10% for both CITP and PIIINP assays. Serum MMP-9 and TIMP-1 levels were measured using two-site sandwich ELISAs (R & D Systems, Inc., Minneapolis, MN) per manufacturer’s protocol. The MMP-9 assay (DMP900, sensitivity <0.156 ng/ml) was designed to measure both the Pro-92 kDa and the active 82 kDa form of MMP-9 in human serum. The TIMP-1 assay (DTM100, sensitivity was <0.08 ng/ml) measures all forms of serum TIMP-1. CV from duplicate measurements were <5% for both MMP-9 and TIMP-1 assays. Commercially available immunoassay was used for quantitative brain natriuretic peptide (BNP) determination on an ADVIA Centaur analyzer system according to the manufacturer’s recommendation. Sensitivity of the assay was <2.0–5000 pg/ml. Normal BNP value at our laboratory was ≤100 pg/ml.
Two-dimensional echocardiography and M-mode imaging
Two-dimensional echocardiography and M-mode imaging were performed at baseline and at weeks 8 and 16. The results were analyzed by a cardiologist in a blinded fashion at Baylor College of Medicine.
Six-minute walk test
The 6MWT was performed at each study visit using a standardized protocol in accordance to the American Thoracic Society statement.11 The total distance walked was recorded and rounded to the nearest meter.12
Statistical data analysis
The primary end-point of the study was to determine the change in biomarker levels (PIIINP, MMP-9, and CITP levels, and ratio of MMP-9/TIMP-1) in patients treated with low dose of spironolactone as compared to placebo. We tested for carry over effects using repeated measure analysis of variance (ANOVA) with treatment group, order of treatment, and group by order interaction in the model. Secondary outcomes included change in six-min walk distance (6MWD), and composite end-point from baseline to weeks 8 and 16. Biomarker levels were drawn three times (baseline, week 8, and week 16) during the study period for each subject. Composite end-point was defined as greater than 10% increase in walk distance, improvement by at least one functional class and absence of clinical worsening.13 Clinical worsening was defined as hospitalization for worsening PAH, all-cause death, addition of prostacyclin therapy, lung transplantation, or atrial septostomy.14,15
Data are presented as mean SD, range, or percentage. Statistical analysis was performed on all patients who achieved week 16, and analysis was intent-to-treat and a p value <0.05 was considered significant. Comparisons were made using paired or unpaired t tests (normally distributed data) or nonparametric analysis, and Chi-square test (for the change in walk distance, WHO FC), as appropriate. Changes in echocardiographic measures and BNP levels are presented descriptively. Traditional repeated measures ANOVA as well as mixed model ANOVA models were used to evaluate changes in biomarker levels. All analyses were performed using available data; no imputation was performed.
Study dose rationale
Addition of low dose spironolactone (non-natriuretic dose) to ACE inhibitor has been shown to improve heart function in the Randomized Aldactone Evaluation Study (RALES) and improve morbidity and mortality in severe CHF.16 The dose of spironolactone used was 25–75 mg daily (minimally natriuretic dose) and these subjects were on maximum ACE inhibitors. In CHF trials, despite normal circulating aldosterone levels, a beneficial effect of spironolactone was seen on morbidity and mortality.16
Sample size rationale
The half-life of spironolactone is short and in healthy volunteers taking 100 mg daily of spironolactone for 15 days the half-life was 1 h. The changes seen in PIIINP in a study by MacFadyen et al. was a difference of approximately 1 µg/L between spironolactone (50–100 mg/day) and placebo at week 8.17 The standard deviation was noted to be 1, requiring 18 per group. This is based on power of 0.80 and alpha of 0.05. Based on this data, we would require 25 subjects in each arm assuming an attrition rate of 20% (drop out and missing data points) as seen in RALES study.16 Hence, our study was adequately powered to test differences in PIIINP levels.
Results
Study population
A total of 46 consecutive PAH patients were screened, 42 patients who met the inclusion and exclusion criteria were enrolled (Fig. 2). Thirty-five patients completed the study. In general, patients were middle-aged and the majority were females with no significant renal or liver impairment. The main clinical and hemodynamic characteristics are outlined in Tables 2 and 3. Two patients were WHO FC I, 21 were WHO FC II, and 12 were WHO FC III at the time of enrollment. Background and concomitant medications are outlined in Table 3.
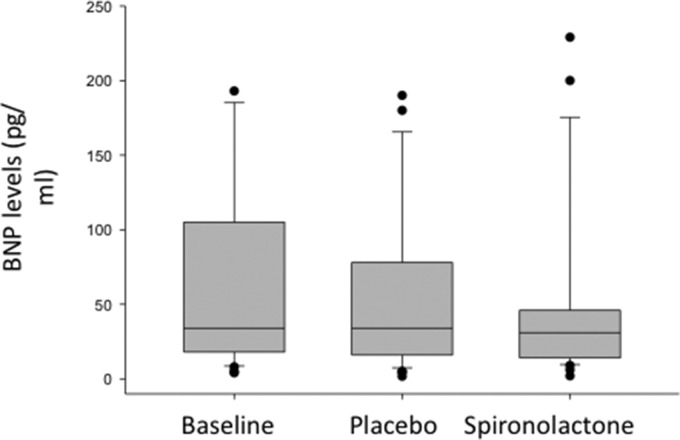
Fig. 2.
BNP levels at baseline and in placebo and spironolactone patients.
BNP: brain natriuretic peptide.
Table 2.
Baseline clinical characteristics, and functional and exercise capacity of enrolled patients.
| Variables | Baseline characteristics |
|---|---|
| N | 42 |
| Age (yrs) | 45 ± 15 |
| Gender (female, n) | 36 |
| Duration of disease (yrs) | 4.5 ± 4.1 |
| JVD present (Y/N) | 12/30 |
| Edema (Y/N) | 23/19 |
| BSA (m2) | 1.77 ± 0.20 |
| Pulse pressure (mm Hg) | 38 ± 11 |
| Race (n) | |
| Caucasian | 24 |
| Hispanic | 10 |
| African American | 6 |
| Asian | 2 |
| Etiology (n) | |
| IPAH | 19 |
| CVD | 12 |
| CHD | 6 |
| HIV | 1 |
| Hereditary | 4 |
| WHO FC (I, II/III), n | 3/24/15 |
| Six-min walk distance, m | 437 ± 109 |
| Borg dyspnea score | 2.5 ± 1.7 |
BSA: body surface area; BUN: blood urea nitrogen; CCB: calcium channel blocker; CHD: congenital heart disease; CVD: collagen vascular disease; GFR: glomerular filtration rate; IPAH: idiopathic pulmonary arterial hypertension; JVD: jugular vein distention; PDE-5 inh: phosphodiesterase 5 inhibitor; PG: prostacyclin analog; WHO FC: World Health Organization functional classification.
Table 3.
Hemodynamics and medications of enrolled patients.
| Echocardiographic parameters | |
|---|---|
| RVSP (mm Hg) | 65 ± 26 |
| RAP (mm Hg) | 8 ± 4 |
| CI (L.min.m2) | 4.32 ± 0.94 |
| TAPSE (cm) | 2.44 ± 0.55 |
| Pericardial effusion (Y/N) | 4/38 |
| PAH medications |
No. of patients |
| PG | 2 |
| ERA | 5 |
| PDE-5 inh | 1 |
| ERA + PDE-5 inh | 17 |
| ERA, PG | 5 |
| PDE-5 inh + PG | 1 |
| ERA + PDE-5 inh + PG | 8 |
| CCB | 3 |
| Concomitant medications | |
| CCB | 7 |
| Digoxin | 16 |
| Coumadin | 12 |
| Diuretic (loop diuretic) | 15 |
CCB: calcium channel blocker; CI: cardiac index; ERA: endothelin receptor blocker; PDE-5 inh: phosphodiesterase 5 inhibitor; PG: prostacyclin analog; RAP: right atrial pressure; RVSP: right ventricular systolic pressure; TAPSE: tricuspid annular plane systolic excursion.
Dosing
At week 8, treatment arms of the patients were blindly switched (placebo or spironolactone) as per randomization protocol. The 50 mg dose of spironolactone was well-tolerated and none of the patients had to discontinue study or study drug due to side-effects. Compliance was above 80% during the study.
Primary endpoint
Biomarker levels were checked at baseline, week 8, and week 16 study visits. There was no change in PIIINP, MMP-9 and TIMP-1 levels, and MMP-9/TIMP-1 ratio at week 8 and week 16 as compared to baseline values in placebo arm and treatment arm (Table 4).
Table 4.
Biomarker levels at baseline, in placebo, and spironolactone-treated patients.
| MMP-9 (ng/ml) | TIMP-1 (ng/ml) | MMP/TIMP | PIIINP (ng/ml) | Aldosterone (pg/ml) | |
|---|---|---|---|---|---|
| Placebo | 267 ± 193 | 161 ± 44 | 1.64 ± 1.01 | 11.28 ± 3.95 | 10 ± 12 |
| Spironolactone | 264 ± 142 | 151 ± 32 | 1.80 ± 0.99 | 13.48 ± 9.66 | 22 ± 23 |
| P value | NS | NS | NS | NS | 0.001 |
MMP-9: matrix metalloproteinsase 9; PIIINP: amino-terminal propeptide of procollagen type III levels; TIMP-1: tissue inhibitor of metalloproteinase 1.
Changes in biomarker levels and electrolyte levels
There was no difference in electrolyte levels, renal function, and liver function tests over the course of the study as outlined in Table 5. Sodium, potassium, creatinine, AST, and ALT remains unchanged at week 4, 8, 12, and 16 compared to baseline (Table 5). BNP level was 74 ± 95 pg/ml and aldosterone level was 7 ± 8 ng/dl at baseline. BNP levels remained unchanged at week 8 (at crossover) and at end of study (p = 0.72) as compared to baseline (Fig. 2), whereas aldosterone level increased in the treatment arm (10 ± 12 versus 22 ± 23 ng/dl, P = 0.001).
Table 5.
Electrolyte levels at baseline, in placebo, and spironolactone-treated patients.
| AST (U/L) | ALT (U/L) | BUN (mg/dl) | Creatinine (mg/dl) | GFR (ml/min/1.73 m2) | Na (mEq/L) | K (mEq/L) | |
|---|---|---|---|---|---|---|---|
| Placebo | 27 ± 12 | 23 ± 14 | 14 ± 3 | 0.83 ± 0.17 | 85 ± 20 | 142 ± 3 | 4.33 ± 0.36 |
| Spironolactone | 25 ± 14 | 21 ± 14 | 13 ± 4 | 0.85 ± 0.18 | 82 ± 20 | 141 ± 3 | 4.38 ± 0.37 |
| P value | NS | NS | NS | NS | NS | 0.041 | NS |
AST: aspartate aminotransferase; ALT: alanine aminotransferase; GFR: glomerular filtration rate; K: potassium; Na: sodium.
Changes in exercise capacity and echocardiographic parameters
The baseline 6MWD was 436 ± 115 m at baseline and no change in walk distance was noted at weeks 8 and 16 as compared to baseline values (p = 0.372) (Table 6). Borg dyspnea score remained unchanged at weeks 8 and 16 as compared to baseline (p = 0.552) and lowest oxygen saturation was 87 ± 6%, 83 ± 10%, and 86 ± 9% at baseline, week 8, and week 16 respectively. There was no change in the noninvasive assessment over the course of the study (Table 7).
Table 6.
Exercise and functional parameters at baseline, in placebo, and spironolactone-treated patients.
| 6MWD (meters) | Borg dyspnea score | Max HR (b/min) | Minimal Oxygen saturation (%) | WHO Functional class | BMI (kg/m2) | Mean arterial pressure (mmHg) | |
|---|---|---|---|---|---|---|---|
| Placebo | 434 ± 113 | 2 ± 2 | 119 ± 29 | 83 ± 10 | 2 ± 1 | 28 ± 5 | 85 ± 12 |
| Spironolactone | 441 ± 107 | 2 ± 2 | 116 ± 20 | 86 ± 9 | 2 ± 1 | 27 ± 7 | 86 ± 9 |
| P value | NS | NS | NS | NS | NS | NS | NS |
6MWD: six-minute walk distance; BMI: body mass index; Max HR: maximum heart rate; WHO FC: World Health Organization functional classification.
Table 7.
Hemodynamic parameters at baseline, in placebo and spironolactone-treated patients.
| RA Areas (cm2) | RAP (mm Hg) | TR Peak Velocity (msec) | RVSP (mmHg) | TAPSE (cm) | RV S’ Prime (cm/sec) | RVOT Acc Time AT(msec) | CI (L/min.m2) | Pericardial effusion (present) | |
|---|---|---|---|---|---|---|---|---|---|
| Placebo | 18 ± 1 | 7 ± 0.5 | 3.7 ± 0.14 | 65 ± 5 | 1.91 ± 0.1 | 10.95 ± 0.39 | 85 ± 3.09 | 2.5 ± 0.1 | 6 |
| Spironolactone | 19 ± 1 | 7 ± 0.6 | 3.7 ± 0.14 | 66 ± 5 | 1.85 ± 0.1 | 11.27 ± 0.41 | 88 ± 3.06 | 2.4 ± 0.1 | 6 |
| P value | NS | NS | NS | NS | NS | NS | NS | NS | NS |
CI: cardiac index; RAP: right atrial pressure; RV: right ventricle; RVOT: right ventricular outflow tract; RVSP: right ventricular systolic pressure; S : myocardial systolic excursion velocity; TAPSE: tricuspid annular plane systolic excursion; TR: tricuspid regurgitation.
Composite end-point and clinical worsening
The composite end-point, defined as greater than 10% increase in walk distance, improvement by at least one functional class and absence of clinical worsening was not met during the study period. There were no hospitalizations for worsening PAH, addition of prostacyclin therapy, lung transplantation, or atrial septostomy during the study period. There was one death unrelated to PAH or disease worsening.
Safety
Three patients withdrew consent after enrolling in the study, two were lost to follow-up (did not show up for their study appointment), one patient died of unrelated to study medication (hemoptysis), and one patient terminated early due to unrelated medical condition (hyperthyroidism). The side-effect reported included shortness of breath, edema, congestion, cough, and palpitations; however, there was no difference between the study periods as outlined in Table 8. None of the patients complained of breast pain or developed gynecomastia during the duration of this study.
Table 8.
Adverse events reported by >3% of patients.
| Adverse event | Placebo | Spironolactone |
|---|---|---|
| Shortness of breath | 11 | 10 |
| Edema | 10 | 10 |
| Congestion | 10 | 10 |
| Cough | 7 | 4 |
| Palpitations | 6 | 7 |
Discussion
In this cross-over study, each patient received spironolactone and placebo in a randomize order for at least eight weeks. The primary endpoint was change in collagen biomarker level, and this study was powered to detect changes in PIIINP as previously shown in heart failure studies.13–17 In our study, collagen biomarker levels remained unchanged at weeks 8 and 16. This lack of change could be attributed to the short duration of spironolactone exposure and a longer duration of treatment may be warranted. Nevertheless, this proof of concept study showed that spironolactone was well tolerated by PAH patients with no worsening of renal or liver function albeit these patients had normal baseline renal and liver functions at study enrollment.
Spironolactone is an aldosterone receptor blocker that competitively binds to the MR and is activated by aldosterone. MR blockage reduces cerebral, renal, and coronary vascular inflammation in response to elevated aldosterone concentration in animals. Spironolactone has multiple effects on vascular remodeling in humans.18 Administration of spironolactone for one month improved forearm blood flow in response to acetylcholine in chronic heart failure patients, indicating improved endothelial dysfunction.18 Furthermore, the circulating concentration of PIIINP, a marker of vascular collagen turnover, was reduced by spironolactone in stable CHF patients.19,20 In the RALES, adding spironolactone (non-diuretic dose) to the standard therapy of ACE inhibitor and loop diuretic for 12 weeks improved heart failure in severe systolic heart failure patients, treatment for 24 months reduced morbidity and mortality.21 In the RALES study, many subjects were on an ACE inhibitor and treatment with 25–75 mg of spironolactone was shown to be effective.21 Although the circulating aldosterone level was normal in these trials, a beneficial effect of aldosterone receptor blockage was seen on morbidity and mortality. It is proposed that the effects of aldosterone depend on the underlying oxidative states and that in cases of pathological vascular condition accompanied by high oxidative stress as seen in PAH; aldosterone receptor blockage may be beneficial.
Role of spironolactone in vascular remodeling
Elevated aldosterone levels were previously shown in PAH subjects with no evidence of left heart dysfunction to be associated with disease severity.4,9 Local production of aldosterone has been documented in human pulmonary arteries and in smooth muscle and endothelial cells cultivated from human pulmonary arteries.9 Furthermore, aldosterone administration in rats was shown to increase collagen deposition in pulmonary arteries.8 Pro-fibrotic effects of aldosterone are mediated through activation of TGF-beta-Smad2/3 signaling.22 Circulating levels of PIIINP, a marker of vascular collagen synthesis, was shown to be reduced by spironolactone in patients with stable CHF.19 Together, these data indicate that RAAS and ongoing vascular fibrosis may play an important role in the pathogenesis of PAH.
In CHF, subjects on maximum ACE-inhibitor, with evidence of complete inhibition of vascular converting enzyme, had elevated circulating aldosterone levels or “aldosterone escape”.23 Addition of low dose spironolactone (non-natriuretic dose) to ACE inhibitor was shown to improve heart function in the RALES trial and improve morbidity and mortality in severe CHF. It is important to note that in these trials, the dose of spironolactone was 25–75 mg daily (minimally natriuretic dose) and these subjects were on maximum ACE inhibitors. In CHF trials, despite normal circulating aldosterone levels, a beneficial effect of spironolactone was seen on morbidity and mortality. RAAS is the target of pharmacological manipulation with success in left heart failure.21 However, the role of aldosterone receptor blockage in PAH remains unexplored.
Spironolactone dose and duration of exposure
The spironolactone dose used in our study was 50 mg per day and spironolactone dose reported in the retrospective database analysis of AIRES data (Ambrisentan in Patients With Moderate to Severe Pulmonary Arterial Hypertension) was 19–54 mg daily (mean dose was 31 mg in the ambrisentan group and 39 mg in the placebo group).24 It is important to realize that the analysis had only 10 patients in the ambrisentan + spironolactone group, those patients were sicker (higher FC, worse walk) than in the ambrisentan only group. In addition, the ARIES study was an intervention with a drug (ambrisentan and Endothelin A receptor blocker) which has been demonstrated to increase aldosterone levels, and hence the addition of an aldosterone receptor antagonist would be expected to mitigate otherwise adverse clinical outcomes. In addition and importantly one of the first studies done to assess the impact of aldosterone blockade on cardiovascular outcomes reported reduction in markers of collagen turnover, with an eight-week exposure preceded by a four-week run in stabilization period.17
PAH patients had to be on stable PAH meds for four weeks before study enrollment and no change in background medication for >2 weeks could enroll. As this was a double-blind cross-over study with the primary objective change indices of collagen metabolism, and the patients served as their own controls, we used a shorter duration of exposure. Incidentally, most of the patients were on stable PAH meds for longer than four weeks.
Forty-two patients were enrolled in our study, 35 patients completed the study, and the completion rate was 83%. Four patients were screen failure (one did not qualify due normal hemodynamics, one had recent changes in medications, and two refused due to conflict in schedule). Three patients withdrew consent after signing consent form. One patient due to time commitment (started a new job and also started school). Second patient withdrew consent as felt more tired and fatigued after eight weeks of medication. It was explained to the patient that this was a crossover study and same study medication will not be continued; however, patient still withdrew consent and no further study-related procedures were undertaken. Unblinding after study completion showed that this patient was on the active drug for those eight weeks (that is spironolactone 50 mg daily). Third patient withdrew consent after signing consent form due to conflict with dates, and no study-related procedures were undertaken.
Spironolactone and collagen biomarkers
In our study, no change in collagen biomarkers level was seen, although in the stable patient with CHF, circulating levels of PIIINP, a marker of vascular collagen synthesis, was shown to be reduced by spironolactone. It could be the duration of exposure to spironolactone is too short and a longer exposure may be needed. A longer duration of study will be able to address this. The dose of spironolactone used may be low and a higher dose may need to be used; however, in both heart failure trials (RALES and Ephesus), the mean spironolactone dose was 37.5 mg, which is similar to the dose that was used in this study.
In the RALES, adding spironolactone (non-diuretic dose) to the standard therapy of ACE inhibitor and loop diuretic for 12 weeks improved heart failure in severe systolic heart failure patients16; treatment with 25 mg daily of spironolactone for 24 months reduced morbidity and mortality.21 Although the circulating aldosterone level was normal in these trials, a beneficial effect of aldosterone receptor blockage was seen.16,25
Electrolyte levels
Hyperkalemia is a known side-effect of spironolactone treatment that was not seen in our patients. No changes in renal function or sodium level was noted.
Limitation of the study
In most studies, patients have to be on stable PAH medications for 12 weeks. However, in our study, PAH patients had to be on stable PAH meds for four weeks before study enrollment.
No change in PAH medications was allowed and no change in background medication for >2 weeks could enroll. We used four weeks stable PAH meds as this was a single-center study and most of these were on stable PAH meds for longer than four weeks.
We did not see an effect on collagen metabolism in our study. Part of the reason may be that though the data would suggest that the impact on collagen metabolism in primary cardiac disorders can be detected at eight weeks, it may well be that the collagen metabolism changes in the pulmonary vascular bed are either (1) not affected in a meaningful way by aldosterone antagonists at all or that (2) higher doses are needed to impact the vascular bed or that (3) longer duration of exposure is required. All we can conclude is that doses higher than those used in the RALES study and in MacFadyen’s study on collagen metabolism in heart failure did not clinically or metabolically measurably impact PH patients. A study looking at a longer duration of spironolactone exposure in PAH patients may be needed to explore this possibility.
Conclusions
Our study failed to show changes in collagen biomarker levels with spironolactone treatment in PAH patients. However, the use of spironolactone was well tolerated.
Acknowledgements
The authors thank Ms. Janice Brister for providing administrative support, Praveen Konasagar in patient recruitment, and Yana Kisarova for technical support.
Author contributions
ZS and AF were involved in the study design, conduct of the study, data analysis, and manuscript preparation. AB was involved in study design, echocardiographic measurements, data review, and manuscript preparation. AD and ME were involved in study design, data analysis, and manuscript preparation. BS was involved in statistical analysis and manuscript preparation.
Conflict of interest
The author(s) declare that there is no conflict of interest related to the subject of this study.
Ethical approval
This study was approved by institutional review board of Baylor College of Medicine Houston Methodist Hospital and registered at www.clinicaltrials.gov (NCT01468571).
Funding
This study was funded by a NIH-NHLBI -K23 grant to ZS.
ORCID iD
Zeenat Safdar https://orcid.org/0000-0002-8984-8205
References
- 1.Eddahibi S, Morrell N, d’Ortho MP, et al. Pathobiology of pulmonary arterial hypertension. Eur Respir J 2002; 20: 1559–1572. [DOI] [PubMed] [Google Scholar]
- 2.Nijst P, Verbrugge FH, Martens P, et al. Plasma renin activity in patients with heart failure and reduced ejection fraction on optimal medical therapy. J Renin Angiotensin Aldosterone Syst 2017; 18: 1470320317729919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brilla CG. Aldosterone and myocardial fibrosis in heart failure. Herz 2000; 25: 299–306. [DOI] [PubMed] [Google Scholar]
- 4.Safdar Z, Thakur A, Singh S, et al. Circulating aldosterone levels and disease severity in pulmonary arterial hypertension. J Pulm Respir Med 2015; 5: pii: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey A, Garg S, Matulevicius SA, et al. Effect of mineralocorticoid receptor antagonists on cardiac structure and function in patients with diastolic dysfunction and heart failure with preserved ejection fraction: a meta-analysis and systematic review. J Am Heart Assoc 2015; 4: e002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitt B, Pfeffer MA, Assmann SF, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med 2014; 370: 1383–1392. [DOI] [PubMed] [Google Scholar]
- 7.Safdar Z, Tamez E, Chan W, et al. Circulating collagen biomarkers as indicators of disease severity in pulmonary arterial hypertension. JACC Heart Fail 2014; 2: 412–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Man FS, Tu L, Handoko ML, et al. Dysregulated renin-angiotensin-aldosterone system contributes to pulmonary arterial hypertension. Am J Respir Crit Care Med 2012; 186: 780–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maron BA, Opotowsky AR, Landzberg MJ, et al. Plasma aldosterone levels are elevated in patients with pulmonary arterial hypertension in the absence of left ventricular heart failure: a pilot study. Eur J Heart Fail 2013; 15: 277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Handoko ML, de Man FS, Allaart CP, et al. Perspectives on novel therapeutic strategies for right heart failure in pulmonary arterial hypertension: lessons from the left heart. Eur Respir Rev 2010; 19: 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 12.Kawut SM, Bagiella E, Shimbo D, et al. Rationale and design of a phase II clinical trial of aspirin and simvastatin for the treatment of pulmonary arterial hypertension: ASA-STAT. Contemp Clin Trials 2011; 32: 280–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olschewski H, Simonneau G, Galie N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347: 322–329. [DOI] [PubMed] [Google Scholar]
- 14.Barst RJ, Langleben D, Frost A, et al. Sitaxsentan therapy for pulmonary arterial hypertension. Am J Respir Crit Care Med 2004; 169: 441–447. [DOI] [PubMed] [Google Scholar]
- 15.Rubin LJ, Badesch DB, Barst RJ, et al. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med 2002; 346: 896–903. [DOI] [PubMed] [Google Scholar]
- 16.Effectiveness of spironolactone added to an angiotensin-converting enzyme inhibitor and a loop diuretic for severe chronic congestive heart failure (the Randomized Aldactone Evaluation Study [RALES]). Am J Cardiol 1996; 78: 902–907. [DOI] [PubMed]
- 17.MacFadyen RJ, Barr CS, Struthers AD. Aldosterone blockade reduces vascular collagen turnover, improves heart rate variability and reduces early morning rise in heart rate in heart failure patients. Cardiovasc Res 1997; 35: 30–34. [DOI] [PubMed] [Google Scholar]
- 18.Farquharson CA, Struthers AD. Spironolactone increases nitric oxide bioactivity, improves endothelial vasodilator dysfunction, and suppresses vascular angiotensin I/angiotensin II conversion in patients with chronic heart failure. Circulation 2000; 101: 594–597. [DOI] [PubMed] [Google Scholar]
- 19.Zannad F, Alla F, Dousset B, et al. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the randomized aldactone evaluation study (RALES). Rales Investigators. Circulation 2000; 102: 2700–2706. [DOI] [PubMed] [Google Scholar]
- 20.Tsutamoto T, Wada A, Maeda K, et al. Effect of spironolactone on plasma brain natriuretic peptide and left ventricular remodeling in patients with congestive heart failure. J Am Coll Cardiol 2001; 37: 1228–1233. [DOI] [PubMed] [Google Scholar]
- 21.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341: 709–717. [DOI] [PubMed] [Google Scholar]
- 22.Yan Y, Wang C, Lu Y, et al. Mineralocorticoid receptor antagonism protects the aorta from vascular smooth muscle cell proliferation and collagen deposition in a rat model of adrenal aldosterone-producing adenoma. J Physiol Biochem 2018; 74: 17–24. [DOI] [PubMed] [Google Scholar]
- 23.Pitt B. “Escape” of aldosterone production in patients with left ventricular dysfunction treated with an angiotensin converting enzyme inhibitor: implications for therapy. Cardiovasc Drugs Ther 1995; 9: 145–149. [DOI] [PubMed] [Google Scholar]
- 24.Maron BA, Waxman AB, Opotowsky AR, et al. Effectiveness of spironolactone plus ambrisentan for treatment of pulmonary arterial hypertension (from the [ARIES] study 1 and 2 trials). Am J Cardiol 2013; 112: 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pitt B, Williams G, Remme W, et al. The EPHESUS trial: eplerenone in patients with heart failure due to systolic dysfunction complicating acute myocardial infarction. Eplerenone Post-AMI Heart Failure Efficacy and Survival Study. Cardiovasc Drugs Ther 2001; 15: 79–87. [DOI] [PubMed] [Google Scholar]