Abstract
Recent decades mark a great progress in the treatment of HIV infection. What was once a deadly disease is now a chronic infection. However, HIV-infected patients are prone to develop comorbidities, which severely affect their daily functions. For example, a large population of patients develop a variety of neurological and cognitive complications, called HIV associated neurological disorders (HAND). Despite efficient repression of viral replication in the periphery, evidence shows that the virus can remain active in the central nervous system (CNS). This low level of replication is believed to result in a progression of neurocognitive dysfunction in infected individuals. Insufficient viral inhibition in the brain results from the inability of several treatment drugs in crossing the blood-brain barrier (BBB) and reaching therapeutic concentrations in the CNS. The current manuscript discusses several strategies that are being developed to enable therapeutics to cross the BBB, including bypassing BBB, inhibition of efflux transporters, the use of active transporters present at the BBB, and nanotechnology. The increased concentration of therapeutics in the CNS is desirable to prevent viral replication; however, potential side effects of anti-retroviral drugs need also to be taken into consideration.
Keywords: HIV, Blood brain barrier, anti-retroviral drugs, central nervous system, viral reservoir, nanoparticles
1. INTRODUCTION
Natural progression of HIV disease is associated with a gradual exhaustion of the immune system and a rise in complications such as opportunistic infections or other comorbidities. HIV can also cross the blood brain barrier and infect the central nervous system (CNS), which results in a wide array of complications, ranging from HIV associated dementia (HAD) to asymptomatic neurocognitive impairment (ANI) [1]. The introduction of highly active anti-retroviral therapy (HAART) has drastically reduced the severity of neurological diseases, but has not reduced their prevalence [2]. This indicates a need for a comprehensive treatment approach to ameliorate current therapies to target HIV replication in the CNS.
The blood brain barrier (BBB) is a highly selective barrier that restricts the passage of elements from the circulatory system to the CNS. This restriction prevents toxic molecules, viruses, bacteria and inflammatory cells from reaching the CNS, which could damage the brain. While BBB is important in preventing CNS infection, it also hinder the treatment of brain pathology. Crossing the BBB has proved to be a major obstacle in treatment of a variety of brain diseases, from viral and bacterial infections to cancer or brain metastasis [3, 4].
The successful delivery of drugs to the CNS is highly dependent on the structure of the molecule. In the treatment of HIV, several anti-retroviral drugs have been analyzed to identify their ability to cross the BBB. While some demonstrate a relatively high CNS penetration-effectiveness (CPE), a high proportion of therapeutics show low to poor CPE (see table 1). This hinders the efficiency of treatment and increases the probability of drug resistance due to CNS HIV replication at sub-optimal drug levels [5].
Table 1:
CNS penetrating efficiency (CPE) of anti-retroviral drugs used in HIV treatment.
| CNS penetrating efficiency (CPE) | |||||
|---|---|---|---|---|---|
| Drug Class | 4 | 3 | 2 | 1 | Non-classified |
| Nucleoside Reverse Transcriptase Inhibitors | Zidovudine | Abacavir | Didanosine | Tenofovir | |
| Emtricitabine | Stavudine | Zalcitabine | |||
| Lamivudine | |||||
| Nonnucleoside Reverse Transcriptase Inhibitors | Nevirapine | Delavirdine | Etravirine | Rilpivirine: CSF/Plasma ratio 1.2–1.6% but above IC50 | |
| Efavirenz | |||||
| Protease | Indinavir/r | Darunavir/r | Atazanavir | Nelfinavir | |
| Inhibitors | Fosamprenavir/r | Atazanavir/r | Ritonavir | ||
| Indinavir | Fosamprenavir/r | Saquinavir | |||
| Lopinavir/r | Saquinavir/r | ||||
| Tipranavir/r | |||||
| Entry/Fusion | Vicriviroc | Maraviroc | Enfuvirtide | ||
| Inhibitors | |||||
| Integrase | Raltegravir | Dolutrgravir: CSF/Plasma ratio 0.467–0.546% but above IC50 | |||
| Inhibitors | |||||
| Elvitegravir: | |||||
| No data (clinical study underway UCSD, Letendre S.) | |||||
1.1. Blood brain barrier
The BBB is mainly composed of brain microvascular endothelial cells (BMEC) that form a highly sealed layer around the brain circulatory system to control the exchange of charged molecules of more than 400 Da [6]. This restricts the passage of proteins and nutrients, but also of drugs used in the treatment of neuroinfections. The tightness on this barrier is controlled by multiple proteins implicated in the formation of tight junctions. The main effectors in the BBB tight junctions are claudins (especially claudin-5), which are transmembrane proteins that are implicated in sealing of paracellular space. This structure is also composed of intracellular proteins which regulate tight junction tightness and its link with the cytoskeleton. Proteins such as zona occludens-1, 2, and 3, along with cingulin, act as adaptor molecules between membrane proteins and the cytoskeleton. In addition, occludin, junctional adhesion molecules (JAMs), and adherens junction proteins (PECAM-1, VE-cadherin and caveolin-1) play a role in tight junction regulation [7].
Due to the highly restrictive nature of BBB, several transport mechanisms are present to supply the CNS with nutrients and preserve tissue homeostasis. Several transporters are expressed on the surface of BMEC. GLUT1 is responsible for the transport of glucose and is tightly regulated in response to metabolic needs in the CNS, as demonstrated by the distinct and dynamic distribution of this transporter on both sides of the endothelium [8]. In addition, several amino acid transporters are expressed on both apical and basolateral sides of BMEC [9]. Ion equilibrium across the BBB is maintained using ion transporters for sodium, potassium and chloride. Several transporters in the BBB also function to protect the CNS from toxic substances. Some amino acid transporters are exclusively expressed on the basolateral side to remove excess amino acids from the CNS [10]. BMEC also express ABC transporters, such as P-glycoprotein 1 (P-gp), that pump drugs and other harmful substances back into the circulation [11]. The harmonious action of these transporters preserves CNS microenvironment integrity, supplies it with nutrients and prevents the entry of toxic molecules.
The layer formed by brain endothelial cells is surrounded by a basement membrane that primary functions to maintain the integrity of the BBB. The luminal layer consists of collagen (mainly isoform IV) and laminins (mainly α4β1γ1 and α5β1γ1 to a lesser degree). The abluminal layer is associated with the brain parenchyma and is produced by astrocytes. It is mainly composed of laminin α1β1γ1 and α2β1γ1. The equilibrium between the luminal and abluminal layers is essential to restrict leukocyte migration [12]. The two layers are linked by the small matrix proteins called nidogen and perlecan [13–15]. The ensemble maintains BBB integrity by providing an anchor substrate for cells of the neurovascular unit (BMEC, astrocytes and pericytes) and a region for cell-cell interaction [16, 17]. This anchoring is imparted using integrins, which can result in intracellular signaling by the FAK and MAPK pathways, affecting cellular proliferation and differentiation [18].
On the brain (i.e., parenchymal) side of the basement membrane, astrocytes and pericytes are the main components of the BBB. They are adjacent to BMEC and have an important role in regulating TJ tightness and the basement membrane integrity. Astrocytes are in direct contact with BMEC using their end-feet projections and cover almost the entire brain side of the BBB [19]. This expanse is discontinuous in only few areas to allow interactions of other cells, such as microglial, neurons and other glial cells, with the BBB. Astrocytes act as a second layer of restriction to molecules transported across this layer and also secrete factors of their own (TGF-β, bFGF and GDNF) to influence BMEC functions [20]. Disruption of this cell-cell communication, often observed in infections and inflammation, can lead to BBB disruption, highlighting the importance of this mechanism in the maintenance of its integrity [21].
Another cells of the neurovascular unit are pericytes. They play an important role in cerebral blood flow via their contractile ability, and are important in maintaining BBB integrity by affecting differentiation of BMEC and angiogenesis [22–24]. Pericytes also influence maturation of brain microvessels. The loss of pericytes results in the accumulation of toxic molecules in the CNS, arteriovenous malformation, and promotes the development of neurodegenerative disease [25, 26]. Evidence demonstrates that pericytes have a regulatory effect on transcytosis, TJ integrity, and vessel structure, which could all be linked to an impact on BBB integrity.
1.2. BBB and HIV infection
There are over 35 million people in the world living with AIDS, and of those around 1.5 million per year succumb to AIDS related illnesses [27]. Disease burden was highly alleviated by the introduction of HAART in 1990s, shifting HIV from a deadly disease to a chronic infection. While some complications related to HIV infections, such as immunodepletion, can be prevented, HIV-infected patients still demonstrate a high incidence of various comorbidities, including neurological disorders [1, 28–30].
HIV is a neuroinvasive virus that can cross into the CNS causing inflammation and neurotoxicity. The crossing of the virus across the BBB is still not fully elucidated; however, most evidence points to a “Trojan horse” model, in which infected immune cells migrate to the CNS, releasing virus into the brain tissue allowing the subsequent infection of microglial cells and astrocytes [31–33]. Our recent studies indicate that BBB pericytes are also permissible to HIV infection [34, 35]. While HIV-associated dementia (HAD) is rare in patients under HAART, milder neurodegenerative diseases, such as asymptomatic neurocognitive impairment (ANI) and mild neurocognitive disorder (MND), are still present in 40 to 60% of patients [36, 37]. Typical symptoms of MND are confusion, forgetfulness, and problems with cognition and movement, effecting daily life and work duties. The main cause of HIV associated neurocognitive disorders (HAND) is mainly associated with HIV-encephalitis; however, other factors, such as neurotoxic viral proteins or BBB disruption can play a significant role in cognitive decline and HAND progression.
HIV-induced disruption of the BBB is an important part neuropathogenesis induced by the virus. While HIV does not infect endothelial cells, it can directly infect astrocytes and pericytes, i.e., cells important in maintaining BBB integrity [35, 38, 39]. This process affects a variety of cellular functions important for the maintenance of the barrier integrity, such as the secretion of growth factors and tight junction regulation. The presence of cell-cell communication channels, formed by connexin43 and gap junctions, extends the reach of HIV infection, affecting a wider area and bystander cells. In addition, the ensuing immune responses exacerbate BBB disruption by the secretion of matrix metalloproteinases (MMPs) [40] and decreased expression of tight junction proteins due to pro-inflammatory molecules, such as TNF-α, IL-1β or IFNγ [41–43].
Several HIV proteins exhibit high level of toxicity, which may also induce vascular and neuronal pathology. Exposure of neurons to gp120, even at picomolar levels, is highly toxic and has been linked to HIV-associated sensory neuropathy [44, 45]. Furthermore, gp120 can bind the viral co-receptors, CCR5 and CXCR4, present on BMEC and lead to an increase in monolayer permeability due to downregulation of tight junction proteins and an increase in the levels of MMPs [46]. HIV Tat is another viral protein that has potent toxicity. It can affect BBB integrity and TJ assembly in BMEC, via a process that has been linked to signaling via small GTPases [47]. In addition, Tat exposure can lead to elevated intracellular ROS levels and cause apoptosis [48]. Finally, viral proteins Nef and Vpr have also been shown to be associated with BBB permeability and neurotoxicity [49, 50].
2. TARGETING THE CNS RESERVOIRS
The confined nature of the CNS is highly effective at protecting it from pathogens. However, in the event of failure of this system and established brain infection, the BBB becomes an obstacle that can severely obstruct treatment efficacy. While therapeutic levels of drugs can be achieved in the plasma, several factors can lead to a low degree of penetration into the CNS leading to hindered viral inhibition. As a result, the CNS can act as a viral reservoir where HIV can replicate and increasing the number of latently infected cells [51]. In the event of HAART interruption, virus may cross back into circulation and restore high levels of viremia. Furthermore, the sub-optimal concentrations of antiretroviral drugs (ARVd) in the brain can result in the selection of resistant mutations that lead to loss of treatment efficacy [52]. Finally, HIV replication in the brain stimulates neurodegeneration and cognitive disorders. These facts highlight the need for drugs that can efficiently cross the BBB to achieve therapeutic concentrations in the CNS to prevent HIV replication.
The efficiency of ARVd in crossing the BBB varies greatly. Efavirenz and atazanavir demonstrate low CSF concentrations, averaging 0.5% and 1% of plasma levels, respectively [53, 54]. In comparison, nevirapine can reach CSF concentration that represent 29–63 % of plasma levels [55, 56]. To further enhance this problem, the ratio of CSF to plasma drug concentration can vary greatly between individuals and over time, in part in association with BBB permeability [57].
It has been demonstrated that treatment of patients with drugs demonstrating low BBB penetration is associated with higher prevalence of neurocognitive disorders. A CHARTER study of 300 individuals demonstrated that 26% of patients with undetectable HIV RNA levels (below 2 copies per ml) had detectable CSF viremia [58]. This study also indicated that patients treated with drug regimen with low BBB penetration levels demonstrate poorer performance on neuropsychological tests. These findings indicate that uncontrolled low levels of CNS HIV replication could lead to nervous system injury leading to HAND. This report is supported by other studies that evaluated patients who developed neurocognitive disorders despite stable antiretroviral treatment and undetectable blood HIV RNA levels [59, 60].
3. STRATEGIES IN CROSSING THE BBB
Several factors can influence CNS drug concentration. Drug efflux pumps, such as P-gp and organic anion transporters can actively shuttle drugs out of the CNS. In addition, several characteristics of the drugs can impact BBB penetration. Molecules highly bound by plasma proteins are less likely to cross the BBB. On the other hand, low molecular weight and hydrophobicity are factors that promote BBB penetration, while ionization has a negative effect.
Multiple mechanisms can play a role in a drug’s ability to cross into the brain parenchyma. They include paracellular aqueous pathway, transcellular lipophilic pathway, transport proteins, receptor mediated transcytosis and adsorptive transcytosis.
3.1. Efflux pump
Numerous drugs, including ARVds, are able to cross the BBB, but are actively pumped out from the brain parenchyma by efflux transporters. For example, protease inhibitors (PI) are mostly large lipophilic drugs that can cross into the brain but bind with high affinity to P-gp [61]. One strategy to overcome this mechanism is to add into the treatment regimen ritonavir (another PI), which demonstrates even higher affinity for P-go and thus reduces PI translocation [62, 63]. An alternate method is to block these transport proteins by co-administration of specific inhibitors along with treatment. Several of such compounds are being developed and are in various stages of clinical development. The use of the first generation of P-gp inhibitors, such as verapamil and cyclosporine A, was compromised by their low affinity and toxicity. The use of the second generation inhibitor valspodar successfully increased treatment efficacy of paclitaxel, who is a substrate of P-gp, and significantly reduced tumor size in a nude mouse model of glioblastoma [64]. However, the translation to clinical trial had limited success [65]. The use of third generation P-gp inhibitors proved much more successful. For examples, elacridar increased brain concentration of the drug paclitaxel 5-folds and reduced tumor size by 90% [66]. Inhibition of other transporters such as MRP (by sulfinpyrazone and probenecid) and BCRP (by fumitremorgin C) was reported, but their effectiveness in clinical settings has yet to be demonstrated. While promising, these inhibitors demonstrated limited success up to now. In addition, the potential side effects of long term administration of these compounds are unknown, but need to be evaluated given the important role played the efflux transporters in brain homeostasis [67, 68]. A potential alternative is to bypass the efflux transporters without inhibition. Several strategies are being developed, with the most promising being based on masking the drug and transferring it across the brain endothelium without exposure to the ABC transporters. One such mechanism uses immunoliposomes which are coupled with molecules that actively transport it across the BBB to the brain parenchyma.
Testing for transporter activity is useful as a screening tool for identification of drugs with high CNS penetration. However, inconsistencies are observed between BBB models both in vitro and in vivo. These problems, additionally coupled with the population variations in expression and polymorphism of efflux transporters, makes the prediction of drug efficacy difficult and not fully reliable [69, 70].
3.2. Increasing BBB translocation
Because several mechanisms are present at the BBB to actively transport substrates across the BBB, a possible approach is to employ these intrinsic transporters to actively import therapeutics into the CNS. A well explored strategy is to use the transferrin transporter, normally used for iron transport into the CNS [71]. The conjugation of a drug to monoclonal antibody against this receptor has been used experimentally both in vitro and in vivo [72–74]. The strategy demonstrated a significant increase in drug delivery to the CNS in a brain tumor model, leading to a significant reduction in tumor size and increased animal survival [75]. Despite its efficacy, the feasibility of this method is compromised by potential side effects linked to hemolytic anemia associated with the antibody [76]. A new approach, based on a non-competitive peptide that binds to the transferrin receptor, demonstrated low toxicity while retaining the translocation capacity [77–79]. However, it should be added that the binding to the transferrin receptor is influenced by coating density on the therapeutic agent [80].
Other receptors present at the BBB have also been used to increase translocation of therapeutics into the CNS. The low density lipoprotein receptor-related protein-1 (LRP-1) has been demonstrated as a suitable candidate for the translocation of IgG antibody to the CNS, through a process that does not involve vesicle acidification [81]. A monoclonal antibody against the insulin receptor (HIRMAb) coupled with an enzyme was successfully transported to the CNS [82]. In addition, studies have screened potential candidates for receptor mediated transcytosis and identified several target receptors, such as basign, Glut1 and CD98hc in mice [83], transthyretin [84], melanotransferrin [85, 86], apoE [87], RAGE [88], Fcγ [89] and SCAR [90].
In addition to using active transporters, therapeutic agents can also be coupled with cell-penetrating peptides (CPPs) to translocate across the BBB by triggering endocytosis. Several of these peptides have been identified and characterized for their ability to deliver cargo to the CNS, mostly in vitro [91–93]. The first and the most studied group of these peptides was derived from Antp (antennapedia transcription factor from Drosophila Melanogaster) and from the HIV protein TAT [94–96]. A second group of CPP peptides was identified from chimeric molecules, such as transpotan, composed of a segment of galanin and mastroparan, a wasp venom [97]. The third group of this family has been composed of synthetic peptides identified mainly through phage display. The effectiveness for CNS delivery has been demonstrated for several CPPs, such as Tat, RDP, FGF4, RVG, Penetratin, SynB1/3 and Angiopep. They were able to mediate successful delivery of proteins, nucleic acids and/or small molecules [98–104].
The use of nanoparticles also proved to be a suitable method to increase BBB penetration. Nanoparticles can be used in conjunction with the above mentioned approaches to target drug delivery to the CNS or alone to exploit an increase in BBB permeability observed in brain diseases. Several types of nanoparticles can be used, such as nanotubes, liposomes, solid lipid nanoparticles, nanospheres, nanocapsules, polymeric mycelles and dendrimers. They vary greatly in structure and composition. Several of these strategies have been successfully used to deliver compounds to the CNS [105–108]. Dendrimers have demonstrated their ability to translocate small molecules, such as the chemotherapeutic agent doxorubicin, and nucleic acids across the BBB using the transferrin receptor. The strategy resulted in a 2–3 fold increase in CNS concentration as compared to free drug and increased survival time in a mouse brain tumor model [109, 110]. Regarding HIV treatment, a group observed that encapsulation of atazanavir in solid lipid nanoparticles increases uptake by endothelial cells up to 3 folds [111]. Another group tested delivery of stavudine, delavirdine and saquinavir (nuncleoside reverse transcriptase inhibitor (NRTI), non-nucleoside reverse transcriptase inhibitor (NNRTI) and PI respectively) linked to several nanoparticle carriers, observing enhanced delivery across an in vitro BBB model by up to 16 folds compared to drug alone [112]. In a SCID rat model of intracranial HIV-1 infection, it was demonstrated that coupling of zidovudine to a nanogel matrix increased treatment efficacy, leading to lower HIV levels in the CNS [113]. Finally, enfuvirtide, a fusion inhibitor that does not cross the BBB, when coupled with iron oxide nanoparticles coated with amphiphilic polymer, significantly increased translocation and anti-viral activity [114].
Varieties of magnetic nanocarriers (MNCs) have been developed in recent years for target-specific drug delivery. As such, BBB translocation ability of magnetic (Fe3O4/Fe2O3) nanocarrier for anti-HIV and anti-addiction efficacy has been intensively studied [114–122]. However, drug release from this nanocarrier is manually uncontrollable, and depends on pathology-specific cellular responses (e.g. variation temperature, pH, intracellular Ca2+ level, etc.). To overcome this constraint, a novel electro-magnetic carrier (MENCs) was developed. Unlike MNC, MENC possess both magnetic and electric fields at physiological temperature range. The alternating current (AC) trigger on these particles breaks the symmetry of charge distribution i.e. ionic bonding between drugs and nanocarriers which provide control over drug release as and when required [123–126]. A ~3 fold higher transendothelial translocation of AZTTP could be achieved using MENCs, and HIV-p24 inhibition efficacy of AC-triggered released drug remained unaffected [123]. Similarly, mRNAs could be delivered across BBB using MENCs [125], and mice undergoing MENCs treatment to brain did not show signs of negative neuro-modulation [126]. Thus, MENCs possess unique ability to provide control over field-mediated drug release and can be applied to treating many CNS disorders, including HIV infection.
3.3. Bypassing the Blood-brain barrier
A potential way to circumvent the obstacles of the BBB is to enter the CNS using alternative routes of delivery. Several experiments, conducted mainly in rodents, demonstrated that intranasal drug administration increases uptake of drugs to the brain parenchyma as opposed to oral or peritoneal delivery [127–129]. The effectiveness of this delivery has been observed even for large and charged molecules such as insulin [130, 131]. The drugs are routed to the CNS along the olfactory pathway by the nasal cavity extension of the subarachnoid space. While this technique demonstrates promising results, a high variability is observed in various published reports [132–134]. In addition, the differences in nasal physiology between rodents and human make the translation of this technique difficult [135].
Direct delivery to the CNS using physical methods such as intracerebral or intrathecal administration has proved to be effective in several clinical trials, raising a potential of implanting an infusion system to facilitate repeated delivery [136–139]. While this technique bypasses any barriers to drug delivery, its implementation in a large population such as the HIV cohort is not feasible. In addition, the lifelong need for therapy in HIV patients makes the maintenance of this method very challenging and complications have been observed [140, 141].
Finally, a possible delivery route through the BBB is to disrupt it, enabling drugs to cross into the CNS. Several approaches have been tested; however, they rely mainly on osmotic methods that dilate tight junctions by inducing cell shrinkage using chemicals such as mannitol and polydixylitol [142–144]. While they are currently used in the clinic, their delivery methods strongly affects their efficiency in disrupting the BBB. In addition, these compounds may act nonspecifically. A new technique that is rising in popularity for CNS delivery is the use of focused ultrasounds (FUS). This non-invasive method was first discovered in the 1950s [145] but its employment in drug delivery was first studies in the 1990s [146]. The local disruption of the BBB is achieved by focusing ultrasounds at a specific site of the brain and injecting microbubbles into the circulation [147]. When they reach the target area, there is an increase in reagents crossing the BBB due to sonoporation. While this method has been extensively studied in rodents, the translation to non-human primates proved challenging since the thicker skull made it harder for acoustic pressure to reach the brain. While a group was able to use this technique for effective delivery of agents to the CNS [148], there remains the need for further testing to evaluate long term safety and tissue damage [149]. In addition, successful implementation of this treatment approach in a large population may not be feasible.
4. DRUG EFFICACY AND TOXICITY IN CNS CELLS
An important consideration for ARVd efficacy in the CNS is metabolism of cells involved in HIV infection of the brain, which can be substantially different from the cellular targets of HIV infection in the periphery, namely T cells and macrophages. Cells susceptible to HIV infection in the CNS are primarily microglial cells, perivascular monocytes, and astrocytes [35, 150, 151]. It is well known that a cell type plays a central role in determining the inhibitory concentration (IC) of drugs. For example, relatively low levels of nucleotides present in monocytes or astrocytes can increase the effectiveness of NRTIs and NNRTIs [152]. At the same time, a lower activity of cellular kinases needed for the phosphorylation of NRTIs, such as lamivudine or zidovudine, can reduce the effectiveness of these drugs [153].
The microenvironment in the CNS is also quite different than in the serum, affecting drug efficacy. Drugs that are highly protein bound, for example PI and NNRTIs, have diminished activity in the presence of serum [154]. On the other hand, CSF albumin concentration (8–50 mg/L) is lower than in blood (34–54 g/L), allowing for increased efficacy of these therapeutics. In fact, it was shown that darunavir is mainly unbound in CSF [155]. All of these factors need to be taken into consideration when identifying the concentrations needed in the CNS to inhibit HIV replication. This is critical because subtherapeutic levels of drugs allow the development and selection of drug resistance variants of HIV, affecting treatment of infection both in the brain as in periphery.
On the other hand, the neurotoxicity of ARVds also needs to be taken into account when discussing their entry into the CNS. The lack of drug elimination by liver and kidneys once ARVds cross the BBB can lead to their accumulation. This phenomenon can be further amplified by lower activities of enzymes that degrade these compounds in cellular targets of the CNS. For example, a correlation has been observed between efavirenz-associated toxicity and the presence of specific cytochrome alleles, such as CYP2B6*6, implicated in its elimination [156]. This association has also been linked to alterations of mitochondrial functions, disruption of autophagy, and cell stress responses in neurons [157, 158] and other cell types [159, 160]. Moreover, efavirenz and PIs disrupt glucose metabolism [28, 161], and drugs, such as zidovudine and ritonavir, can induce the production of reactive oxygen species, leading to increased cellular oxidation [162, 163]. All these observations highlight the importance of balancing the need for CNS delivery of ARVds with potential side effects that could worsen neurological complications associated with the infection.
5. CONCLUSION
The development of HAND in a large proportion of HIV infected patients highlights the need for comprehensive treatment approaches that can prevent viral replication on both sides of the BBB. Limited activation of specific NRTIs in the brain requires enhanced delivery to reach inhibitory concentrations. At the same time, careful consideration must be applied to identify potential consequences of higher levels of drugs in the CNS. Indeed, neurotoxicity of specific ARVds can be observed at the levels that are therapeutic in the periphery.
Several methods are being explored to overcome the challenges posed by the restrictions of the BBB (see figure 1). While the disruption of the barrier function or bypassing the BBB can enhance drug delivery to the CNS, these procedures are frequently invasive and may not be feasible. On the other hand, modifications of the ARVds or their linkage to other molecules or nanoparticles can result in increased delivery into the brain. Several of such approaches have proven their efficacy in animal models; however, comprehensive clinical studies are needed to determine their applicability to HIV treatment.
Figure 1: Delivery across the blood brain barrier.
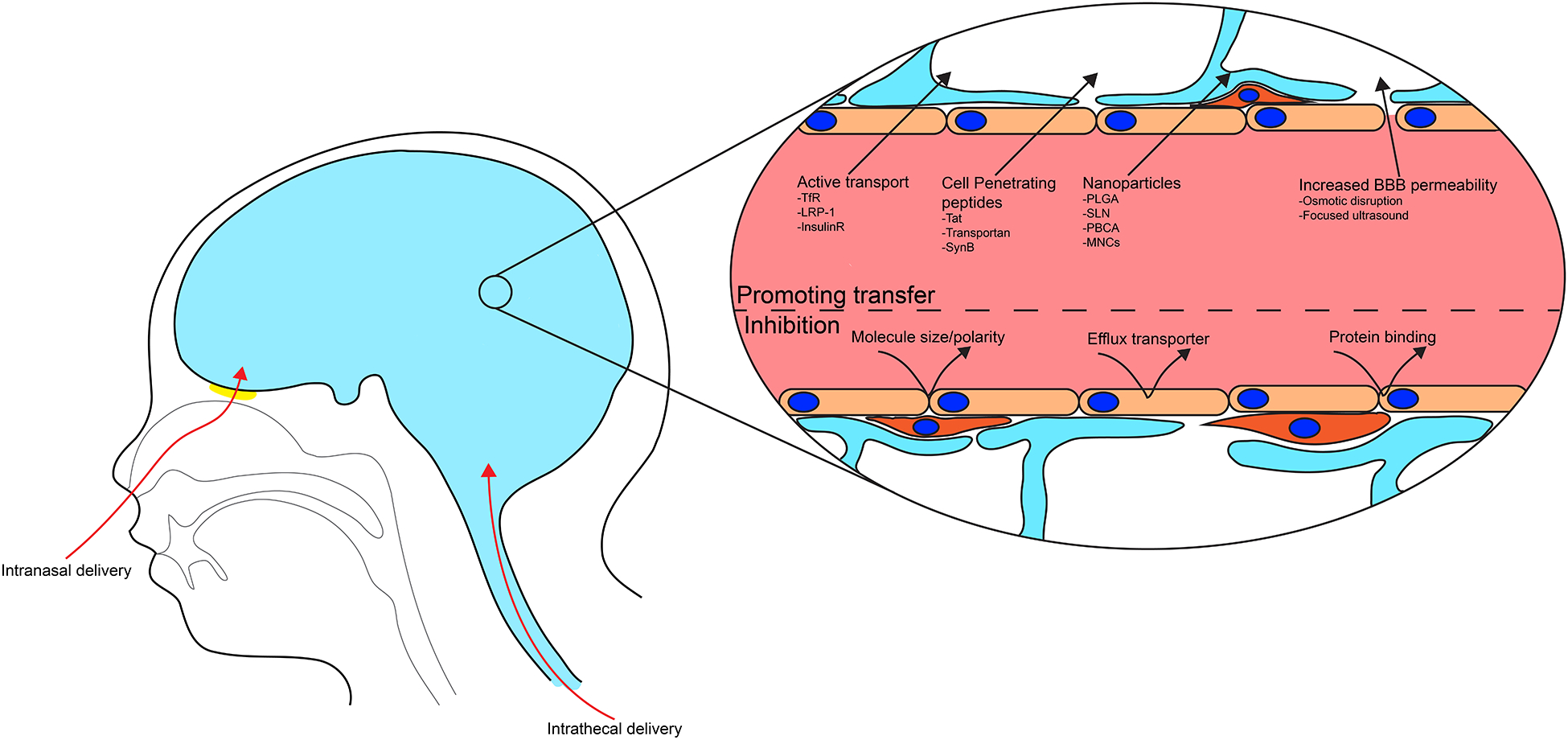
The ability of a drug to reach the CNS is highly dependent on its properties. Molecule size, polarity, and protein binding are factors restricting the passage. Furthermore, a high affinity for efflux transporters will contribute to removing drugs from the CNS. To overcome these obstacles, several mechanisms are targeted, including bypassing BBB, inhibition of efflux transporters, nanotechnology, the use of cell penetrating peptides, and taking advantages from active transporters present at the BBB.
Table 2.
Summary of strategies to overcome BBB restrictions for CNS delivery
| Physical | Direct delivery to the CNS | Intrathecal injection |
| Intracerebral injection | ||
| Targeted delivery | Intranasal delivery | |
| BBB disruption | Osmotic disruption | |
| Mannitol | ||
| Polydixytol | ||
| Focused ultrasound | ||
| Efflux transporter inhibition | Inhibition | P-glycoprotein |
| Verapramil | ||
| Cyclosporin A | ||
| Valspodar | ||
| Elacridar | ||
| MRP | ||
| Sulfinpyrazone | ||
| Probenecid | ||
| BCRP | ||
| Fumitremorgin C | ||
| Allosteric inhibition | Ritonavir | |
| Therapeutic making | Immunoliposome | |
| Drug coating/modification | Receptor mediated transport | Transferin receptor |
| Insulin receptor | ||
| Lipoprotein receptor | ||
| Cell penetrating peptide | Antennapeptide | |
| Tat | ||
| Transportan | ||
| Penatratin | ||
| Angiopep | ||
| SynB1/3 | ||
| Nanoparticles | Nanotube | |
| Liposome | ||
| Solid lipid nanoparticles | ||
| Nanospheres | ||
| Nanocapsule | ||
| Dendrimers | ||
| Polymeric mycells | ||
| Magnetic nanocarriers |
Acknowledgements
This work was supported, in whole or in part, by National Institutes of Health Grants DA039576, DA027569, DA040537, HL126559, MH098891, and MH072567 and by the Miami Center for AIDS Research funded by NIH Grant MH063022.
List abbreviation
- ANI
Asymptomatic neurocognitive impairment
- ARVd
Anti-retroviral drug
- BBB
Blood brain barrier
- BMEC
Brain microvascular endothelial cells
- CNS
Central nervous system
- CPE
CNS penetrating efficiency
- CPP
Cell penetrating peptide
- FUS
Focused ultra-sound
- HAART
Highly active anti-retroviral therapy
- HAD
HIV associated dementia
- HAND
HIV associated neurocognitive disorder
- IC
Inhibitory concentration
- JAMs
Junctional adhesion molecules
- LRP-1
Lipoprotein receptor-related protein-1
- MENC
novel electro-magnetic carrier
- MMPs
Matrix metalloproteases
- MNC
Magnetic nanocarrier
- MND
Mild neurocognitive disorder
- NNRTI
non-nucleoside reserve transcriptase inhibitor
- NRTI
nucleoside reverse transcriptase inhibitor
- PI
Protease inhibitor
Footnotes
Conflict of Interest
Nothing to report
References
- [1].Clifford DB, Ances BM. HIV-associated neurocognitive disorder. Lancet Infect Dis, 2013; 13: 976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS, 2007; 21: 1915–21. [DOI] [PubMed] [Google Scholar]
- [3].Tan YC, Gill AK, Kim KS. Treatment strategies for central nervous system infections: an update. Expert Opin Pharmacother, 2015; 16: 187–203. [DOI] [PubMed] [Google Scholar]
- [4].Weidle UH, Niewohner J, Tiefenthaler G. The Blood-Brain Barrier Challenge for the Treatment of Brain Cancer, Secondary Brain Metastases, and Neurological Diseases. Cancer Genomics Proteomics, 2015; 12: 167–77. [PubMed] [Google Scholar]
- [5].Ferretti F, Gisslen M, Cinque P, Price RW. Cerebrospinal Fluid HIV Escape from Antiretroviral Therapy. Curr HIV/AIDS Rep, 2015; 12: 280–8. [DOI] [PubMed] [Google Scholar]
- [6].Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx, 2005; 2: 3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Keaney J, Campbell M. The dynamic blood-brain barrier. FEBS J, 2015; 282: 4067–79. [DOI] [PubMed] [Google Scholar]
- [8].Cornford EM, Hyman S. Localization of brain endothelial luminal and abluminal transporters with immunogold electron microscopy. NeuroRx, 2005; 2: 27–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hawkins RA, O’Kane RL, Simpson IA, Vina JR. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr, 2006; 136: 218S–26S. [DOI] [PubMed] [Google Scholar]
- [10].Aminoff MJ, Daroff RB. Encyclopedia of the neurological sciences. Academic Press/Elsevier: Waltham, MA: 2014. [Google Scholar]
- [11].Qosa H, Miller DS, Pasinelli P, Trotti D. Regulation of ABC efflux transporters at blood-brain barrier in health and neurological disorders. Brain Res, 2015; 1628: 298–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sixt M, Engelhardt B, Pausch F, Hallmann R, Wendler O, Sorokin LM. Endothelial cell laminin isoforms, laminins 8 and 10, play decisive roles in T cell recruitment across the blood-brain barrier in experimental autoimmune encephalomyelitis. J Cell Biol, 2001; 153: 933–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Daneman R, Prat A. The blood-brain barrier. Cold Spring Harb Perspect Biol, 2015; 7: a020412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Roberts J, Kahle MP, Bix GJ. Perlecan and the blood-brain barrier: beneficial proteolysis? Front Pharmacol, 2012; 3: 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development, 2004; 131: 1619–28. [DOI] [PubMed] [Google Scholar]
- [16].Hill J, Rom S, Ramirez SH, Persidsky Y. Emerging roles of pericytes in the regulation of the neurovascular unit in health and disease. J Neuroimmune Pharmacol, 2014; 9: 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sa-Pereira I, Brites D, Brito MA. Neurovascular unit: a focus on pericytes. Mol Neurobiol, 2012; 45: 327–47. [DOI] [PubMed] [Google Scholar]
- [18].Engelhardt B. beta1-integrin/matrix interactions support blood-brain barrier integrity. J Cereb Blood Flow Metab, 2011; 31: 1969–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia, 2010; 58: 1094–103. [DOI] [PubMed] [Google Scholar]
- [20].Cheslow L, Alvarez JI. Glial-endothelial crosstalk regulates blood-brain barrier function. Curr Opin Pharmacol, 2016; 26: 39–46. [DOI] [PubMed] [Google Scholar]
- [21].Watkins S, Robel S, Kimbrough IF, Robert SM, Ellis-Davies G, Sontheimer H. Disruption of astrocyte-vascular coupling and the blood-brain barrier by invading glioma cells. Nat Commun, 2014; 5: 4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Attwell D, Mishra A, Hall CN, O’Farrell FM, Dalkara T. What is a pericyte? J Cereb Blood Flow Metab, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fernandez-Klett F, Priller J. Diverse functions of pericytes in cerebral blood flow regulation and ischemia. J Cereb Blood Flow Metab, 2015; 35: 883–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kornfield TE, Newman EA. Regulation of blood flow in the retinal trilaminar vascular network. J Neurosci, 2014; 34: 11504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kofler NM, Cuervo H, Uh MK, Murtomaki A, Kitajewski J. Combined deficiency of Notch1 and Notch3 causes pericyte dysfunction, models CADASIL, and results in arteriovenous malformations. Sci Rep, 2015; 5: 16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sagare AP, Bell RD, Zhao Z, Ma Q, Winkler EA, Ramanathan A, Zlokovic BV. Pericyte loss influences Alzheimer-like neurodegeneration in mice. Nat Commun, 2013; 4: 2932. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [27].WHO. Global report: UNAIDS report on the global AIDS epidemic 2013. Joint United Nations Programme on HIV/AIDS (UNAIDS): Geneva: 2013. [Google Scholar]
- [28].da Cunha J, Maselli LM, Stern AC, Spada C, Bydlowski SP. Impact of antiretroviral therapy on lipid metabolism of human immunodeficiency virus-infected patients: Old and new drugs. World J Virol, 2015; 4: 56–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lambert CT, Sandesara PB, Hirsh B, Shaw LJ, Lewis W, Quyyumi AA, Schinazi RF, Post WS, Sperling L. HIV, highly active antiretroviral therapy and the heart: a cellular to epidemiological review. HIV Med, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Waheed S, Atta MG. Predictors of HIV-associated nephropathy. Expert Rev Anti Infect Ther, 2014; 12: 555–63. [DOI] [PubMed] [Google Scholar]
- [31].Joseph SB, Arrildt KT, Sturdevant CB, Swanstrom R. HIV-1 target cells in the CNS. J Neurovirol, 2015; 21: 276–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Burdo TH, Lackner A, Williams KC. Monocyte/macrophages and their role in HIV neuropathogenesis. Immunol Rev, 2013; 254: 102–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology, 1985; 147: 231–6. [DOI] [PubMed] [Google Scholar]
- [34].Castro V, Bertrand L, Luethen M, Dabrowski S, Lombardi J, Morgan L, Sharova N, Stevenson M, Blasig IE, Toborek M. Occludin controls HIV transcription in brain pericytes via regulation of SIRT-1 activation. FASEB J, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Nakagawa S, Castro V, Toborek M. Infection of human pericytes by HIV-1 disrupts the integrity of the blood-brain barrier. J Cell Mol Med, 2012; 16: 2950–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Griffin TZ, Kang W, Ma Y, Zhang M. The HAND Database: a gateway to understanding the role of HIV in HIV-associated neurocognitive disorders. BMC Med Genomics, 2015; 8: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group C. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology, 2010; 75: 2087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Dewhurst S, Bresser J, Stevenson M, Sakai K, Evinger-Hodges MJ, Volsky DJ. Susceptibility of human glial cells to infection with human immunodeficiency virus (HIV). FEBS Lett, 1987; 213: 138–43. [DOI] [PubMed] [Google Scholar]
- [39].Li GH, Anderson C, Jaeger L, Do T, Major EO, Nath A. Cell-to-cell contact facilitates HIV transmission from lymphocytes to astrocytes via CXCR4. AIDS, 2015; 29: 755–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brkic M, Balusu S, Van Wonterghem E, Gorle N, Benilova I, Kremer A, Van Hove I, Moons L, De Strooper B, Kanazir S, Libert C, Vandenbroucke RE. Amyloid beta Oligomers Disrupt Blood-CSF Barrier Integrity by Activating Matrix Metalloproteinases. J Neurosci, 2015; 35: 12766–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Chai Q, He WQ, Zhou M, Lu H, Fu ZF. Enhancement of blood-brain barrier permeability and reduction of tight junction protein expression are modulated by chemokines/cytokines induced by rabies virus infection. J Virol, 2014; 88: 4698–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Chapouly C, Tadesse Argaw A, Horng S, Castro K, Zhang J, Asp L, Loo H, Laitman BM, Mariani JN, Straus Farber R, Zaslavsky E, Nudelman G, Raine CS, John GR. Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain, 2015; 138: 1548–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Rochfort KD, Collins LE, McLoughlin A, Cummins PM. TNF-alpha-mediated disruption of cerebrovascular endothelial barrier integrity in vitro involves the production of proinflammatory IL-6. J Neurochem, 2015. [DOI] [PubMed] [Google Scholar]
- [44].Catani MV, Corasaniti MT, Navarra M, Nistico G, Finazzi-Agro A, Melino G. gp120 induces cell death in human neuroblastoma cells through the CXCR4 and CCR5 chemokine receptors. J Neurochem, 2000; 74: 2373–9. [DOI] [PubMed] [Google Scholar]
- [45].Yuan SB, Shi Y, Chen J, Zhou X, Li G, Gelman BB, Lisinicchia JG, Carlton SM, Ferguson MR, Tan A, Sarna SK, Tang SJ. Gp120 in the pathogenesis of human immunodeficiency virus-associated pain. Ann Neurol, 2014; 75: 837–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Louboutin JP, Strayer DS. Blood-brain barrier abnormalities caused by HIV-1 gp120: mechanistic and therapeutic implications. ScientificWorldJournal, 2012; 2012: 482575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhong Y, Zhang B, Eum SY, Toborek M. HIV-1 Tat triggers nuclear localization of ZO-1 via Rho signaling and cAMP response element-binding protein activation. J Neurosci, 2012; 32: 143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Toborek M, Lee YW, Pu H, Malecki A, Flora G, Garrido R, Hennig B, Bauer HC, Nath A. HIV-Tat protein induces oxidative and inflammatory pathways in brain endothelium. J Neurochem, 2003; 84: 169–79. [DOI] [PubMed] [Google Scholar]
- [49].Saribas AS, Khalili K, Sariyer IK. Dysregulation of autophagy by HIV-1 Nef in human astrocytes. Cell Cycle, 2015; 14: 2899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Ferrucci A, Nonnemacher MR, Wigdahl B. Extracellular HIV-1 viral protein R affects astrocytic glyceraldehyde 3-phosphate dehydrogenase activity and neuronal survival. J Neurovirol, 2013; 19: 239–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fois AF, Brew BJ. The Potential of the CNS as a Reservoir for HIV-1 Infection: Implications for HIV Eradication. Curr HIV/AIDS Rep, 2015; 12: 299–303. [DOI] [PubMed] [Google Scholar]
- [52].Smit TK, Brew BJ, Tourtellotte W, Morgello S, Gelman BB, Saksena NK. Independent evolution of human immunodeficiency virus (HIV) drug resistance mutations in diverse areas of the brain in HIV-infected patients, with and without dementia, on antiretroviral treatment. J Virol, 2004; 78: 10133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Best BM, Koopmans PP, Letendre SL, Capparelli EV, Rossi SS, Clifford DB, Collier AC, Gelman BB, Mbeo G, McCutchan JA, Simpson DM, Haubrich R, Ellis R, Grant I, Group C. Efavirenz concentrations in CSF exceed IC50 for wild-type HIV. J Antimicrob Chemother, 2011; 66: 354–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Best BM, Letendre SL, Brigid E, Clifford DB, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Simpson DM, Ellis R, Capparelli EV, Grant I, Group C. Low atazanavir concentrations in cerebrospinal fluid. AIDS, 2009; 23: 83–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].van Praag RM, van Weert EC, van Heeswijk RP, Zhou XJ, Sommadossi JP, Jurriaans S, Lange JM, Hoetelmans RM, Prins JM. Stable concentrations of zidovudine, stavudine, lamivudine, abacavir, and nevirapine in serum and cerebrospinal fluid during 2 years of therapy. Antimicrob Agents Chemother, 2002; 46: 896–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Antinori A, Perno CF, Giancola ML, Forbici F, Ippolito G, Hoetelmans RM, Piscitelli SC. Efficacy of cerebrospinal fluid (CSF)-penetrating antiretroviral drugs against HIV in the neurological compartment: different patterns of phenotypic resistance in CSF and plasma. Clin Infect Dis, 2005; 41: 1787–93. [DOI] [PubMed] [Google Scholar]
- [57].Tsuchiya K, Hayashida T, Hamada A, Kato S, Oka S, Gatanaga H. Low raltegravir concentration in cerebrospinal fluid in patients with ABCG2 genetic variants. J Acquir Immune Defic Syndr, 2014; 66: 484–6. [DOI] [PubMed] [Google Scholar]
- [58].Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med, 2010; 18: 45–55. [PMC free article] [PubMed] [Google Scholar]
- [59].Dahl V, Peterson J, Fuchs D, Gisslen M, Palmer S, Price RW. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS, 2014; 28: 2251–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Canestri A, Lescure FX, Jaureguiberry S, Moulignier A, Amiel C, Marcelin AG, Peytavin G, Tubiana R, Pialoux G, Katlama C. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clin Infect Dis, 2010; 50: 773–8. [DOI] [PubMed] [Google Scholar]
- [61].Robillard KR, Chan GN, Zhang G, la Porte C, Cameron W, Bendayan R. Role of P-glycoprotein in the distribution of the HIV protease inhibitor atazanavir in the brain and male genital tract. Antimicrob Agents Chemother, 2014; 58: 1713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Marzolini C, Mueller R, Li-Blatter X, Battegay M, Seelig A. The brain entry of HIV-1 protease inhibitors is facilitated when used in combination. Mol Pharm, 2013; 10: 2340–9. [DOI] [PubMed] [Google Scholar]
- [63].Drewe J, Gutmann H, Fricker G, Torok M, Beglinger C, Huwyler J. HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem Pharmacol, 1999; 57: 1147–52. [DOI] [PubMed] [Google Scholar]
- [64].Fellner S, Bauer B, Miller DS, Schaffrik M, Fankhanel M, Spruss T, Bernhardt G, Graeff C, Farber L, Gschaidmeier H, Buschauer A, Fricker G. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J Clin Invest, 2002; 110: 1309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Thomas H, Coley HM. Overcoming multidrug resistance in cancer: an update on the clinical strategy of inhibiting p-glycoprotein. Cancer Control, 2003; 10: 159–65. [DOI] [PubMed] [Google Scholar]
- [66].Kemper EM, van Zandbergen AE, Cleypool C, Mos HA, Boogerd W, Beijnen JH, van Tellingen O. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin Cancer Res, 2003; 9: 2849–55. [PubMed] [Google Scholar]
- [67].Planting AS, Sonneveld P, van der Gaast A, Sparreboom A, van der Burg ME, Luyten GP, de Leeuw K, de Boer-Dennert M, Wissel PS, Jewell RC, Paul EM, Purvis NB Jr., Verweij J. A phase I and pharmacologic study of the MDR converter GF120918 in combination with doxorubicin in patients with advanced solid tumors. Cancer Chemother Pharmacol, 2005; 55: 91–9. [DOI] [PubMed] [Google Scholar]
- [68].Kuppens IE, Witteveen EO, Jewell RC, Radema SA, Paul EM, Mangum SG, Beijnen JH, Voest EE, Schellens JH. A phase I, randomized, open-label, parallel-cohort, dose-finding study of elacridar (GF120918) and oral topotecan in cancer patients. Clin Cancer Res, 2007; 13: 3276–85. [DOI] [PubMed] [Google Scholar]
- [69].Bruhn O, Cascorbi I. Polymorphisms of the drug transporters ABCB1, ABCG2, ABCC2 and ABCC3 and their impact on drug bioavailability and clinical relevance. Expert Opin Drug Metab Toxicol, 2014; 10: 1337–54. [DOI] [PubMed] [Google Scholar]
- [70].Liu L, Collier AC, Link JM, Domino KB, Mankoff DA, Eary JF, Spiekerman CF, Hsiao P, Deo AK, Unadkat JD. Modulation of P-glycoprotein at the Human Blood-Brain Barrier by Quinidine or Rifampin Treatment: A Positron Emission Tomography Imaging Study. Drug Metab Dispos, 2015; 43: 1795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Moos T, Rosengren Nielsen T, Skjorringe T, Morgan EH. Iron trafficking inside the brain. J Neurochem, 2007; 103: 1730–40. [DOI] [PubMed] [Google Scholar]
- [72].Pardridge WM. Blood-brain barrier drug delivery of IgG fusion proteins with a transferrin receptor monoclonal antibody. Expert Opin Drug Deliv, 2015; 12: 207–22. [DOI] [PubMed] [Google Scholar]
- [73].Clark AJ, Davis ME. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc Natl Acad Sci U S A, 2015; 112: 12486–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Johnsen KB, Moos T. Revisiting nanoparticle technology for blood-brain barrier transport: Unfolding at the endothelial gate improves the fate of transferrin receptor-targeted liposomes. J Control Release, 2016; 222: 32–46. [DOI] [PubMed] [Google Scholar]
- [75].Kim SS, Rait A, Kim E, DeMarco J, Pirollo KF, Chang EH. Encapsulation of temozolomide in a tumor-targeting nanocomplex enhances anti-cancer efficacy and reduces toxicity in a mouse model of glioblastoma. Cancer Lett, 2015; 369: 250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Couch JA, Yu YJ, Zhang Y, Tarrant JM, Fuji RN, Meilandt WJ, Solanoy H, Tong RK, Hoyte K, Luk W, Lu Y, Gadkar K, Prabhu S, Ordonia BA, Nguyen Q, Lin Y, Lin Z, Balazs M, Scearce-Levie K, Ernst JA, Dennis MS, Watts RJ. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci Transl Med, 2013; 5: 183ra57, 1–12. [DOI] [PubMed] [Google Scholar]
- [77].Wangler C, Nada D, Hofner G, Maschauer S, Wangler B, Schneider S, Schirrmacher E, Wanner KT, Schirrmacher R, Prante O. In vitro and initial in vivo evaluation of (68)Ga-labeled transferrin receptor (TfR) binding peptides as potential carriers for enhanced drug transport into TfR expressing cells. Mol Imaging Biol, 2011; 13: 332–41. [DOI] [PubMed] [Google Scholar]
- [78].Prades R, Oller-Salvia B, Schwarzmaier SM, Selva J, Moros M, Balbi M, Grazu V, de La Fuente JM, Egea G, Plesnila N, Teixido M, Giralt E. Applying the retro-enantio approach to obtain a peptide capable of overcoming the blood-brain barrier. Angew Chem Int Ed Engl, 2015; 54: 3967–72. [DOI] [PubMed] [Google Scholar]
- [79].Lee JH, Engler JA, Collawn JF, Moore BA. Receptor mediated uptake of peptides that bind the human transferrin receptor. Eur J Biochem, 2001; 268: 2004–12. [DOI] [PubMed] [Google Scholar]
- [80].Wiley DT, Webster P, Gale A, Davis ME. Transcytosis and brain uptake of transferrin-containing nanoparticles by tuning avidity to transferrin receptor. Proc Natl Acad Sci U S A, 2013; 110: 8662–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tian X, Nyberg S, P SS, Madsen J, Daneshpour N, Armes SP, Berwick J, Azzouz M, Shaw P, Abbott NJ, Battaglia G. LRP-1-mediated intracellular antibody delivery to the Central Nervous System. Sci Rep, 2015; 5: 11990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Boado RJ, Ka-Wai Hui E, Zhiqiang Lu J, Pardridge WM. Insulin receptor antibody-iduronate 2-sulfatase fusion protein: pharmacokinetics, anti-drug antibody, and safety pharmacology in Rhesus monkeys. Biotechnol Bioeng, 2014; 111: 2317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zuchero YJ, Chen X, Bien-Ly N, Bumbaca D, Tong RK, Gao X, Zhang S, Hoyte K, Luk W, Huntley MA, Phu L, Tan C, Kallop D, Weimer RM, Lu Y, Kirkpatrick DS, Ernst JA, Chih B, Dennis MS, Watts RJ. Discovery of Novel Blood-Brain Barrier Targets to Enhance Brain Uptake of Therapeutic Antibodies. Neuron, 2016; 89: 70–82. [DOI] [PubMed] [Google Scholar]
- [84].Kim SY, Choi ES, Lee HJ, Moon C, Kim E. Transthyretin as a new transporter of nanoparticles for receptor-mediated transcytosis in rat brain microvessels. Colloids Surf B Biointerfaces, 2015; 136: 989–96. [DOI] [PubMed] [Google Scholar]
- [85].Kuo YC, Chao IW. Conjugation of Melanotransferrin Antibody on Solid Lipid Nanoparticles for Mediating Brain Cancer Malignancy. Biotechnol Prog, 2015. [DOI] [PubMed] [Google Scholar]
- [86].Demeule M, Poirier J, Jodoin J, Bertrand Y, Desrosiers RR, Dagenais C, Nguyen T, Lanthier J, Gabathuler R, Kennard M, Jefferies WA, Karkan D, Tsai S, Fenart L, Cecchelli R, Beliveau R. High transcytosis of melanotransferrin (P97) across the blood-brain barrier. J Neurochem, 2002; 83: 924–33. [DOI] [PubMed] [Google Scholar]
- [87].Herz J, Marschang P. Coaxing the LDL receptor family into the fold. Cell, 2003; 112: 289–92. [DOI] [PubMed] [Google Scholar]
- [88].Deane R, Wu Z, Zlokovic BV. RAGE (yin) versus LRP (yang) balance regulates alzheimer amyloid beta-peptide clearance through transport across the blood-brain barrier. Stroke, 2004; 35: 2628–31. [DOI] [PubMed] [Google Scholar]
- [89].Zlokovic BV, Skundric DS, Segal MB, Lipovac MN, Mackic JB, Davson H. A saturable mechanism for transport of immunoglobulin G across the blood-brain barrier of the guinea pig. Exp Neurol, 1990; 107: 263–70. [DOI] [PubMed] [Google Scholar]
- [90].Panzenboeck U, Balazs Z, Sovic A, Hrzenjak A, Levak-Frank S, Wintersperger A, Malle E, Sattler W. ABCA1 and scavenger receptor class B, type I, are modulators of reverse sterol transport at an in vitro blood-brain barrier constituted of porcine brain capillary endothelial cells. J Biol Chem, 2002; 277: 42781–9. [DOI] [PubMed] [Google Scholar]
- [91].Zou LL, Ma JL, Wang T, Yang TB, Liu CB. Cell-penetrating Peptide-mediated therapeutic molecule delivery into the central nervous system. Curr Neuropharmacol, 2013; 11: 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Stalmans S, Bracke N, Wynendaele E, Gevaert B, Peremans K, Burvenich C, Polis I, De Spiegeleer B. Cell-Penetrating Peptides Selectively Cross the Blood-Brain Barrier In Vivo. PLoS One, 2015; 10: e0139652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kang T, Gao X, Chen J. Harnessing the capacity of cell-penetrating peptides for drug delivery to the central nervous system. Curr Pharm Biotechnol, 2014; 15: 220–30. [DOI] [PubMed] [Google Scholar]
- [94].Lo SL, Wang S. An endosomolytic Tat peptide produced by incorporation of histidine and cysteine residues as a nonviral vector for DNA transfection. Biomaterials, 2008; 29: 2408–14. [DOI] [PubMed] [Google Scholar]
- [95].Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem, 1997; 272: 16010–7. [DOI] [PubMed] [Google Scholar]
- [96].Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the Antennapedia homeodomain is receptor-independent. J Biol Chem, 1996; 271: 18188–93. [DOI] [PubMed] [Google Scholar]
- [97].Pooga M, Hallbrink M, Zorko M, Langel U. Cell penetration by transportan. FASEB J, 1998; 12: 67–77. [DOI] [PubMed] [Google Scholar]
- [98].Rousselle C, Clair P, Smirnova M, Kolesnikov Y, Pasternak GW, Gac-Breton S, Rees AR, Scherrmann JM, Temsamani J. Improved brain uptake and pharmacological activity of dalargin using a peptide-vector-mediated strategy. J Pharmacol Exp Ther, 2003; 306: 371–6. [DOI] [PubMed] [Google Scholar]
- [99].Rousselle C, Clair P, Lefauconnier JM, Kaczorek M, Scherrmann JM, Temsamani J. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol Pharmacol, 2000; 57: 679–86. [DOI] [PubMed] [Google Scholar]
- [100].Kilic E, Kilic U, Hermann DM. TAT-GDNF in neurodegeneration and ischemic stroke. CNS Drug Rev, 2005; 11: 369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Jo D, Liu D, Yao S, Collins RD, Hawiger J. Intracellular protein therapy with SOCS3 inhibits inflammation and apoptosis. Nat Med, 2005; 11: 892–8. [DOI] [PubMed] [Google Scholar]
- [102].Hwang do W, Son S, Jang J, Youn H, Lee S, Lee D, Lee YS, Jeong JM, Kim WJ, Lee DS. A brain-targeted rabies virus glycoprotein-disulfide linked PEI nanocarrier for delivery of neurogenic microRNA. Biomaterials, 2011; 32: 4968–75. [DOI] [PubMed] [Google Scholar]
- [103].Fu A, Wang Y, Zhan L, Zhou R. Targeted delivery of proteins into the central nervous system mediated by rabies virus glycoprotein-derived peptide. Pharm Res, 2012; 29: 1562–9. [DOI] [PubMed] [Google Scholar]
- [104].Che C, Yang G, Thiot C, Lacoste MC, Currie JC, Demeule M, Regina A, Beliveau R, Castaigne JP. New Angiopep-modified doxorubicin (ANG1007) and etoposide (ANG1009) chemotherapeutics with increased brain penetration. J Med Chem, 2010; 53: 2814–24. [DOI] [PubMed] [Google Scholar]
- [105].Serramia MJ, Alvarez S, Fuentes-Paniagua E, Clemente MI, Sanchez-Nieves J, Gomez R, de la Mata J, Munoz-Fernandez MA. In vivo delivery of siRNA to the brain by carbosilane dendrimer. J Control Release, 2015; 200: 60–70. [DOI] [PubMed] [Google Scholar]
- [106].Peluffo H, Unzueta U, Negro-Demontel ML, Xu Z, Vaquez E, Ferrer-Miralles N, Villaverde A. BBB-targeting, protein-based nanomedicines for drug and nucleic acid delivery to the CNS. Biotechnol Adv, 2015; 33: 277–87. [DOI] [PubMed] [Google Scholar]
- [107].Zhang TT, Li W, Meng G, Wang P, Liao W. Strategies for transporting nanoparticles across the blood-brain barrier. Biomater Sci, 2015. [DOI] [PubMed] [Google Scholar]
- [108].Tang X, Liang Y, Zhu Y, Xie C, Yao A, Chen L, Jiang Q, Liu T, Wang X, Qian Y, Wei J, Ni W, Dai J, Jiang Z, Hou W. Anti-transferrin receptor-modified amphotericin B-loaded PLA-PEG nanoparticles cure Candidal meningitis and reduce drug toxicity. Int J Nanomedicine, 2015; 10: 6227–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Huang S, Li J, Han L, Liu S, Ma H, Huang R, Jiang C. Dual targeting effect of Angiopep-2-modified, DNA-loaded nanoparticles for glioma. Biomaterials, 2011; 32: 6832–8. [DOI] [PubMed] [Google Scholar]
- [110].He H, Li Y, Jia XR, Du J, Ying X, Lu WL, Lou JN, Wei Y. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials, 2011; 32: 478–87. [DOI] [PubMed] [Google Scholar]
- [111].Chattopadhyay N, Zastre J, Wong HL, Wu XY, Bendayan R. Solid lipid nanoparticles enhance the delivery of the HIV protease inhibitor, atazanavir, by a human brain endothelial cell line. Pharm Res, 2008; 25: 2262–71. [DOI] [PubMed] [Google Scholar]
- [112].Kuo YC, Su FL. Transport of stavudine, delavirdine, and saquinavir across the blood-brain barrier by polybutylcyanoacrylate, methylmethacrylate-sulfopropylmethacrylate, and solid lipid nanoparticles. Int J Pharm, 2007; 340: 143–52. [DOI] [PubMed] [Google Scholar]
- [113].Gerson T, Makarov E, Senanayake TH, Gorantla S, Poluektova LY, Vinogradov SV. Nano-NRTIs demonstrate low neurotoxicity and high antiviral activity against HIV infection in the brain. Nanomedicine, 2014; 10: 177–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Fiandra L, Colombo M, Mazzucchelli S, Truffi M, Santini B, Allevi R, Nebuloni M, Capetti A, Rizzardini G, Prosperi D, Corsi F. Nanoformulation of antiretroviral drugs enhances their penetration across the blood brain barrier in mice. Nanomedicine, 2015; 11: 1387–97. [DOI] [PubMed] [Google Scholar]
- [115].Wen X, Wang K, Zhao Z, Zhang Y, Sun T, Zhang F, Wu J, Fu Y, Du Y, Zhang L, Sun Y, Liu Y, Ma K, Liu H, Song Y. Brain-targeted delivery of trans-activating transcriptor-conjugated magnetic PLGA/lipid nanoparticles. PLoS One, 2014; 9: e106652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Saiyed ZM, Gandhi NH, Nair MP. Magnetic nanoformulation of azidothymidine 5’-triphosphate for targeted delivery across the blood-brain barrier. Int J Nanomedicine, 2010; 5: 157–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Sagar V, Pilakka-Kanthikeel S, Pottathil R, Saxena SK, Nair M. Towards nanomedicines for neuroAIDS. Rev Med Virol, 2014; 24: 103–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Sagar V, Pilakka-Kanthikeel S, Atluri VS, Ding H, Arias AY, Jayant RD, Kaushik A, Nair M. Therapeutical Neurotargeting via Magnetic Nanocarrier: Implications to Opiate-Induced Neuropathogenesis and NeuroAIDS. J Biomed Nanotechnol, 2015; 11: 1722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Raymond AD, Diaz P, Chevelon S, Agudelo M, Yndart-Arias A, Ding H, Kaushik A, Jayant RD, Nikkhah-Moshaie R, Roy U, Pilakka-Kanthikeel S, Nair MP. Microglia-derived HIV Nef+ exosome impairment of the blood-brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J Neurovirol, 2015. [DOI] [PubMed] [Google Scholar]
- [120].Pilakka-Kanthikeel S, Atluri VS, Sagar V, Saxena SK, Nair M. Targeted brain derived neurotropic factors (BDNF) delivery across the blood-brain barrier for neuro-protection using magnetic nano carriers: an in-vitro study. PLoS One, 2013; 8: e62241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Jayant RD, Atluri VS, Agudelo M, Sagar V, Kaushik A, Nair M. Sustained-release nanoART formulation for the treatment of neuroAIDS. Int J Nanomedicine, 2015; 10: 1077–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Ding H, Sagar V, Agudelo M, Pilakka-Kanthikeel S, Atluri VS, Raymond A, Samikkannu T, Nair MP. Enhanced blood-brain barrier transmigration using a novel transferrin embedded fluorescent magneto-liposome nanoformulation. Nanotechnology, 2014; 25: 055101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Nair M, Guduru R, Liang P, Hong J, Sagar V, Khizroev S. Externally controlled on-demand release of anti-HIV drug using magneto-electric nanoparticles as carriers. Nat Commun, 2013; 4: 1707. [DOI] [PubMed] [Google Scholar]
- [124].Kaushik A, Jayant RD, Sagar V, Nair M. The potential of magneto-electric nanocarriers for drug delivery. Expert Opin Drug Deliv, 2014; 11: 1635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Sagar V, Pilakka-Kanthikeel S, Ding H, Atluri VS, Jayant RD, Kaushik A, Nair M. Novel magneto electric nanodelivery of “microRNA mimic” across blood-brain barrier: Implications to cocaine modulation on HIV-associated neurocognitive disorders. Journal of NeuroImmune Pharmacology, 2014; 9: 49. [Google Scholar]
- [126].Sagar V, Kaushik A, Roy U, Jayant RD, Atluri VS, Pilakka-Kanthikeel S, El-Haage N, Nair M. Effect of Magneto-electric nanoparticle on deep brain motor coordination activity. Journal of NeuroImmune Pharmacology, 2015; 10: S99–S100. [Google Scholar]
- [127].Zhuang X, Teng Y, Samykutty A, Mu J, Deng Z, Zhang L, Cao P, Rong Y, Yan J, Miller D, Zhang HG. Grapefruit-derived Nanovectors Delivering Therapeutic miR17 Through an Intranasal Route Inhibit Brain Tumor Progression. Mol Ther, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Parikh RH, Patel RJ. Nanoemulsions for Intranasal Delivery of Riluzole to Improve Brain Bioavailability: Formulation Development and Pharmacokinetic Studies. Curr Drug Deliv, 2015. [DOI] [PubMed] [Google Scholar]
- [129].Chauhan MB, Chauhan NB. Brain Uptake of Neurotherapeutics after Intranasal versus Intraperitoneal Delivery in Mice. J Neurol Neurosurg, 2015; 2. [PMC free article] [PubMed] [Google Scholar]
- [130].Hanson LR, Frey WH 2nd. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci, 2008; 9 Suppl 3: S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Born J, Lange T, Kern W, McGregor GP, Bickel U, Fehm HL. Sniffing neuropeptides: a transnasal approach to the human brain. Nat Neurosci, 2002; 5: 514–6. [DOI] [PubMed] [Google Scholar]
- [132].Aly AE, Waszczak BL. Intranasal gene delivery for treating Parkinson’s disease: overcoming the blood-brain barrier. Expert Opin Drug Deliv, 2015; 12: 1923–41. [DOI] [PubMed] [Google Scholar]
- [133].in ‘t Veen JP, van den Berg MP, Romeijn SG, Verhoef JC, Merkus FW. Uptake of fluorescein isothiocyanate-labelled dextran into the CSF after intranasal and intravenous administration to rats. Eur J Pharm Biopharm, 2005; 61: 27–31. [DOI] [PubMed] [Google Scholar]
- [134].Zhang H, Meng J, Zhou S, Liu Y, Qu D, Wang L, Li X, Wang N, Luo X, Ma X. Intranasal Delivery of Exendin-4 Confers Neuroprotective Effect Against Cerebral Ischemia in Mice. AAPS J, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol, 2006; 34: 252–69. [DOI] [PubMed] [Google Scholar]
- [136].Muenzer J, Hendriksz CJ, Fan Z, Vijayaraghavan S, Perry V, Santra S, Solanki GA, Mascelli MA, Pan L, Wang N, Sciarappa K, Barbier AJ. A phase I/II study of intrathecal idursulfase-IT in children with severe mucopolysaccharidosis II. Genet Med, 2016; 18: 73–81. [DOI] [PubMed] [Google Scholar]
- [137].Hasnat MJ, Rice JE. Intrathecal baclofen for treating spasticity in children with cerebral palsy. Cochrane Database Syst Rev, 2015; 11: CD004552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Hamza M, Doleys DM, Saleh IA, Medvedovsky A, Verdolin MH, Hamza M. A Prospective, Randomized, Single-Blinded, Head-to-Head Long-Term Outcome Study, Comparing Intrathecal (IT) Boluses With Continuous Infusion Trialing Techniques Prior to Implantation of Drug Delivery Systems (DDS) for the Treatment of Severe Intractable Chronic Nonmalignant Pain. Neuromodulation, 2015; 18: 636–48; discussion 649. [DOI] [PubMed] [Google Scholar]
- [139].Chen BK, Staff NP, Knight AM, Nesbitt JJ, Butler GW, Padley DJ, Parisi JE, Dietz AB, Windebank AJ. A safety study on intrathecal delivery of autologous mesenchymal stromal cells in rabbits directly supporting Phase I human trials. Transfusion, 2015; 55: 1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Kurnutala LN, Kim D, Sayeed H, Sibai N. Persistent Spinal Headache After Removal of Intrathecal Drug Delivery System: A Case Report and Review of Literature. Anesth Pain Med, 2015; 5: e29786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Kratzsch T, Stienen MN, Reck T, Hildebrandt G, Hoederath P. Catheter-tip Granulomas Associated with Intrathecal Drug Delivery--A Two-Center Experience Identifying 13 Cases. Pain Physician, 2015; 18: E831–40. [PubMed] [Google Scholar]
- [142].Shin BJ, Burkhardt JK, Riina HA, Boockvar JA. Superselective intra-arterial cerebral infusion of novel agents after blood-brain disruption for the treatment of recurrent glioblastoma multiforme: a technical case series. Neurosurg Clin N Am, 2012; 23: 323–9, ix–x. [DOI] [PubMed] [Google Scholar]
- [143].Gonzales-Portillo GS, Sanberg PR, Franzblau M, Gonzales-Portillo C, Diamandis T, Staples M, Sanberg CD, Borlongan CV. Mannitol-enhanced delivery of stem cells and their growth factors across the blood-brain barrier. Cell Transplant, 2014; 23: 531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Garg P, Pandey S, Seonwoo H, Yeom S, Choung YH, Cho CS, Choung PH, Hoon Chung J. Hyperosmotic polydixylitol for crossing the blood brain barrier and efficient nucleic acid delivery. Chem Commun (Camb), 2015; 51: 3645–8. [DOI] [PubMed] [Google Scholar]
- [145].Lynn JG, Zwemer RL, Chick AJ, Miller AE. A New Method for the Generation and Use of Focused Ultrasound in Experimental Biology. J Gen Physiol, 1942; 26: 179–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Bednarski MD, Lee JW, Callstrom MR, Li KC. In vivo target-specific delivery of macromolecular agents with MR-guided focused ultrasound. Radiology, 1997; 204: 263–8. [DOI] [PubMed] [Google Scholar]
- [147].Timbie KF, Mead BP, Price RJ. Drug and gene delivery across the blood-brain barrier with focused ultrasound. J Control Release, 2015; 219: 61–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Downs ME, Buch A, Karakatsani ME, Konofagou EE, Ferrera VP. Blood-Brain Barrier Opening in Behaving Non-Human Primates via Focused Ultrasound with Systemically Administered Microbubbles. Sci Rep, 2015; 5: 15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Downs ME, Buch A, Sierra C, Karakatsani ME, Teichert T, Chen S, Konofagou EE, Ferrera VP. Long-Term Safety of Repeated Blood-Brain Barrier Opening via Focused Ultrasound with Microbubbles in Non-Human Primates Performing a Cognitive Task. PLoS One, 2015; 10: e0125911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Watkins BA, Dorn HH, Kelly WB, Armstrong RC, Potts BJ, Michaels F, Kufta CV, Dubois-Dalcq M. Specific tropism of HIV-1 for microglial cells in primary human brain cultures. Science, 1990; 249: 549–53. [DOI] [PubMed] [Google Scholar]
- [151].Gorry PR, Ong C, Thorpe J, Bannwarth S, Thompson KA, Gatignol A, Vesselingh SL, Purcell DF. Astrocyte infection by HIV-1: mechanisms of restricted virus replication, and role in the pathogenesis of HIV-1-associated dementia. Curr HIV Res, 2003; 1: 463–73. [DOI] [PubMed] [Google Scholar]
- [152].Aquaro S, Svicher V, Schols D, Pollicita M, Antinori A, Balzarini J, Perno CF. Mechanisms underlying activity of antiretroviral drugs in HIV-1-infected macrophages: new therapeutic strategies. J Leukoc Biol, 2006; 80: 1103–10. [DOI] [PubMed] [Google Scholar]
- [153].Gray LR, Tachedjian G, Ellett AM, Roche MJ, Cheng WJ, Guillemin GJ, Brew BJ, Turville SG, Wesselingh SL, Gorry PR, Churchill MJ. The NRTIs lamivudine, stavudine and zidovudine have reduced HIV-1 inhibitory activity in astrocytes. PLoS One, 2013; 8: e62196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Avery LB, Zarr MA, Bakshi RP, Siliciano RF, Hendrix CW. Increasing extracellular protein concentration reduces intracellular antiretroviral drug concentration and antiviral effect. AIDS Res Hum Retroviruses, 2013; 29: 1434–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Croteau D, Rossi SS, Best BM, Capparelli E, Ellis RJ, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur J, McCutchan JA, Morgello S, Simpson DM, Grant I, Letendre S, Group C. Darunavir is predominantly unbound to protein in cerebrospinal fluid and concentrations exceed the wild-type HIV-1 median 90% inhibitory concentration. J Antimicrob Chemother, 2013; 68: 684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Sanchez Martin A, Cabrera Figueroa S, Cruz Guerrero R, Hurtado LP, Hurle AD, Carracedo Alvarez A. Impact of pharmacogenetics on CNS side effects related to efavirenz. Pharmacogenomics, 2013; 14: 1167–78. [DOI] [PubMed] [Google Scholar]
- [157].Purnell PR, Fox HS. Efavirenz induces neuronal autophagy and mitochondrial alterations. J Pharmacol Exp Ther, 2014; 351: 250–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Apostolova N, Funes HA, Blas-Garcia A, Alegre F, Polo M, Esplugues JV. Involvement of nitric oxide in the mitochondrial action of efavirenz: a differential effect on neurons and glial cells. J Infect Dis, 2015; 211: 1953–8. [DOI] [PubMed] [Google Scholar]
- [159].Polo M, Alegre F, Funes HA, Blas-Garcia A, Victor VM, Esplugues JV, Apostolova N. Mitochondrial (dys)function - a factor underlying the variability of efavirenz-induced hepatotoxicity? Br J Pharmacol, 2015; 172: 1713–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Bertrand L, Toborek M. Dysregulation of Endoplasmic Reticulum Stress and Autophagic Responses by the Antiretroviral Drug Efavirenz. Mol Pharmacol, 2015; 88: 304–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Sinxadi PZ, McIlleron HM, Dave JA, Smith PJ, Levitt NS, Haas DW, Maartens G. Plasma Efavirenz Concentrations Are Associated With Lipid and Glucose Concentrations. Medicine (Baltimore), 2016; 95: e2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Jiang B, Hebert VY, Li Y, Mathis JM, Alexander JS, Dugas TR. HIV antiretroviral drug combination induces endothelial mitochondrial dysfunction and reactive oxygen species production, but not apoptosis. Toxicol Appl Pharmacol, 2007; 224: 60–71. [DOI] [PubMed] [Google Scholar]
- [163].Auclair M, Afonso P, Capel E, Caron-Debarle M, Capeau J. Impact of darunavir, atazanavir and lopinavir boosted with ritonavir on cultured human endothelial cells: beneficial effect of pravastatin. Antivir Ther, 2014; 19: 773–82. [DOI] [PubMed] [Google Scholar]


