To the editor:
Ibrutinib, approved by the US Food and Drug Administration (FDA), is an inhibitor of Bruton tyrosine kinase (BTK).1-5 Ibrutinib use is associated with atrial fibrillation (AF), with an incidence of 5% to 6% after 18 months on therapy4-6 and up to 16% with longer follow-up.2,7 Prompted by an episode of unexplained ventricular tachycardia (VT) in a patient taking ibrutinib, we hypothesized that ibrutinib use may be associated with ventricular arrhythmias (VAs). To further investigate, we gathered 4 cases of VAs in ibrutinib-treated patients, mined the FDA Adverse Event Reporting System (FAERS) for additional reports of VAs in patients receiving ibrutinib, and estimated incidence rates of VAs in clinical trials of ibrutinib.
Individual patients were enrolled on data collection protocols approved by institutional review boards or collected from publicly available data. We obtained adverse events (AEs) for ibrutinib reported to the FAERS from the fourth quarter of 2013 (ibrutinib was approved by the FDA in November 2013) to the fourth quarter of 2015. Additional details were requested for AE reports with the following preferred search terms: ventricular arrhythmia, ventricular fibrillation (VF), Brugada syndrome, right bundle branch block, cardiac arrest, cardiac death, cardiac fibrillation, cardiorespiratory arrest, conduction disorder, sudden death, sudden cardiac death, ventricular extrasystoles, and ventricular tachycardia. If dates of ibrutinib discontinuation, cardiac events, or death were redacted, we used the date when the patient was last seen alive or when the event was filed. Patients for whom there was not enough information to determine the timing of ibrutinib administration or for whom ibrutinib was discontinued before the event were excluded. Clinical trial data were drawn from published results. Median time on therapy was assumed to be mean time on therapy to calculate total time on therapy for the group of interest. Statistical analysis was performed by using STATA 14 (STATA, College Station, TX).
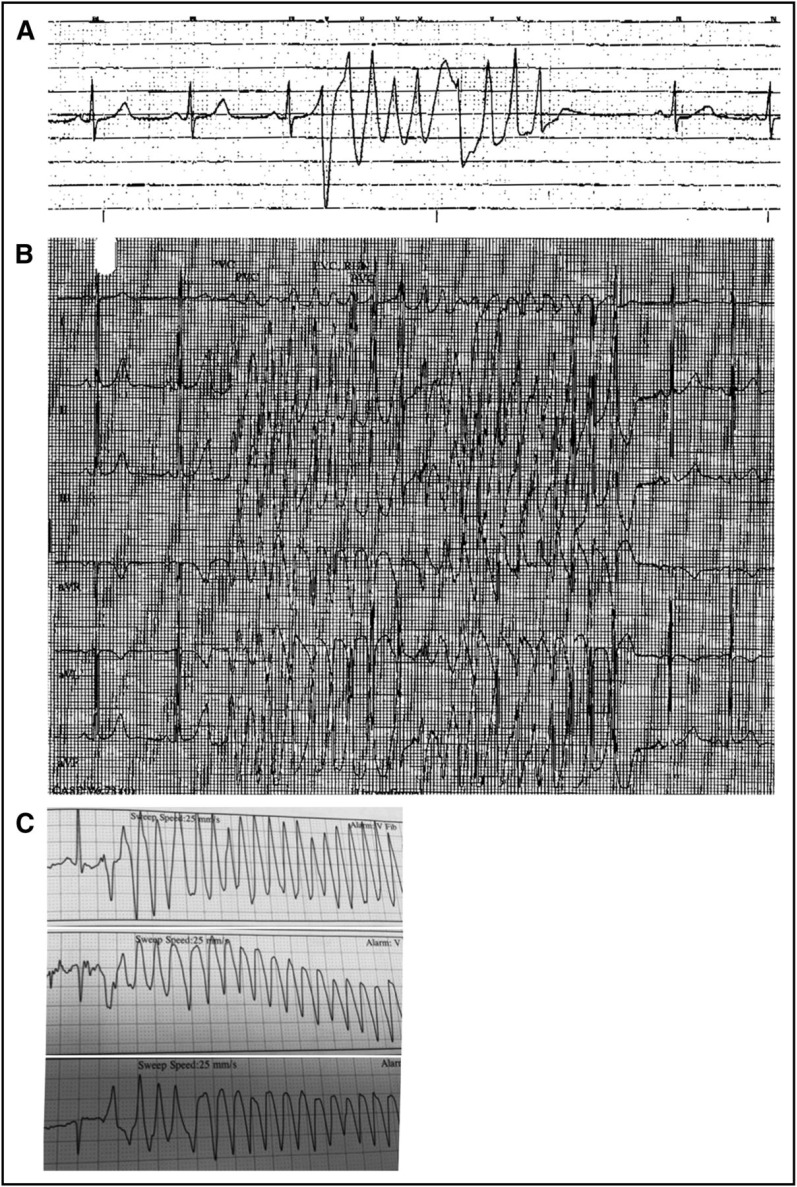
Our index patient was a 60-year-old man who had chronic lymphocytic leukemia (CLL) and no prior cardiac history. He was a vigorous exerciser with resting sinus bradycardia. Two months after beginning ibrutinib, he reported new palpitations. He experienced syncope 86 days after initiating ibrutinib. Frequent premature ventricular contractions (PVCs) and nonsustained VT were identified (Figure 1A). An exercise stress test was negative for ischemic changes, but polymorphic VT occurred when he transferred from stretcher to treadmill (Figure 1B). Cardiac catheterization and echocardiogram were normal. Electrophysiologic studies induced PVCs originating from the moderator band but could not ablate the focus; therefore, he was started on antiarrhythmics and a cardioverter-defibrillator was implanted. Ibrutinib was resumed at discharge. Attempted downtitration of his quinidine later resulted in an increase in PVCs. He has since been maintained on ibrutinib, quinidine, and metoprolol for 28 months with occasional nonsustained VT on device interrogation.
Figure 1.
Ventricular tachycardia captured in patients on ibrutinib. (A) A brief run of nonsustained ventricular tachycardia is seen on telemetry shortly after a syncopal episode in a patient who had been taking ibrutinib 420 mg per day for 86 days. (B) A 20-beat run of polymorphic ventricular tachycardia experienced by the same patient with syncope while receiving ibrutinib. (C) Polymorphic ventricular tachycardia identified in a patient reporting presyncope after 28 days receiving ibrutinib 420 mg per day.
A second patient was a 55-year-old man with refractory CLL, primary sclerosing cholangitis, and no prior cardiac disease. He had a witnessed collapse 366 days after initiating ibrutinib and was resuscitated. While he was hospitalized, he had an R-on-T phenomenon resulting in polymorphic VT followed by VF. Defibrillation restored sinus rhythm. Coronary angiography, echocardiogram, cardiac magnetic resonance imaging, and genetic testing for inherited sodium channelopathies all failed to identify any cardiac abnormalities. A third patient was a 53-year-old man with CLL, AF, coronary artery disease, and a 30-pack-year smoking history. Nineteen days after initiating ibrutinib, he reported palpitations and presyncope. Holter monitoring showed frequent ventricular ectopic beats. Nine days later, he was found to have polymorphic VT (Figure 1C). A fourth instance of VF while taking ibrutinib may have been triggered by an acute ischemic event. Ibrutinib was stopped for all patients; in 3 patients, ibrutinib was resumed 10 to 50 days later; two patients had recurrent VAs (details are provided in supplemental Table 1, available on the Blood Web site).
We queried the FAERS for cases of VAs or sudden death in patients taking ibrutinib. This database contains AEs submitted by health care professionals, consumers, and manufacturers. We analyzed the preferred terms listed above, and further limited our study population to patients without concomitant acute illness. We identified 7 additional instances of VT/VF and 6 sudden deaths (supplemental Table 1). Ten of 13 patients had no prior cardiac history. No patients were taking other medications known to induce cardiac arrhythmias. For all patients, the median time to event from ibrutinib initiation was 65 days (range, 6-698 days) and the median age was 61 years (range, 49-85 years). In all patients identified in the FAERS database who underwent additional cardiac workup, no clear cause could be identified.
In published clinical trials of ibrutinib that enrolled approximately 1000 total participants, we identified 10 cases of sudden death or cardiac arrest (Table 1). Incidence rates of sudden death as a function of time on therapy were calculated. The weighted average of the incidence rates was 788 events per 100 000 person-years. In comparison, rates of sudden cardiac death for 65-year-olds are in the range of 200 to 400 events per 100 000 person-years,8-11 which were exceeded in all but 1 of the ibrutinib studies. Four studies (RESONATE,3 RESONATE-2,5 HELIOS,12 and RAY13) had control arms that did not contain ibrutinib, but only the HELIOS study publicly reported all grade 3 to 5 AEs, which allowed for direct comparison of incidence rates between 2 randomized populations. The aggregate number of grade ≥ 3 VAs, cardiac arrests, and sudden deaths in HELIOS was 7 in the ibrutinib-containing arm and 0 in the placebo-containing arm (supplemental Table 2). Assuming these events happened in separate patients, this corresponds to 1,991 events per 100 000 person-years versus 0 events per 100 000 person-years, respectively (exact two-sided P = .025).
Table 1.
Ibrutinib studies with reported sudden deaths/cardiac arrests
| Studies* | No. of Patients | Median time on therapy (months) | Age (y) | No. of sudden deaths/cardiac arrests in ibrutinib arm | Incidence per 100 000 patient-years (95% CI) | Reference | |
|---|---|---|---|---|---|---|---|
| Median | Range | ||||||
| OSU experience: NCT01105247, NCT01217749, NCT01589302, NCT01578707 (RESONATE) | 308 | 20 | 65 | 26-91 | 1 | 194.8 (4.9-1085.4) | 22 |
| NCT01722487 (RESONATE-2) | 135 | 17.4 | 73 | 65-89 | 2 | 1021.7 (123.7-3690.8) | 5 |
| MDACC experience: NCT01105247, NCT01520519, NCT01752426, NCT01578707 (RESONATE) | 127 | 13 | 61 | 36-83 | 2 | 1453.7 (176.1-5252.1) | 23 |
| NCT01500733 (Phase 2 NHLBI) | 51 | 24† | 62 | 33-82 | 1 | 980.4 (24.8-5462.4) | 24 |
| Swedish Compassionate Use | 95 | 10.2† | 69 | 42-86 | 1 | 1238.4 (31.4-6899.9) | 25 |
| NCT01611090 (HELIOS) | 287 | 14.7 | 64 | 31-86 | 3 | 853.3 (176.0-2493.7) | 12 |
CI, confidence interval; MDACC, MD Anderson Cancer Center; NHLBI, National Heart, Lung, and Blood Institute; OSU, The Ohio State University.
Some of these long term follow-up studies report on patients from the same trials, but they report only on nonoverlapping sets of patients treated at the home institution.
Median follow-up time. Median time on therapy was not provided.
Ibrutinib is an arrhythmogenic molecule, although the arrhythmogenic mechanism is not well understood. A prior study has shown no effect of ibrutinib on QTc length,14 and patients with monitoring at the time of the event did not display a prolonged QTc (Figure 1). In cultured cardiomyocytes, ibrutinib triggers abnormal action potentials (both early and delayed afterdepolarizations) and increases late sodium current (INaL), ultimately leading to enhanced automaticity.15 The kinase responsible for these changes is unknown and may not be BTK16; ibrutinib inhibits 19 kinases with a half maximal inhibitory concentration (IC50) <100 nM.17
In randomized trials to date, any increased risk of VAs or sudden death has been outweighed by the benefits of treating the underlying disease, as shown by the improvement in overall survival in RESONATE3 and RESONATE-2.5 However, it is unknown whether this favorable risk-benefit balance will be maintained as ibrutinib treatment is expanded to populations that may be older, have more underlying cardiac disease, or have lower-risk CLL. For example, a case of VT has been reported in a trial examining ibrutinib in asymptomatic early-stage CLL,18 and 2 recent case reports identified VT in ibrutinib-treated patients with a history of AF19 and cardiomyopathy.20 In addition, ibrutinib studies currently have short follow-up, and the risk-benefit ratio may change with extended therapy.4 Our study is limited by its retrospective approach, the self-reported nature of FAERS data, and lack of access to primary trial data. Not all studies of ibrutinib have reported sudden deaths,21 and focusing only on studies that report sudden deaths may overestimate their incidence. Nevertheless, future trials of ibrutinib should report VAs and sudden deaths. Whether more specific BTK inhibitors might mitigate these concerns ultimately depends on whether the cause is attributable to on-target BTK or off-target non-BTK inhibition. Meanwhile, clinicians should inquire about symptoms of VAs in ibrutinib-treated patients, have a low threshold for cardiac workup if they are present, and consider the possibility of VAs when weighing the risk-benefit ratio of ibrutinib therapy.
Supplementary Material
The online version of this article contains a data supplement.
Authorship
Acknowledgments: The authors thank Jeff Ishizuka for assistance in obtaining events from the FAERS.
This study was supported by the American Cancer Society Grant No. RSG-13-002-01-CCE, the National Comprehensive Cancer Network, the Melton Family Fund for CLL Research, and the Susan and Gary Rosenbach Fund for Lymphoma Research (J.R.B.).
Contribution: B.L.L. and J.R.B. conceived and designed the study; all authors collected and/or analyzed data, drafted and provided critical review of the manuscript, and provided final approval of the manuscript.
Conflict-of-interest disclosure: J.R.B. served as a consultant for Gilead Sciences, Infinity Pharmaceuticals, Janssen Pharmaceuticals, Pharmacyclics, Roche/Genentech, Astra-Zeneca, and Abbvie. J.A.J. served as a consultant for Gilead Sciences, Janssen Pharmaceuticals, Pharmacyclics, AbbVie, and Genentech. E.D.J. served as a consultant for Pharmacyclics, Janssen Pharmaceuticals, and Infinity Pharmaceuticals. J.C.B. received institutional research support and served as a consultant for Pharmacyclics, AbbVie, and Gilead Sciences. V.B. served as a consultant for Gilead Sciences, Janssen Pharmaceuticals, Lundbeck, and AbbVie. R.J.G. received institutional research support from AstraZeneca, Kowa American, Novartis, and Pfizer Pharmaceuticals. J.J.M. served as a consultant for Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Jennifer R. Brown, Dana-Farber Cancer Institute, Mayer Building Room 226, 450 Brookline Ave, Boston, MA 02215; e-mail: jennifer_brown@dfci.harvard.edu.
References
- 1.Wang ML, Rule S, Martin P, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang ML, Blum KA, Martin P, et al. Long-term follow-up of MCL patients treated with single-agent ibrutinib: updated safety and efficacy results. Blood. 2015;126(6):739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Byrd JC, Brown JR, O’Brien S, et al. ; RESONATE Investigators . Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med. 2014;371(3):213-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrd JC, Furman RR, Coutre SE, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood. 2015;125(16):2497-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger JA, Tedeschi A, Barr PM, et al. ; RESONATE-2 Investigators . Ibrutinib as Initial Therapy for Patients with Chronic Lymphocytic Leukemia. N Engl J Med. 2015;373(25):2425-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leong DP, Caron F, Hillis C, et al. The risk of atrial fibrillation with ibrutinib use: a systematic review and meta-analysis. Blood. 2016;128(1):138-140. [DOI] [PubMed] [Google Scholar]
- 7.Farooqui M, Valdez J, Soto S, Bray A, Tian X, Wiestner A. Atrial fibrillation in CLL/SLL patients on ibrutinib [abstract]. Blood. 2015;126(23). Abstract 2933. [Google Scholar]
- 8.Becker LB, Han BH, Meyer PM, et al. Racial differences in the incidence of cardiac arrest and subsequent survival. The CPR Chicago Project. N Engl J Med. 1993;329(9):600-606. [DOI] [PubMed] [Google Scholar]
- 9.Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate-based review in a large U.S. community. J Am Coll Cardiol. 2004;44(6):1268-1275. [DOI] [PubMed] [Google Scholar]
- 10.Rothwell PM, Coull AJ, Silver LE, et al. ; Oxford Vascular Study . Population-based study of event-rate, incidence, case fatality, and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet. 2005;366(9499):1773-1783. [DOI] [PubMed] [Google Scholar]
- 11.Straus SM, Bleumink GS, Dieleman JP, van der Lei J, Stricker BH, Sturkenboom MC. The incidence of sudden cardiac death in the general population. J Clin Epidemiol. 2004;57(1):98-102. [DOI] [PubMed] [Google Scholar]
- 12.Chanan-Khan A, Cramer P, Demirkan F, et al. ; HELIOS investigators . Ibrutinib combined with bendamustine and rituximab compared with placebo, bendamustine, and rituximab for previously treated chronic lymphocytic leukaemia or small lymphocytic lymphoma (HELIOS): a randomised, double-blind, phase 3 study. Lancet Oncol. 2016;17(2):200-211. [DOI] [PubMed] [Google Scholar]
- 13.Dreyling M, Jurczak W, Jerkeman M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: an international, randomised, open-label, phase 3 study. Lancet. 2016;387(10020):770-778. [DOI] [PubMed] [Google Scholar]
- 14.Loury D, Sukbuntherng J, Clow F, James DF, Kunkel LA. Open label evaluation of ECG in patients with chronic lymphocytic leukemia (CLL) receiving ibrutinib monotherapy. J Clin Oncol. 2013;31(13 suppl). Abstract 7057. [Google Scholar]
- 15.Yang T, Moslehi JJ, Roden DM. Proarrhythmic effects of ibrutinib, a clinically approved inhibitor of Bruton’s tyrosine kinase (BTK) used in cancer therapy [abstract]. Circulation. 2015;132(Suppl 3). Abstract A14587. [Google Scholar]
- 16.McMullen JR, Boey EJ, Ooi JY, Seymour JF, Keating MJ, Tam CS. Ibrutinib increases the risk of atrial fibrillation, potentially through inhibition of cardiac PI3K-Akt signaling. Blood. 2014;124(25):3829-3830. [DOI] [PubMed] [Google Scholar]
- 17.Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci USA. 2010;107(29):13075-13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Langerbeins P, Bahlo J, Rhein C, et al. Ibrutinib in early stage CLL: preliminary safety results of a placebo-controlled phase III study [abstract]. Blood. 2015;126(23). Abstract 2934. [Google Scholar]
- 19.Tomcsányi J, Nényei Z, Mátrai Z, Bózsik B. Ibrutinib, an approved tyrosine kinase inhibitor as a potential cause of recurrent polymorphic ventricular tachycardia. JACC Clin Electrophysiol. 2016;2(7):847-849. [DOI] [PubMed] [Google Scholar]
- 20.Wallace N, Wong E, Cooper D, Chao H. A case of new-onset cardiomyopathy and ventricular tachycardia in a patient receiving ibrutinib for relapsed mantle cell lymphoma. Clin Case Rep. 2016;4(12):1120-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Brien S, Jones JA, Coutre SE, et al. Ibrutinib for patients with relapsed or refractory chronic lymphocytic leukaemia with 17p deletion (RESONATE-17): a phase 2, open-label, multicentre study. Lancet Oncol. 2016;17(10):1409-1418. [DOI] [PubMed] [Google Scholar]
- 22.Maddocks KJ, Ruppert AS, Lozanski G, et al. Etiology of Ibrutinib Therapy Discontinuation and Outcomes in Patients With Chronic Lymphocytic Leukemia. JAMA Oncol. 2015;1(1):80-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain P, Keating M, Wierda W, et al. Outcomes of patients with chronic lymphocytic leukemia after discontinuing ibrutinib. Blood. 2015;125(13):2062-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farooqui MZ, Valdez J, Martyr S, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: a phase 2, single-arm trial. Lancet Oncol. 2015;16(2):169-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Winqvist M, Asklid A, Andersson PO, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: data from 95 consecutive patients treated in a compassionate use program. A study from the Swedish Chronic Lymphocytic Leukemia Group. Haematologica. 2016;101(12):1573-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.