Abstract
Objective
To compare the efficacy of platinum- and non-platinum-based regimens as first-line treatment for advanced triple-negative breast cancer (TNBC) and analyze the relationship between their efficacy and BRCA gene status.
Methods
Retrospectively analyze clinical data of 220 patients diagnosed pathologically with advanced TNBC and treated at the Department of Breast Oncology, Peking University Cancer Hospital from 2013 to 2018 and evaluate the efficacy of chemotherapy. A total of 114 patients had BRCA1/2 gene tested by next generation sequencing (NGS) using peripheral blood, and we analyzed the correlation between their efficacy and BRCA1/2 gene status.
Results
Non-platinum-based chemotherapy (NPCT) was administered to 129 and platinum-based chemotherapy (PBCT) to 91 study patients. The clinical benefit rate (CBR) and median progression-free survival (PFS) were not statistically different between NPCT and PBCT groups. The median overall survival (OS) was 30.0 and 22.5 months for PBCT and NPCT group, respectively [P=0.090, hazard ratios (HR)=0.703]. BRCA status was assessed in 114 patients, 14 of whom had deleterious germline BRCA1/2 (gBRCA) mutations (seven in each group). In PBCT group, the CBR was 85.7% and 35.1% for patients with and without deleterious gBRCA mutations, respectively (P=0.039). The median PFS were 14.9 and 5.3 months and median OS were 26.5 and 15.5 months for patients with and without deleterious gBRCA mutations, respectively (P=0.001, P=0.161, respectively). Patients in PBCT group had significantly greater rates of grade 3−4 anemia (5.5%vs. 0%) and thrombocytopenia (8.8% vs. 0%), whereas palmar-plantar erythrodysesthesia (12.4% vs. 0%) and peripheral neuropathy (8.6% vs. 1.1%) occurred more frequently in NPCT group.
Conclusions
Platinum-based regimens are more effective in patients with deleterious gBRCA mutations, but no difference in patients without BRCA gene mutations, so non-platinum is an option in patients without BRCA gene mutations considering the toxicity and side effect. And we recommend that patients with advanced TNBC should have BRCA gene test.
Keywords: Advanced breast cancer, triple negative, BRCA mutation , next-generation sequencing, platinum, efficacy
Introduction
Triple-negative breast cancer (TNBC) is negative for expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2), and accounts for an estimated 15% of breast cancers (1). It is associated with a poor clinical outcome and high relapse rate (2). Because of the absence of hormonal receptors and HER2, chemotherapy is the mainstay of treatment of TNBC (3,4). TNBC shows a higher prevalence of BRCA1/2 mutations than other subtypes of breast cancer. It has been reported that 11%−20% of patients with TNBC carry germlineBRCA1/2 (gBRCA) mutations (3,5,6). BRCA1 and BRCA2 are tumor suppressor genes (7). They play an important role in homologous recombination (HR) repair, which is responsible for repairing interstrand crosslinks (ICL) and double strand breaks (DSB) (8,9). Mutations in BRCA1/2 genes result in genome instability and lead to development of malignancy (9,10). Individuals harboring BRCA mutations have an increased risk of developing breast cancer, which is often of triple negative phenotype. TNBC accounts for 70% of breast cancers with BRCA1 mutations and 16%−23% of those withBRCA2 mutations (11). While most patients with sporadic TNBC do not have BRCA1 mutations, evidence exists of BRCA1 pathway dysfunction in these tumors, a state defined as BRCAness (12,13). The mechanism of platinum-based therapy, which is an effective treatment for TNBC (14), is the generation of both intrastrand crosslinks and ICL, which inhibit DNA replication and transcription and induce DSB, eventually leading to cell death (8,14,15). Several studies have investigated the role of PBCT in metastatic TNBC; however, their results are conflicting. The CBCSG006 trial (16) showed that cisplatin plus gemcitabine is superior to paclitaxel plus gemcitabine as first-line therapy for patients with metastatic TNBC. However, according to the TNT trial (17), carboplatin is not more active than docetaxel in an unselected cohort, being more active than docetaxel only in patients with germline-mutated BRCA1/2 breast cancer. Moreover, the use of platinum-based regimens is limited because of their adverse effects and drug resistance associated with DNA damage repair (DDR) (8,14,18). Adverse effects of cisplatin, such as nephrotoxicity, neurotoxicity, and ototoxicity and of carboplatin, namely myelosuppression, limit their therapeutic effect of prolonging longevity (18). Because of DDR, tumors show intrinsic or acquired drug resistance to platinum-based regimens (8). To improve efficacy and decrease unnecessary use of platinum drugs, we retrospectively analyzed the clinical data of 220 patients with advanced breast cancer who had pathologically confirmed TNBC and were treated at the Department of Breast Oncology, Peking University Cancer Hospital from January 2013 to October 2018. We compared the efficacy of platinum- and non-platinum-based first-line therapy for advanced TNBC and analyzed the factors affecting the efficacy of these regimens.
Materials and methods
Patients
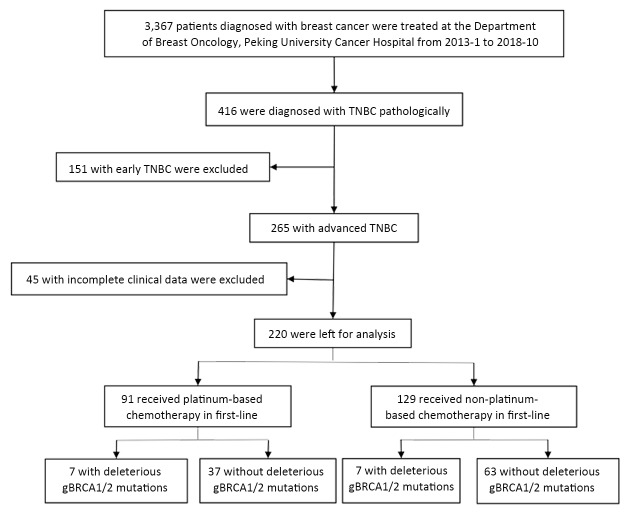
From January 2013 to October 2018, 3,367 patients diagnosed with breast cancer were treated at the Department of Breast Oncology, Peking University Cancer Hospital, 416 of whom (12.4%) were diagnosed with TNBC pathologically, 265 of whom having advanced stage disease. The inclusion criteria were as follows: 1) pathological diagnosis of advanced TNBC; 2) Eastern Cooperative Oncology Group (ECOG) score ≤2; 3) received at least two cycles of treatment or underwent at least one response evaluation; and 4) had measurable lesions. Patients with incomplete clinical data (n=45) were excluded, leaving 220 patients for analysis. The screening process is showed in Figure 1 . Their clinical data were collected from medical records. Informed consent was obtained from all patients before commencement of treatment.
1. Screening process.
Evaluation
Efficacy was analyzed according to clinical benefit rate (CBR), progression-free survival (PFS) and overall survival (OS). Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 by computed tomography scan or magnetic resonance imaging. Evaluations were performed every 6−12 weeks or whenever the patient’s condition changed. CBR was defined as the proportion of patients who achieved CR, PR, or SD for at least 24 weeks. PFS was defined as the time from starting treatment to identification of disease progression or death from any cause. OS was defined as the time from starting first-line treatment to death from any cause. Patients who survived without progression, died from any cause or were lost from follow-up were censored at the date of last follow-up (31 October 2018) or of their last contact. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.
Follow-up
We followed up by regular inpatient, outpatient or telephone every 8−12 weeks. Every follow-up period, the clinicians would record the result of computed tomography scan or magnetic resonance imaging as well as the adverse events of treatment. The last follow-up date was 31 October 2018.
DNA extraction
Peripheral blood was collected in ethylene diamine tetraacetic acid (EDTA) Vacutainer tubes and processed within 3 h. Genomic DNA was extracted from peripheral blood lymphocytes (PBLs) by using a DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Next-generation sequencing (NGS)
Sequence of BRCA1/2 gene has been enriched and sequenced by high throughput platform. All exons, 20 base pairs proximal to the 5’ end and 10 base pairs distal to the 3’ end of each exon were analyzed. Detected variations included single point mutations and small indels. Clinically important (pathogenic or likely pathogenic) mutations identified by the high throughput DNA sequencing method were verified by Sanger DNA sequencing analysis.
The variants were classified into the following five categories according to American College of Medical Genetics and Genomics (ACMG) Standards and Guidelines: pathogenic, likely pathogenic, benign, likely benign, and uncertain significance (19). In this study, pathogenic and likely pathogenic mutations were treated as deleterious gBRCA mutations.
Statistical analysis
Statistical analyses were carried out using IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA). Categorical data are presented as numbers and percentages and continuous data as medians and ranges. Pearson’s χ2 or Fisher’s exact tests were used for comparison of categorical variables. PFS and OS were estimated using the Kaplan-Meier method and compared using the log-rank test. A multivariate Cox regression model was also performed to compute hazard ratios (HR) and 95% confidence interval (95% CI) and adjusting for prognostic variables. All P values were two sided and P<0.05 was considered to denote statistical significance.
Results
Patient characteristics
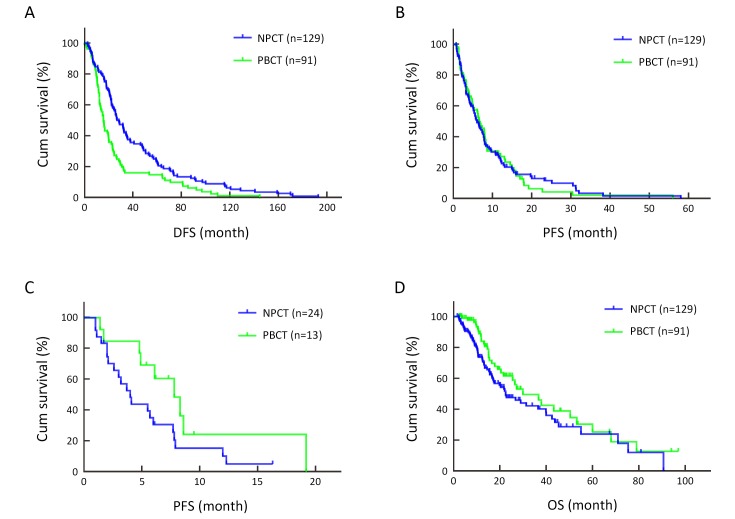
Non-platinum-based chemotherapy (NPCT) was administered to 129 (58.6%) of the 220 patients and platinum-based chemotherapy (PBCT) to the remaining 91 (41.4%) as first-line therapy. Their baseline characteristics are presented in Table 1 . The overall median age was 49 (range, 21−75) years old, being 51 (25−75) years old in the NPCT and 46 (21−66) years old in the PBCT group. The median disease-free survival (DFS) was 19.9 (95% CI: 16.4−23.4) months. Patients in the PBCT group were younger at onset and had shorter DFS than those in the NPCT group (P=0.002). The median DFS was 24.3 (0−192.8 months, 95% CI: 20.6−28.0) months in the NPCT group and 14.3 (0−144.6 months, 95% CI: 11.9−16.7) months in the PBCT group (P=0.002) (Figure 2A ). However, there were no significant differences in other baseline characteristics between these two groups.
1. Baseline characteristics of patients according to type of first-line therapy.
| Characteristics | NPCT (n=129) [n (%)] | PBCT (n=91) [n (%)] | P |
| DFS, disease-free survival; 95% CI, 95% confidence interval; NPCT, non-platinum-based chemotherapy; PBCT, platinum-based chemotherapy; *, Including digestive tract, pancreatic and prostate cancer and blood system tumors; **, Including lung, liver and brain metastases. | |||
| Age (year) | |||
| Median (range) | 51 (25−75) | 46 (21−66) | 0.001 |
| ≤50 | 64 (49.6) | 62 (68.1) | 0.006 |
| >50 | 65 (50.4) | 29 (31.9) | |
| Family history | |||
| Breast/ovarian cancer | 12 (9.3) | 9 (9.9) | 0.416 |
| Other cancers* | 16 (12.4) | 17 (18.7) | |
| No | 101 (78.3) | 65 (71.4) | |
| Histology of primary tumor | |||
| Invasive ductal carcinoma | 113 (87.6) | 79 (86.8) | 0.063 |
| Invasive lobular carcinoma | 8 (6.2) | 1 (1.1) | |
| Others | 8 (6.2) | 11 (12.1) | |
| Diagnosed triple negative | |||
| Primary tumor | 111 (86.0) | 79 (86.8) | 0.870 |
| Metastatic sites | 18 (14.0) | 12 (13.2) | |
| Tumor grade | |||
| I | 1 (0.8) | 0 (0) | 0.624 |
| II | 49 (38.0) | 30 (33.0) | |
| III | 44 (34.1) | 33 (36.3) | |
| Unknown | 35 (27.1) | 28 (30.8) | |
| Ki67 index | |||
| ≤14% | 12 (9.3) | 9 (9.9) | 0.459 |
| 15%−50% | 50 (38.8) | 26 (28.6) | |
| >50% | 45 (34.9) | 39 (42.9) | |
| Unknown | 22 (17.1) | 17 (18.7) | |
| DFS (month) | |||
| Median (95% CI) | 24.3 (20.6−28.0) | 14.3 (11.9−16.7) | 0.002 |
| ≤24 | 66 (51.2) | 69 (75.8) | 0.000 |
| >24 | 63 (48.8) | 22 (24.2) | |
| Metastatic site | |||
| Node | 56 (43.4) | 43 (47.3) | 0.573 |
| Bone | 31 (24.0) | 20 (22.0) | 0.722 |
| Chest wall | 23 (17.8) | 18 (19.8) | 0.714 |
| Lung | 41 (31.8) | 30 (33.0) | 0.853 |
| Liver | 24 (18.6) | 13 (14.3) | 0.399 |
| Brain | 6 (4.7) | 2 (2.2) | 0.554 |
| Other sites | 13 (10.1) | 20 (22.0) | 0.015 |
| Number of metastases | |||
| 1 | 88 (68.2) | 52 (57.1) | 0.153 |
| 2 | 22 (17.1) | 25 (27.5) | |
| ≥3 | 19 (14.7) | 14 (15.4) | |
| Visceral metastasis** | |||
| No | 72 (55.8) | 51 (56.0) | 0.973 |
| Yes | 57 (44.2) | 40 (44.0) | |
2. DFS, first-line PFS and OS according to treatment group. (A) DFS according to treatment group (P=0.002); (B) First-line PFS according to treatment group (P=0.907); (C) First-line PFS in patients with liver metastases according to treatment group (P=0.078); (D) OS from start of treatment of patients according to treatment group (P=0.090). DFS, disease-free survival; PFS, progression-free survival; OS, overall survival.
BRCA gene detection
BRCA1/2 gene testing was performed on 35 patients in Beijing Genomics Institution (BGI) clinical laboratories, on 33 patients in Peking University Cancer Hospital, on 61 patients in Huidu Shanghai clinical laboratory, and on 13 patients in other centers (some patients were tested more than once). The results are summarized in Table 2 . BRCA1/2 gene testing was performed at least twice in different centers in 25 of them and the concordance was 100% (Table 3 ). Thus, we analyzed CBR, PFS and OS using a combination of all these results.
2. Sources and results of BRCA gene testing .
| Results | BGI | Hospital* | Huidu** | Other centers |
| BGI, Beijing Genomics Institution; *, Peking University Cancer Hospital; **, Huidu Shanghai clinical laboratory. | ||||
| Total | 35 | 33 | 61 | 13 |
| Positive | 4 | 5 | 8 | 2 |
| Negative | 31 | 28 | 53 | 11 |
3. Concordance of BRCA findings from different sources .
| No. of patients | BGI | Hospital* | Huidu** | Other centers |
| BGI, Beijing Genomics Institution; VUS, variant of uncertain significance in detected site; *, Peking University Cancer Hospital; **, Huidu Shanghai clinical laboratory. | ||||
| 16 | Negative | − | Negative | − |
| 31 | Negative | Negative | − | − |
| 32 | Negative | − | Negative | − |
| 47 | − | Negative | Negative | − |
| 49 | Negative | Negative | Negative | − |
| 55 | Negative | − | Negative | − |
| 58 | Negative | Negative | Negative | − |
| 61 | Negative | − | Negative | − |
| 83 | Positive | Positive | Positive | − |
| 87 | Negative | − | Negative | − |
| 105 | − | Positive | Positive | − |
| 107 | − | − | Positive | Positive |
| 123 | − | Negative | Negative | − |
| 125 | Negative | − | Negative | − |
| 130 | − | VUS | VUS | − |
| 143 | − | Negative | Negative | − |
| 180 | − | Negative | Negative | − |
| 181 | VUS | VUS | − | − |
| 186 | − | Negative | Negative | − |
| 189 | − | VUS | VUS | − |
| 195 | − | Negative | Negative | − |
| 210 | Positive | Positive | − | − |
| 211 | − | − | Negative | Negative |
| 213 | − | Negative | Negative | − |
| 217 | − | Negative | Negative | − |
Signature of BRCA mutation
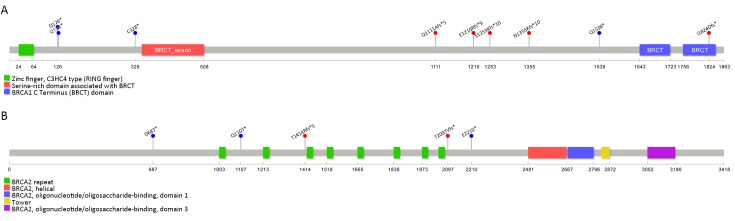
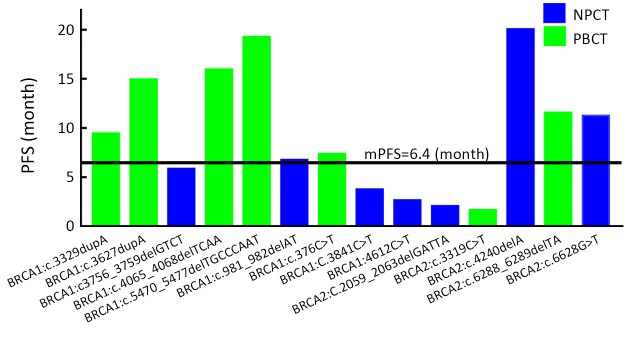
In all, 114 patients underwent BRCA1/2 gene testing, 14 (12.3%) of whom were found to have deleterious gBRCA mutations, seven in the NPCT and seven in the PBCT group. Nine had mutations of uncertain significance, the rest being (likely) benign mutations or wild type. These findings are presented in Table 4 and the mutation sites in Figure 3 . The PFS of patients with deleterious gBRCA mutations is compared with that (6.4 months) of all 220 patients in Figure 4 .
4. Summary of BRCA mutations .
| Variables | NPCT (n=70) | PBCT (n=44) |
| VUS, variant of uncertain significance in detected site; NPCT, non-platinum-based chemotherapy; PBCT, platinum-based chemotherapy. | ||
| Positive | 7 | 7 |
| Negative | 63 | 37 |
| (Likely) benign/Wild type | 62 | 29 |
| VUS | 1 | 8 |
3. Locations of deleterious germline BRCA1/2 (gBRCA) mutations. (A) BRCA1; (B) BRCA2.
4. Progression-free survival (PFS) of patients with deleterious germline BRCA1/2 (gBRCA) mutations according to treatment group. mPFS, median PFS.
In the NPCT group, patients with BRCA2 mutation sites in c.4240delA and c.6628G>T had longer PFS. When these two patients were excluded, the PFS was 3.7 (95% CI: 1.4−6.0) months for patients with deleteriousgBRCA mutations in the NPCT group; this does not differ significantly from the PFS (5.1 months) of patients without such mutations (P=0.220). The OS for the five patients with gBRCA mutations in the NPCT group could not be calculated because there were too few data.
Response and survival
The median follow-up time after recurrence or metastasis was 14.3 (range, 1.7−97.0) months. Overall, the CBR was 48.1% (62/129) for the NPCT and 51.6% (47/91) for the PBCT group; this difference is not significant (P=0.600). There was also no significant difference in median PFS (6.0 months, 95% CI: 4.6−7.4 for the NPCT and 6.6 months, 95% CI: 5.1−8.1 for the PBCT group) (P=0.907) (Figure 2B ). CBR and median PFS did not differ significantly in these two groups within the subgroups of age of onset (≤50 years vs. >50 years), DFS (≤24 months vs. >24 months), and visceral metastases. CBR and median PFS were also analyzed according to site of metastases. The PBCT group tended to have better outcomes in patients with liver and chest wall metastases; however, neither difference was statistically significant ( Figure 2C ). Only eight patients developed brain metastases, two of whom had received PBCT. There were too few patients with brain metastases to compare CBR and PFS between the groups. CBR and median PFS are shown in Table 5 . The median OS was around 7.5 months longer in the PBCT (30.0 months, 95% CI: 17.5−42.4) than NPCT group (22.5 months, 95% CI: 14.4−30.7); this difference was not statistically significant (P=0.090, HR=0.703, 95% CI: 0.466−1.059) (Figure 2D ).
5. Efficacy according to clinically important factors, including type of chemotherapy regimen.
| Variables | CBR [% (n/N)] | PFS (95% CI) | |||||
| NPCT (n=129) | PBCT (n=91) | P | NPCT (n=129) | PBCT (n=91) | P | ||
| DFS, disease-free survival; NPCT, non-platinum-based chemotherapy; PBCT, platinum-based chemotherapy; CBR, clinical benefit rate; PFS, progression-free survival; 95% CI, 95% confidence interval. | |||||||
| Total | 48.1 (62/129) | 51.6 (47/91) | 0.600 | 6.0 (4.6−7.4) | 6.6 (5.1−8.1) | 0.907 | |
| Age of onset (year) | |||||||
| ≤50 | 53.1 (34/64) | 56.5 (35/62) | 0.708 | 6.4 (4.9−7.9) | 7.3 (5.9−8.7) | 0.950 | |
| >50 | 43.1 (28/65) | 41.4 (12/29) | 0.878 | 5.7 (2.3−9.1) | 4.5 (1.7−7.3) | 0.713 | |
| DFS (month) | |||||||
| ≤24 | 31.8 (21/66) | 44.9 (31/69) | 0.118 | 4.5 (3.4−5.6) | 6.2 (4.4−8.0) | 0.333 | |
| >24 | 65.1 (41/63) | 72.7 (16/22) | 0.511 | 7.9 (7.0−8.8) | 8.6 (0.8−16.4) | 0.965 | |
| Visceral metastasis | 47.4 (27/57) | 50.0 (20/40) | 0.798 | 6.0 (4.2−7.8) | 6.7 (4.0−9.4) | 0.939 | |
| Non-visceral metastasis | 48.6 (35/72) | 52.9 (27/51) | 0.636 | 6.5 (4.7−8.3) | 6.6 (3.6−9.6) | 0.866 | |
| Lung metastasis | 48.8 (20/41) | 46.7 (14/30) | 0.860 | 6.0 (3.5−8.5) | 6.2 (3.0−9.4) | 0.687 | |
| Liver metastasis | 37.5 (9/24) | 61.5 (8/13) | 0.188 | 4.0 (2.6−5.4) | 7.8 (5.0−10.6) | 0.078 | |
| Chest wall metastasis | 34.8 (8/23) | 55.6 (10/18) | 0.183 | 5.0 (2.1−7.9) | 6.6 (2.1−11.1) | 0.096 | |
| Bone metastasis | 51.6 (16/31) | 55.0 (11/20) | 0.813 | 5.7 (1.3−10.1) | 8.0 (6.6−9.4) | 0.980 | |
| Lymph node metastasis | 48.2 (27/56) | 48.8 (21/43) | 0.951 | 7.3 (4.9−9.7) | 6.2 (4.4−8.0) | 0.765 | |
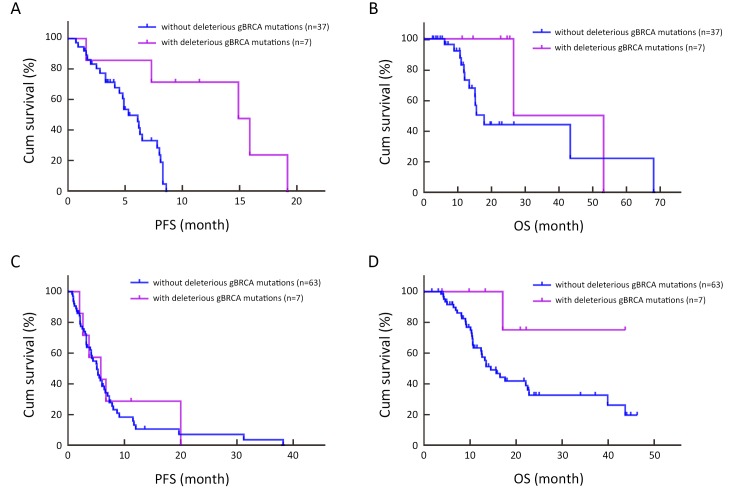
Additionally, subgroup analyses were performed. In the PBCT group, the CBR was 85.7% (6/7) for patients with deleterious gBRCA mutations and 35.1% (13/37) for those without; these response rates differ significantly (P=0.039). The median PFS was significantly longer in patients with BRCA mutations; namely, 14.9 months (95% CI: 6.9−22.9) for patients with deleteriousgBRCA mutations vs. 5.3 months (95% CI: 4.0−6.6) for those without (P=0.001) (Figure 5A ). By multivariate Cox regression model after adjusting for age of onset, DFS, tumor grade and visceral metastasis, the risk of progression was reduced by 88.7% for patients with deleterious gBRCA mutations compared to those without (P=0.008, HR=0.113, 95% CI: 0.023−0.566) (Table 6 ). The median OS was nearly 11 months longer in patients with deleterious gBRCA mutations (26.5 months vs. 15.5 months, 95% CI: 10.9−20.2); however, this difference was not statistically significant (P=0.161) (Figure 5B , Table 7 ).
5. First-line progression-free survival (PFS) of patients with or without deleterious germline BRCA1/2 (gBRCA) mutations in subgroup analysis. (A) First-line PFS of patients with or without deleterious gBRCA mutations in platinum-based chemotherapy (PBCT) group (P=0.001); (B) Overall survival (OS) for platinum-based treatment of patients with or without deleterious gBRCA mutations in PBCT group (P=0.161); (C) First-line PFS of patients with or without deleterious gBRCA mutations in non-platinum-based chemotherapy (NPCT) group (P=0.677); (D) OS from non-platinum-based treatment of patients with or without deleterious gBRCA mutations in NPCT group (P=0.075).
6. Multivariate Cox analyses for DFS in PBCT group.
| Variables | HR (95% CI) | P |
| DFS, disease-free survival; PBCT, platinum-based chemotherapy; gBRCA, germline BRCA1/2; HR, hazard ratio; 95% CI, 95% confidence interval. | ||
| Age of onset (≤50 vs. >50) (year) | 0.576 (0.233−1.420) | 0.230 |
| DFS (≤24 vs. >24) (month) | 0.391 (0.096−1.596) | 0.191 |
| Tumor grade (III vs. II) | 1.740 (0.545−5.557) | 0.350 |
| Visceral metastasis (visceral vs. non-visceral) | 0.823 (0.368−1.839) | 0.635 |
| gBRCA mutation (with vs. without) | 0.113 (0.023−0.566) | 0.008 |
7. CBR, PFS and OS with or without deleterious gBRCA mutations according to type of chemotherapy .
| Results | PBCT (n=44) | NPCT (n=70) | |||||
| With (n=7) | Without (n=37) | P | With (n=7) | Without (n=63) | P | ||
| CBR, clinical benefit rate; PFS, progression-free survival; OS, overall survival; gBRCA, germline BRCA1/2; PBCT, platinum-based chemotherapy; NPCT, non-platinum-based chemotherapy; 95% CI, 95% confidence interval. | |||||||
| CBR | 85.7% (6/7) | 35.1% (13/37) | 0.039 | 57.1% (4/7) | 39.7% (25/63) | 0.627 | |
| PFS [median (95% CI)] (month) | 14.9 (6.9−22.9) | 5.3 (4.0−6.6) | 0.001 | 5.8 (1.2−10.4) | 5.1 (3.8−6.4) | 0.677 | |
| OS [median (95% CI)] (month) | 26.5 (−) | 15.5 (10.9−20.2) | 0.161 | − | 14.5 (10.4−18.6) | 0.075 | |
In the NPCT group, CBR, PFS and OS were quite close between patients with and without deleterious gBRCA mutations. The CBR was 57.1% (4/7) and 39.7% (25/63) for patients with and without deleterious gBRCA mutations, respectively (P=0.627), the median PFS was 5.8 months (95% CI: 1.2−10.4) and 5.1 months (95% CI: 3.8−6.4) for patients with and without deleteriousgBRCA mutations, respectively (P=0.677) (Figure 5C ), and the median OS was 14.5 months (95% CI: 10.4−18.6) for patients without deleteriousgBRCA mutations (Figure 5D ). Only one of the seven patients with deleterious gBRCA mutations died; thus, there were too few data to calculate the OS (Table 7 ).
Adverse events
Adverse events were recorded in 180 patients, the most frequent is leucopenia, the incidence of which was similar in the two groups. Leucopenia occurred in 44.3% of the patients in the NPCT group and in 41.8% of the patients in the PBCT group. Other adverse events of any grade that occurred in at least 15% of patients in either group were neutropenia (20.2% and 26.4%), fatigue (22.5% and 15.4%) and nausea (13.2% and 15.4%). Significantly more patients in the PBCT group had grade 3−4 anemia (which occurred in 0% of the patients in the NPCT group and 5.5% of the patients in the PBCT group) and thrombocytopenia (0% and 8.8%), whereas significantly more patients had palmar-plantar erythrodysesthesia (PPE) (12.4% and 0%) and peripheral neuropathy (8.6% and 1.1%) in the NPCT group. There were no treatment-related deaths (Table 8 ).
8. Drug-related adverse events.
| Adverse events | NPCT (n=129) [n (%)] | PBCT (n=91) [n (%)] | |||||
| Grade 1−2 | Grade 3 | Grade 4 | Grade 1−2 | Grade 3 | Grade 4 | ||
| ALT, alanine aminotransferase; AST, aspartate aminotransferase; PPE, palmar-plantar erythrodysesthesia; NPCT, non-platinum-based chemotherapy; PBCT, platinum-based chemotherapy; NA, not applicable. No grade 5 adverse events were observed. | |||||||
| Hematological | |||||||
| Leucopenia | 38 (29.5) | 14 (10.9) | 5 (3.9) | 24 (26.4) | 11 (12.1) | 3 (3.3) | |
| Neutropenia | 11 (8.5) | 13 (10.1) | 2 (1.6) | 15 (16.5) | 6 (6.6) | 3 (3.3) | |
| Febrile neutropenia | NA | 2 (1.6) | 0 (0) | NA | 2 (2.2) | 0 (0) | |
| Anemia | 4 (3.1) | 0 (0) | 0 (0) | 3 (3.3) | 4 (4.4) | 1 (1.1) | |
| Thrombocytopenia | 2 (1.6) | 0 (0) | 0 (0) | 3 (3.3) | 5 (5.5) | 3 (3.3) | |
| Laboratory-assessed items | |||||||
| Increased ALT/AST | 6 (4.7) | 0 (0) | 0 (0) | 9 (9.9) | 0 (0) | 0 (0) | |
| Increased bilirubin | 3 (2.3) | 0 (0) | 0 (0) | 3 (3.3) | 0 (0) | 0 (0) | |
| Non-hematological | |||||||
| Nausea | 16 (12.4) | 1 (0.8) | NA | 14 (15.4) | 0 (0) | NA | |
| Vomiting | 9 (7.0) | 1 (0.8) | 0 (0) | 4 (4.4) | 1 (1.1) | 0 (0) | |
| Anorexic | 19 (14.7) | 0 (0) | 0 (0) | 6 (6.6) | 0 (0) | 0 (0) | |
| Diarrhea | 5 (3.9) | 0 (0) | 0 (0) | 4 (4.4) | 0 (0) | 0 (0) | |
| Abdominal distension | 3 (2.3) | 0 (0) | NA | 5 (5.5) | 0 (0) | NA | |
| Constipation | 5 (3.9) | 0 (0) | 0 (0) | 3 (3.3) | 0 (0) | 0 (0) | |
| Fatigue | 27 (20.9) | 2 (1.6) | NA | 13 (14.3) | 1 (1.1) | NA | |
| Hyperhidrosis | 15 (11.6) | 0 (0) | NA | 9 (9.9) | 0 (0) | NA | |
| Weight loss | 3 (2.3) | 0 (0) | NA | 1 (1.1) | 0 (0) | NA | |
| Insomnia | 13 (10.1) | 1 (0.8) | NA | 10 (11.0) | 0 (0) | NA | |
| Pain | 13 (10.1) | 0 (0) | NA | 5 (5.5) | 0 (0) | NA | |
| Alopecia | 17 (13.2) | NA | NA | 10 (11.0) | NA | NA | |
| PPE | 16 (12.4) | 0 (0) | NA | 0 (0) | 0 (0) | NA | |
| Pruritus | 8 (6.2) | 0 (0) | NA | 4 (4.4) | 0 (0) | NA | |
| Hyperpigmentation | 7 (5.4) | NA | NA | 5 (5.5) | NA | NA | |
| Dyspnea | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Palpitations | 3 (2.3) | NA | NA | 0 (0) | NA | NA | |
| Stomatitis | 8 (6.2) | 0 (0) | 0 (0) | 2 (2.2) | 0 (0) | 0 (0) | |
| Peripheral neuropathy | 10 (7.8) | 1 (0.8) | 0 (0) | 1 (1.1) | 0 (0) | 0 (0) | |
| Headache | 1 (0.8) | 0 (0) | NA | 0 (0) | 0 (0) | NA | |
| Dizziness | 2 (1.6) | 0 (0) | NA | 1 (1.1) | 0 (0) | NA | |
Discussion
In this study, we found that PBCT and NPCT achieved similar overall CBR, PFS and OS in our cohort of patients with advanced TNBC. However, in the PBCT group, patients with deleterious gBRCA mutations had higher CBR and longer PFS, and a non-significant tendency to longer OS than those without such mutations. No such differences were observed in the NPCT group. Additionally, we calculated the prevalence of deleterious gBRCA mutations and summarized the types of these mutations.
The prevalence of BRCA1/2 mutation is low level among patients with sporadic cancer and healthy individuals, but is higher in patients with risk factors such as TNBC and positive family history (20,21). In a Chinese cohort, Xie et al. found that the rate of BRCA1/2 gene mutations in breast cancer was 5.3% overall, being the highest at 11.2% in those with triple negative disease (6). Patients with TNBC and a family history of breast and/or ovarian cancer have a higher rate of BRCA gene mutations, approximately 12.7% (20). In our study, the overall rate was 12.3% (14/114) in patients with TNBC and 23.5% (4/17) in patients with a family history of breast or ovarian cancer, these rates are comparable to those reported previously. However, the rate in patients with a family history of breast or ovarian cancer was higher than previously reported, and this discrepancy was possibly attributable to the small sample size.
Over 2,000 different mutations in BRCA1/2 genes have been reported (22). Breast cancer risk varies by type and location of BRCA1/2 mutations (23). Most deleterious mutations lead to truncated proteins that are nonfunctional (22,24). The BRCA1 protein contains a RING domain, a nuclear localization sequence (NLS), a CHK2 phosphorylation site on S988, a coiled-coil domain, and a BRCT domain (23,25). BRCA2 protein contains eight BRC repeats that bind RAD51, the DNA-binding domain that may facilitate BRCA2 binding to both single- and double-stranded DNA, an NLS and a cyclin-dependent kinase (CDK) phosphorylation site that also binds RAD51 (23,25). Mutations in the RING domain (c.72-192), the coiled coil domain (c.3759-3819, c.4191-4272), and the BRCT domain (c.4926-5169, c.5268-5526) of BRCA1 are associated with high risk of breast cancer (23). Additionally, mutations in BRC repeats (c.3006-6255), the DNA-binding domain (c.7437-8001) and OB folds (c.8010-8400, c.9156-9570) have an impact on BRCA2 function (22,23). Mutations in these domains result in homologous recombination deficiency (HRD) and may result in increased sensitivity to platinum-based regimens. In this study, 114 patients had BRCA gene testing by using NGS; the identified deleterious mutations are summarized in Table 9 , Figure 3 . All deleterious mutations were located upstream or in the middle of the domain named above and were either frameshift or nonsense, which result in truncated proteins and loss of normal function.
9. Summary of deleterious gBRCA mutations .
| No. of patients | Gene | Location | Mutation type | AA change |
| gBRCA, germline BRCA1/2. | ||||
| 193 | BRCA1 | c.3329dupA | Frameshift | Q1111Afs*5 |
| 126 | BRCA1 | c.3627dupA | Frameshift | E1210Rfs*9 |
| 105 | BRCA1 | c.3756_3759delGTCT | Frameshift | S1253Rfs*10 |
| 198 | BRCA1 | c.4065_4068delTCAA | Frameshift | N1355Kfs*10 |
| 210 | BRCA1 | c.5470_5477delTGCCCAAT | Frameshift | I1824Dfs* |
| 107 | BRCA1 | c.981_982delAT | Nonsense | C328* |
| 157 | BRCA1 | c.376C>T | Nonsense | Q126* |
| 216 | BRCA1 | c.3841C>T | Nonsense | Q128* |
| 98 | BRCA1 | c.4612C>T | Nonsense | Q1538* |
| 10 | BRCA2 | c.2059_2063delGATTA | Frameshift | D687* |
| 215 | BRCA2 | c.3319C>T | Nonsense | Q1107* |
| 83 | BRCA2 | c.4240delA | Frameshift | T1414Rfs*5 |
| 136 | BRCA2 | c.6288_6289delTA | Frameshift | T2097Vfs* |
| 113 | BRCA2 | c.6628G>T | Nonsense | E2210* |
The prevalence and spectrum of BRCA1 and BRCA2 mutations are heterogeneous in diverse groups of individuals. For example, Ashkenazi Jews are prone to well-described founder mutations in BRCA1 (187delAG and 5385insC) and BRCA2 (6174delT) (22). These founder mutations constitute more than 90% of mutations in Ashkenazi Jews but occur less frequently in other populations (26). One of our 14 patients with deleterious mutations was found to have BRCA1 c.5470_5477delTGCCCAAT and another patient was found to carry BRCA1 c.981_982delAT. These two mutations occur frequently in Chinese individuals, suggesting that they are also potential founder mutations in Chinese population (20). We also detected three deleterious mutations on BRCA2 (c.4240delA, c.6288_6289delTA, and c.6628G>T), which resulted in nonfunctional truncated proteins but were rarely reported.BRCA2 c.4240delA is a deletion of “A” at the 4240th nucleotide of BRCA2 gene which results in a frameshift mutation and premature truncation of the BRCA2 protein. The gene with this mutation can only encode 1,417 amino acids while the wild type one can encode 3,418 amino acids. Similarly, BRCA2 c.6288_6289delTA is a frameshift mutation, resulting in the change of the amino acid 2097 from Thr to Val and consequently a premature truncated protein. This variant is not reported in Clinvar ( http://www.ncbi.nlm.nih.gov/clinvar), 1000 genomes ( http://www.1000genomes.org), NHLBI-ESP 6500 exome project ( http://evs.gs.washington.edu/EVS), and the Exome Aggregation Consortium databases ( http://exac.broadinstitute.org/). We classified it as likely pathogenic according to ACMG Guidelines. With a further review of the patient’s family history of cancer, the patient’s mother had ovarian cancer supporting that this mutation is deleterious. BRCA2 c.6628G>T is a nonsense mutation, leading to a truncated BRCA2 protein at amino acid 2210 (Glu). While this variant is not reported in Clinvar, BIC (Breast Cancer Information Core; http://research.nhgri.nih.gov/bic/), and UMD-BRCA2 (Universal Mutation Database; http://www.umd.be/BRCA2/) databases, it has been reported pathogenic with one case in LOVD database (Leiden Open Variation Database; http://www.lovd.nl) and one case in Japan (27). In our study, the patient also reported a family history of breast and liver cancer. These three mutations may be warranted for further screening to determine if they are specific to Chinese or Asian population.
Multiple genes, including BRCA1/2, ATM, RAD51 and BRIP1, are involved in HR (28); in this study, we mainly studied the BRCA1/2 gene. BRCA gene mutations can lead to HRD, resulting in failure to repair DNA double strand breaks and thus increasing sensitivity to agents aimed at DNA (5,10,29). Although most patients with sporadic TNBC do not have BRCA1 mutations, there is evidence of BRCA1 pathway dysfunction in these tumors (13). Platinum-based regimens are effective treatments for advanced breast cancer and damage DNA by cross-linking with DNA, thereby killing tumor cells (30). Therefore, platinum-based regimens should be more effective in TNBC, especially TNBC with BRCA gene mutations (30,31).
Clinical trials have shown that the use of platinum-based regimens as neoadjuvant chemotherapy can improve the pathologic complete response (pCR) rates of patients with BRCA gene mutations and thus improve the OS (32,33). The CBCSG006 trial in patients with advanced TNBC (16) found that cisplatin plus gemcitabine was superior to paclitaxel plus gemcitabine as first-line therapy (PFS, 7.73 months vs. 6.47 months, P=0.009). However, in our study, we identified no overall difference in efficacy between platinum- and non-platinum-based therapy (CBR, 51.6% vs. 48.1%, P=0.600, median PFS, 6.6 months vs. 6.0 months, P=0.907). However, among patients receiving platinum-based regimens, the CBR and PFS were statistically superior in patients with deleterious gBRCA mutations, namely, 85.7% vs. 35.1%, respectively, P=0.039, and 14.9 months vs. 5.3 months, respectively, P=0.001. Additionally, the TNT trial (17) found that platinum-based regimens achieve a better objective response rate (68% vs. 33.3%, P=0.03) and PFS (6.8 months vs. 4.4 months, P=0.002) in patients with BRCA gene mutations. We found no such difference in the NPCT group, suggesting that patients with advanced TNBC and deleterious gBRCA mutations gain more benefit from platinum-based regimens.
The median PFS of platinum-based regimens tended to be better in patients with liver and chest wall metastases; however, the difference between the PBCT and NPCT groups was not significant (P=0.078) (Figure 2C ). Whether the efficacy of platinum-based regimens is related to metastatic sites and how to screen the patients who will benefit needs further study.
Limited information is available about OS. In the TNT trial (17), OS did not differ significantly between carboplatin and docetaxel either overall or in the BRCA subgroup. The CBCSG006 trial updated their survival data in 2018 (34) and reported identifying no statistical difference in overall OS between the cisplatin plus gemcitabine vs. paclitaxel plus gemcitabine arms, and no significant correlation between gBRCA1/2 status and OS. The median follow-up time of our study was 14.3 (range, 1.7−97.0) months. As the median OS of TNBC after recurrence is about 9 months (35), the follow-up time of our study had covered the median OS in most TNBC patients. In our study, there was no statistically significant difference in OS, although OS tended to be longer in patients who received PBCT and harbored deleterious gBRCA mutations. Given that OS is influenced by many factors, including adverse effects of treatment and subsequent treatment, further investigation in larger cohorts is needed.
In our study, the most frequent adverse event was leucopenia and the PBCT group had significantly more grade 3−4 anemia and thrombocytopenia, which is in concordance with the results of the CBCSG006 trial (16). PPE and peripheral neuropathy occurred more frequently in the non-platinum-based group; these are associated with use of capecitabine.
The main limitation of this study is inherent to its retrospective design. Additionally, some clinical information, such as adverse effects, were missing because they were not documented in the medical records; these data may have influenced the identified associations to some extent.
Conclusions
Platinum-based regimens are more effective in patients with deleterious gBRCA mutations, but no difference in patients without BRCA gene mutations, so non-platinum is an option in patients without BRCA gene mutations considering the toxicity and side effect. And we recommend that patients with advanced TNBC should have BRCA gene test.
Acknowledgements
The authors wish to acknowledge Dr. Shidong Jia (Huidu Shanghai Medical Sciences) for his scientific support and program endorsement.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Foulkes WD, Smith IE, Reis-Filho JS Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 2.Wu N, Zhang J, Zhao J, et al Precision medicine based on tumorigenic signaling pathways for triple-negative breast cancer. Oncol Lett. 2018;16:4984–96. doi: 10.3892/ol.2018.9290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chalakur-Ramireddy NKR, Pakala SB Combined drug therapeutic strategies for the effective treatment of Triple Negative Breast Cancer. Biosci Rep. 2018;38:Pii:BSR20171357. doi: 10.1042/BSR20171357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neophytou C, Boutsikos P, Papageorgis P Molecular mechanisms and emerging therapeutic targets of triple-negative breast cancer metastasis. Front Oncol. 2018;8:31. doi: 10.3389/fonc.2018.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hahnen E, Hauke J, Engel C, et al Germline mutations in triple-negative breast cancer. Breast Care (Basel) 2017;12:15–9. doi: 10.1159/000455999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Meng H, Yao L, et al Germline mutations in cancer susceptibility genes in a large series of unselected breast cancer patients. Clin Cancer Res. 2017;23:6113–9. doi: 10.1158/1078-0432.CCR-16-3227. [DOI] [PubMed] [Google Scholar]
- 7.Godet I, Gilkes DM BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integr Cancer Sci Ther. 2017:4. doi: 10.15761/ICST.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rocha CRR, Silva MM, Quinet A, et al DNA repair pathways and cisplatin resistance: an intimate relationship. Clinics (Sao Paulo) 2018;73:e478s. doi: 10.6061/clinics/2018/e478s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saether NH, Skuja E, Irmejs A, et al Platinum-based neoadjuvant chemotherapy in BRCA1-positive breast cancer: a retrospective cohort analysis and literature review. Hered Cancer Clin Pract. 2018;16:9. doi: 10.1186/s13053-018-0092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Li H, Shao B, et al Identification of recurrent BRCA1 mutation and its clinical relevance in Chinese triple-negative breast cancer cohort. Cancer Med. 2017;6:547–54. doi: 10.1002/cam4.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stevens KN, Vachon CM, Couch FJ Genetic susceptibility to triple-negative breast cancer. Cancer Res. 2013;73:2025–30. doi: 10.1158/0008-5472.CAN-12-1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koshy N, Quispe D, Shi R, et al Cisplatin-gemcitabine therapy in metastatic breast cancer: Improved outcome in triple negative breast cancer patients compared to non-triple negative patients. Breast. 2010;19:246–8. doi: 10.1016/j.breast.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Temian DC, Pop LA, Irimie AI, et al The epigenetics of triple-negative and basal-like breast cancer: current knowledge. J Breast Cancer. 2018;21:233–43. doi: 10.4048/jbc.2018.21.e41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birkbak NJ, Li Y, Pathania S, et al Overexpression of BLM promotes DNA damage and increased sensitivity to platinum salts in triple-negative breast and serous ovarian cancers. Ann Oncol. 2018;29:903–9. doi: 10.1093/annonc/mdy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slyskova J, Sabatella M, Ribeiro-Silva C, et al Base and nucleotide excision repair facilitate resolution of platinum drugs-induced transcription blockage. Nucleic Acids Res. 2018;46:9537–49. doi: 10.1093/nar/gky764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu XC, Zhang J, Xu BH, et al Cisplatin plus gemcitabine versus paclitaxel plus gemcitabine as first-line therapy for metastatic triple-negative breast cancer (CBCSG006): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2015;16:436–46. doi: 10.1016/S1470-2045(15)70064-1. [DOI] [PubMed] [Google Scholar]
- 17.Tutt A, Tovey H, Cheang MCU, et al Carboplatin in BRCA1/2-mutated and triple-negative breast cancer BRCAness subgroups: the TNT Trial. Nat Med. 2018;24:628–37. doi: 10.1038/s41591-018-0009-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen X, Wang J, Fu Z, et al Curcumin activates DNA repair pathway in bone marrow to improve carboplatin-induced myelosuppression. Sci Rep. 2017;7:17724. doi: 10.1038/s41598-017-16436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Richards S, Aziz N, Bale S, et al Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lang GT, Shi JX, Hu X, et al The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: Screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer. 2017;141:129–42. doi: 10.1002/ijc.30692. [DOI] [PubMed] [Google Scholar]
- 21.National Health Commission of The People’s Republic of China Chinese guidelines for diagnosis and treatment of breast cancer 2018 (English version) Chin J Cancer Res. 2019;31:259–77. doi: 10.21147/j.issn.1000-9604.2019.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karami F, Mehdipour P A comprehensive focus on global spectrum of BRCA1 and BRCA2 mutations in breast cancer. Biomed Res Int. 2013;2013:928562. doi: 10.1155/2013/928562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rebbeck TR, Mitra N, Wan F, et al Association of type and location of BRCA1 and BRCA2 mutations with risk of breast and ovarian cancer. JAMA. 2015;313:1347–61. doi: 10.1001/jama.2014.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S, Parmigiani G Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25:1329–33. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roy R, Chun J, Powell SN BRCA1 and BRCA2: different roles in a common pathway of genome protection. Nat Rev Cancer. 2011;12:68–78. doi: 10.1038/nrc3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yurgelun MB, Elaine H, Garber JE Population-wide screening for germline BRCA1 and BRCA2 mutations: too much of a good thing? J Clin Oncol. 2015;33:3092–5. doi: 10.1200/JCO.2015.60.8596. [DOI] [PubMed] [Google Scholar]
- 27.Arai M, Yokoyama S, Watanabe C, et al Genetic and clinical characteristics in Japanese hereditary breast and ovarian cancer: first report after establishment of HBOC registration system in Japan. J Hum Genet. 2018;63:447–57. doi: 10.1038/s10038-017-0355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Foulkes WD, Shuen AY In brief: BRCA1 and BRCA2. J Pathol. 2013;230:347–9. doi: 10.1002/path.4205. [DOI] [PubMed] [Google Scholar]
- 29.Schneider BP, Winer EP, Foulkes WD, et al Triple-negative breast cancer: risk factors to potential targets. Clin Cancer Res. 2008;14:8010–8. doi: 10.1158/1078-0432.CCR-08-1208. [DOI] [PubMed] [Google Scholar]
- 30.Sparano JA Defining a role and predicting benefit from platinum-based therapy in breast cancer: an evolving story. J Clin Oncol. 2015;33:1–3. doi: 10.1200/JCO.2014.57.7890. [DOI] [PubMed] [Google Scholar]
- 31.Narod SA BRCA mutations in the management of breast cancer: the state of the art. Nat Rev Clin Oncol. 2010;7:702–7. doi: 10.1038/nrclinonc.2010.166. [DOI] [PubMed] [Google Scholar]
- 32.Byrski T, Gronwald J, Huzarski T, et al Pathologic complete response rates in young women with BRCA1-positive breast cancers after neoadjuvant chemotherapy. J Clin Oncol. 2010;28:375–9. doi: 10.1200/JCO.2008.20.7019. [DOI] [PubMed] [Google Scholar]
- 33.Cortazar P, Zhang L, Untch M, et al Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 34.Zhang J, Lin Y, Sun XJ, et al Biomarker assessment of the CBCSG006 trial: a randomized phase III trial of cisplatin plus gemcitabine compared with paclitaxel plus gemcitabine as first-line therapy for patients with metastatic triple-negative breast cancer. Ann Oncol. 2018;29:1741–7. doi: 10.1093/annonc/mdy209. [DOI] [PubMed] [Google Scholar]
- 35.Dent R, Trudeau M, Pritchard KI, et al Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–34. doi: 10.1158/1078-0432.ccr-06-3045. [DOI] [PubMed] [Google Scholar]